Abstract
Mechanisms underlying the variation in human life expectancy are largely unknown, but lipid metabolism and especially lipoprotein size was suggested to play an important role in longevity. We have performed comprehensive lipid phenotyping in the Leiden Longevity Study (LLS). By applying multiple logistic regression analysis we tested for the first time the effects of parameters in lipid metabolism (i.e., classical serum lipids, lipoprotein particle sizes, and apolipoprotein E levels) on longevity independent of each other. Parameters in lipid metabolism were measured in offspring of nonagenarian siblings from 421 families of the LLS (n = 1,664; mean age, 59 years) and in the partners of the offspring as population controls (n = 711; mean age, 60 years). In the initial model, where lipoprotein particles sizes, classical serum lipids and apolipoprotein E were included, offspring had larger low-density lipoprotein (LDL) particle sizes (p = 0.017), and lower triglyceride levels (p = 0.026), indicating that they displayed a more beneficial lipid profile. After backwards regression only LDL size (p = 0.014) and triglyceride levels (p = 0.05) were associated with offspring from long-lived families. Sex-specific backwards regression analysis revealed that LDL particle sizes were associated with male longevity (increase in log odds ratio (OR) per unit = 0.21; p = 0.023). Triglyceride levels (decrease OR per unit = 0.22; p = 0.01), but not LDL particle size, were associated with female longevity. Due to the analysis of a comprehensive lipid profile, we confirmed an important role of lipid metabolism in human longevity, with LDL size and triglyceride levels as major predicting factors.
Keywords: Human longevity, Triglycerides, HDL cholesterol, LDL cholesterol, Lipoprotein particle size, Apolipoprotein E
Introduction
Over the last century, life expectancy has dramatically increased in western societies (Oeppen and Vaupel 2002); nevertheless, inter-individual differences in life expectancy remain. For example, family members of nonagenarian siblings and centenarians have a 30% survival benefit for three generations compared with their birth cohort (Perls et al. 2007; Schoenmaker et al. 2006). Offspring of nonagenarians siblings and centenarians are characterized by a lower prevalence of several major diseases, including diabetes, hypertension, coronary artery disease (CAD) (Adams et al. 2008; Westendorp et al. 2009), and strokes (Terry et al. 2004), implying that these differences in life expectancy are associated with a familial, possible genetic, factor that influences the risk of metabolic diseases.
The mechanisms underlying the variation in human life expectancy are still largely unknown but various studies suggest involvement of lipid metabolism. Specifically, low plasma apoE levels in elderly apolipoprotein E gene (APOE) ε3/ε3 carriers are associated with a decreased mortality risk (Mooijaart et al. 2006) and in comparison with same-aged controls, offspring of centenarians or nonagenarian siblings had a tendency for higher plasma levels of high-density lipoprotein cholesterol (HDL-C), lower levels of low-density lipoprotein cholesterol (LDL-C) (Barzilai et al. 2001), and larger lipoprotein particle sizes (Barzilai et al. 2003; Heijmans et al. 2006). Studies into longevity performed so far, often analyzed a subset of blood lipid parameters only and were relatively small. Their findings thus require confirmation in larger studies, particularly because they could not account for the correlation between the various lipid parameters. The importance of the latter is illustrated by the disappearance of the association of HDL particle size with coronary artery disease and familial longevity after adjustment for triglyceride and/or apolipoprotein B levels (Barzilai et al. 2003; El Harchaoui et al. 2009; Heijmans et al. 2006).
To improve the understanding of the association between lipid metabolism and human longevity, we performed multiple analyses of classical blood lipid parameters, lipoprotein particle sizes and apoE levels in long-lived families from the Leiden Longevity Study. We compared 1,664 offspring of nonagenarian sibships, who were shown to have a life-long survival advantage (Schoenmaker et al. 2006) and a decreased incidence of metabolic and cardiovascular disease (Westendorp et al. 2009), with 711 similarly aged partners as controls. Since men and women differ in lipid metabolism and life expectancy (Freedman et al. 2004), we also stratified for gender in our analysis.
Materials and methods
Study design and subjects
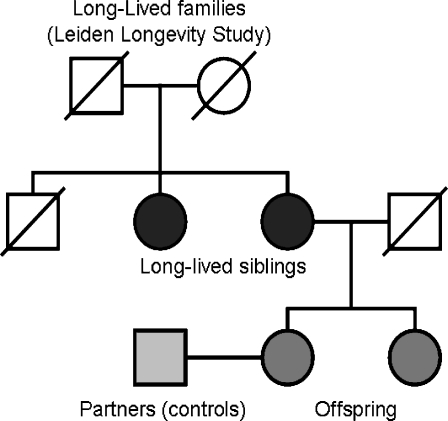
The Leiden Longevity Study (Fig. 1) included 421 Caucasian families consisting of long-lived siblings together with their offspring and the spouses of the offspring (Schoenmaker et al. 2006). Long-lived families were recruited if at least two long-lived siblings were alive and willing to participate. Males were considered long-lived if 89 years or older and females 91 years or older. The mean age of the long-lived siblings was 93.4 ± 2.6 years. In 2001, less than 0.5% of the Dutch population fulfilled these criteria. The Medical Ethical Committee of the Leiden University Medical Centre approved the study and informed consent was obtained from all subjects.
Fig. 1.
Schematic representation of the Leiden Longevity Study. The Leiden longevity study consists of 982 long-lived siblings (males >89 years; females >91 years), their offspring (n = 1,671), and the partners of the offspring (n = 745)
Previously, lipoprotein particle sizes have been analyzed in 165 families from the Leiden Longevity Study using a 400-MHz proton NMR analyzer at LipoScience (Heijmans et al. 2006). Between June 2007 and September 2007, lipoprotein particle sizes were measured in the remaining 255 families using the same method as described in (Heijmans et al. 2006). In short this technique takes advantage of the natural proton NMR spectroscopy “signatures” emitted by lipoprotein particles of different sizes. This information can be used to calculate the concentration of each lipoprotein particle subclass. Next, mean particle sizes (diameter in nanometers) were determined by weighting the relative mass percentage of each subclass according to its diameter (Otvos 2002). Together with the previous study, lipoprotein particle sizes were available for 2,416 individuals consisting of the offspring (n = 1,671) of long-lived siblings and the partners of the offspring as controls (n = 745). Between November 2006 and May 2008, additional information on self-reported bodily measures was collected for the offspring and the controls, and in addition information on medication use was requested from the participants' pharmacist (Westendorp et al. 2009).
Biochemical analyses
In all subjects non-fasting venous blood samples were taken. Lipid levels (triglycerides, total cholesterol, and HDL-C) were determined in serum samples using fully automated equipment (the Hitachi Modular or the Cobas Integra 800 both from Roche, Almere, the Netherlands) (Rozing et al. 2009). LDL-C levels were calculated using the Friedewald formula (LDL-C = total cholesterol—HDL-C—(triglcyerides/2.2); unit mmol/L) and set to missing if plasma triglyceride levels exceeded 4.52 mmol/L (Heijmans et al. 2006). ApoE was determined in serum samples using a human apoE-specific sandwich ELISA (Mooijaart et al. 2006; van Vlijmen et al. 1994).
APOEε2/ε3/ε4 genotyping
DNA was isolated using standard techniques (QIAamp blood maxi kit, QIAGEN, (Venlo, the Netherlands) high salt extraction). For determining APOEε2/ε3/ε4 genotypes, two SNPs in the APOEε2/ε3/ε4 gene were genotyped using two Taqman SNP genotyping assays with the following assayIDs: C_904973_10 (rs7412) and C_3084793_20 (rs429358) (APPLIED BIOSYSTEMS, Foster City, USA). The assay was run on a 7900HT (APPLIED BIOSYSTEMS) according to manufacturer's specification and genotypes were called using the Sequence Detection Software version 2.2 (APPLIED BIOSYSTEMS). The genotype call rate for the APOEε2/ε3/ε4 polymorphism was 98.7%.
Statistical analysis
Continuous variables were tested for normality; if appropriate, they were logarithmically transformed to obtain a normal distribution. Differences in lipid parameters between offspring and their controls were assessed using logistic regression, adjusted for age, sex, and the interaction between them. Robust standard errors were used to adjust for dependencies within families. To determine the effects of lipid parameters in more detail and independent of each other, multiple logistic regression was used. The lipid variables LDL particle size, HDL particle size, LDL-C, HDL-C, triglyceride levels, and ApoE levels were included in the initial starting model together with the covariates age, sex, and the interaction between. Using a backward regression procedure, the lipid parameter with the highest p value was removed each round until a model only including lipid variables independently associating with familial longevity was obtained. We also stratified for sex. Differences between offspring and controls for genotype distributions of rs5882 and the APOEε2/ε3/ε4 polymorphism were evaluated using the chi-square test. Only subjects with complete measurements for all the biochemical variables were included in the analysis. STATA/SE version 8.0 for Windows was used for data analysis.
Results
To test the association of blood lipid parameters with human longevity, we studied individuals for whom complete measurements were available (total cholesterol, HDL-C, LDL-C, triglycerides levels, ApoE, mean HDL, and LDL particle sizes). Compared with controls (n = 711), offspring of long-lived siblings (n = 1,664) had lower triglyceride levels (1.67 vs. 1.75 mmol/L; p = 0.001), larger mean HDL particle sizes (9.05 vs. 9.03 nm; p = 0.01), larger mean LDL particle sizes (21.32 vs. 21.22 nm; p < 10−3), and a smaller total cholesterol/HDL-C ratio (4.08 vs. 4.20; p = 0.004). These observations were independent of age, sex, and the interaction between them (Table 1). Further adjustment for self-reported body mass index (BMI), which was available in 619 controls and 1,399 offspring, did not affect these associations (triglyceride levels, p = 0.01; HDL particle size, p = 0.03; LDL particle size, p < 10−3; total cholesterol/HDL-C ratio, p = 0.03). As shown in Table 1, no differences were observed for apoE levels for the complete group, even when the analysis was restricted to APOE ε3/ε3 carriers only. We observed that the genotype distribution for the APOE polymorphism differs between offspring and controls (ε2/ε2 = 0.4, ε2/ε3 = 10.2, ε2/ε3 = 3.2, ε3/ε3 = 62.3, ε3/ε4 = 21.3 and ε4/ε4 = 2.5; ε2/ε2 = 1.0, ε2/ε3 = 13.3, ε2/ε3 = 2.3, ε3/ε3 = 60.8, ε3/ε4 = 21.2, and ε4/ε4 = 1.2, respectively, p = 0.019).
Table 1.
Baseline characteristics of the Leiden Longevity Study population
| Leiden Longevity Study | Controls | Offspring | Controls vs. offspring |
|---|---|---|---|
| Mean (SD) | Mean (SD) | p Value | |
| Number of individuals | 711 | 1,664 | – |
| Females N (%) | 409 (57.5) | 898 (54.0) | |
| Lipid lowering medication N (%)a | 68 (9.56) | 109 (6.55) | 0.010 |
| Males | 32 (10.60) | 60 (7.83) | 0.170 |
| Females | 36 (8.80) | 49 (5.46) | 0.030 |
| Age (year) | 58.87 ± 7.45 | 59.40 ± 6.53 | 0.046 |
| Males | 61.55 ± 7.33 | 59.26 ± 6.50 | <10−3 |
| Females | 56.88 ± 6.91 | 59.52 ± 6.56 | <10−3 |
| Body mass indexb | 25.56 ± 3.67 | 25.28 ± 3.46 | 0.055d |
| Males | 25.84 ± 3.27 | 25.64 ± 2.86 | 0.486e |
| Females | 25.34 ± 3.94 | 24.97 ± 3.86 | 0.060e |
| Triglyceride (mmol/L)c | 1.75 ± 0.86 | 1.67 ± 0.82 | 0.001d |
| Males | 1.93 ± 0.89 | 1.86 ± 0.85 | 0.311e |
| Females | 1.62 ± 0.81 | 1.51 ± 0.76 | 0.001e |
| HDL cholesterol (mmol/L) | 1.43 ± 0.46 | 1.46 ± 0.44 | 0.053d |
| Males | 1.25 ± 0.36 | 1.29 ± 0.37 | 0.104e |
| Females | 1.57 ± 0.48 | 1.60 ± 0.45 | 0.240e |
| LDL cholesterol (mmol/L) | 3.33 ± 0.92 | 3.34 ± 0.99 | 0.750d |
| Males | 3.27 ± 0.94 | 3.30 ± 0.94 | 0.997e |
| Females | 3.37 ± 0.94 | 3.37 ± 1.03 | 0.701e |
| Total cholesterol/HDL ratioc | 4.20 ± 1.39 | 4.08 ± 1.28 | 0.004d |
| Males | 4.64 ± 1.49 | 4.47 ± 1.29 | 0.080e |
| Females | 3.88 ± 1.21 | 3.75 ± 1.17 | 0.018e |
| HDL particle size (nm) | 9.03 ± 0.51 | 9.05 ± 0.50 | 0.010d |
| Males | 8.79 ± 0.43 | 8.83 ± 0.44 | 0.172e |
| Females | 9.21 ± 0.50 | 9.25 ± 0.46 | 0.031e |
| LDL particle size (nm) | 21.22 ± 0.83 | 21.32 ± 0.81 | <10−3d |
| Males | 20.86 ± 0.76 | 20.99 ± 0.78 | 0.023e |
| Females | 21.49 0.78 | 21.60 ± 0.73 | 0.010e |
| ApoE (mg/dL)c | 2.81 ± 0.98 | 2.82 ± 0.99 | 0.967d |
| Males | 2.72 ± 0.92 | 2.76 ± 0.97 | 0.424e |
| Females | 2.87 ± 1.02 | 2.87 ± 1.01 | 0.481e |
| APOEε3/ε3 carriers | 441 (62.03) | 989 (59.44) | – |
| Males | 198 (65.56) | 443 (57.83) | – |
| Females | 243 (59.41) | 546 (60.80) | – |
| ApoE (mg/dL) in APOEε3/ε3 carriers | 2.71 ± 0.73 | 2.68 ± 0.79 | 0.356 |
| Males | 2.66 ± 0.72 | 2.61 ± 0.81 | 0.495 |
| Females | 2.80 ± 0.74 | 2.73 ± 0.77 | 0.104 |
Values are presented as means ± standard deviations or as numbers (percentages) from the total population. To correct for dependencies within families, robust standard errors were used
aLipid lowering medication includes 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors (statin), fibrates, niacin, bile acid sequestrants, and Niemann-Pick C1-like 1 protein inhibitors
bBody mass index was calculated based on self reported height and weight in 1,867 participants of the LLS, consisting of 822 males and 1,045 females
cLogarithmically transformed to obtain a normal distribution
dAdjusted for medication use, age, sex, and the interaction between age and sex
eAdjusted for age
Sex-specific analysis revealed that female offspring had lower triglyceride levels (1.51 mmol/L vs. 1.62 mmol/L, p = 0.001), a lower cholesterol/HDL-C ratio (3.75 vs. 3.88, p = 0.018), larger LDL particle sizes (21.60 nm vs. 21.49 nm, p = 0.01), and larger HDL particle sizes (9.25 nm vs. 9.21 nm, p = 0.031) compared with controls. After adjusting for BMI, HDL particle size and the cholesterol/HDL-C ratio were no longer significant different between offspring and controls (data not shown). In males however, offspring only had larger LDL sizes (20.99 nm vs. 20.86, p = 0.023) compared to offspring and for the cholesterol/HDL-C ratio a trend was observed were offspring had a lower ratio compared to controls (4.47 vs. 4.64, p = 0.080). Adjustment for BMI did not affect these results (data not shown).
These univariate analyses do not account for the strong correlations that exist between the various lipid parameters. For example, the Pearson's correlation coefficient between LDL particle size and triglyceride levels is −0.487, and the correlation between HDL-C and LDL particle size is 0.661 (Table 2). To assess the association between longevity and the lipid parameters in more detail and independent of each other, we used multiple logistic regression where the starting model included the all the available lipid parameters, except for total cholesterol and the ratio LDL-C/HDL-C which were not included because they are highly correlated with LDL-C and HDL-C, respectively, which introduces multicollinearity. In this initial model (Table 3), the lipid parameters LDL size (increase in log OR per unit = 0.21; p = 0.017) and triglyceride levels (decrease in log OR per unit = 0.26; p = 0.026) were significantly different between offspring and controls independent from the other lipid variables. After backwards regression, were age, sex, and the interaction between age and sex were not removed to account for the study design, the final model included LDL particle size (increase in log OR per unit = 0.16; p = 0.014) and triglyceride levels (decrease in log OR per unit = 0.21; p = 0.05).
Table 2.
Pearson correlation coefficientsa between the lipid parameters in the Leiden Longevity Study
| HDL size | TG | CHOL | HDL-C | LDL-C | Chol/HDL | ApoE | |
|---|---|---|---|---|---|---|---|
| LDL size | |||||||
| Total population | 0.793 | −0.470 | 0.067 | 0.624 | −0.016** | −0.606 | −0.074 |
| Men | 0.778 | −0.489 | −0.027** | 0.633 | −0.077* | −0.621 | −0.141 |
| Women | 0.734 | −0.375 | 0.015 | 0.518 | −0.076 | −0.527 | −0.077 |
| HDL size | |||||||
| Total population | – | −0.550 | −0.005** | 0.742 | −0.143 | −0.754 | −0.065 |
| Men | – | −0.519 | −0.092 | 0.736 | −0.150 | −0.741 | −0.169 |
| Women | – | −0.525 | −0.075 | 0.683 | −0.194 | −0.741 | −0.051** |
| TG | |||||||
| Total population | – | – | 0.327 | −0.372 | 0.191 | 0.614 | 0.233 |
| Men | – | – | 0.378 | −0.393 | 0.150 | 0.616 | 0.306 |
| Women | – | – | 0.390 | −0.290 | 0.244 | 0.587 | 0.209 |
| CHOL | |||||||
| Total population | – | – | – | 0.364 | 0.924 | 0.317 | 0.188 |
| Men | – | – | – | 0.255 | 0.922 | 0.389 | 0.169 |
| Women | – | – | – | 0.364 | 0.924 | 0.360 | 0.198 |
| HDL-C | |||||||
| Total population | – | – | – | – | 0.129 | −0.743 | 0.021** |
| Men | – | – | – | – | 0.119* | −0.765 | −0.087* |
| Women | – | – | – | – | 0.126 | −0.715 | 0.053** |
| LDL-C | |||||||
| Total population | – | – | – | – | – | 0.486 | 0.115 |
| Men | – | – | – | – | – | 0.508 | 0.078* |
| Women | – | – | – | – | – | 0.559 | 0.103 |
| CHOL/HDL | |||||||
| Total population | – | – | – | – | – | – | 0.129 |
| Men | – | – | – | – | – | – | 0.195 |
| Women | – | – | – | – | – | – | 0.116 |
| ApoE | |||||||
| Total population | – | – | – | – | – | – | – |
| Men | – | – | – | – | – | – | – |
| Women | – | – | – | – | – | – | – |
LDL size mean LDL lipoprotein particle size, HDL size mean HDL lipoprotein particle size, TG triglycerides, CHOL cholesterol, HDL-C HDL cholesterol, LDL-C LDL cholesterol, CHOL/HDL ratio between total cholesterol and HDL cholesterol, ApoE apolipoprotein E
*0.01 < p < 0.05; **p > 0.05
aSignificant at p < 0.01 (2-tailed), unless otherwise indicated
Table 3.
Multiple variate logistic regression analysis with all measured lipid variables included
| Total population (N = 2,375) | Males (N = 1,068) | Females (N = 1,307) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Betaa | SE | p Value | Betaa | SE | p Value | Betaa | SE | p Value | |
| Age (year) | 0.062 | 0.010 | <10−3 | −0.050 | 0.012 | <10−3 | 0.066 | 0.010 | <10−3 |
| Sex | 6.901 | 1.066 | <10−3 | – | – | – | – | – | – |
| Sex × age | −0.112 | 0.018 | <10−3 | – | – | – | – | – | – |
| LDL size (nm) | 0.207 | 0.087 | 0.017 | 0.268 | 0.140 | 0.055 | 0.178 | 0.117 | 0.127 |
| HDL size (nm) | −0.124 | 0.201 | 0.537 | −0.283 | 0.333 | 0.395 | −0.064 | 0.259 | 0.805 |
| LDL-C (mmol/L) | −0.001 | 0.053 | 0.992 | −0.031 | 0.080 | 0.697 | 0.024 | 0.074 | 0.741 |
| HDL-C (mmol/L) | 0.010 | 0.177 | 0.953 | 0.235 | 0.322 | 0.466 | −0.080 | 0.219 | 0.713 |
| Triglycerides (mmol/L)b | −0.257 | 0.115 | 0.026 | −0.039 | 0.171 | 0.821 | −0.417 | 0.164 | 0.011 |
| ApoE (mmol/L)b | 0.117 | 0.163 | 0.473 | 0.240 | 0.226 | 0.287 | 0.000 | 0.203 | 1.000 |
| Constant | −6.210 | 1.784 | 0.000 | 0.425 | 2.898 | 0.883 | −6.092 | 2.391 | 0.011 |
People using lipid lowering medication were excluded from analysis. Robust standard errors were used to account for dependencies within families in all analysis
LDL size mean LDL lipoprotein particle size, HDL size mean HDL lipoprotein particle size, HDL-C HDL cholesterol, LDL-C LDL cholesterol, ApoE apolipoprotein E
aChange in log OR per unit
bLogarithmically transformed to obtain a normal distribution
Sex-specific multiple regression indicated differences between men and women. In the initial multivariate logistic regression model, none of the lipid parameters was significantly different in male offspring compared with controls, but for LDL size a trend was observed (p = 0.055) (Table 3). After backwards regression, male offspring had a larger LDL particle size than controls (increase in log OR per unit = 0.21; p = 0.023), whereas no differences in triglyceride levels were observed. For female offspring, in the initial starting model, only triglyceride levels, were lower among female offspring (decrease in log OR per unit = 0.42; p = 0.011) (Table 3). After backwards regression, no difference in LDL size were observed in female offspring as compared with controls, whereas triglyceride levels were significantly lower among female offspring (decrease in log OR per unit = 0.22; p = 0.01).
The valine allele of rs5882 in the cholesterol ester transfer protein gene was previously found to be associated with larger LDL and HDL particle sizes (Barzilai et al. 2003). The association of the valine allele with larger LDL size was replicated in a linear regression analysis in controls and offspring combined (p = 0.008; adjusted for age, sex, and triglyceride levels), but this allele was not associated with HDL size (p = 0.200). The polymorphism, however, did not explain the difference in LDL size between controls and offspring (increase in log OR per unit = 0.16; p = 0.009 after accounting for rs5882, as compared with increase in log OR per unit = 0.17; p = 0.014 without rs5882). Nor was the valine allele associated with the offspring group (p = 0.131).Similar results were obtained for man and women separately.
Discussion
We investigated the association of classical serum lipid levels, apoE levels and lipoprotein particle sizes with human longevity, in a study that was sufficiently large to account for correlations among lipid parameters and to test for differences between sexes. Our main finding is that both LDL particle size and triglyceride levels are associated with human longevity independent of other lipid parameters. Further analysis indicated that LDL particle size is particularly associated with male, whereas triglyceride levels are associated with female longevity.
Our study (N = 2,375) confirms the association of LDL particle size previously observed in a smaller study (N = 474) (Barzilai et al. 2003). The differences in the final end model were comparable with the findings from Barzilai for males (0.21 nm increase), but not for females (in the final model only triglyceride levels were significantly different between offspring and controls). Remarkable, an association of triglyceride levels with longevity was not reported before. In one study, only a trend was observed (Heijmans et al. 2006) and in another study triglycerides were not mentioned in relation to longevity (Barzilai et al. 2003). In a very small study where fasting samples were used, no association between longevity and triglyceride levels were found (Barzilai et al. 2001). Since we used non-fasting samples and had a large study population, this could explain why we did found an association with triglyceride levels and longevity. Our results did not confirm the previously reported association of LDL and HDL cholesterol levels (Barzilai et al. 2001) and HDL particle size (Barzilai et al. 2003). For HDL particle size we did observe an association in a univariate analysis, but a multiple analysis that accounted for the correlation between lipid parameters showed that this result depended on other blood lipid parameters in particular triglyceride levels (Heijmans et al. 2006).
Because the APOE genotype was previously found to confound the association between ApoE level and all-cause mortality in elderly subjects (Mooijaart et al. 2006), it was necessary to stratify the data according to APOE genotype. Still we did not find an association of apoE level with longevity. However, we did find that the beneficial APOEε2 allele was more common in the offspring compared with controls, which might explain why offspring have a more beneficial lipid profile compared with controls (Mooijaart et al. 2006).
Since offspring of nonagenarian siblings from the Leiden Longevity Study have a lower prevalence of late-onset metabolic diseases (Westendorp et al. 2009), it could be hypothesized that low triglyceride levels and a larger mean LDL particle size are protective against diseases like diabetes type II and CAD. High triglyceride levels are a component of the metabolic syndrome, which is associated with a higher risk for cardiovascular diseases (CVD) and type II diabetes (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001). Triglyceride levels itself are also independently associated with a higher risk for CVD (Sarwar et al. 2007). Elevated triglyceride levels are associated with increased levels of remnants lipoproteins, which might initiate and promote the development of atherosclerotic plaques (Nordestgaard et al. 2007). Mean LDL particle size is determined by summing the size-weighted relative contribution made by each subspecies (i.e., small, medium and large LDL particles) to the total concentration of LDL particles (Otvos et al. 1992). Thus, people with a small mean LDL particle size have relatively more small LDL particles as compared with people with a large mean LDL particle sizes. Small LDL particle sizes are associated with type II diabetes (Festa et al. 2005), metabolic syndrome (Gentile et al. 2008; Kathiresan et al. 2006), insulin resistance (Garvey et al. 2003; Goff et al. 2005), incident CVD (Mora et al. 2009), and future CAD (El Harchaoui et al. 2007). Possible atherosclerosis could be induced by those small LDL particles, as indicated in a study of two knockout mice strains with similar cholesterol levels, were the Ldr−/−Apob100/100 mice had a larger number of smaller lipoprotein proteins and more atherosclerosis than the Apoe−/−Apob100/100 mice (Veniant et al. 2008). Thus from literature it is clear that LDL particle size and triglyceride levels have been contributing to cardiovascular diseases.
LDL particle size seems to be particularly associated with male longevity whereas triglyceride levels are associated with female longevity, indicating that the role of lipid metabolism in longevity may be different in men and women. In women it was found that LDL particle size associated with incident CVD, however in that study LDL particle size was comparable but not superior to standard lipid levels in predicting CVD (Mora et al. 2009). This could support our finding that triglyceride levels eliminated the association between LDL size and longevity in women. Moreover, in a meta-analysis of population based prospective studies it was concluded that high triglyceride levels are a larger risk factor for cardiovascular disease in women than in men (Hokanson and Austin 1996). But in a more recent meta-analysis, it was found that the effect of triglyceride levels on cardiovascular disease did not differ between men and women (Sarwar et al. 2007). On the other hand, men are characterized by smaller and denser LDL particles and higher triglyceride levels than women (Freedman et al. 2004; Lemieux et al. 2002), which is also the case in our study. In addition, men have higher hepatic lipase activity compared with women, resulting in a smaller mean LDL particle size (Carr et al. 2001; Magkos et al. 2009). Thus different plasma concentrations of enzymes involved in intravascular lipid remodeling between men and women (Magkos et al. 2009) may explain our observed sex differences regarding LDL particle size and familial longevity. But further research on lipid enzymes difference between men and women in relation to longevity is needed to confirm this hypothesis.
This study has several strong points. Firstly, due to the large sample size it was possible to adjust for the correlations among the lipid parameters and to stratify for gender. Secondly, since controls are the partners of the offspring of long-lived siblings, they did not differ on any major indicators of lifestyle, including current smoking, self-reported BMI, and level of education (Westendorp et al. 2009). A weak point is that the NMR spectroscopy-based lipid parameters were not determined in a single measurement which introduced batch effects, for which it was necessary to adjust during analysis. We choose not to correct for the treatment of high cholesterol levels with lipid lowering medication, which mostly consist of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor (statin) treatment (93.8%), because per individual it is unknown if statin treatment reduces moderate to severe hypercholesterolemia to above or below population average lipid levels. Since the offspring group is using lesser lipid lowering medication than the controls, this might bias our results in such a way that we underestimate the effect of lipid metabolism on longevity. Also, the use of non-fasting samples introduced noise in our data-set, which may lead to an underestimation of the effects found our study.
Our study confirms the link between lipid metabolism and human longevity and identifies LDL particle size and triglyceride levels as the key lipid parameters. To gain more insight into why there are differences between males and females, it is necessary to include information on lipid enzymes, genetic and expression data of genes known to be involved in lipid metabolism in future studies investigating the relation between lipid metabolism and longevity in humans.
Acknowledgements
We wish to thank all the participants of the Leiden Longevity Study for their contribution. We are grateful to Marja Kersbergen and Margo van Schie for determining apoE plasma levels and standard lipid parameters.
This study is supported by grants from Innovation Oriented research Program IOP on genomics (SenterNovem; IGE5007), the Dutch Heart Foundation (NHS2006B195), the Netherlands Consortium for Healthy Ageing (050 60 810), and by a stimulation grant (05040202, Healthy Ageing) from the Netherlands Genomics Initiative (NGI)/Netherlands Organization for scientific research (NWO).
Footnotes
LUMC (AAM Vaarhorst, PE Slagboom, BT Heijmans, M Beekman, JN Kok, JW Jukema), WU (Y Lu, EJM Feskens, M Muller), RIVM (JMA Boer, MET Dollé), UNIMAAS/UHM (MMJ van Greevenbroek, APM Gorgels), and the VU (DI Boomsma)
References
- Adams ER, Nolan VG, Andersen SL, Perls TT, Terry DF. Centenarian offspring: start healthier and stay healthier. J Am Geriatr Soc. 2008;56(11):2089–2092. doi: 10.1111/j.1532-5415.2008.01949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Gabriely I, Gabriely M, Iankowitz N, Sorkin JD. Offspring of centenarians have a favorable lipid profile. J Am Geriatr Soc. 2001;49(1):76–79. doi: 10.1046/j.1532-5415.2001.49013.x. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Atzmon G, Schechter C, Schaefer EJ, Cupples AL, Lipton R, Cheng S, Shuldiner AR. Unique lipoprotein phenotype and genotype associated with exceptional longevity. JAMA. 2003;290(15):2030–2040. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- Carr MC, Hokanson JE, Zambon A, Deeb SS, Barrett PH, Purnell JQ, Brunzell JD. The contribution of intraabdominal fat to gender differences in hepatic lipase activity and low/high density lipoprotein heterogeneity. J Clin Endocrinol Metab. 2001;86(6):2831–2837. doi: 10.1210/jc.86.6.2831. [DOI] [PubMed] [Google Scholar]
- El Harchaoui K, Steeg WA, Stroes ES, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJ, Khaw KT, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. J Am Coll Cardiol. 2007;49(5):547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- El Harchaoui K, Arsenault BJ, Franssen R, Despres JP, Hovingh GK, Stroes ES, Otvos JD, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. High-density lipoprotein particle size and concentration and coronary risk. Ann Intern Med. 2009;150(2):84–93. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the insulin resistance atherosclerosis study. Circulation. 2005;111(25):3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D'Agostino RB, Wilson PW, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham study. Clin Chem. 2004;50(7):1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–462. doi: 10.2337/diabetes.52.2.453. [DOI] [PubMed] [Google Scholar]
- Gentile M, Panico S, Jossa F, Mattiello A, Ubaldi S, Marotta G, Pauciullo P, Rubba P. Small dense LDL particles and metabolic syndrome in a sample of middle-aged women. Findings from progetto atena. Clin Chim Acta. 2008;388(1–2):179–183. doi: 10.1016/j.cca.2007.10.033. [DOI] [PubMed] [Google Scholar]
- Goff DC, Jr, D'Agostino RB, Jr, Haffner SM, Otvos JD. Insulin resistance and adiposity influence lipoprotein size and subclass concentrations. Results from the insulin resistance atherosclerosis study. Metabolism. 2005;54(2):264–270. doi: 10.1016/j.metabol.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Beekman M, Houwing-Duistermaat JJ, Cobain MR, Powell J, Blauw GJ, Ouderaa F, Westendorp RG, Slagboom PE. Lipoprotein particle profiles mark familial and sporadic human longevity. PLoS Med. 2006;3(12):e495. doi: 10.1371/journal.pmed.0030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk. 1996;3(2):213–219. doi: 10.1097/00043798-199604000-00014. [DOI] [PubMed] [Google Scholar]
- Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D'Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the framingham heart study. Circulation. 2006;113(1):20–29. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- Lemieux I, Pascot A, Lamarche B, Prud'homme D, Nadeau A, Bergeron J, Despres JP. Is the gender difference in LDL size explained by the metabolic complications of visceral obesity? Eur J Clin Investig. 2002;32(12):909–917. doi: 10.1046/j.1365-2362.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- Magkos F, Mohammed BS, Mittendorfer B. Plasma lipid transfer enzymes in non-diabetic lean and obese men and women. Lipids. 2009;44(5):459–464. doi: 10.1007/s11745-009-3285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooijaart SP, Berbee JF, Heemst D, Havekes LM, Craen AJ, Slagboom PE, Rensen PC, Westendorp RG. ApoE plasma levels and risk of cardiovascular mortality in old age. PLoS Med. 2006;3(6):e176. doi: 10.1371/journal.pmed.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119(7):931–939. doi: 10.1161/CIRCULATIONAHA.108.816181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48(3–4):171–180. [PubMed] [Google Scholar]
- Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992;38(9):1632–1638. [PubMed] [Google Scholar]
- Perls T, Kohler IV, Andersen S, Schoenhofen E, Pennington J, Young R, Terry D, Elo IT. Survival of parents and siblings of supercentenarians. J Gerontol A Biol Sci Med Sci. 2007;62(9):1028–1034. doi: 10.1093/gerona/62.9.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozing MP, Westendorp RG, Frolich M, Craen AJ, Beekman M, Heijmans BT, Mooijaart SP, Blauw GJ, Slagboom PE, Heemst HD. Human insulin/IGF-1 and familial longevity at middle age. Aging (Albany, NY) 2009;1(8):714–722. doi: 10.18632/aging.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10, 158 incident cases among 262, 525 participants in 29 Western prospective studies. Circulation. 2007;115(4):450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- Schoenmaker M, Craen AJ, Meijer PH, Beekman M, Blauw GJ, Slagboom PE, Westendorp RG. Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet. 2006;14(1):79–84. doi: 10.1038/sj.ejhg.5201508. [DOI] [PubMed] [Google Scholar]
- Terry DF, Wilcox MA, McCormick MA, Perls TT. Cardiovascular disease delay in centenarian offspring. J Gerontol A Biol Sci Med Sci. 2004;59(4):385–389. doi: 10.1093/gerona/59.4.m385. [DOI] [PubMed] [Google Scholar]
- Vlijmen BJ, Maagdenberg AM, Gijbels MJ, Boom H, HogenEsch H, Frants RR, Hofker MH, Havekes LM. Diet-induced hyperlipoproteinemia and atherosclerosis in apolipoprotein E3-Leiden transgenic mice. J Clin Invest. 1994;93(4):1403–1410. doi: 10.1172/JCI117117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veniant MM, Beigneux AP, Bensadoun A, Fong LG, Young SG. Lipoprotein size and susceptibility to atherosclerosis-insights from genetically modified mouse models. Curr Drug Targets. 2008;9(3):174–189. doi: 10.2174/138945008783755629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp RG, Heemst D, Rozing MP, Frolich M, Mooijaart SP, Blauw GJ, Beekman M, Heijmans BT, Craen AJ, Slagboom PE. Nonagenarian siblings and their offspring display lower risk of mortality and morbidity than sporadic nonagenarians: the Leiden Longevity Study. J Am Geriatr Soc. 2009;57(9):1634–1637. doi: 10.1111/j.1532-5415.2009.02381.x. [DOI] [PubMed] [Google Scholar]