Abstract
It is well established that the cerebral cortex undergoes extensive remodeling in aging. In this study, we used behaviorally characterized rats to correlate age-related morphological changes with cognitive impairment. For this, young and aged animals were tested in the Morris water maze to evaluate their cognitive performance. Following behavioral characterization, the animals were perfused and a combination of intracellular labeling and immunohistochemistry was applied. Using this approach, we characterized the dendritic morphology of cortical pyramidal neurons as well as the pattern of glutamatergic and GABAergic appositions on their cell bodies and dendrites. We focused on the association region of the parietal cortex (LtPA) and the medial prefrontal cortex (mPFC) for their involvement in the Morris water maze task. We found an age-related atrophy of distal basal dendrites that did not differ between aged cognitively unimpaired (AU) and aged cognitively impaired animals (AI). Dendritic spines and glutamatergic appositions generally decreased from young to AU and from AU to AI rats. On the other hand, GABAergic appositions only showed a trend towards a decrease in AU rats. Collectively, the data show that the ratio of excitatory/inhibitory inputs was only altered in AI animals. When cortical cholinergic varicosities were labeled on alternate sections, we found that AI animals also had a significant reduction of cortical cholinergic boutons compared to AU or young animals. In aged animals, the density of cortical cholinergic varicosities correlated with the excitatory/inhibitory ratio. Our data suggests that both cholinergic atrophy and an imbalance towards inhibition may contribute to the observed age-associated behavioral impairment.
Keywords: intracellular labeling, immunocytochemistry, GABA, glutamate, image analysis, cognitive decline
Introduction
The cerebral cortex has long been known to be involved in cognitive processing. There is extensive evidence that shows a fine balance between excitatory and inhibitory currents is crucial for information processing in the cerebral cortex (Hausser and Clark, 1997; Le et al., 2008; Miura et al., 2007). This excitatory and inhibitory synaptic transmission is altered by numerous neuromodulators (e.g. acetylcholine) that have been shown to be important for proper cognitive function (Gu, 2002; Sarter and Bruno, 1997). Normal aging has detrimental effects on cognition and is associated with cortical remodeling (for review see (Dickstein et al., 2007; Morrison and Hof, 2002). Contrary to what was believed previously, normal aging is not associated with extensive cell loss in the rat (Merrill et al., 2001; Pugnaloni et al., 1998) or human (Haug and Eggers, 1991; Terry et al., 1987) cortex. However there is abundant evidence in the literature showing neuronal atrophy and synaptic loss in humans, primates and rodents (Duan et al., 2003; Jacobs et al., 2001; Uylings and de Brabander, 2002; Wong et al., 2000). In experimental animals, a widely used and accepted paradigm to evaluate cognitive function is the Morris water maze (MWM) which assesses spatial learning and memory (Brandeis et al., 1989; McNamara and Skelton, 1993; Morris, 1984). Lesion studies have demonstrated that the parietal and prefrontal cortices play a crucial role in spatial memory formation (Gottlieb, 2002; Kolb et al., 1997; Ragozzino et al., 1998; Sutherland et al., 1988). As the water maze task depends on the function of both the hippocampus and the cerebral cortex, the systems consolidation model of memory formation states that memories are first encoded by the hippocampus and then consolidated in the cerebral cortex (Maviel et al., 2004). Consistently, lesions or inactivation of the mPFC impair memory consolidation in the hours following a single day of water maze training (Kraemer et al., 1996; Leon et al., 2010). As well, lesions affecting the parietal cortex significantly impair the retention of the platform location following training (Elliott et al., 1989). Aged rats show altered performances in the MWM, accompanied by an age-related atrophy of pyramidal neurons as well as synaptic loss in the above-mentioned cortical regions (Majdi et al., 2007; Markham and Juraska, 2002; Wong et al., 1998a; Wong et al., 1998b). By means of whole cell patch clamp recordings on acute cortical slices from behaviorally characterized young and aged animals, it has recently been shown that pyramidal neurons from lamina V of the parietal cortex of aged cognitively impaired animals had an altered ratio of mEPSC and mIPSC (Wong et al., 2006a). More precisely, aged cognitively unimpaired rats had a decrease in the frequency of both excitatory and inhibitory mPSC. On the other hand, aged cognitively impaired rats had decreased mEPSCs while the frequency of mIPSCs was comparable to that of young animals. According to quantal analysis, since the frequencies were altered but the amplitudes of the currents were unchanged, the data suggests alterations in synaptic numbers as opposed to changes in receptor levels. Alternatively, differences in the average release probability of apposing presynaptic boutons could also lead to the observed physiological changes. A detailed morphological analysis of excitatory and inhibitory appositions on pyramidal neurons can provide valuable information regarding the mechanisms driving the imbalance towards inhibition. In our lab, we have been using an approach that allows the simultaneous visualization of dendrites from intracellularly-labeled cortical pyramidal neurons and the appositions on them from presynaptic boutons of specific neurotransmitter systems. By exploiting this combination of immunocytochemistry and intracellular fillings on fixed tissue, we were able to acquire morphological data from a higher number of animals in many cortical regions. Our objective was to quantify the age-related dendritic atrophy and to verify if it correlated with cognitive loss.
Moreover, we investigated whether there was a change in the excitatory and inhibitory appositions on morphologically characterized cortical pyramidal neurons that would explain the previously described imbalance towards inhibition. The advantage to our approach is that we can sacrifice all the animals at the same time following behavioral characterization and, as the tissue is fixed, we have sufficient time to analyze morphological parameters from many neurons across cortical regions. We were mainly interested in the association region of the parietal cortex (LtPA) and the medial prefrontal cortex (mPFC) as both structures have been shown to be involved in the cognitive processing associated with performing the MWM (Kolb et al., 1997; Sutherland et al., 1982) or during memory consolidation and recall (Leon et al., 2009). We were specifically interested in the morphological and synaptic analysis of cortical pyramidal neurons since they are the main output system of the cortex (Ramón y Cajal, 1909; Rockland and Pandya, 1979) and their age-related morphological and physiological changes are better documented than interneurons. We also wanted to discriminate between pyramidal neurons from lamina V and lamina III as their circuitry and connectivity are different (Bannister, 2005; Callaway, 2002). Furthermore, since different dendritic compartments have a different physiology and receive different inputs (Bannister, 2005; Berghuis et al., 2004a; Thomson and Deuchars, 1997), we compared synaptic densities on proximal and distal basal dendrites as well as on proximal and distal apical dendrites. For example, a combination of physiological recordings, intracellular labeling and immunocytochemistry has demonstrated that the perisomatic region of pyramidal neurons has the highest density of inhibitory appositions and that most of those synapses are made by fast spiking, paravalbumin positive interneurons. Conversely, inhibitory synapses made on more distal dendrites generally come from adapting, cholecystokinin positive interneurons (Berghuis et al., 2004a).
Materials and methods
Twelve young (6 months old) and 93 aged (24 months old) animals were used for the behavioral characterization. All animals were male Fischer 344 rats obtained from the National Institute for Aging (NIH). For this study, brains from six young and twelve aged animals were used. All procedures were approved by the McGill University Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care and of the NIH.
Behavioral testing
Young and aged rats were tested using a modified version of the Morris water maze. In short, the water maze consists of a circular pool filled with water made opaque with a non-toxic colorant and is kept at 26°C. To reduce stress associated with the water maze, the animals were habituated to the pool by swimming for 30 seconds in the absence of the platform. On the following day, a platform was hidden two centimeters below the surface and remained at the same location throughout experimental days. Visual cues were placed on the walls of the experimental room and the rats were put in the pool three times per day for five consecutive days. The animals were given 120 seconds to find the hidden platform on which they remained for 10 seconds before they were taken out of the pool, dried and put back in their cages. A video camera tracking system was used to record the swim path (HVS Image, Buckingham, UK). The time required for each rat to reach the platform, defined as latency, the distance swam during each trial and the average swim speed were measured. On the sixth experimental day, to control for visual, locomotor and motivational impairments, the platform was made visible by raising it above the water and rats with longer escape latencies (significantly different from the average latency of the young group) were excluded from the study. For the mentioned behavioral experiment, a total of 4 animals were excluded on day 6. Aged animals showing latencies greater than two standard deviations relative to young rats, during testing days 3-5, were considered as aged cognitively impaired (AI). Aged animals showing latencies within one standard deviation of young rats were considered as aged cognitively unimpaired (AU). Aged rats displaying an intermediate latency between one standard deviation and two standard deviations were eliminated from the study to better differentiate between the AI and AU populations. Since no group showed significantly different swim speed, the escape latencies were proportional to the swim path lengths. The results of this behavioral testing are published elsewhere (Majdi et al., 2009).
Perfusions
Following behavioral testing, the animals were deeply anaesthetized with Equithesin (6.5 mg chloral hydrate and 3 mg sodium pentobarbital in a volume of 0.3 ml, i.p., per 100 g body weight) and perfused through the left ventricle with cold saline. The brains were removed, cut in small blocks and fixed overnight at 4°C in 0.1M phosphate buffer (PB) solution containing 4% formaldehyde obtained from paraformaldehyde (pH 7.4).
Intracellular injections
Following fixation, thick tissue slices (250 μm) were cut with a Vibratome and kept in PBS. Sections containing the mPFC and the LtPA regions of the cerebral cortex were placed in an iontophoretic chamber mounted on a rig equipped with a fluorescence microscope, a micromanipulator and a current generator. A sharp microelectrode was used to inject Lucifer yellow (LY; di-lithium salt, Sigma, dissolved at 8% in 0.1M Tris buffer pH 7.4) into neurons of lamina V and lamina III of the selected cortical regions. Briefly, the sharp electrode was inserted in the cell body of the neuron and a continuous current of 5-10 nA was applied until the tip of the dendrites appeared bright when observed with fluorescence microscopy. Ten to 20 neurons were injected in both lamina V and lamina III per section, and four sections were used for each cortical region per animal. The remaining thick sections were cryoprotected and stored at −20°C in a solution containing 37.5% ethylene glycol and 37.5% sucrose in PBS.
Lucifer Yellow immunocytochemistry and cell reconstruction
Following intracellular injections, the LY fluorescence was bleached by exposure to a bright light. The cortical slices were incubated with a Rabbit anti-Lucifer yellow polyclonal antibody (generous donation of Dr. De Felipe, Instituto Cajal, Madrid, Spain) diluted 1:400 000 in stock solution (2% bovine serum albumin; Sigma, St. Louis), 1% Triton X-100 (Fisher, Ottawa) and 5% sucrose in 0.1 M phosphate buffer for 48 hours. The sections were washed with PBS and incubated with a rhodamine red X conjugated donkey anti rabbit secondary antibody (1:200, Jackson; Medicorp, Montreal) in PBS with 5% normal donkey serum (NDS) (Jackson Immunoresearch; sold by Medicorp, Montreal) for 2 hours. The sections were washed with PBS and temporarily mounted on a glass slide and coverslipped with PBS. The neurons were reconstructed by confocal microscopy with the appropriate laser settings for the rhodamine red X fluorochrome. Z stacks were acquired with a 40x water immersion objective (Zeiss Achroplan, NA 0.80). The neurons with the most dendrites in the plane of sectioning were imaged. Five neurons were selected and reconstructed in both lamina III and lamina V for each section on which intracellular injections were performed. The experimenter was blinded to the animal group from which the cells came, and selected the neurons to be reconstructed based on the quality of the fill (ie bright dendritic ends) and their orientation in the plane of section.
Immunocytochemical labeling of glutamatergic and GABAergic presynaptic elements
Following imaging for dendritic arborizations, the thick sections were put in a solution of 30% sucrose in 0.1M PB at 4°C overnight. The thick sections were then re-sectioned at 20 μm with a cryostat. Free floating sections were recovered in PBS, blocked for 1 hour in 10% NDS in PBS+T. The sections were then incubated overnight at 4°C with either a mouse monoclonal anti-VGluT1 (1:100, Medi Mabs, Montreal) the characterization of which is published in (Bell et al., 2006) or a mouse monoclonal anti-GAD 65 (1:1000, Millipore, Billerica) in 5% NDS in PBS+T. On the following day, the sections were washed with PBS and incubated with an Alexa Fluor 647 conjugated donkey anti-mouse IgG secondary antibody (1:800, Invitrogen, Burlington) for 2 hours. Following this incubation, the sections were washed in PBS, mounted on gelatin subbed slides, air dried and coverslipped with Aqua Polymount (Polysciences; Warrington, PA).
Imaging of synaptic appositions
The synaptic appositions were imaged using a Zeiss LSM 510 confocal microscope (Zeiss Canada), equipped with two different Helium-Neon (HeNe) lasers. A 63x oil immersion objective (Zeiss Panapo; NA 1.40) was used with a multi-track scanning method to completely separate the detection of the Alexa 647 and Rhodamine Red-X signals. This method allows complete separation of settings regarding lasers, filter combinations and detection thresholds for each channel. Z stacks were acquired with a small pinhole (0.73μm; optical section < 0.3μm) in order to estimate as accurately as possible the number of appositions of GAD65 or VGluT1 immunopositive (IR) presynaptic elements on intracellularely filled post-synaptic structures. The dendritic segments were selected, by an experimenter blinded to their animal group of origin, based on the quality of the fill (brightness of the LY signal) and the intensity of the immunolabeling for presynaptic elements around the segment.
Immunocytochemical labeling of cholinergic presynaptic elements
Following the more time sensitive intracellular injections and cell reconstructions, the remaining thick sections that were stored at −20°C and contained the ACC or the LtPA region were immersed in a solution of 30% sucrose in 0.1M PB for 1 hour to wash out the ethylene glycol from the storage solution. Following this, the thick sections were re-sectioned at 20μm with a cryostat and collected in PBS. To reduce background, tissues were incubated with 0.1% hydrogen peroxide in PBS for 20 minutes. The sections were washed with PBS and blocked in a solution of 10% normal rabbit serum (NRS) in PBS+T for 1 hour. The sections were then incubated overnight at 4°C in a solution containing a polyclonal goat anti-VAChT antibody (1:20 000, Millipore, Billerica) and 5% NRS in PBS+T. The sections were washed with PBS, and incubated for 2 hours in a biotinylated rabbit anti-goat secondary antibody (1:200, Vector) with 5% NRS in PBS. The sections were washed with PBS and incubated for 1 hour with the A and B reagents (4μl of A + 4μl of B per milliliter of PBS, ABC Vectastain Elite kit). The sections were washed in PBS and incubated for 5 minutes in a solution of 3-3′diaminobenzidine (Sigma, 5mg for 10ml of PBS). Hydrogen peroxide was then added to the solution to reach a final concentration of 0.01%. The reaction was terminated after 10 minutes by the addition of PBS. The sections were washed in PBS, mounted on gelatin-subbed slides, air dried, dehydrated in ascending alcohols and xylene, then coverslipped with Entellan (EMD, Gibbstown). VAChT-IR boutons were imaged by bright field microscopy using a Zeiss Axioplan 2 imaging microscope equipped with a 40x Plan-Fluotar oil-immersion objective. Images were acquired with a high-resolution colour digital camera using the Zeiss Axiovision software. Five pictures were taken in both lamina V and lamina III for each section. Four sections were imaged for both the ACC and LtPA cortical regions.
Quantification and data analysis
For dendritic length analysis, branching points were located on a 3D representation of the neuron made by the LSM software. Total dendritic lengths for basal dendrites of each branching order were measured on a 2D projection image (in TIFF format) with an MCID Elite image analysis software (Imaging Research Inc., St. Catharines, ON, Canada). All imaged neurons were quantified, the data was pooled for each animal and variations between groups were analyzed with a one way ANOVA followed by a Tukey’s post-hoc test.
For synaptic appositions, the confocal z stacks were opened with the Metamorph software (version 7.0; Molecular Devices, Sunnyvale, CA) using the 3D viewer function. Both channels (corresponding to LY and VGluT or GAD65) were thresholded and an isosurface shell was used. Dendritic segments were selected, their lengths were measured and the spines and synaptic appositions were manually counted by a blinded experimenter. To quantify the synaptic appositions on the cell body, a single optical slice (thinner than 0.7μm) that contained the largest cross sectional area of the soma was selected. The perimeter was measured and the appositions were counted. For both glutamatergic and GABAergic appositions, an average of 3 cell bodies and 10 dendritic segments per compartment (basal-proximal, basal-distal, apical-proximal, and apical-distal) were quantified for both lamina V and lamina III of the ACC and the LtPA1 regions. The data for each compartment and each neurotransmitter system was grouped per animal. The differences between groups were analyzed with a one way ANOVA followed by a Tukey’s post-hoc test.
For cholinergic presynaptic bouton counts, the micrographs were exported as TIFF files and analyzed with the M5 MCID image analysis software. The quantification procedure is explained in greater detail elsewhere (Wong et al., 1998a). All pictures for each region studied were pooled for each animal, and the differences between groups were analyzed with a one way ANOVA followed by a Tukey’s post-hoc test.
Results
Pyramidal cell morphology
The quantification of total dendritic lengths for each branching order revealed that aging is accompanied by a preferential reduction of the most distal dendritic compartments (Figures 1 and 2). However, of the cortical regions analyzed, these changes reached statistical significance only in lamina V of the LtPA (Figure 2A) and lamina III of the mPFC (Figure 2B). More precisely, there was a statistically significant reduction in the total lengths of quaternary order dendrites in both AU and AI rats when compared to young (Y) (p<0.05), but there was no difference when AU rats were compared to AI (Figure 2).
Figure 1. Analysis of basal dendritic lengths according to branching order.
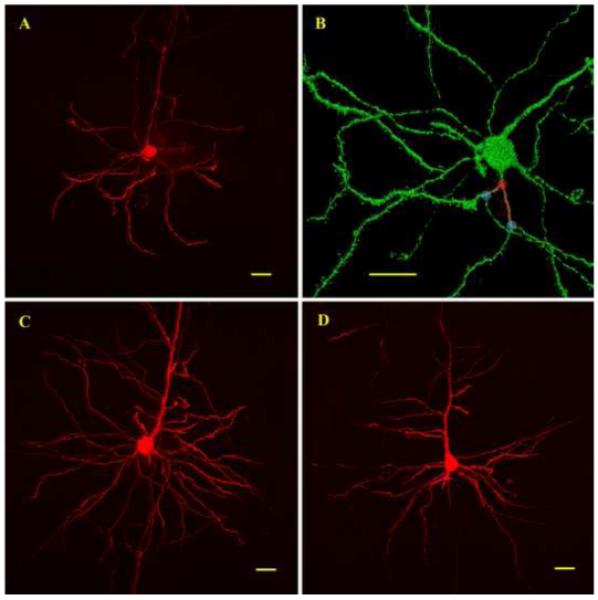
(A) 2D projection of a Lucifer yellow filled cortical pyramidal neuron imaged by confocal microscopy. (B) Same neuron as in (A) visualized with the 3D viewer of the Metamorph 7.0 software. Branching points were located on a 3D representation of the neuron. Red circles indicate examples of branching points separating primary order dendrites from secondary order dendrites. Blue circles indicate examples of branching points separating secondary order dendrites from tertiary order dendrites. Once the branching points were located, total dendritic lengths were measured for each branching order on a 2D projection. (C) Confocal image showing a young neuron with a complex basal dendritic tree. (D) Confocal image showing an aged neuron with less distal dendritic surface. Scale bars = 20μm.
Figure 2. Quantification of basal dendritic lengths.
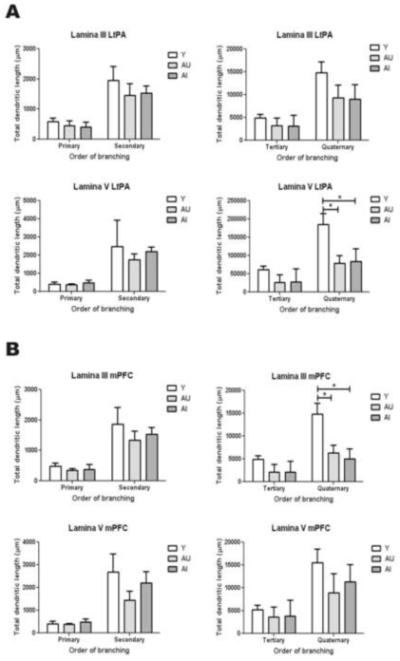
Total dendritic lengths were quantified for each branching order using the MCID system. (A) Quantification of dendritic lengths of cortical pyramidal neurons of the LtPA. Note that a significant age-related attrition was observed on quaternary order basal dendrites from neurons having their cell body in LV. There was no observable difference between AU and AI. (B) Quantification of dendritic lengths of cortical pyramidal neurons from the mPFC. Note that a significant age-related attrition was observed on quaternary order basal dendrites from neurons having their cell body in LIII but not LV. There was no detectable difference between AU and AI. * = p< 0.05
Changes in synaptic patterns
In order to investigate changes in synaptic patterns in the three experimental groups, we quantified the density of dendritic spines as well as the density of excitatory and inhibitory appositions on the cell body, proximal basal, distal basal, proximal apical and distal apical dendrites of pyramidal neurons in LV and LII/III of the LtPA and mPFC (Figures 3-5). In these experiments, we considered primary and secondary order dendrites as proximal; tertiary and quaternary order were considered as distal dendrites. Statistically significant changes were only found on the distal basal dendrites (Figures 3C and 5).
Figure 3. Quantification of spine densities on dendrites from cortical pyramidal neurons.

(A) Confocal image showing a pyramidal neuron filled with Lucifer Yellow observed with the 63x objective and a digital zoom of 2x. Note that dendritic spines are visible under this magnification. (B) 3D reconstruction of the region framed in A made with the Metamorph 7.0 software. (C) Quantification of spine densities on distal basal dendrites. The data was analyzed with a one way ANOVA. Differences between groups were analyzed with a Tukey’s post hoc test. * = p< 0.05
Figure 5. Quantification of synaptic appositions on distal basal dendrites.

The density of excitatory (VGluT1-IR) and inhibitory (GAD65-IR) appositions were measured and ratios of excitatory / inhibitory (E/I) appositions were calculated. Note that excitatory appositions tend to decrease from Y to AI as inhibitory appositions tend to decrease only in AU as densities measured in AI are comparable to Y. Altered ratios of E/I were found in LV of the LtPA and in both LIII and LV of the mPFC. * = p< 0.05.
Changes in dendritic spines
We quantified the densities of dendritic spines on proximal (primary and secondary) and distal (tertiary and quaternary) dendrites of pyramidal neurons. Although overall there was a trend for a reduction in spine densities when comparing Y and AI animals, this difference only reached statistical significance in layer V of the LtPA (Figure 3C). Differences between AU and AI did not attain significance in any of the cortical areas or branching orders studied (Figure 3C).
Changes in excitatory VGluT1 immunoreactive appositions
There was a trend towards a decrease in the density of VGluT1-IR appositions (mostly on dendritic spines - Figure 4A-C and 5) when comparing Y and AI animals. Significant differences between Y and AI were only detected on distal basal dendrites in lamina V of the LtPA and mPFC. Significant differences between Y and AU were found on distal basal dendrites in lamina V of the LtPA (Figure 5). No statistically significant differences between AU and AI were detected (Figure 5). As was observed for dendritic spines, the density of VGluT1-IR appositions was consistently higher on distal dendrites than on proximal dendrites. VGluT1-IR appositions were very sparse on the cell body.
Figure 4. Confocal analysis of synaptic appositions in a neurotransmitter specific manner.
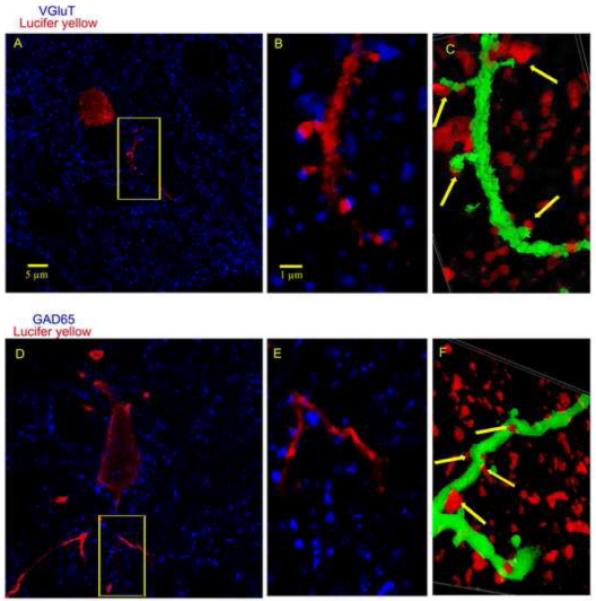
(A) Confocal image showing Lucifer Yellow and VGluT1 immunoreactivity. (B) Enlargement of the framed area in (A). Note that VGluT1-IR boutons were sometimes found in close proximity to LY-IR dendrites. (C) 3D reconstruction of the double labeling observed in (B). Arrows indicate putative glutamatergic appositions. Note that most of the appositions were on dendritic spines. (D) Confocal image showing Lucifer Yellow and GAD65 immunoreactivities. (E) Enlargement of the framed area in (D). Note that GAD65-IR boutons were sometimes found in close proximity to dendrites. (F) 3D reconstruction of the double labeling seen in (E). Arrows indicate putative GABAergic appositions.
Changes in inhibitory GAD 65 immunoreactive appositions
As expected, GAD 65-IR appositions occurred on dendritic shafts, not spines (Figure 4D-E). As opposed to dendritic spine and VGluT1-IR apposition densities, the density of GAD 65-IR appositions did not decrease from Y to AI. Instead, there was a constant trend where the density of GAD 65-IR appositions was lower in AU than in Y whereas the density in AI was generally comparable to Y (Figure 5). However, none of these differences attained significance. Since the trend was different between excitatory and inhibitory appositions, we calculated the ratios of excitatory/inhibitory (E/I) appositions and compared them between groups. Importantly, we found that the ratios were significantly altered favoring inhibitory appositions only in AI animals in lamina V of the LtPA as well as in laminae V and II/III of the mPFC (Figure 5). The ratios were not significantly altered in the other cortical areas studied (data not shown).
Changes in cortical cholinergic bouton counts
We quantified the densities of cortical cholinergic boutons (Figure 6), consistent with a previous study investigating changes in the overall cortical neuropile (Bruno et al., 2004), and found significantly decreased bouton counts only in aged-impaired animals (Figure 7). Of the regions studied, statistically significant reductions in AI animals were observed in both LV and LIII of the LtPA and in LIII of the mPFC (Figure 7). Interestingly, in cortical regions where both a significant cholinergic atrophy and an imbalance towards inhibition was observed in aged animals, the E/I ratio positively correlated with the cholinergic bouton density (Figure 8).
Figure 6. Analysis of cortical cholinergic boutons.

(A) Example of a micrograph showing VAChT immunoreactivity in the rat cortex. (B) Same image as in (A) following the ‘target accent’ transformation made by the MCID image analysis software, to facilitate subsequent quantification. (C) Same image as in (A) and (B) following quantification. Purple dots represent elements that meet the brightness threshold and size criteria set with the MCID software. The puncta were counted by the software to measure the bouton density, with segmentation limits set up to eliminate the counting of cut, non-varicose axons. (D) VAChT immunoreactivity in LV of the LtPA of a young animal. (E) VAChT immunoreactivity in the cortex of a AU animal. Note the highly conserved number of VAChT-IR boutons. (F) VAChT immunoreactivity in an AI animal. Note the apparent reduction in VAChT-IR boutons compared to Y and AU.
Figure 7. Quantification of cholinergic bouton densities.

VAChT-IR boutons were counted in the LtPA and mPFC. Note that in LIII and LV of the LtPA cortex, there was a significant decrease of cholinergic boutons in AI when compared to Y or AU (p< 0.05). In LIII of the mPFC cortex, AI animals had significantly less boutons than AU (p< 0.05).
Figure 8. Relationship between cholinergic loss and the imbalance towards inhibition.

In LV of the LtPA and in LIII of the mPFC of AI, we observed both an imbalance towards inhibition on distal basal dendrites and an overall cholinergic loss. When E/I ratios were plotted against cholinergic bouton counts, we found a significant positive correlation between the 2 factors (p< 0.001 in the LtPA and p< 0.01 in the mPFC).
Discussion
The present study investigated for the first time the changes in immunocytochemically characterized input to intracellularly labeled neocortical pyramidal neurons in aged-impaired compared to aged-unimpaired and young rats. Our findings show a change in the ratio of inputs favoring inhibition in specific layers of two cortical areas.
Changes in dendritic lengths
Due to technical limitations, we were restricted to quantifying dendritic lengths in the basal dendritic compartment. When cutting the brains with the Vibratome, the level at which the cutting plane was parallel to the normal alignment of the pyramidal neurons varied from animal to animal. Given that apical dendrites travel a long distance through several cortical laminae, the changes in total apical dendritic lengths were mostly representative of the angle at which the brains were sectioned as opposed to biological variations between animals. For a similar reason, in the basal compartment, we quantified branching orders from 1 to 4 because we found that the higher orders varied according to the plane of sectioning as well. We report an age-related attrition of quaternary order basal dendrites from neurons found in LV of the LtPA and LIII of the mPFC. No other significant changes were observed between AU and AI (as shown in Figure 2). The age-related atrophy of cortical pyramidal neurons in humans and rats has been reported elsewhere (de Brabander et al., 1998; Jacobs et al., 1997; Jacobs et al., 2001; Vaughan, 1977; Wong et al., 2000). Interestingly, the current study, using data from behaviorally characterized animals, suggests that this attrition is not the main factor contributing to cognitive impairment. It should be kept in mind that pyramidal neurons from AU and AI could still have different features in the more distal compartments or on apical dendrites which escaped this study because of technical limitations.
The mechanisms leading to dendritic atrophy are still unclear. It is interesting to note that, in our data sets, we could only detect significant changes in those cortical areas that had the most pronounced loss of presynaptic elements. In one of our recent publications,, we also demonstrate a more prominent loss of synapses in LV of the LtPA cortex and LIII of the mPFC (Majdi et al., 2007), although the study only looked at overall synaptic populations in the neuropile without considering inputs to pyramidal neurons. It is possible that the loss of presynaptic elements could lead to the loss of post-synaptic dendritic elements. There is some evidence that pyramidal neurons depend on BDNF for trophism and phenotypic conservation (Horch and Katz, 2002; McAllister et al., 1996; McAllister et al., 1995).,Whether the trophic/survival effects of BDNF on dendritic morphology are mediated via direct effects on the post-synaptic neuron, or by its effects on the apposing synapses that can be potentiated by BDNF (Kang and Schuman, 1995; Korte et al., 1995; Patterson et al., 1992), remain to be elucidated (McAllister et al., 1999). More precisely, glutamatergic synaptic activity leads to the entry of calcium through NMDA and some subtypes of AMPA receptors (Burnashev, 1996; Isaac et al., 2007). Intracellular calcium then leads to calcium-dependant kinase activation, such as CAMK and MAPK that promote survival and growth (Redmond, 2008). Activation of metabotropic glutamate receptors (mGluR) could also be trophic for pyramidal neurons (Balazs, 2006). A recent review by Mattson discusses how the glutamatergic system is involved in the maintenance of central neurons (Mattson, 2008). Following this hypothesis, the fact that we did not see significant differences in the density of glutamatergic appositions on distal basal dendrites from AU and AI could explain why there was no difference in total dendritic length between these two groups.
Changes in dendritic spines
Even if we did not report significant differences in spine density between AU and AI, it should be kept in mind that we did not account for spine morphology in our analysis. Interestingly, a recent publication has found a correlation between the head volume of thin spines and cognitive scores in monkeys (Dumitriu et al., 2010). Therefore, it is possible that dendritic spines from AU and AI may differ morphologically
Changes in glutamatergic appositions
We observed a generalized trend where glutamatergic appositions on distal basal dendrites were higher in Y, decreased slightly in AU and decreased further in AI (see Figure 5). It is thought that activity at the synapse is necessary for its maintenance via the entry of calcium in the presynaptic bouton (Gomez and Zheng, 2006; Spira et al., 2001) and the activity-dependant release of BDNF by the post-synaptic cell (Lykissas et al., 2007). It was reported that large lamina V cortical pyramidal neurons from aged animals have a significant decrease in the frequency of miniature excitatory post synaptic currents but increased action potential-dependent synaptic inputs (Wong et al., 2000). In the study from Wong et al. (2000), the decreased frequency of miniature events also suggests an anatomical loss of excitatory appositions. The increase of excitatory post synaptic potentials in the absence of tetrodotoxin, suggests that the remaining appositions are more active. Based on this physiological data, coupled with our finding that some dendritic compartments did not lose excitatory appositions, it is likely that there is an age associated remodeling of excitatory circuits where some become less active and atrophic, as others compensate and become more active. As well, some studies have reported changes in axonal transport in aging (Niewiadomska et al., 2005); reviewed in (Galbraith and Gallant, 2000). Therefore, a failure in proper retrograde transport of BDNF could also lead to a reduction in pre-synaptic glutamatergic boutons.
Changes in GABAergic appositions
Following quantification of inhibitory appositions on cortical pyramidal neurons, we found that the density of appositions also changed on distal basal dendrites of neurons having their cell body in LV of the LtPA or in LV or II/III of the mPFC. The observed trends were different from those in the glutamatergic system, as shown in Figure 5. In most cases, the density of inhibitory appositions was decreased only in AU when compared to Y as AI had levels which were similar to Y. When ratios of E/I appositions on distal basal dendrites were calculated, we found that the ratios were conserved when AU rats were compared to Y. On the other hand, in some of the areas studied, the ratios were significantly decreased when AI rats were compared to Y or AU rats (Figure 5). This data, suggesting an imbalance towards inhibition, supports the previously described changes in miniature post synaptic currents seen in aged rats that were impaired at the Morris water maze (Wong et al., 2006b). Such an imbalance towards inhibition can be detrimental to proper cognitive function as the ability to induce LTP can be reduced by GABAB and GABAA receptor-mediated mechanisms (McDonnell et al., 2007; Steele and Mauk, 1999). As well, pharmacological potentiation of inhibitory currents was found to significantly impair spatial learning and memory retrieval (Deng et al., 2009; Jo et al., 2007; Wang and Cai, 2008). It is interesting to note that the aged cognitively impaired Fischer 344 rats, as measured by olfactory discrimination learning, had their behavioral impairment reversed with a GABAB antagonist (Lasarge et al., 2009). The data from this pharmacological manipulation supports the idea that the imbalance towards inhibition in AI animals is not just correlative but participates in the observed cognitive impairment. The fact that a particular cortical environment in AI animals leads to a more pronounced degeneration of one system and a sparing of another might appear counter intuitive. In the following part of the discussion, we will focus on potential mechanisms that could explain a selective degeneration of the excitatory glutamatergic system.
The relationship between neurotrophins and the excitatory and inhibitory systems
It has been demonstrated that, in culture systems and in adult tissue, BDNF and NT3 exert trophic effects on the glutamatergic system (for review see (Lessmann, 1998). On the other hand, the effect of these particular neurotrophins on mature inhibitory neurons has not been well described. Some data suggests a trophic role of these neurotrophins on inhibitory synapses (Baldelli et al., 2002; Berghuis et al., 2004b; Marty et al., 2000; Ohba et al., 2005; Seil and Drake-Baumann, 2000a; Seil and Drake-Baumann, 2000b), but the evidence is mostly derived from in vitro experiments or developmental studies. Interestingly, the few papers investigating the role of BDNF and NT3 in adult tissue seem to demonstrate that both these neurotrophins actually inhibit the inhibitory interneurons (Frerking et al., 1998; Tanaka et al., 1997), resulting in desinhibition. Some groups have reported that the effects of neurotrophins on inhibitory neurons are age dependant, in that they are trophic in juvenile tissue and promote depression in mature tissue (Gao and van den Pol, 1999; Mizoguchi et al., 2003). According to this data, BDNF and NT3 would both favor excitatory neurotransmission, having opposite effects on inhibitory systems, which may be of particular importance in epilepsy. It has been demonstrated that excessive neuronal activity leads to a substantial release of neurotrophins that correlates with a sprouting of excitatory synapses and a decrease in inhibitory synapses (Ernfors et al., 1991; Isackson et al., 1991; Ribak et al., 1979). It is possible that in normal aging, if the neurotrophic system is compromised, the opposite could happen, meaning the glutamatergic synapses would be downregulated, as the GABAergic synapses would be more active and either sprout or be protected from attrition.
Aging, cognitive status and the cholinergic system
In behaviorally characterized aged rats, our lab has previously characterized the relationship between cortical cholinergic atrophy and cognitive impairment (Bruno et al., 2004). The present study includes different cortical regions and demonstrates that the most pronounced cholinergic loss occurs in LIII of the mPFC and both LIII and LV of the LtPA (Figures 6 and 7). We also show that AI rats have both an imbalance towards inhibition and reduced numbers of cholinergic boutons. Given this interesting fact, we performed regression analyses in LV of the LtPA and in LIII of the mPFC, where both cholinergic counts and E/I ratios were altered, to look for potential relationships between these two changes. The data shows that in aged animals, the cortical cholinergic counts positively correlate with the ratio of E/I appositions on distal basal dendrites (Figure 8). It is important to keep in mind that this finding does not necessarily imply a causal relationship, but suggests a mechanistic relationship linking the ratio of E/I appositions to the well known age-related cholinergic atrophy.
Potential impact of the cholinergic system on excitatory/inhibitory balance
As described in previous paragraphs, presynaptic elements are dynamic and their activity levels can potentially regulate their steady state numbers (Mattson, 2008). Acetylcholine has the potential to make glutamatergic neurons more excitable (Desai and Walcott, 2006; McCormick and Prince, 1985; Metherate et al., 1988) and it has recently been shown that it could inhibit inhibitory synaptic activity (Kruglikov and Rudy, 2008; Salgado et al., 2007). As further discussed below, there is a likelihood that a loss of cholinergic function would affect excitatory and inhibitory systems differentially, favoring the survival or sprouting of inhibitory synapses, and depressing and pruning the excitatory synapses.
Potential impact of increased inhibition on the cholinergic system
Our lab has recently shown that NGF, the neurotrophin responsible for the phenotypic maintenance of cortical cholinergic synapses, is released in an activity-dependant manner (Bruno and Cuello, 2006). It is possible that a degeneration of excitatory synapses with maintenance of inhibitory synapses, would lead to less neuronal activity and consequently less release of proNGF. The neuronal activity and the neurotrophin release in AI are issues that deserve to be further investigated.
Conclusions
Using a combination of cognitive screening, intracellular filling and immunocytochemistry, we were able to demonstrate that a cholinergic loss accompanied by a decrease in the ratio of glutamatergic/GABAergic appositions on distal basal dendrites from cortical pyramidal neurons. Both of these changes correlated with poorer cognitive performance in aged rats. Regression analysis revealed a positive correlation between the density of cortical cholinergic varicosities and the ratio of E/I appositions in aged rats.
Acknowledgements
This work was supported by the National Institutes of Health grant R01AG020529 (ARS and ACC) and the Canadian Institutes of Health Research grants MOP-79411 to ARS and MOP-62735 to ACC. ACC is the holder of the McGill University Charles E. Frosst Merck Chair of Pharmacology. ACC is grateful for the support from Dr. Alan Frosst and the Frosst family. We would also like to thank Dr. Javier DeFelipe for sharing some of his expertise regarding intracellular injections. We are grateful to Manon St-Louis, Adriana Ducatenzeiler and Vanessa Partridge for their technical guidance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors do not have financial interests or other situations which would incur a conflict of interest with this study.
References
- Balazs R. Trophic effect of glutamate. Curr. Top. Med. Chem. 2006;6:961–968. doi: 10.2174/156802606777323700. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Novara M, Carabelli V, Hernandez-Guijo JM, Carbone E. BDNF up-regulates evoked GABAergic transmission in developing hippocampus by potentiating presynaptic N- and P/Q-type Ca2+ channels signalling. Eur. J. Neurosci. 2002;16:2297–2310. doi: 10.1046/j.1460-9568.2002.02313.x. [DOI] [PubMed] [Google Scholar]
- Bannister AP. Inter- and intra-laminar connections of pyramidal cells in the neocortex. Neurosci. Res. 2005;53:95–103. doi: 10.1016/j.neures.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Bell KF, Ducatenzeiler A, Ribeiro-da-Silva A, Duff K, Bennett DA, Cuello AC. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol. Aging. 2006;27:1644–1657. doi: 10.1016/j.neurobiolaging.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Ibanez RM, Ernfors P, Harkany T. Turning the heterogeneous into homogeneous: studies on selectively isolated GABAergic interneuron subsets. Int. J. Dev. Neurosci. 2004a;22:533–543. doi: 10.1016/j.ijdevneu.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Dobszay MB, Sousa KM, Schulte G, Mager PP, Hartig W, Gorcs TJ, Zilberter Y, Ernfors P, Harkany T. Brain-derived neurotrophic factor controls functional differentiation and microcircuit formation of selectively isolated fast-spiking GABAergic interneurons. Eur. J. Neurosci. 2004b;20:1290–1306. doi: 10.1111/j.1460-9568.2004.03561.x. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int. J. Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Bruno MA, Clarke PB, Seltzer A, Quirion R, Burgess K, Cuello AC, Saragovi HU. Long-lasting rescue of age-associated deficits in cognition and the CNS cholinergic phenotype by a partial agonist peptidomimetic ligand of TrkA. J Neurosci. 2004;24:8009–8018. doi: 10.1523/JNEUROSCI.1508-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MA, Cuello AC. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. U. S. A. 2006;103:6735–6740. doi: 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N. Calcium permeability of glutamate-gated channels in the central nervous system. Curr. Opin. Neurobiol. 1996;6:311–317. doi: 10.1016/s0959-4388(96)80113-9. [DOI] [PubMed] [Google Scholar]
- Callaway EM. Cell type specificity of local cortical connections. J. Neurocytol. 2002;31:231–237. doi: 10.1023/a:1024165824469. [DOI] [PubMed] [Google Scholar]
- de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur. J. Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- Deng PY, Xiao Z, Yang C, Rojanathammanee L, Grisanti L, Watt J, Geiger JD, Liu R, Porter JE, Lei S. GABA(B) receptor activation inhibits neuronal excitability and spatial learning in the entorhinal cortex by activating TREK-2 K+ channels. Neuron. 2009;63:230–243. doi: 10.1016/j.neuron.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Walcott EC. Synaptic bombardment modulates muscarinic effects in forelimb motor cortex. J. Neurosci. 2006;26:2215–2226. doi: 10.1523/JNEUROSCI.4310-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb. Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott PJ, Garofalo L, Cuello AC. Limited neocortical devascularizing lesions causing deficits in memory retention and choline acetyltransferase activity- -effects of the monosialoganglioside GM1. Neuroscience. 1989;31:63–76. doi: 10.1016/0306-4522(89)90030-4. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Brain-derived neurotrophic factor (BDNF) modulates inhibitory, but not excitatory, transmission in the CA1 region of the hippocampus. J. Neurophysiol. 1998;80:3383–3386. doi: 10.1152/jn.1998.80.6.3383. [DOI] [PubMed] [Google Scholar]
- Galbraith JA, Gallant PE. Axonal transport of tubulin and actin. J. Neurocytol. 2000;29:889–911. doi: 10.1023/a:1010903710160. [DOI] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Neurotrophin-3 potentiates excitatory GABAergic synaptic transmission in cultured developing hypothalamic neurones of the rat. J. Physiol. 1999;518(Pt 1):81–95. doi: 10.1111/j.1469-7793.1999.0081r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat. Rev. Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. Parietal mechanisms of target representation. Curr. Opin. Neurobiol. 2002;12:134–140. doi: 10.1016/s0959-4388(02)00312-4. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol. Aging. 1991;12:336–338. doi: 10.1016/0197-4580(91)90013-a. [DOI] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Horch HW, Katz LC. BDNF release from single cells elicits local dendritic growth in nearby neurons. Nat. Neurosci. 2002;5:1177–1184. doi: 10.1038/nn927. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: Temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J. Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cereb. Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Jo YS, Park EH, Kim IH, Park SK, Kim H, Kim HT, Choi JS. The medial prefrontal cortex is involved in spatial memory retrieval under partial-cue conditions. J. Neurosci. 2007;27:13567–13578. doi: 10.1523/JNEUROSCI.3589-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kolb B, Côté S, Ribeiro-da-Silva A, Cuello AC. Nerve growth factor treatment prevents dendritic atrophy and promotes recovery of function after cortical injury. Neuroscience. 1997;76:1139–1151. doi: 10.1016/s0306-4522(96)00448-4. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer PJ, Brown RW, Baldwin SA, Scheff SW. Validation of a single-day Morris Water Maze procedure used to assess cognitive deficits associated with brain damage. Brain Res. Bull. 1996;39:17–22. doi: 10.1016/0361-9230(95)02028-4. [DOI] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulated by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasarge CL, Banuelos C, Mayse JD, Bizon JL. Blockade of GABA(B) receptors completely reverses age-related learning impairment. Neuroscience. 2009;164:941–947. doi: 10.1016/j.neuroscience.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le RN, Amar M, Moreau A, Baux G, Fossier P. Impaired GABAergic transmission disrupts normal homeostatic plasticity in rat cortical networks. Eur. J. Neurosci. 2008;27:3244–3256. doi: 10.1111/j.1460-9568.2008.06288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon W, Bruno MA, Allard S, Cuello AC. Inhibition of ERK signalling pathway in the prefrontal cortex impairs recent memory consolidation. 2009 [Google Scholar]
- Leon WC, Bruno MA, Allard S, Nader K, Cuello AC. Engagement of the PFC in consolidation and recall of recent spatial memory. Learn. Mem. 2010;17:297–305. doi: 10.1101/lm.1804410. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen. Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Lykissas MG, Batistatou AK, Charalabopoulos KA, Beris AE. The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovasc. Res. 2007;4:143–151. doi: 10.2174/156720207780637216. [DOI] [PubMed] [Google Scholar]
- Majdi M, Ribeiro-da-Silva A, Cuello AC. Cognitive impairment and transmitter-specific pre- and postsynaptic changes in the rat cerebral cortex during ageing. Eur. J Neurosci. 2007;26:3583–3596. doi: 10.1111/j.1460-9568.2007.05966.x. [DOI] [PubMed] [Google Scholar]
- Majdi M, Ribeiro-da-Silva A, Cuello AC. Variations in excitatory and inhibitory postsynaptic protein content in rat cerebral cortex with respect to aging and cognitive status. Neuroscience. 2009;159:896–907. doi: 10.1016/j.neuroscience.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol. Aging. 2002;23:579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- Marty S, Wehrle R, Sotelo C. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J. Neurosci. 2000;20:8087–8095. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc. Natl. Acad. Sci. USA. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. Suppression of LTP-like plasticity in human motor cortex by the GABAB receptor agonist baclofen. Exp. Brain Res. 2007;180:181–186. doi: 10.1007/s00221-006-0849-0. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain Res. Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J. Comp Neurol. 2001;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- Metherate R, Tremblay N, Dykes RW. The effects of acetylcholine on response properties of cat somatosensory cortical neurons. J. Neurophysiol. 1988;59:1231–1252. doi: 10.1152/jn.1988.59.4.1231. [DOI] [PubMed] [Google Scholar]
- Miura K, Tsubo Y, Okada M, Fukai T. Balanced excitatory and inhibitory inputs to cortical neurons decouple firing irregularity from rate modulations. J. Neurosci. 2007;27:13802–13812. doi: 10.1523/JNEUROSCI.2452-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi Y, Ishibashi H, Nabekura J. The action of BDNF on GABA(A) currents changes from potentiating to suppressing during maturation of rat hippocampal CA1 pyramidal neurons. J. Physiol. 2003;548:703–709. doi: 10.1113/jphysiol.2003.038935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Selective vulnerability of corticocortical and hippocampal circuits in aging and Alzheimer’s disease. Prog. Brain Res. 2002;136:467–486. doi: 10.1016/s0079-6123(02)36039-4. [DOI] [PubMed] [Google Scholar]
- Niewiadomska G, Baksalerska-Pazera M, Riedel G. Altered cellular distribution of phospho-tau proteins coincides with impaired retrograde axonal transport in neurons of aged rats. Ann. N. Y. Acad. Sci. 2005;1048:287–295. doi: 10.1196/annals.1342.026. [DOI] [PubMed] [Google Scholar]
- Ohba S, Ikeda T, Ikegaya Y, Nishiyama N, Matsuki N, Yamada MK. BDNF locally potentiates GABAergic presynaptic machineries: target-selective circuit inhibition. Cereb. Cortex. 2005;15:291–298. doi: 10.1093/cercor/bhh130. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices: A stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 mRNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- Pugnaloni A, Pallotti F, Genova ML, Zucchini C, Amati S, Tesei M, Biagini G, Lenaz G. Histomorphometric studies in rat cerebral cortex: normal aging and cell loss. Cell Mol. Biol. (Noisy. -le-grand) 1998;44:597–604. [PubMed] [Google Scholar]
- Ragozzino ME, Adams S, Kesner RP. Differential involvement of the dorsal anterior cingulate and prelimbic-infralimbic areas of the rodent prefrontal cortex in spatial working memory. Behav. Neurosci. 1998;112:293–303. doi: 10.1037//0735-7044.112.2.293. [DOI] [PubMed] [Google Scholar]
- Ramón y, Cajal S. Histologie du Système Nerveux de l’Homme et des Vertébrés. Vol. 1. A.Maloine; Paris: 1909. Translated by L. Azoulay. [Google Scholar]
- Redmond L. Translating neuronal activity into dendrite elaboration: signaling to the nucleus. Neurosignals. 2008;16:194–208. doi: 10.1159/000111563. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Harris AB, Vaughn JE, Roberts E. Inhibitory, GABAergic nerve terminals decrease at sites of focal epilepsy. Science. 1979;205:211–214. doi: 10.1126/science.109922. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Pandya DN. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Salgado H, Bellay T, Nichols JA, Bose M, Martinolich L, Perrotti L, Atzori M. Muscarinic M2 and M1 receptors reduce GABA release by Ca2+ channel modulation through activation of PI3K/Ca2+ -independent and PLC/Ca2+ -dependent PKC. J. Neurophysiol. 2007;98:952–965. doi: 10.1152/jn.00060.2007. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP. Cognitive functions of cortical acetylcholine: Toward a unifying hypothesis. Brain Res. Rev. 1997;23:28–46. doi: 10.1016/s0165-0173(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. Neurotrophins and activity-dependent inhibitory synaptogenesis. Prog. Brain Res. 2000a;128:219–229. doi: 10.1016/S0079-6123(00)28019-9. [DOI] [PubMed] [Google Scholar]
- Seil FJ, Drake-Baumann R. TrkB receptor ligands promote activity-dependent inhibitory synaptogenesis. J. Neurosci. 2000b;20:5367–5373. doi: 10.1523/JNEUROSCI.20-14-05367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira ME, Oren R, Dormann A, Ilouz N, Lev S. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell Mol. Neurobiol. 2001;21:591–604. doi: 10.1023/A:1015135617557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele PM, Mauk MD. Inhibitory control of LTP and LTD: stability of synapse strength. J. Neurophysiol. 1999;81:1559–1566. doi: 10.1152/jn.1999.81.4.1559. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial Mapping: Definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neurosci. Lett. 1982;31:271–276. doi: 10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 1988;8:1863–1872. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Saito H, Matsuki N. Inhibition of GABAA synaptic responses by brain-derived neurotrophic factor (BDNF) in rat hippocampus. J. Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann. Neurol. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cereb. Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Vaughan DW. Age-related deterioration of pyramidal cell basal dendrites in rat auditory cortex. J. Comp Neurol. 1977;171:501–515. doi: 10.1002/cne.901710406. [DOI] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Reversible disconnection of the hippocampal-prelimbic cortical circuit impairs spatial learning but not passive avoidance learning in rats. Neurobiol. Learn. Mem. 2008;90:365–373. doi: 10.1016/j.nlm.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Wong TP, Campbell PM, Ribeiro-da-Silva A, Cuello AC. Synaptic numbers across cortical laminae and cognitive performance of the rat during ageing. Neuroscience. 1998a;84:403–412. doi: 10.1016/s0306-4522(97)00485-5. [DOI] [PubMed] [Google Scholar]
- Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De KY. Loss of presynaptic and postsynaptic structures is accompanied by compensatory increase in action potential-dependent synaptic input to layer V neocortical pyramidal neurons in aged rats. J Neurosci. 2000;20:8596–8606. doi: 10.1523/JNEUROSCI.20-22-08596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De KY. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci. Lett. 2006a;397:64–68. doi: 10.1016/j.neulet.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Wong TP, Marchese G, Casu MA, Ribeiro-da-Silva A, Cuello AC, De KY. Imbalance towards inhibition as a substrate of aging-associated cognitive impairment. Neurosci Lett. 2006b;397:64–68. doi: 10.1016/j.neulet.2005.11.055. [DOI] [PubMed] [Google Scholar]
- Wong TP, Marchese G, De Koninck Y, Ribeiro-da-Silva A, Cuello AC. Whole cell recordings from layer V pyramidal neurons in the neocortex of young and aged rats: changes in morphological properties and synaptic inputs. Soc. Neurosci. Abstr. 1998b;24:825. [Google Scholar]


