Abstract
Natural Killer (NK) cells recognize target cells through activating receptors, many of which rely on the transmembrane adaptors DAP10, DAP12 and FcRγ to deliver intracellular signals. Because these adaptors initiate distinct signaling pathways, they dictate the type of response mediated by receptor engagement. DAP10, for example, primarily triggers cytotoxicity, whereas DAP12 induces both cytotoxicity and IFN-γ secretion. In mice, NKG2D signals through both DAP10 and DAP12, which broadens and modulates the type of response engendered by encounter with ligand. Although initial studies indicated that Ly49H and Ly49D recruit only DAP12, a recent report suggested that they also associate with DAP10 in transfected cells. We asked whether this association occurs and is functionally significant under physiologic conditions. Our data demonstrate that DAP10 does associate with Ly49H and Ly49D in primary NK cells. While this association contributes slightly to cell surface expression of both receptors, it has no significant impact on Ly49H-mediated control of murine cytomegalovirus infection. Thus, while many activating NK cell receptors are promiscuous in terms of adaptor association, our data indicate that the functional consequences of such promiscuity may vary widely and may not be evident in all cases.
Keywords: signaling, innate immunity, cytomegalovirus
Introduction
Natural Killer (NK) cells display a vast array of activating receptors, many of which recognize MHC class I or MHC class I-like proteins. In mice, Ly49H recognizes the MHC class I-like molecule m157 encoded by murine cytomegalovirus (MCMV) [1, 2] whereas, Ly49D detects allogeneic MHC class I [3]. NKG2D recognizes a variety of MHC class I-like molecules that are induced by cellular injury - MIC and ULBP in humans, and RAE, H60 and MULT1 in mice [4]. Through these receptors, NK cells recognize and eliminate allogeneic, virally infected and transformed cells. Ly49H, Ly49D and NKG2D are the ligand-binding subunits of receptor complexes, which include transmembrane adaptor proteins that are required for cell surface expression of the complex and delivery of intracellular signals. Each adaptor initiates a distinct signaling pathway that dictates the type of response induced by receptor engagement of ligand [5-7].
The adapter DAP12 contains an immunoreceptor tyrosine-based activation motif (ITAM), which, upon phosphorylation, acts as docking site for the protein tyrosine kinases Syk and ZAP70. This signaling pathway triggers both cytotoxicity and secretion of interferon-gamma (IFN-γ)[5-7]. The adapter DAP10 contains a YxNM motif that recruits phosphatidylinositol-3-kinase (PI-3K)[8, 9] and the Grb2-Vav complex [5, 10, 11]. This signaling pathway is sufficient for inducing cytotoxicity, but is inefficient in triggering cytokine production [12].
Receptor-adaptor pairing depends on compatibility of the transmembrane and cytoplasmic domains. Initial studies indicated that Ly49H and Ly49D associate with DAP12 [13, 14], whereas NKG2D associates with DAP10 [15]. Subsequently, it became evident that each receptor may not be anchored to a single adaptor. Mice express two isoforms of NKG2D that are generated by alternative splicing and differ only in their cytoplasmic domains. Consequently, the two isoforms are identical in terms of ligand binding but have disparate affinities for DAP10 and DAP12; the long form (NKG2D-long) associates exclusively with DAP10, whereas the short form (NKG2D-short) recruits both DAP10 and DAP12 [16, 17]. Because of this promiscuity, NKG2D-short is capable of activating a broader spectrum of signaling molecules than NKG2D-long, triggering both cytotoxicity and IFN-γ production. Whether this additional flexibility is limited to NKG2D or is shared by other activating receptors has not been fully investigated.
Recent studies have suggested that Ly49H and Ly49D, like NKG2D, associate with both DAP12 and DAP10, at least in transfected cells [18]. Detectable cell surface expression of Ly49D in DAP12-deficient NK cells [19] and residual Ly49D-mediated cytotoxicity in DAP12-loss of function NK cells [20] also support the hypothesis that Ly49D can associate with an alternative adaptor. However, whether DAP10 associates with Ly49D and Ly49H in primary cells and contributes to their function in vivo has not been determined.
To address this directly, we assessed the relative contributions of DAP10 and DAP12 to Ly49H and Ly49D function in vitro and in vivo using mice deficient for either DAP10-/-, DAP12-/- or both DAP10 and DAP12 (DAP10/DAP12-/-). We demonstrate that cell surface expression of Ly49H and Ly49D is somewhat reduced on NK cells lacking DAP10. However, DAP10 does not significantly contribute to Ly49H-mediated control of murine cytomegalovirus (MCMV) infection. Thus, although Ly49D and Ly49H can associate with DAP10 in NK cells, this promiscuity does not significantly alter their function in vitro or in vivo. We conclude that while many activating NK cell receptors may be somewhat promiscuous in terms of adaptor association, the degree of promiscuity clearly varies widely.
Results and discussion
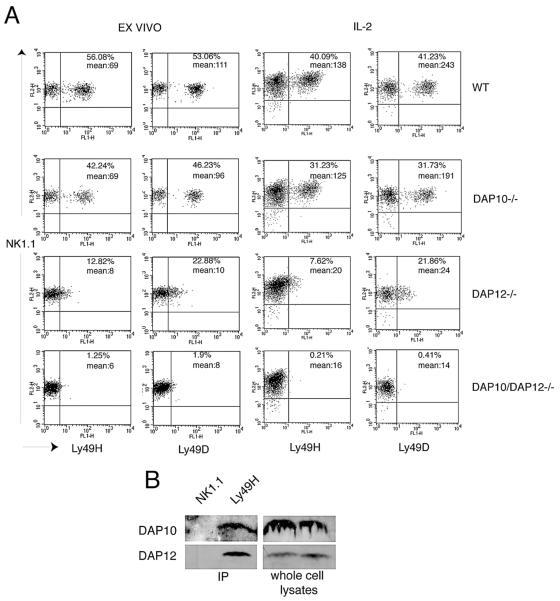
DAP10 contributes to Ly49D and Ly49H expression
Because recent studies suggested that Ly49H and Ly49D associate with both DAP12 and DAP10 in transfected cells [18], we assessed Ly49D and Ly49H expression in DAP10-/-, DAP12-/- and DAP10/DAP12-/- mice. WT, DAP10-/-, DAP12-/- and DAP10/DAP12-/- NK cells isolated ex-vivo or cultured in IL-2 were stained with anti-Ly49H and anti-Ly49D antibodies. As previously reported [19], lack of DAP12 resulted in a marked reduction of Ly49D and Ly49H expression in all NK cells, although expression was not completely abrogated (Fig. 1). Conversely, lack of DAP10 caused a slight reduction in Ly49H and Ly49D expression. Ly49H and Ly49D expression was completely abolished in NK cells lacking both DAP10 and DAP12 (Fig. 1A). Moreover, immunoprecipitation of Ly49H from IL-2 cultured NK cells followed by immunoblotting with anti-DAP10 and anti-DAP12 antibodies confirmed that Ly49H recruits DAP10 as well as DAP12 (Fig. 1B). We conclude that Ly49D and Ly49H rely primarily on DAP12 for cell surface expression in NK cells, although both also associate with DAP10 to a small extent.
Fig. 1. DAP10 contributes to Ly49H and Ly49D expression.
(A) NK cells from WT, DAP10-/-, DAP12-/- and DAP10/DAP12-/- mice were purified from spleen and stained ex vivo or after culture in IL-2 (LAK) with anti-Ly49D and anti-Ly49H antibodies. Ly49D and Ly49H expression on gated NK1.1+ CD3- cells is shown. Results are representative of at least 3 independent experiments. (B) 15×106 IL-2 cultured NK cells were lysed and immunoprecipitated with anti-Ly49H or anti-NK1.1 as a control. Immunoprecipitates were analyzed by immunoblotting with anti-DAP10 and anti-DAP12 antibodies. The right panels show immunoblots of whole cell lysates with anti-DAP10 and anti-DAP12 antibodies to control for the amount of protein.
No evidence for DAP10-dependent Ly49D- and Ly49H-mediated cytotoxicity and IFN-γ production in vitro
To determine whether DAP10 contributes to Ly49H- and Ly49D-mediated cytotoxicity, we compared the ability of WT, DAP10-/-, DAP12-/- and DAP10/DAP12-/- NK cells cultured in IL-2 for 5-7 days to kill Baf/3 cells transfected with either the Chinese Hamster class I molecule Hm1-C4, which engages Ly49D, or m157, which engages Ly49H. Lack of DAP10 did not affect Ly49H- and Ly49D-mediated cytotoxicity (Fig. 2A-B). Additionally, Ly49D- and Ly49H-mediated cytotoxicity was abrogated to the same extent in DAP12-/- and DAP10/DAP12-/- NK cells. Consistent with these results, lysis of Chinese hamster ovary (CHO) cells, which express Hm1-C4, was entirely DAP12- dependent and DAP10-independent (Fig. 2C).
Fig. 2. Ly49H- and Ly49D-mediated cytotoxicity and IFN-γ production in vitro are DAP10-independent.
(A-C) WT, DAP10-/-, DAP12-/- and DAP10/DAP12-/- NK cells cultured in IL-2 for 5-7 days were challenged with parental Baf/3 cells, Baf/3 cells transfected with m157 (A) or Hm1-C4 in the presence or absence of an anti-Ly49D antibody (B), or CHO cells in the presence or absence of an anti-Ly49D antibody (C). Cytotoxicity was measured by standard chromium release assay. Results are representative of at least 3 independent experiments. (D) WT and DAP12-/- NK cells were cultured in IL-2 for 3 days and tested for cytolytic activity against Baf/3 cells transfected with m157 or CHO cells in the presence or absence of an anti-Ly49D or anti-Ly49H antibody. Results are representative of at least 3 independent experiments. (E) DAP10-/-, DAP12-/-, DAP10/DAP12-/- and WT NK cells cultured in IL-2 for 5-7 days were stimulated with plastic-coated anti-Ly49D, -Ly49H, -NKG2D and - NK1.1 antibodies. After 16 hours of stimulation, cell culture supernatants were assayed by cytometric beads array for IFN-γ production. Data presented are mean values ± SD from 5 independent experiments.
In contrast to our results, a previous report indicated that NK cells derived from DAP12 loss-of-function mice retain some ability to kill Chinese hamster ovary (CHO) cells [20]. Because these results were obtained using NK cells cultured in IL-2 for three days rather than 5-7 days as in our experiments, we also tested the lytic capacity of NK cells at day three of culture. Again, lack of DAP12 completely abrogated Ly49H- and Ly49D-mediated cytotoxicity (Fig. 2D). We conclude that, despite the association of Ly49D and Ly49H with DAP10, Ly49D and Ly49H-mediated lysis of target cells in vitro depends exclusively on DAP12.
Because NK cells are also a source of IFN-γ, we asked whether lack of DAP10 affects Ly49D- or Ly49H-mediated production of IFN-γ. We cultured WT, DAP10- /-, DAP12-/- and DAP10/DAP12-/- NK cells in IL-2, then seeded them in wells coated with antibodies that bind Ly49D, Ly49H, NKG2D or NKR-P1C (NK1.1 in C57BL/6 mice) and measured cytokine production. Ly49D and Ly49H-mediated IFN-γ production was unaffected by lack of DAP10 and, like cytotoxicity, was equally abrogated by lack of DAP12 or both DAP10 and DAP12 (Fig. 2D). This strongly implies that Ly49D- and Ly49H-effector functions rely mainly on DAP12. NKG2D stimulation induced very little IFN-γ production, which was strongly impaired by the lack of both DAP12 and DAP10. NKR-P1C signals through FcRγ [21], independent of both DAP12 and DAP10. Consistent with this, NKR-P1C-mediated cytokine production was not reduced in the absence of DAP10, DAP12 or both DAP10 and DAP12; somewhat surprisingly, it was slightly augmented although the differences were not statistically significant (Fig. 2D).
Role of DAP10 and DAP12 in controlling MCMV infection
Effective NK cell recognition of murine cytomegalovirus (MCMV)-infected cells depends on binding of Ly49H to the m157 viral glycoprotein [1, 2]. Upon Ly49H- m157 interaction, NK cells become activated and proliferate [22]. As effector NK cells, they can both kill MCMV-infected cells and secrete IFN-γ, which triggers both innate and adaptive immune responses. To investigate the impact of DAP10 and DAP12 on NK cell-mediated control of MCMV, WT, DAP10-/-, DAP12-/- and DAP10/DAP12-/- mice were infected with MCMV and sacrificed 4 days post-infection to measure viral titers in the spleens and livers. A group of WT and DAP10-/- mice was administered BrdU intraperitoneally during MCMV infection to measure spleen NK cell proliferation. NK cell proliferation (data not shown) and viral titers in the spleens were similar in WT and DAP10-/- mice (Fig. 3). Spleen viral titers were higher in DAP12-/- than WT mice, as previously reported [23]. Although splenic viral titers were slightly higher in DAP10/DAP12-/- than DAP12-/- mice, this difference was not statistically significant with the number of mice used. Together, these results show that DAP10 has no significant role in NK cell-mediated elimination of MCMV infected cells in vivo. In contrast to previous reports [23], viral titers in the livers of DAP12-/- mice were not significantly lower than those of WT mice. Additionally, lack of DAP10, either alone or in combination with lack of DAP12, did not increase susceptibility to MCMV infection. These results suggest that MCMV clearance in the liver depends only minimally on Ly49H-signaling and may involve other signaling pathways. Accordingly, we have recently shown that mice that lack PLCγ2 can control MCMV in the liver just like WT mice, although Ly49H is incapable of triggering cytotoxicity or IFN-γ secretion [24].
Fig. 3. Control of MCMV infection is DAP10-independent.
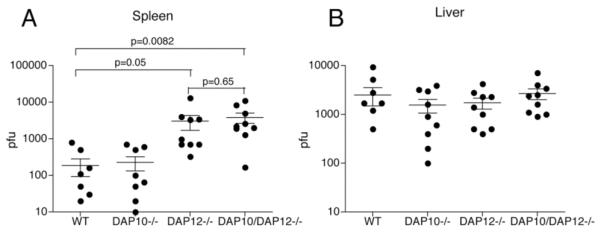
(A) Age-matched WT, DAP10-/-, DAP12-/- and DAP10/DAP12-/- mice were infected i.p. with 5 × 104 PFU of MCMV. Four days post infection, mice were sacrificed and viral loads determined in the spleen (A) and liver (B) by plaque assay. Data presented are mean values ± SD from 3 independent experiments. Although DAP12-/- mice had higher viral titers in the spleen than WT mice, the difference only borders on statistical significance. In contrast, the difference between viral titers in the spleens of DAP10/DAP12-/- and WT mice is highly statistically significant.
In conclusion, our results show that although Ly49H and Ly49D associate predominantly with DAP12 on NK cells, DAP10 is recruited by a small proportion of these receptors. DAP10 can clearly mediate cell surface expression of Ly49D and Ly49H on NK cells, however no functional correlate of this association is evident in vitro or in vivo. The limited contribution of DAP10 to Ly49D and Ly49H signaling contrasts with its major role in NKG2D signaling [5, 6]. Because multiple activating receptors in NK cells and myeloid cells can potentially recruit DAP12 and/or DAP10, it is likely that these receptors recruit them, although to differing degrees, depending on the conformational compatibility of their transmembrane and cytosolic domains with that of DAP12 and DAP10. Differing degrees of promiscuity for each receptor may further tune NK cell responses as a whole.
Material and Methods
Mice
DAP10-/- and DAP12-/- mice have been described [17, 25]. DAP10/DAP12-/- mice were obtained from Dr. T. Takai. All mice are on C57BL/6 background, facilitated by genome-wide SSLP typing at 10cM intervals by the Rheumatic Disease Core Center’s speed congenic lab at Washington University School of Medicine. Animal studies were approved by the Washington University Animal Studies Committee.
Cell cultures, antibodies and flow cytometry
NK cells were purified from spleens by positive selection with DX5 microbeads (Miltenyi Biotech). Cells were expanded in IL-2 (1000U/ml) for 7 days. Ex-vivo purified cells and IL-2 cultured cells were stained with antibodies specific for NK1.1, CD3, Ly49D (Pharmingen) and Ly49H (3D10) [22]. Before labeling, cells were always preincubated with mAb 2.4G2 (ATCC) to block Fc-receptors.
Immunoblot
Spleen NK cells were isolated, cultured 5-8 days in rIL-2 (1000U/ml), lysed with in lysis buffer (1% β-maltoside, 50 Mm Tris-HCl, pH 8.0, 150 mM NaCl, 1mM EDTA, 1.5mM MgCl2, 10% glycerol, plus protease inhibitors) and immunoprecipitated with anti-NK1.1 and anti-Ly49H antibodies. Precipitated proteins were fractionated by SDS-PAGE, transferred to nitrocellulose membranes and probed with anti-DAP10 and anti-DAP12 antibodies, which have been described [17, 25]. To confirm that all substrates were adequately immunoprecipitated, whole cell lysates were reprobed with anti-DAP10 and anti- DAP12 antibodies.
Cytotoxicity assays
Cytotoxicity against Baf/3, Baf/3-m157, Baf/3-C-4 and CHO target cells was performed by standard chromium release assay using NK cells purified with DX5 microbeads (Miltenyi). NK cells were cultured in rIL-2 (1000U/ml) for 3-5 days as previously described [17].
IFN-γ measurement
IFN-γ was quantified by cytometric bead array (BDTM CBA Mouse Inflammation Kit). NK cells isolated with DX5 microbeads were plated into 96 well plates coated with anti-NK1.1, anti-Ly49D, anti-Ly49H and anti-NKG2D (A10, BD) (50 μg/ml). Before stimulation, cells were preincubated with mAb 2.4G2 (ATCC) to block Fc-receptors. Supernatants were collected after 15h and IFN-γ release was measured according to the manufacturer’s directions.
Mouse infections and plaque assays
C57BL/6 and knockout mice were housed in a biosafety level 2 facility at Washington University in accordance with all federal and university policies. MCMV (ATCC VR-1399) was grown and titered on NIH 3T12 fibroblasts (ATCC CCL 164). Salivary gland-passaged stocks (sgMCMV) were prepared by infecting BALB/c mice with 105 PFU of tissue culture-passaged MCMV, and salivary glands were harvested and homogenized in DMEM (Invitrogen Life Technologies) with 10% BCS (HyClone). Mice were infected with 5 × 104 PFU of sgMCMV i.p., and organs were harvested 3 days postinfection and frozen in 1 ml of DMEM at -80°C. Titers were determined by plaque assay on 3T12 fibroblasts. After mechanical disruption by bead-beating (BioSpec), serial 10-fold dilutions were made and plated in duplicate on 3T12 monolayers in six-well plates (Costar). After infection in minimal medium at 37°C for 1 h, monolayers were overlaid with 2 ml of 2% DMEM-0.5% Noble agar and incubated for 6 days (37°C, 5% CO2) with one overlay of 2 ml at 3 days. At 6 days, plates were overlaid with 2.5% neutral red in DMEM-0.5% Noble agar. Plaques were counted 12 h later. Statistical significance of differences in viral titers between groups of mice was determined by unpaired t test using the Prism software. For intracellular proliferation experiments, age-matched WT and DAP10-/- mice were infected i.p. with 5 × 104 PFU of MCMV. On days 4 and 6 after infection, mice were injected with 2mg of BrdU and sacrificed 3 h later. Cell preparation were depleted of red blood cells and incubated for 10 min with 2.4G2 culture supernatant to block non-specific binding of the antibody. To stain incorporated BrdU, cells were first surface-stained with biotinylated 3D10 followed by PE-streptavidin and allophycocyanin-PK136. Cells were fixed, permeabilized, treated with DNase and stained with FITC-anti-BrdU (BrdU Flow kit).
Acknowledgments
We would like to thank Sandeep K. Tripathy for sharing the Ly49H antibody and Tammy P. Cheng for providing MCMV stocks. This paper was supported by National Institutes of Health Grant R01AI056139-05.
Glossary
Abbreviation
- MCMV
Murine cytomegalovirus
- CHO cells
chinese hamster ovary cells
References
- 1.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 2.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa H, Iizuka K, Poursine-Laurent J, Shastri N, Yokoyama WM. A ligand for the murine NK activation receptor Ly-49D: activation of tolerized NK cells from beta 2-microglobulin-deficient mice. J Immunol. 2002;169:126–136. doi: 10.4049/jimmunol.169.1.126. [DOI] [PubMed] [Google Scholar]
- 4.Eagle RA, Traherne JA, Ashiru O, Wills MR, Trowsdale J. Regulation of NKG2D ligand gene expression. Hum Immunol. 2006;67:159–169. doi: 10.1016/j.humimm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tassi I, Klesney-Tait J, Colonna M. Dissecting natural killer cell activation pathways through analysis of genetic mutations in human and mouse. Immunol Rev. 2006;214:92–105. doi: 10.1111/j.1600-065X.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SK, Ortaldo JR, McVicar DW. The ever-expanding Ly49 gene family: repertoire and signaling. Immunol Rev. 2001;181:79–89. doi: 10.1034/j.1600-065x.2001.1810106.x. [DOI] [PubMed] [Google Scholar]
- 8.Giurisato E, Cella M, Takai T, Kurosaki T, Feng Y, Longmore GD, Colonna M, Shaw AS. Phosphatidylinositol 3-kinase activation is required to form the NKG2D immunological synapse. Mol Cell Biol. 2007;27:8583–8599. doi: 10.1128/MCB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D-mediated signaling requires a DAP10- bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7:524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 10.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 11.Graham DB, Cella M, Giurisato E, Fujikawa K, Miletic AV, Kloeppel T, Brim K, Takai T, Shaw AS, Colonna M, Swat W. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177:2349–2355. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- 12.Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, Lanier LL, Colucci F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 13.Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly- 49D and Ly-49H associate with mouse DAP12 and form activating receptors. J Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 14.Mason LH, Willette-Brown J, Anderson SK, Gosselin P, Shores EW, Love PE, Ortaldo JR, McVicar DW. Characterization of an associated 16-kDa tyrosine phosphoprotein required for Ly-49D signal transduction. J Immunol. 1998;160:4148–4152. [PubMed] [Google Scholar]
- 15.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 16.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 17.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 18.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 19.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 20.Tomasello E, Desmoulins PO, Chemin K, Guia S, Cremer H, Ortaldo J, Love P, Kaiserlian D, Vivier E. Combined natural killer cell and dendritic cell functional deficiency in KARAP/DAP12 loss-of-function mutant mice. Immunity. 2000;13:355–364. doi: 10.1016/s1074-7613(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 21.Arase N, Arase H, Park SY, Ohno H, Ra C, Saito T. Association with FcRgamma is essential for activation signal through NKR-P1 (CD161) in natural killer (NK) cells and NK1.1+ T cells. J Exp Med. 1997;186:1957–1963. doi: 10.1084/jem.186.12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dokun AO, Kim S, Smith HR, Kang HS, Chu DT, Yokoyama WM. Specific and nonspecific NK cell activation during virus infection. Nat Immunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 23.Sjolin H, Tomasello E, Mousavi-Jazi M, Bartolazzi A, Karre K, Vivier E, Cerboni C. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J Exp Med. 2002;195:825–834. doi: 10.1084/jem.20011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tassi I, Presti R, Kim S, Yokoyama WM, Gilfillan S, Colonna M. Phospholipase C-gamma 2 is a critical signaling mediator for murine NK cell activating receptors. J Immunol. 2005;175:749–754. doi: 10.4049/jimmunol.175.2.749. [DOI] [PubMed] [Google Scholar]
- 25.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]