Abstract
Objective
To determine if a simplified model for predicting pre-eclampsia can be developed by combining first trimester serum analytes, PAPP-A and free β-hCG, and maternal characteristics.
Methods
A retrospective cohort study of patients seen for first-trimester aneuploidy screening from 2003–2009. The 5th, 10th, 90th and 95th percentiles for the analyte-MoMs for our population were determined and evaluated for association with pre-eclampsia. Univariate and backward stepwise logistic regression analyses were performed and the area under the ROC curves (AUC) used to determine the best models for predicting pre-eclampsia.
Results
Among 4,020 women meeting the inclusion criteria, outcome data was available for 3,716 (93%). There were 293 cases of pre-eclampsia. The final model identified a history of pre-gestational diabetes (aOR 2.6, 95% CI 1.7–3.9), chronic hypertension (aOR 2.6, 95% CI 1.7–3.9), maternal BMI >25 (aOR 2.5, 95% CI 1.9–3.4), African American race (aOR 1.8, 95% CI 1.3–2.6), and PAPP-A MoM <10th percentile (aOR 1.6, 95% CI 1.1–2.4) to be significant predictors of pre-eclampsia. (AUC= 0.70, 95% CI 0.65–0.72)
Conclusion
Low first-trimester PAPP-A levels are associated with the development of pre-eclampsia; however, the model was only modestly efficient in its predictive ability.
Keywords: pre-eclampsia, PAPP-A, free β-hCG, first trimester screening
Introduction
Pre-eclampsia remains a major cause of both perinatal and maternal morbidity and mortality. (Confidential Enquiries, 2001) The etiology of pre-eclampsia is not completely understood, and currently, there are no reliable tests to identify an at-risk population. (Yu et al., 2006) Given the high prevalence of pre-eclampsia and its associated morbidities, early identification of women at high risk for developing this disorder could heighten awareness for both patients and providers and possibly prompt physicians to employ increased clinical surveillance.
The association of low first trimester levels of pregnancy-associated plasma protein A (PAPP-A) with adverse pregnancy outcomes, including pre-eclampsia, has previously been demonstrated in multiple studies. (Ong et al., 2000; Smith et al., 2002; Yaron et al., 2002; Tul et al., 2003; Dugoff et al., 2004; Krantz et al., 2004; Spencer et al., 2006; Spencer et al., 2008; Spencer et al., 2008; Cowans et al., 2007; Spencer et al., 2008) One of these studies also found that a decreased level of free beta human chorionic gonadotropin (β-hCG) in the first trimester was associated with pre-eclampsia.(Ong et al., 2000) In the majority of these reports, abnormal levels of these first trimester markers yielded a low positive predictive value for the development of pre-eclampsia with overall weak statistical significance.
The objectives of this study were to 1) evaluate the association between abnormal levels of first trimester serum analytes, PAPP-A and free β-hCG, and the subsequent development of pre-eclampsia in our patient population and 2) develop an integrated model for the prediction of pre-eclampsia using a combination of these first trimester serum markers and maternal characteristics.
Methods
This was a retrospective cohort study of all patients with singleton gestations who underwent first trimester screening for aneuploidy between 2003 and 2009 at Washington University Medical Center in St. Louis. Approval from the institutional review board at our institution was obtained. First trimester screening was performed between 11 weeks and 0 days and 13 weeks and 6 days gestation. Ultrasound examinations were performed by experienced obstetrical sonographers with Certification in Competence in the 11–13 weeks scan by the Fetal Medicine Foundation. All images were reviewed by certified maternal-fetal medicine specialists. All pregnancies were dated by last menstrual period (LMP) if consistent with crown-rump length (CRL) measurements (±7 days) or by the CRL measurement if it was not consistent with menstrual dating. Cases of aneuploidy were excluded from the study.
The prenatal diagnosis database at our institution was used to extract maternal demographics, obstetrical history, ultrasound findings, serum analyte levels and neonatal outcome information. Pregnancy outcome information is routinely obtained by dedicated nurse coordinators at our institution. For the greater than 90% of patients delivering within our health care system, outcome data is extracted from our perinatal computerized database. The small remainder of patients are given a standardized form to be returned after delivery providing the details about their pregnancy outcome. The standardized follow-up sheet includes details about pregnancy complications, delivery indications, and neonatal outcomes, including chromosomal and structural abnormalities. When a form is not returned within four weeks of the expected date of delivery, the patient is contacted by phone by the coordinator. In cases where the patient cannot be contacted, the outcome information is obtained from her referring physician.
Pre-eclampsia was defined as blood pressure of >140 mm Hg systolic and/or 90 mm Hg diastolic on two or more occasions with proteinuria of 0.3 gm or more in a 24-hour urine specimen occurring after 20 weeks gestation in a woman with previously normal blood pressure. If a 24-hour urine sample was not available, proteinuria was diagnosed as 1+ or greater on urine dipstick. (ACOG, 2002) In patients with chronic hypertension, superimposed pre-eclampsia was diagnosed in cases of acute onset proteinuria and worsening hypertension as determined by the patient’s physician. (ACOG, 2001) PAPP-A and free β-hCG levels were converted to multiples of the median (MoM), adjusting for gestational age, ethnicity, maternal weight, insulin-dependent diabetes, history of anti-convulsant use, and history of a previous fetal neural tube defect by the commercial labs used for first trimester screening at our institution. (Genzyme and GeneCare). The 5th, 10th, 90th and 95th percentiles for the analyte-MoMs for our population were determined and evaluated for association with pre-eclampsia. Pearson chi-squared or Fisher’s exact tests were used for the comparison of categorical variables, and Student’s t- tests were used for continuous variables. Univariate analysis was initially performed to determine variables with a significant association with pre-eclampsia. Backward stepwise logistic regression was then used to determine the final prediction model for pre-eclampsia. Variables were included in the model based on a significant P value (<0.10) in the univariate analysis. The variables with the highest non-significant P values were then sequentially removed until the area under the ROC curve (AUC) was optimized or when the further removal of variables resulted in a fall in the AUC.
A scoring system to evaluate the efficiency of the model for the prediction of pre-eclampsia was then developed, allocating a score=1 for each positive variable. The sensitivity, specificity and likelihood ratios (LR), both positive and negative, and their 95% confidence intervals (CI) were then determined for each potential score. Statistical analyses were performed using STATA software package, version 10, Special Edition (College Station, TX).
Results
Of the 4,035 women in our cohort, outcome data was available for 3,741 (93%) patients. After excluding 25 patients with aneuploidy, 3,716 patients were included in the analysis. The demographic distribution of our population and univariate analysis of maternal characteristics is shown in Table 1. The mean maternal age of patients in our cohort was 34.8 ± 4.3 years, and the majority were of Caucasian race. 293 (7.9%) patients were classified as having pre-eclampsia. Maternal characteristics that were identified in the univariate analysis as significant risk factors for the development of pre-eclampsia included African American race, maternal age <25 years old, both overweight and obese maternal BMI, maternal drug use, pre-gestational diabetes and chronic hypertension.
Table 1.
Demographics of the cohort and univariate analysis for the association between maternal characteristics and pre-eclampsia
| Demographic | Pre-eclampsia (n=293) % |
Normal (n-3,423) % |
RR (95% CI) |
p-value |
|---|---|---|---|---|
| Maternal Age (yrs) | ||||
| <25 | 20 (6.8) | 99 (2.9) | 2.2 (1.5–3.3) | 0.002 |
| 25–34 | 95 (32.4) | 1,064 (31.1) | Reference | - |
| 35–39 | 144 (49.1) | 1,868 (54.6) | 0.8 (0.6–1.0) | 0.07 |
| ≥40 | 34 (11.6) | 392 (11.4) | 1.0 (0.7–1.4) | 0.93 |
| Maternal Race | ||||
| Caucasian | 214 (73.0) | 2,828 (82.6) | Reference | - |
| African American | 63 (21.5) | 283 (8.3) | 2.7 (2.1–3.4) | <0.001 |
| Asian | 4 (1.4) | 92 (2.7) | 0.5 (0.2–1.4) | 0.17 |
| Hispanic | 1 (0.3) | 56 (1.6) | 0.2 (0.0–1.5) | 0.08 |
| Other | 11 (3.7) | 164 (4.8) | 0.9 (0.4–1.7) | 0.70 |
| Chronic HTN | 47 (16.0) | 121 (3.5) | 3.9 (3.0–5.2) | <0.001 |
| Pre-gestational DM | 28 (9.5) | 66 (1.9) | 4.0 (2.9–5.6) | <0.001 |
| Tobacco Use | 21 (7.2) | 182 (5.3) | 1.3 (0.9–2.0) | 0.19 |
| Alcohol Use | 60 (20.4) | 768 (22.4) | 0.9 (0.7–1.1) | 0.35 |
| Illicit Drug Use | 7 (2.4) | 21 (0.6) | 3.1 (1.6–6.0) | 0.009 |
| Maternal BMI (kg/m2) | ||||
| <18.5 | 3 (1.0) | 87 (2.5) | 0.4 (0.1–1.3) | 0.11 |
| 18.5–24.9 | 72 (24.6) | 1666 (48.7) | Reference | - |
| 25–29.9 | 78 (26.6) | 742 (21.7) | 1.3 (1.0–1.7) | 0.04 |
| ≥30 | 108 (36.9) | 581 (17.0) | 2.7 (2.1–3.4) | <0.001 |
| Unknown* | 32 (10.9) | 347 (10.1) | 1.1 (0.7–1.5) | 0.67 |
HTN=hypertension; DM=diabetes mellitus; BMI=body mass index; RR=relative risk; CI=confidence interval
Indicates patients for which either height, weight, or both were missing from patient questionnaire
The results of the association between maternal serum analytes and pre-eclampsia using various thresholds of both PAPP-A and free β-hCG are shown in Table 2. There was no statistically significant association between free β-hCG and pre-eclampsia across all defined thresholds. PAPP-A MoM <10th percentile (RR 1.6, 95% CI 1.2–2.2) and PAPP-A MoM <5th percentile (RR 1.6, 95% CI 1.1–2.4) were found to be significantly associated with pre-eclampsia. There was no significant association between high levels of PAPP-A and pre-eclampsia.
Table 2.
Univariate analysis for first trimester serum analytes and their association with pre-eclampsia
| MoM | Percentile | PEC n=293 (%) |
Normal n=3423 (%) |
RR (95% CI) |
P-value |
|---|---|---|---|---|---|
| PAPP-A | |||||
| <0.46 | <5th | 23 (7.8) | 158 (4.6) | 1.6 (1.1–2.4) | 0.01 |
| <0.58 | <10th | 45 (15.3) | 318 (9.3) | 1.6 (1.2–2.2) | 0.001 |
| >2.31 | >90th | 33 (11.3) | 336 (9.8) | 1.1 (0.8–1.6) | 0.47 |
| >2.83 | >95th | 15 ( 5.1) | 170 (4.9) | 1.0 (0.6–1.7) | 0.94 |
| Free β-hCG | |||||
| <0.47 | <5th | 17 (5.8) | 169 (4.9) | 1.2 (0.7–1.8) | 0.53 |
| <0.56 | <10th | 29 (9.9) | 310 (9.0) | 1.1 (0.7–1.5) | 0.68 |
| >1.99 | >90th | 28 (9.5) | 340 (9.9) | 0.9 (0.6–1.4) | 0.78 |
| >2.38 | >95th | 13 (4.4) | 165 (4.8) | 0.9 (0.5–1.5) | 0.73 |
PEC=pre-eclampsia; RR=relative risk; CI=confidence interval; MoM=multiples of the median
Using backward stepwise logistic regression, a final model to predict the risk of pre-eclampsia was developed. The model identified a history of pre-gestational diabetes (aOR 2.6, 95% CI 1.7–3.9), chronic hypertension (aOR 2.6, 95% CI 1.7–3.9), maternal BMI >25 (aOR 2.5, 95% CI 1.9–3.4), African American race (aOR 1.8, 95% CI 1.3–2.6), and PAPP-A MoM <10th percentile (aOR 1.6, 95% CI 1.1–2.4) to be significant predictors of pre-eclampsia. (AUC=0.70, 95% CI 0.65–0.72) (Figure 1) The regression equation for the model is as follows: Log Odds of Pre-eclampsia = −3.25 + 0.51 x PAPP-A (1 if <10th%ile, 0 if >10th%ile) + 0.93 x BMI (1 if >25, 0 if ≤25) + 0.94 x cHTN (1 if cHTN present, 0 if absent) + 0.97 x DM (1 if DM present, 0 if absent) + 0.61 x African American Race (1 if African American, 0 if other race); R2=0.07, p<0.001. Including PAPP-A as a continuous variable rather than a categorical variable in the model did not alter its predictive efficiency. (AUC 0.69 vs. 0.70) A scoring system was then developed based on the significant factors identified in the final model (score=1 for each positive factor). A score of ≥2 was identified as the best predictor for pre-eclampsia with a sensitivity of 36.4% (95% CI 30.6–42.6), specificity of 86.8% (95% CI 85.6–88.0), and LR+ of 2.8 (95% CI 2.3–3.3). (Table 3)
Figure 1.
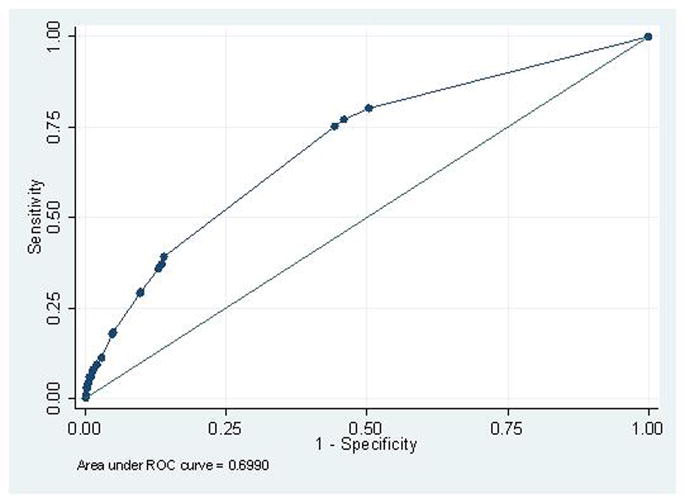
Table 3.
Scoring system for the prediction of pre-eclampsia
| Score | Sensitivity (95% CI) |
Specificity (95% CI) |
Correctly Classified | LR+ (95% CI) |
LR- (95% CI) |
|---|---|---|---|---|---|
| ≥1 | 80.2% (74.8–84.9) |
49.6% (47.8–51.4) |
52.0% | 1.6 (1.5–1.7) |
0.40 (0.31–0.51) |
| ≥2 | 36.4% (30.6–42.6) |
86.8% (85.6–88.0) |
82.8% | 2.8 (2.3–3.3) |
0.73 (0.67–0.80) |
| ≥3 | 9.3% (6.1–13.5) |
98.0% (97.4–98.5) |
91.0% | 4.6 (2.9–7.3) |
0.92 (0.89–0.96) |
| ≥4 | 3.5% (1.6–6.5) |
99.7% (99.5–99.9) |
92.1% | 13.1 (5.1–33.6) |
0.97 (0.95–0.99) |
| 5 | 0.8% (0.1–2.8) |
99.9% (99.8–100.0) |
92.1% | 23.3 (2.1–255.7) |
1.0 (0.98–1.01) |
LR= likelihood ratio; CI=confidence interval
Discussion
Results from our study confirm the previously established association between first-trimester low levels of PAPP-A and the subsequent development of pre-eclampsia; however, we demonstrated no association between extreme levels of free β-hCG and pre-eclampsia. In a large, multicenter study of 34,271 women, Dugoff et al. demonstrated that women with low first-trimester PAPP-A levels were significantly more likely to experience pre-eclampsia along with other adverse obstetric outcomes. A pattern of increased risk was noted as the level of PAPP-A became more extreme. Free β-hCG was not found to be associated with pre-eclampsia in that study. (Dugoff et al., 2004) Similar were the results of a study of 5,584 patients which demonstrated a significant association between low PAPP-A levels and pregnancy complications including pre-eclampsia. In contrast to our results, this study also reported a significant association between first trimester free β-hCG levels ≤10th percentile and the subsequent development of pre-eclampsia. (Ong et al., 2000)
Individually, low levels of PAPP-A have a low sensitivity to predict the future risk of developing pre-eclampsia. This factor has been combined with other parameters including uterine artery Doppler flow studies to improve the predictive capability; however, there remains a need to develop better methods for early detection. (Spencer et al., 2005) Our study aimed to combine first trimester PAPP-A levels with other maternal characteristics including pre-gestational diabetes, chronic hypertension, African American race, and maternal BMI >25 to improve the yield of predicting pre-eclampsia risk. Although PAPP-A MoM levels are already adjusted for maternal weight, ethnicity, and maternal insulin-dependent diabetes prior to being reported by the lab, maternal BMI >25, African American race, and a history of pre-gestational diabetes were found to be independent predictors of pre-eclampsia in our analysis and, therefore, were included in our model. Our prediction model generated an AUC of 0.70 (95% CI 0.65–0.72), indicating an only modest efficiency for the prediction of pre-eclampsia. In order to further refine the model, the scoring system was created, identifying a score ≥2 as the best predictor for pre-eclampsia (LR+ 2.8). Using this particular threshold, a balance between sensitivity and specificity is met. A score ≥2 yielded a sensitivity of 36.4% and a specificity of 86.8%, while a score of ≥3 yielded a higher specificity of 98.0% at the price of a lower sensitivity of 9.3%. Given the high morbidity and mortality associated with pre-eclampsia, the best screening test would identify as many patients as possible; therefore, the higher false positive rate (13.2%) of a score ≥2 is acceptable. A recent study by Poon et al. also created a prediction model for the risk of pre-eclampsia by combining first trimester serum analytes and Doppler flow indices after assigning patient-specific a priori risks based on historic factors and biophysical parameters. This model had a detection rate of 88.5% for early pre-eclampsia and 46.7% for late pre-eclampsia at a 5% false positive rate. While this model was able to predict those at highest risk for disease, the more narrow false positive rate may have excluded those at more moderate risk for disease. (Poon et al., 2010) Close surveillance of patients at more moderate risk for disease may also be clinically useful. Our model may aid in the identification of this moderate risk group. Placental developmental and functional impairment during the first trimester appears to affect the development of hypertension later in pregnancy. Low levels of PAPP-A at 10–14 weeks may be a marker of impaired placentation and a smaller placental mass. (Ong et al., 2000) The paracrine effects of insulin-like growth factors (IGF) are thought to control the invasion of trophoblasts into the decidua. As PAPP-A is a protease for IGF binding proteins (IGFBP), low PAPP-A is associated with high levels of IGFBP. This consequently results in a lowering of free IGF, leading to impaired invasion of the trophoblasts into the maternal decidua. This hypothesis provides biologic plausibility for the association of low first trimester PAPP-A and the development of pre-eclampsia later in pregnancy. (Dugoff et al., 2004; Kniss et al., 1994; Clemmons, 1998; Irwin et al., 1999)
Strengths of our study include our robust genetic database with dedicated outcome coordinators responsible for obtaining accurate and complete outcome data. We were also able to confidently exclude aneuploidy cases from our analysis given that all chromosomal abnormalities were confirmed by chorionic villus sampling, amniocentesis or postnatal newborn testing. Our study is not without limitations, including its retrospective design. Our relatively small sample size also prevented us from performing a sub-analysis of the association between first trimester analytes and varying degrees of pre-eclampsia severity. Our model also lacked some of the rigorous parameters such as well-characterized patient-specific a priori risks, maternal mean arterial pressure, uterine artery Doppler indices and new first-trimester analytes such as those included in the report by Poon et al mentioned above. (Poon et al., 2010) Our model will therefore be more useful in settings that have similar limitations as ours. Finally, when a patient delivered outside of our healthcare system, the diagnosis of pre-eclampsia was obtained by patient self-report or from a referring provider’s office. Although this may limit the reliability of the diagnosis, this accounts for <10% of patients within our cohort and, therefore, is unlikely to substantially alter our results.
In conclusion, our findings demonstrate that low first-trimester PAPP-A levels are associated with the development of pre-eclampsia; however, our model displayed only modest predictive efficiency. Further investigation of additional factors that will improve the prediction model are warranted in order to develop a tool that will more accurately identify an at-risk population and potentially initiate a protocol of heightened clinical surveillance.
Footnotes
This abstract was presented, in part, as a poster presentation at the annual meeting of the Society for Maternal Fetal Medicine, January 31, 2009, San Diego, CA.
References
- 1.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #33. Washington DC: American College of Obstetricians and Gynecologists; 2002. Diagnosis of management of pre-eclampsia and eclampsia. [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin #29. Washington DC: American College of Obstetricians and Gynecologists; 2001. Chronic hypertension in pregnancy. [Google Scholar]
- 3.Clemmons DR. Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol. 1998;140:19–24. doi: 10.1016/s0303-7207(98)00024-0. [DOI] [PubMed] [Google Scholar]
- 4.Cowans NJ, Spencer K. First trimester ADAM12 and PAPP-A as markers of intrauterine fetal growth restriction through their roles in the insulin-like growth factor system. Prenat Diagn. 2007;27:264–71. doi: 10.1002/pd.1665. [DOI] [PubMed] [Google Scholar]
- 5.Confidential enquiries. The National Institute for Clinical Excellence, Scottish Executive Health Department; Department of Health, Social Services and Public Safety, Northern Ireland. The fifth report of the confidential enquiries into maternal deaths in the United Kingdom 1997–1999. London: RCOG Press; 2001. pp. 76–93. [Google Scholar]
- 6.Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: A population based screening study (The FASTER Trial) Am J Obstet Gynecol. 2004;191:1446–51. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 7.Irwin JC, Suen LF, Martina NA, et al. Role of the IGF system in trophoblast invasion and pre-eclampsia. Hum Reprod. 1999;14:90–6. doi: 10.1093/humrep/14.suppl_2.90. [DOI] [PubMed] [Google Scholar]
- 8.Kniss DA, Shubert PJ, Zimmerman PD, et al. Insulin-like growth factors: their regulation of glucose and amino acid transport in placental trophoblasts isolated from first trimester chorionic villi. J Reprod Med. 1994;39:249–56. [PubMed] [Google Scholar]
- 9.Krantz D, Goetzl L, Simpson JL, et al. Association of extreme first-trimester free human chorionic gonadotropin-beta, pregnancy-associated plasma protein A, and nuchal translucency with intrauterine growth restriction and other adverse pregnancy outcomes. Am J Obstet Gynecol. 2004;191:1452–8. doi: 10.1016/j.ajog.2004.05.068. [DOI] [PubMed] [Google Scholar]
- 10.Ong CYT, Liao AW, Spencer K, et al. First trimester maternal serum free β human chorionic gonadotrophin and pregnancy associated plasma protein A as predictors of pregnancy complications. BJOG. 2000;107:1265–70. doi: 10.1111/j.1471-0528.2000.tb11618.x. [DOI] [PubMed] [Google Scholar]
- 11.Poon LCY, Akolekar R, Lachmann R, et al. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35:662–70. doi: 10.1002/uog.7628. [DOI] [PubMed] [Google Scholar]
- 12.Smith GCS, Stenhouse EJ, Crossley JA, et al. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, pre-eclampsia and stillbirth. J Clin Endocrinol Metab. 2002;87:1762–7. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]
- 13.Spencer K, Yu CK, Cowans NJ, et al. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;10:949–53. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 14.Spencer K, Yu CK, Savvidou M, et al. Prediction of pre-eclampsia by uterine artery Doppler ultrasonography and maternal serum pregnancy-associated plasma protein A, free beta-human chorionic gonadotropin, activin A and inhibin A at 22 + 0 to 24 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27:658–63. doi: 10.1002/uog.2676. [DOI] [PubMed] [Google Scholar]
- 15.Spencer K, Cowans NJ, Molina F, et al. First trimester biochemical markers of aneuploidy and the prediction of pre-term or very early pre-term delivery. Ultrasound Obstet Gynecol. 2008;31:147–52. doi: 10.1002/uog.5163. [DOI] [PubMed] [Google Scholar]
- 16.Spencer K, Cowans NJ, Avgidou K, et al. First trimester ultrasound and biochemical markers of aneuploidy and the prediction of small-for-gestational age fetuses. Ultrasound Obstet Gynecol. 2008;31:15–19. doi: 10.1002/uog.5165. [DOI] [PubMed] [Google Scholar]
- 17.Spencer K, Cowans NJ, Nicolaides KH. Low levels of maternal serum PAPP-A in the first trimester and the risk of pre-eclampsia. Prenat Diagn. 2008;28:7–10. doi: 10.1002/pd.1890. [DOI] [PubMed] [Google Scholar]
- 18.Tul N, Punsenjak S, Osredkar J, et al. Predicting complications of pregnancy with first-trimester maternal serum free beta-hCG, PAPP-A and inhibin-A. Prenat Diagn. 2003;23:990–6. doi: 10.1002/pd.735. [DOI] [PubMed] [Google Scholar]
- 19.Yaron Y, Heifetz S, Ochshorn Y, et al. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat Diagn. 2002;22:778–82. doi: 10.1002/pd.407. [DOI] [PubMed] [Google Scholar]
- 20.Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH Fetal Medicine Foundation Second Trimester Screening Group. An integrated model for the prediction of pre-eclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low-risk women. Am J Obstet Gynecol. 2006;193:429–36. doi: 10.1016/j.ajog.2004.12.014. [DOI] [PubMed] [Google Scholar]


