Abstract
Aberrant DNA methylation and miRNA expression play important roles in the pathogenesis of pancreatic cancer. While interrogating differentially methylated CpG islands in pancreatic cancer we identified two members of miR-200 family, miR-200a and miR-200b that were hypomethylated and overexpressed in pancreatic cancer. We also identified prevalent hypermethylation and silencing of one of their downstream targets, SIP1 (ZFHX1B, ZEB2), whose protein product suppresses E-cadherin expression and contributes to epithelial mesenchymal transition. In a panel of 23 pancreatic cell lines we observed a reciprocal correlation between miR-200, SIP1 and E-cadherin expression with pancreatic cancer associated fibroblasts showing the opposite expression pattern to most pancreatic cancers. In Panc-1 cells, which express SIP1, have low E-cadherin expression and do not express miR-200a or miR-200b, treatment with miR-200a and miR-200b down regulated SIP1 mRNA and increased E-cadherin expression. However, most pancreatic cancers express miR-200a and miR-200b, but this expression does not affect SIP1 expression as the SIP1 promoter is silenced by hypermethylation and in these cancers E-cadherin is generally expressed. Both miR-200a and miR-200b were significantly elevated in the sera of pancreatic cancer and chronic pancreatitis patients compared to healthy controls (p<0.0001), yielding receiver operating characteristic curve areas of 0.861 and 0.85, respectively. In conclusion, most pancreatic cancers display hypomethylation and overexpression of miR-200a and miR-200b, silencing of SIP1 by promoter methylation and retention of E-cadherin expression. The elevated serum levels of miR-200a and miR-200b in most patients with pancreatic cancer could have diagnostic utility.
Keywords: pancreatic cancer, methylation, miR-200, SIP1, E-cadherin
INTRODUCTION
Pancreatic ductal adenocarcinoma is the 4th leading cause of cancer death in the United States and it has the lowest survival rate for any solid cancer. In 2009, it is estimated that 42,470 Americans will be diagnosed with pancreatic cancer (1) and only about 4% of patients will live 5 years after diagnosis. One important reason for this poor survival is that most patients present with advanced disease; only ~15–20% of patients with pancreatic cancer present with small, resectable cancers. Furthermore, pancreatic cancer remains unresponsive to most chemotherapeutic agents. Hence, there is a great need to understand the biological mechanisms that contribute to pancreatic cancer development and progression so as to develop effective therapies, and to identify more effective markers of pancreatic neoplasia so as to more effectively detect pancreatic cancer and its precursors.
Recent studies demonstrate that some precursors to pancreatic cancer, pancreatic cystic neoplasms, can be visualized by pancreatic imaging. Pancreatic imaging is being used to detect preinvasive neoplasms in patients with a significant family history of pancreatic cancer and those who have an inherited predisposition to develop the disease because of germline mutations in genes such as BRCA2, p16, PALB2 (2–4). A limitation of current screening tests is that pancreatic imaging does not reliably detect the microscopic pancreatic cancer precursors known as pancreatic intraepithelial neoplasias (PanIN)(5). In addition, most patients with pancreatic cancer do not have major risk factors for the disease and are not candidates for screening. Hence, there is a need for sensitive, specific and accurate tests that would facilitate the rapid diagnosis of pancreatic cancer and its precursors. New candidate markers have been described in recent years that have been evaluated in serum (6) and in pancreatic secretions (7) and ductal brushings (8) to detect local pancreatic neoplasia, but more accurate markers are needed.
Although many genes are mutated in pancreatic cancer (9), mutant KRAS is the only gene to be extensively studied as a diagnostic marker (10, 11). Aberrantly methylated genes also have potential as diagnostic markers (7, 8). Aberrant CpG island methylation is an important cause of altered gene function in pancreatic (12–17) and other cancers (12–17). Alterations in the expression of microRNAs have also been identified in pancreatic and other cancers and their deregulation appears to contribute to cancer development and progression (18). Profiles of microRNAs can discriminate cancer from normal tissue and even distinguish different tumor types (19). MicroRNAs are measureable in the circulation (20, 21), raising hopes that the detection of certain overexpressed miRNAs could be useful noninvasive tests for the early detection of cancer. For example, elevated circulating levels of miR-21, miR-141 and miR-92 have been found in patients with certain cancers (20–22).
Recently, we empoloyed Agilent microarrays to identify differentially methylated CpG islands in pancreatic cancers (23), and using this method we now report that the 5’ region of miRNA-200 is aberrant methylated in pancreatic cancer. MiR-200a and miR-200b are encoded within a single non-coding polycistronic transcript (24). The miR-200 family targets two E-boxbinding transcription factors, SIP1 (also known as ZFHX1B and ZEB2) and ZEB1, which are negative regulators of E-cadherin. Downregulation of E-cadherin such as through SIP1 is essential for epithelial mesenchymal transition (EMT), a program by which cells lose their adhesive phenotype and become more mobile, and the miR-200 family has recently implicated as a pivotal regulator of the epithelial mesenchymal transition (25–27). EMT has been implicated in the progression of pancreatic and other cancers (28–30), although some investigators suggest that true EMT is not a general feature of cancers, rather that cancers often retain some features of EMT and should be termed EMT-like (31). Genetic and epigenetic mechanisms are important causes of E-cadherin inactivation but occur only occasionally in pancreatic cancers (9)(15). Interestingly, pancreatic cancers lacking E-cadherin expression have an undifferentiated phenotype (32). Although some investigators suspect that EMT is associated with genetic alterations in cancer-associated stromal cells (33), we find no evidence that pancreatic cancer-associated fibroblasts have chromosomal alterations (34).
In addition to reporting on the aberrant methylation of miR-200a/miR-200b in pancreatic cancer, we find these miRNAs are overexpressed and find that the downstream target of miR-200, SIP1 is commonly methylated in pancreatic cancers. We also investigate the influence of miR-200a and miR-200b on E-cadherin and SIP1 expression and demonstrate that miR-200a and miR-200b are potential markers of pancreatic cancer.
MATERIALS AND METHODS
Cell lines and tissue samples
Fifteen human pancreatic cancer cell lines including A32-1, A38-5, AsPC1, BxPC3, Capan1, Capan2, CFPAC1, MiaPaCa2, Panc-1, Panc2.5, Panc2.8, Panc3.014, Panc215, PL3 and PL8 and 7 pancreatic cancer associated fibroblasts (CAFs) including CAF15, CAF16, CAF18, CAF19, CAF20, CAF21 and CAF22 were studied and grown under recommended conditions. An immortalized cell line derived from normal human pancreatic ductal epithelium (HPDE) and human pancreatic Nestin-expressing cells (HPNE) were provided by Dr. Ming-Sound Tsao (University of Toronto) and Dr. Michel Ouellette (University of Nebraska Medical Center), respectively.
Normal and neoplastic tissues were obtained from pancreatic adenocarcinomas resected at the Johns Hopkins Hospital. Fresh-frozen pancreatic tissues from 9 patients who underwent a pancreatic resection for an intraductal papillary mucinous neoplasm (IPMN) without associated invasive adenocarcinoma, 7 patients for neuroendocrine tumors and 19 patients for invasive ductal adenocarcinoma were microdissected to obtain normal pancreatic tissue for DNA analysis. Their mean age ± SD were 64.0±13.0 years (18 female). DNA was analyzed from 36 pancreatic cancer xenografts (32 pancreatic, 3 distal bile duct, and 1 duodenal cancer) (mean age 67.3±8.8 years, 24 female) established from primary carcinomas (15). Fresh-frozen pancreatic cancer tissues (n=7, mean age 62.4±15.4 years, 4 female) and normal pancreas from patients who had undergone resection for a pancreatic neuroendocrine neoplasm, IPMN and mucinous cystadenoma (n=13, mean age 55.5±20.6 years, 5 female), were microdissected, placed in RNAlater immediately after dissection and stored at −80C°. Frozen pancreatic tissues were microdissected using the PALM microlaser system as previously described (9). We also microdissected formalin-fixed paraffin-embedded tissues for SIP1 and E-cadherin methylation analysis as previously described (35).
Eighty-eight serum samples from Johns Hopkins Hospital were analyzed including 45 preoperative samples from patients with pancreatic ductal adenocarcinoma (64.4±10.0 years, 19 females), 11 preoperative samples from patients with chronic pancreatitis undergoing surgical resection (64.0±12.3 years, 3 females) and 32 healthy controls (44.3±10.2 years, 18 females). All sera were collected using standard procedures and stored at −80°C until analysis. All specimens were collected and analyzed with the approval of the Johns Hopkins Committee for Clinical Investigation.
Bisulfite treatment, Methylation specific PCR (MSP) and Bisulfite-modified sequencing (BMS) were performed as previously described (23).
RNA isolation and real-time PCR
Total RNA was extracted using mirVana miRNA isolation kit (Ambion 1560) for tissues and mirVana PARIS kit (Ambion 1556) for serum (100–400µl), according to manufacturer’s instructions and RT-PCR performed as we previously described (36). Real-time PCR was performed in triplicate. MicroRNAs were amplified after specific reverse transcription using TaqMan microRNA assays and TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems) according to manufacturer’s instructions (Applied Biosystems) and normalized against 18S rRNA for cell lines and miR-16 for serum and microdissected tissues(20, 22, 37, 38). Relative expression was determined using the delta-delta Ct method and a >35 Ct value indicated negative amplification. Primers are shown in Supplemental Table 1.
5-aza-2’-deoxycytidine (5-Aza-dC) and Trichloroacetic acid (TSA) treatment was performed as we previously described (17). For anti-miRNA transfection analysis, 2 days after being treated by 1µM 5-Aza-dC, cells were transfected for 1 day then retreated by 5-Aza-dC for another 24 hours.
Transfection of microRNA precursors and inhibitors
Cells were plated at 5 × 104 cells per well in 12-well plates and transfected with pre-miR miRNA precursors or anti-miR™ miRNA inhibitors (has-miR-200a and has-miR-200b, Ambion) at 40nM for miR-200a, miR-200b and the combination (each 20nM) using siPORT™ NeoFX™ Transfection Agent (Ambion). Cy™3 dye-labeled Pre-miR™ Negative Control #1 and Cy™3 dye-labeled Anti-miR™ Negative Control #1 (Ambion) were used as controls for pre-miRNA and anti-miRNA transfection, respectively. Cells were collected for RT-PCR 48 hours post-transfection. For the multiple transfection experiment, cells were split and re-transfected with additional pre-microRNA every 3–4 days for 18 days.
Immunohistochemistry
The expression of e-cadherin protein was examined by immunohistochemical labeling of formalin-fixed, paraffin-embedded tissue microarray (TMA) sections of pancreatic cancers and normal pancreas from 328 patients who underwent pancreaticoduodenectomy at Johns Hopkins Hospital using previously described methods (32, 39). Tissue sections were incubated with an anti-E-cadherin mouse monoclonal antibody (clone HEDC-1, Zymed Laboratories, San Francisco, 1/10 dilution) for 60 minutes at room temperature. The age (mean±SD) of these patients was 66.5±10.5 years (46% female). Expression patterns were classified as intact, partial loss or total loss of expression with total loss of expression indicating less than 5% of cancer cells expressing and partial when less than 95% of cells expressing e-cadherin.
Statistics
Statistical analysis was performed using the SPSS Statistics 17.0 and Microsoft Excel statistics software. A two-tailed P value <0.05 indicated statistical significance.
RESULTS
MiR-200a and miR-200b are hypomethylated in pancreatic cancer
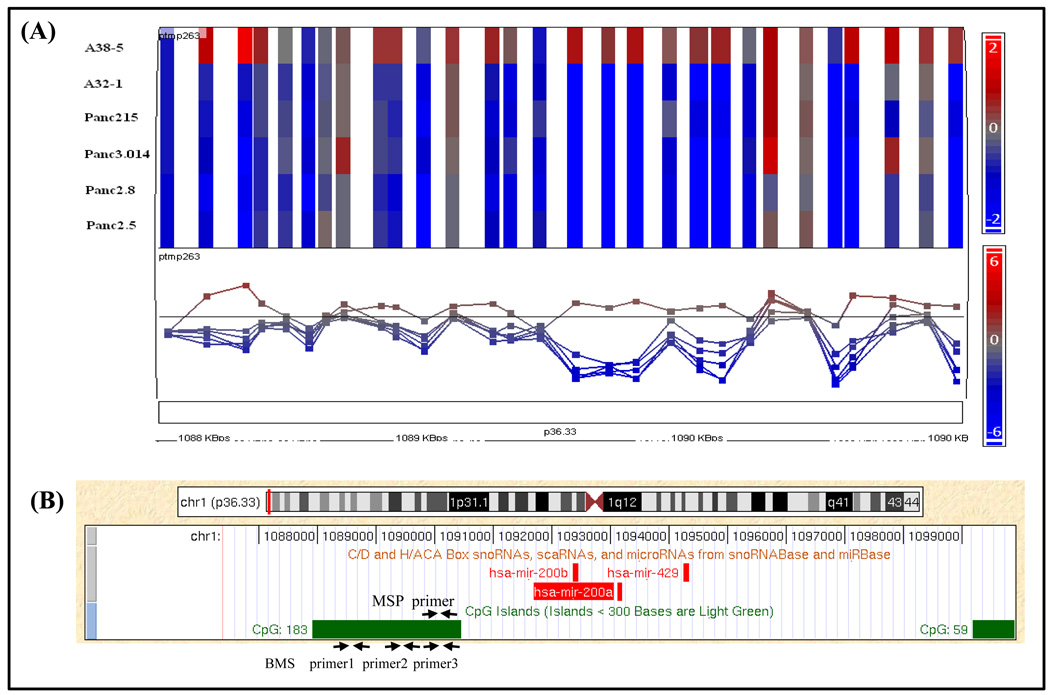
MiR-200a and miR-200b were identified as candidate hypomethylated genes after comparing CpG island methylation profiles of 6 pancreatic cancer lines to normal pancreas using the methylation restriction assay known as methylation CpG island amplification (MCA) and identifying methylation patterns using the Agilent 244K CpG island array (23). On the array a CpG island containing 183 CpG dinucleotides (CGI-183;chr1:1087907–1090447 bps) (2541bp; %GC=65.0%; observed/expected CpGs=69.0%) had probes with elevated log2 Cy3/Cy5 ratios (normal/cancer) indicating hypomethylation in 5 of 6 pancreatic cancer cell lines relative to normal tissues (Figure 1A). This CpG island is located upstream of the putative TSS of the miR-200a/200b locus (Figure 1B) (24).
Figure 1.
MiR-200a and miR-200b hypomethylation in pancreatic cancer (A) Probes within the miR-200 CpG island (CGI-183) indicating reduced log2 Cy3/Cy5 ratios (normal/cancer) in 5 of 6 pancreatic cancer cell lines relative to normal tissues by MCA CpG microarray (23). (B) CGI-183 is located upstream of the miR-200 start site (UCSC database). Primer locations for MSP and BMS are shown.
To validate the hypomethylation of miR-200a and miR-200b in pancreatic cancer, we examined its promoter methylation status in pancreatic cancer and non-neoplastic cell lines using MSP. Eleven of 15 (73.3%) pancreatic cancer cell lines were unmethylated whereas two microdissected normal pancreatic duct samples were predominantly methylated (Table 1 and Supplemental Figure 1). The non-neoplastic cell line, HPNE was completely methylated while HPDE immortalized by HPV16-E6E7 was unmethylated (Table 1 and Supplemental Figure 1A).
Table 1.
Methylation profiles of miR-200a, miR-200b and SIP1 in pancreatic tissues by MSP
| No. | Cell line | miR-200 | SIP1 | No. | XPC | Age | Gender | Diagnosis | miR-200 | SIP1 | No. | NP | Age | Gender | Diagnosis | miR-200 | SIP1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer |
1 | Panc140 | 74 | F | P.C. | U | M | 1 | NP1 | 58 | F | P.C. | P | U | |||
| 1 | A32-1 | U | M | 2 | Panc154 | 77 | F | P.C. | U | M | 2 | NP2 | 81 | M | P.C. | P | U |
| 2 | A38-5 | P | U | 3 | Panc159 | 64 | F | P.C. | U | M | 3 | NP3 | 51 | M | P.C. | P | P |
| 3 | Aspc1 | P | M | 4 | Panc163 | 77 | F | P.C. | U | M | 4 | NP4 | 53 | M | P.C. | P | U |
| 4 | BxPC3 | U | M | 5 | Panc185 | 60 | F | P.C. | U | M | 5 | NP5 | 76 | M | P.C. | P | U |
| 5 | Capan1 | U | M | 6 | Panc194 | 57 | F | P.C. | U | M | 6 | NP6 | 69 | M | P.C. | P | U |
| 6 | Capan2 | U | P | 7 | Panc198 | 69 | F | P.C. | U | M | 7 | NP7 | 75 | M | P.C. | P | U |
| 7 | CFPAC1 | U | U | 8 | Panc215 | 60 | F | P.C. | U | M | 8 | NP8 | 76 | F | P.C. | P | U |
| 8 | MiaPaCa2 | M | M | 9 | Panc219 | 55 | F | P.C. | U | M | 9 | NP9 | 42 | F | P.C. | P | U |
| 9 | Panc-1 | M | U | 10 | Panc253 | 53 | F | P.C. | U | M | 10 | NP10 | 53 | F | P.C. | P | U |
| 10 | Panc2.5 | U | P | 11 | Panc266 | 59 | M | P.C. | U | M | 11 | NP11 | 78 | M | P.C. | P | U |
| 11 | Panc2.8 | U | M | 12 | Panc281 | 66 | F | P.C. | U | P | 12 | NP12 | 74 | F | P.C. | P | U |
| 12 | Panc215 | U | M | 13 | Panc294 | 66 | F | P.C. | U | M | 13 | NP13 | 62 | M | P.C. | P | U |
| 13 | Panc3.014 | U | U | 14 | Panc325 | 70 | F | P.C. | U | M | 14 | NP14 | 71 | M | P.C. | P | U |
| 14 | PL3 | U | M | 15 | Panc354 | 81 | M | P.C. | U | M | 15 | NP15 | 79 | M | P.C. | P | U |
| 15 | PL8 | U | M | 16 | Panc363 | 86 | F | P.C. | U | M | 16 | NP16 | 54 | M | P.C. | P | U |
| 17 | Panc421 | 58 | M | P.C. | U | M | 17 | NP17 | 76 | M | P.C. | P | U | ||||
| Normal | |||||||||||||||||
| 16 | HPDE | U | U | 18 | Panc430 | 73 | F | P.C. | U | P | 18 | NP18 | 70 | F | P.C. | P | U |
| 17 | HPNE | M | U | 19 | JHH10 | 54 | F | P.C. | U | M | 19 | NP19 | 74 | F | P.C. | P | P |
| 18 | midND1 | P | N/A | 20 | JHH11 | 78 | M | P.C. | U | M | 20 | NP20 | 63 | F | P.E.T | P | U |
| 19 | midND2 | P | N/A | 21 | JHH15 | 69 | F | P.C. | U | P | 21 | NP21 | 42 | F | P.E.T | P | U |
| 22 | JHH21 | 80 | M | P.C. | U | M | 22 | NP22 | 39 | M | P.E.T | P | U | ||||
| B.D.C.: bile duct cancer | 23 | JHH24 | 64 | M | P.C. | U | M | 23 | NP23 | 66 | F | P.E.T | P | U | |||
| D.C.: duodenal cancer | 24 | JHH27 | 70 | M | P.C. | U | M | 24 | NP24 | 56 | M | P.E.T | P | U | |||
| IPMN: pancreatic cancer assoc with IPMN | 25 | JHH34 | 57 | F | P.C. | U | ND | 25 | NP25 | 45 | F | P.E.T | P | U | |||
| midND: microdissected normal duct | 26 | Px65 | 63 | F | P.C. | U | P | 26 | NP26 | 41 | F | P.E.T | P | U | |||
| NP: Normal Pancreas | 27 | Px194 | 77 | F | P.C. | U | U | 27 | NP27 | 82 | M | IPMN | P | U | |||
| P.C.: pancreatic cancer | 28 | Px352 | 65 | F | P.C. | U | M | 28 | NP28 | 63 | M | IPMN | P | U | |||
| P.E.T. :pancreatic endocrine tumor | 29 | JHH33 | 60 | M | IPMN | U | M | 29 | NP29 | 57 | M | IPMN | P | U | |||
| XPC: Xenograft of P.C. | 30 | Panc169 | 75 | M | IPMN | U | M | 30 | NP30 | 72 | F | IPMN | P | U | |||
| ND: not determined | 31 | Panc410 | 59 | F | IPMN | U | M | 31 | NP31 | 78 | F | IPMN | P | U | |||
| Methylation | 32 | Panc420 | 68 | M | IPMN | P | M | 32 | NP32 | 72 | F | IPMN | P | U | |||
| Partial methylation | 33 | Panc286 | 69 | F | D.C. | U | M | 33 | NP33 | 56 | F | IPMN | P | U | |||
| Unmethylation | 34 | Panc247 | 82 | M | B.D.C. | U | M | 34 | NP34 | 81 | F | IPMN | P | U | |||
| 35 | Panc287 | 65 | M | B.D.C. | U | M | 35 | NP35 | 72 | F | IPMN | P | U | ||||
| 36 | Panc291 | 62 | F | B.D.C. | U | M | |||||||||||
We next performed bisulfite sequencing to verify the hypomethylation of the miR-200 promoter. We found that 9/11 pancreatic cancer cell lines, 6/6 pancreatic cancer xenografts and the non-neoplastic cell line HPDE were completely unmethylated for the 36 CpG sites sequenced while the non-neoplastic cell line HPNE and two cancer lines MiaPaCa2 and Panc-1 were methylated in all sequenced CpG sites (Supplemental Figure 1B), consistent with MSP data. We next expanded our MSP assay to 36 xenografts of primary pancreatic cancers, 35 normal pancreatic tissues and found that 97.2% (35/36) of pancreatic cancer xenografts were unmethylated while all 35 normal pancreatic tissue samples were methylated. To further analyze normal pancreatic tissue methylation we performed bisulfite sequencing on 10 normal pancreas samples, 6 of which demonstrated predominant methylation at most CpG sites 2 samples with ~50% methylation, and two samples that were predominantly unmethylated (Supplemental Figure 1C).
MiR-200a and miR-200b are overexpressed in pancreatic cancer
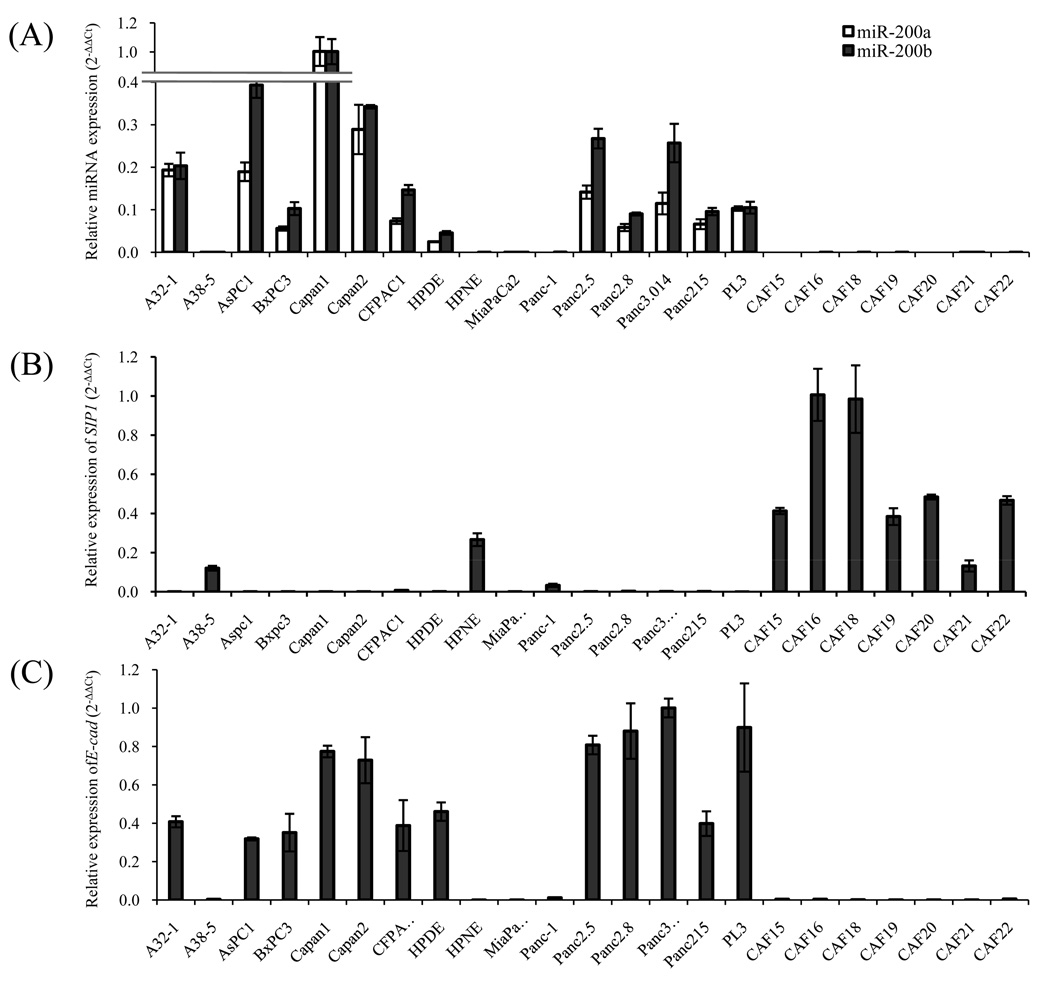
To evaluate whether miR-200a and miR-200b are overexpressed in pancreatic cancers, we measured its expression in pancreatic cell lines and primary pancreatic tissues using qRT-PCR. As shown in Figure 2, compared with the non-neoplastic cell lines HPNE and HPDE, miR-200a and miR-200b were overexpressed in 11/14 pancreatic cancer cell lines. Ten of 11 overexpressing cell lines were completely unmethylated at 5’CpGs while the 12th cell line, AsPC1, was partial methylated (Table 1). MiR-200a and miR-200b were also overexpressed in primary pancreatic cancers compared with normal pancreatic tissues (p<0.001) (Figure 3). Among pancreatic cancer cell lines A38-5, MiaPaCa2 and Panc-1 with low levels of miR-200a and miR-200b (Figure 2), treatment with 5-Aza-dC induced miR-200a and miR-200b by 3-fold or more (Figure 4). Similarly, 5-Aza-dC induced miR-200a and miR-200b expression in HPNE (Figure 4). In contrast, apart from A38-5, treatment of pancreatic cancer cell lines with the HDAC inhibitor, TSA did not alter miR-200a/200b expression (Supplemental Figure 2).
Figure 2.
Expression of miR-200a/miR-200b (A) SIP1 (B) and E-cadherin (C) in pancreatic cancer cell lines relative to non-neoplastic HPNE and CAFs cell lines by real-time PCR. Reference RNAs were used for miR-200a and miR-200b (18s rRNA), SIP1 and E-cadherin (GAPDH). Data are shown as mean±SD of triplicates and are representative of two to three independent experiments.
Figure 3.
Box plot of miR-200a and miR-200b (A) and SIP1 (B) expression in pancreatic cancer tissues by real-time PCR. Boxes represent the inter-quartile range and lines indicate the median value. Reference RNAs were used for miR-200a/miR-200b (MiR-16) and SIP1 (GAPDH).
Figure 4.
QPCR expression of miR-200a, miR-200b, SIP1 and E-cadherin before and after 5-Aza-dC. Data are shown as mean ± SD of triplicates and are representative of two independent experiments. Reference RNAs were used for miR-200a and miR-200b (18s rRNA), SIP1 and E-cadherin (GAPDH). MiR-200a (A) miR-200b (B) (C) SIP1 and (D) E-cadherin.
DNA methylation and expression of SIP1 and effect of miR-200a and miR-200b
We next focused on one of the targets of miR-200, SIP1. SIP1 is an important transcriptional repressor that down regulates multiple genes including E-cadherin and shows aberrant expression in several cancers. Since SIP1 showed hypermethylation in our MCA microarray data, we examined the methylation status of SIP1. We found that the SIP1 promoter was methylated in 73.3% (11/15) of pancreatic cancer cell lines and in 97.1% (34/35) of pancreatic cancer xenografts while 2 non-neoplastic cell lines and 94.3% (33/35) of normal pancreas samples were unmethylated (Table 1). 30 of 35 pancreatic cancer xenografts had complete SIP1 methylation. E-cadherin was also unmethylated in all but one of these xenografts. SIP1 generally displayed a methylation profile inverse to that of miR-200 (Table 1). By qRT-PCR (Figure 2), and by serial analysis of gene expression (SAGE) analysis (9), SIP1 (ZEB2) was expressed in HPNE, but was not expressed in most pancreatic cancer cell lines. Similarly, microdissected pancreatic cancer tissues had significantly lower SIP1 RNA by qRT-PCR than normal pancreas (Figure 3). In contrast, we found abundant SIP1 RNA in 7 of 7 pancreatic cancer associated fibroblasts, which also displayed inverse expression profile to miR-200a and miR-200b (Figure 2). Since Snail and Slug induce SIP1 expression and repress E-cadherin, we examined their expression in pancreatic cancers by SAGE and found SNAIL was minimally expressed and there was no evidence that SNAIL or SLUG expression repressed E-cadherin expression (data not shown).
We found that DNA methylation regulated the expression of SIP1 as treatment with 5-Aza-dC induced SIP1 expression in all 7 cell lines tested (Figure 4). Interestingly, in HPNE cells which have methylated miR-200, induction of miR-200a and miR-200b expression by 5-Aza-dC (Figure 4A/4B) was associated with a 5-fold reduction of SIP1 expression supporting the negative regulation of SIP1 by miR-200 (Figure 4). SIP1 recruits the transcriptional repressor CtBP to inhibit Smad-mediated transcription (40), and since SMAD4 is mutated in ~55% of pancreatic cancers, we determined if loss of SIP1 was more likely pancreatic cancers with wild-type SMAD4, but we did not find any correlation (data not shown).
To test if miR-200 downregulates SIP1 expression in pancreatic cancer cells, we transfected the cell line Panc-1, which is fully methylated at miR-200 and unmethylated at SIP1, with pre-miR-200a or/and pre-miR miR-200b precursors and examined its effects at Day 2 and Day 18 post infection. Compared to control transfected cells, SIP1 mRNA was significantly reduced by miR-200a (p<0.01, p<0.01), miR-200b (p<0.05, p<0.01), and combined miR-200a/200b treatment (p<0.01, p<0.01) both two days after transfection and after 18 days of repeated transfections, respectively (Supplemental Figure 3).
Although microRNAs are not thought to induce epigenetic silencing, since we observed miR-200 overexpression and DNA methylation of SIP1, we considered the possibility that epigenetic inactivation of the SIP1 promoter in pancreatic cancers could be caused by aberrant miR-200 expression. To test this possibility we maintained expression of miR-200 for two weeks by transiently transfecting Panc-1 cells ever y 3–4 days. However, induction of miR-200 expression did not induce any SIP1 promoter methylation by MSP analysis (data not shown).
We also examined if inhibition of miR-200 could increase SIP1 expression. First we treated AsPC-1 cells with antisense inhibitors to miR-200a, miR-200b or both. Since SIP1 is silenced in association with DNA methylation in AsPC-1 cells, treatment with miR-200a, and/or miR-200b did not induce expression of SIP1 in AsPC-1 cells (Supplemental Figure 3). To determine if miR-200a and miR-200b still has the ability to regulate SIP1 expression in cells in which the SIP1 promoter is methylated, we pretreated AsPC1 cells with 5-Aza-dC to induce SIP1 expression. Inhibitors of miR-200a, miR-200b, alone or in combination increased SIP1 expression by 31% (p=0.39), 82% (p<0.05), 128% (p<0.05) compared to negative control transfected cells, respectively (Supplemental Figure 4).
Effect of miR-200 on E-cadherin expression and EMT
Since miR-200 and SIP1 have been identified as regulators of EMT, we examined E-cadherin expression in pancreatic cancers and determined if it was upregulated by miR-200 in pancreatic cancer cells. E-cadherin was expressed in 11/14 pancreatic cancer cell lines at levels approximately at or above the control pancreatic duct line, HPDE, but was not expressed in 7 CAF lines (Figure 2). Of the 3 cell lines lacking E-cadherin expression, MiaPaca2 was completely methylated at the E-cadherin locus associated with gene silencing ((15) and Figure 5), while Panc-1 and A38 express SIP1 and lack miR-200 expression suggesting these pancreatic cancers have lost E-cadherin expression and have undergone EMT as a result of SIP1 expression. Similarly, by SAGE (9) E-cadherin was expressed at or above levels in pancreatic duct epithelial cells in 21 of 24 pancreatic cancers. Indeed, we did find pre-miR-200b and combined pre-miR-200a/200b treatment increased E-cadherin expression in Panc-1 cells (p<0.05, p<0.05) (p<0.05, p<0.01) both at day 2 and at day 18 (after multiple transfections), respectively (Supplemental Figure 3). E-cadherin expression was also reduced in AsPC1 cells both by anti-miR inhibitors of miR-200b and anti-miR-200a and miR-200b, and by 5-Aza-dC treatment (Supplemental Figure 4).
Figure 5.
Serum levels of miR-200a and miR-200b in patients with and without pancreatic disease. Box plots of serum miR-200a (A) and miR-200b (B) levels in patients with pancreatic cancer (PC), patients with chronic pancreatitis (CP) and normal controls (NP). MicroRNA expression levels are normalized to miR-16. Receiver operating characteristic curve (ROC) areas for serum miR-200a (C) and miR-200b (D).
However, we didn’t observe any reversal of EMT morphology (i.e. reversal of fibroblastoid spindles or an increase cell-to-cell contacts) in Panc-1 cells even after sustained suppression of E-cadherin by repeated transfection of miR-200a and miR-200b precursors (Supplemental Figure 3c).
E-cadherin protein expression in primary pancreatic adenocarcinomas
Our results indicate that only a minority of pancreatic cancers cell lines lack E-cadherin expression. Furthermore, we also find that most xenografts of primary pancreatic adenocarcinomas have complete methylation of SIP1 suggesting that SIP1 is not expressed in most pancreatic cancers. But since other mechanisms of EMT could be active in pancreatic cancers, we examined the prevalence of low e-cadherin expression by immunohistochemistry in the resected pancreatic cancer tissues of 328 patients who had undergone pancreaticoduodenectomy. We found intact expression in 188, partial loss in 137, and total loss 7 of e-cadherin expression in 328 pancreatic ductal adenocarcinomas (Supplemental Figure 5). Loss of e-cadherin expression was observed in 2/8 well-differentiated (25%), 65/175 moderately-differentiated (37%), and 74/146 poorly-differentiated (51%) carcinomas (p=0.03, chi-square test).
To examine if DNA methylation was responsible for the loss of e-cadherin, we obtained additional pancreatic cancer tissue from 6 of the 7 primary pancreatic cancers with total loss of e-cadherin. These pancreatic cancers were microdissected and assayed for methylation of E-cadherin and SIP1 by MSP. E-cadherin was methylated in 5 (83.3%) of these 6 pancreatic cancers. In contrast, E-cadherin was partially methylated in only 1 of 6 pancreatic cancer xenografts that had retained e-cadherin expression in its primary pancreatic cancer. SIP1 methylation was detected in 11 of 12 of these pancreatic cancers. These results implicate E-cadherin methylation as the likely cause of absent e-cadherin expression in most of these pancreatic cancers. We did find that one of the 6 pancreatic cancers with absent e-cadherin expression had unmethylated SIP1 suggesting that in this case SIP1 expression likely caused E-cadherin silencing (Supplemental table 2).
Elevated serum miR-200a and miR-200b in patients with pancreatic cancer
We next determined if miR-200a and miR-200b could be detected in serum and if it was more abundant in patients with pancreatic cancer. We measured miR-200a and miR-200b concentrations in 45 patients with pancreatic cancer and 32 healthy controls. Both miR-200a and miR-200b were significantly elevated in sera of patients with pancreatic cancer compared with healthy controls (p<0.001, p<0.001, respectively, Mann-Whitney; Figure 5) (miR-16 was used as a reference). Receiver operating characteristic (ROC) curve indicated serum levels of miR-200a and miR-200b could differentiate patients with pancreatic cancer from healthy controls with ROC curve areas of 0.861 (95% CI=0.774–0.949) and 0.85 (95% CI=0.763–0.938), respectively (Figure 5C/5D). With the cutoff at 0.28 (relative expression value), miR-200a had an 84.4% sensitivity for pancreatic cancer and 87.5% specificity compared to healthy controls. At the cutoff of 0.5 (relative expression value), miR-200b had a sensitivity of 71.1% and specificity of 96.9%. The serum levels of miR-200a and miR-200b in serum samples were significantly correlated (R2= 0.745, p<0.0001, Spearman).
Pancreatitis can mimic pancreatic cancer and many markers of pancreatic cancer are also abnormal in patients with chronic pancreatitis. We measured miR-200a and miR-200b in the serum of 11 patients with chronic pancreatitis and found that serum levels were not significantly different from those observed in patients with pancreatic cancer, (p=0.322, p=0.933, respectively, Mann-Whitney Test) (Figure 5).
DISCUSSION
In this study we find most pancreatic cancers harbor hypomethylation and overexpression of miR-200a and miR-200b and epigenetic silencing of SIP1. In contrast, in many other cancer types, SIP1 is overexpressed (41, 42) and miR-200 is silenced (25–27, 43, 44). DNA methylation has been implicated in the regulation of several microRNAs with most studies identifying hypermethylation of transcriptionally silenced microRNAs (45–47), rather than hypomethylation and overexpression as we found with miR-200. After observing the hypomethylation and overexpression of miR-200 in pancreatic cancers, we chose to focus on the relationship between miR-200 and SIP1 to highlight that this relationship is complicated in pancreatic cancers and distinct from the pattern observed in other cancers. Our evidence indicates that for most pancreatic cancers miR-200 expression is not required to suppress SIP1 because the gene is already silenced by methylation. In many cancer types SIP1 represses the expression of E-cadherin and other genes and is implicated in EMT. The lack of SIP1 expression in most pancreatic cancers indicates that SIP1 does not suppress E-cadherin or mediate EMT in affected pancreatic cancers.
Our results are also consistent with reports that show only a minority of pancreatic cancers completely lack e-cadherin expression (32). However, we do find that many primary pancreatic cancers have focal areas of e-cadherin expression loss and this loss is more common in poorly differentiated cancers, consistent with reports that undifferentiated pancreatic cancers lack e-cadherin expression (32). Such focal loss of e-cadherin expression could represent focal areas of EMT perhaps reflecting tumor microenvironment influences on EMT and since EMT is suspected in the chemoresistance of pancreatic and other cancers, the focal loss of e-cadherin in these cancers could have therapeutic implications (28, 30). Although e-cadherin loss and its functional consequences is a central feature of EMT, other measures of the EMT phenotype such as reorganization of actin filaments could help clarify the significance of pancreatic cancers with weak or focal loss of e-cadherin expression. Our results also indicate that the prevalence of e-cadherin expression loss is greater than the combined prevalence of genetic (9) or DNA methylation induced inactivation of E-cadherin (15) or expression of SIP1, suggesting that there are additional mechanisms for E-cadherin silencing in pancreatic cancers. Indeed, one recent report found that loss of FOXA1/A2 expression could induce EMT in pancreatic cancers (48). In pancreatic cancer cells expressing SIP1, we found that treatment with miR-200a and miR-200b, or epigenetic induction of miR-200a and miR-200b expression can down regulate SIP1 expression. These results suggest that in cancers with epigenetic silencing of miR-200 and expression of SIP1, it may be possible to reverse an EMT phenotype with DNA methyltransferase inhibitors. And in cell lines expressing miR-200a and miR-200b and having a methylated and silenced SIP1 promoter, SIP1 expression could be induced by inhibiting DNA methylation with 5-Aza-dC and further induced by blocking miR-200 expression with antisense to miR-200. These results are consistent with recent studies that demonstrate that members of the miR-200 family can inhibit EMT (25–27, 49). However, although prolonged pre-miR-200 treatment of Panc-1 cells reversed E-cadherin expression, surprisingly it did not alter morphological features of EMT. Perhaps, miR-200 treatment of Panc-1 cells reversed subtle features of EMT besides morphology, or alternatively additional factors could be required to induce EMT in these cells (31). Since SIP1 promoter hypermethylation rather than miR-200 expression is the mechanism for silencing of SIP1 expression in most pancreatic cancers, our results point to as yet unidentified functional consequences of miR-200 overexpression in pancreatic cancers that merit further investigation.
Previous studies have emphasized the expression of SIP1 in pancreatic cancer cells (9,50), but we find only a minority of pancreatic cancers express SIP1. Our results differ from those of Imamichi et al (42) who described elevated SIP1 expression in primary pancreatic cancer tissues which may reflect high levels of SIP1 in contaminating stromal fibroblasts in pancreatic cancer samples. Instead, our results point to a potential advantage to pancreatic cancers of silencing SIP1 perhaps by releasing the transcriptional repression of as yet unidentified downstream targets.
We also found that methylated SIP1 has promising characteristics as a marker of pancreatic neoplasia. By MSP, methylation was detected in over 90% of pancreatic cancers and in less than 10% of pancreatic tissues. We have demonstrated that several aberrantly methylated genes including SPARC, NPTX1, FOXE1 and ppENK are sensitive and specific markers of pancreatic cancer when detected in pancreatic juice and in pancreatic and biliary brushings (7, 8). Further studies are needed to evaluate the role of methylated SIP1 as a marker of pancreatic neoplasia.
We find that both miR-200a and miR-200b are significantly elevated in the serum of patients with resectable pancreatic cancer. Indeed, miR-200a and miR-200b are only occasionally detectable in the serum of healthy controls suggesting it has potential as a diagnostic marker of pancreatic cancer. The elevated serum miR-200a and miR-200b levels in pancreatic cancer are consistent with the elevated levels in pancreatic cancer tissues. MiR-200a and miR-200b levels were also elevated in chronic pancreatitis sera compared to healthy controls which may reflect some expression in normal pancreas released with pancreatitis. Chronic pancreatitis can mimic pancreatic cancer in its clinical presentation and is a common obstacle for pancreatic cancer markers. However, often the clinical suspicion of pancreatic cancer is much higher than that of chronic pancreatitis. In this setting an elevated serum miR-200a/b level would increase the suspicion of pancreatic cancer and warrants further investigations such as pancreatic imaging. Similarly, patients with a strong family history of pancreatic cancer who do not have an increased predisposition to chronic pancreatitis and who undergo screening for pancreatic cancer (4) might benefit from measurement of markers such as miR-200a and miR-200b or other markers that are highly sensitive for pancreatic cancer but not elevated in most other conditions. However, further studies are needed such as in high risk populations to evaluate the performance of these markers.
In conclusion, we find that most pancreatic cancers hypomethylate and overexpressmiR-200a and miR-200b, silence SIP1 by promoter methylation and retain expression of E-cadherin. We also find that most patients with pancreatic cancer have elevated serum levels of miR-200a and miR-200b compared to healthy controls. Further evaluation of the diagnostic utility of these markers is warranted.
Supplementary Material
Acknowledgments
Dr. Goggins is supported by the National Cancer Institute grants (CA62924, CA120432, RC2CA148346), a grant from the Jimmy V foundation and the Michael Rolfe Foundation
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. Epub 2009 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hruban R, Klein A, Eshleman J, Axilbund J, Goggins M. Familial Pancreatic Cancer. Expert Review in Gastroenterology and Hepatology. 2007;1:81–88. doi: 10.1586/17474124.1.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–781. doi: 10.1016/j.cgh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–316. [PMC free article] [PubMed] [Google Scholar]
- 6.Walter K, Hong SM, Nyhan S, et al. Serum fatty acid synthase as a marker of pancreatic neoplasia. Cancer Epidemiol Biomarkers Prev. 2009;18:2380–2385. doi: 10.1158/1055-9965.EPI-09-0144. Epub 009 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsubayashi H, Canto M, Sato N, et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208–1217. doi: 10.1158/0008-5472.CAN-05-2664. [DOI] [PubMed] [Google Scholar]
- 8.Parsi MA, Li A, Li CP, Goggins M. DNA methylation alterations in endoscopic retrograde cholangiopancreatography brush samples of patients with suspected pancreaticobiliary disease. Clin Gastroenterol Hepatol. 2008;6:1270–1278. doi: 10.1016/j.cgh.2008.07.007. Epub 2008 Sep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. Epub 2008 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi C, Fukushima N, Abe T, et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol Ther. 2008;7 doi: 10.4161/cbt.7.3.5362. [DOI] [PubMed] [Google Scholar]
- 11.Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095–1102. doi: 10.1016/j.gie.2008.07.033. Epub 2009 Jan 18. [DOI] [PubMed] [Google Scholar]
- 12.Sato N, Fukushima N, Chang R, Matsubayashi H, Goggins M. Differential and epigenetic gene expression profiling identifies frequent disruption of the RELN pathway in pancreatic cancers. Gastroenterology. 2006;130:548–565. doi: 10.1053/j.gastro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Sato N, Maitra A, Fukushima N, et al. Frequent hypomethylation of multiple genes overexpressed in pancreatic ductal adenocarcinoma. Cancer Res. 2003;63:4158–4166. [PubMed] [Google Scholar]
- 14.Sato N, Matsubayashi H, Abe T, Fukushima N, Goggins M. Epigenetic down-regulation of CDKN1C/p57KIP2 in pancreatic ductal neoplasms identified by gene expression profiling. Clin Cancer Res. 2005;11:4681–4688. doi: 10.1158/1078-0432.CCR-04-2471. [DOI] [PubMed] [Google Scholar]
- 15.Ueki T, Toyota M, Sohn T, et al. Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res. 2000;60:1835–1839. [PubMed] [Google Scholar]
- 16.Omura N, Goggins M. Epigenetics and epigenetic alterations in pancreatic cancer. Int J Clin Exp Pathol. 2009;2:310–326. Epub 2008 Nov 15. [PMC free article] [PubMed] [Google Scholar]
- 17.Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–3742. [PubMed] [Google Scholar]
- 18.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 20.Lawrie CH, Gal S, Dunlop HM, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of colorectal cancer patients: A potential marker for colorectal cancer screening. Gut. 2009 doi: 10.1136/gut.2008.167817. [DOI] [PubMed] [Google Scholar]
- 23.Omura N, Li CP, Li A, et al. Genome-wide profiling of methylated promoters in pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7:1146–1156. doi: 10.4161/cbt.7.7.6208. Epub 2008 Apr 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res. 2008;68:7846–7854. doi: 10.1158/0008-5472.CAN-08-1942. [DOI] [PubMed] [Google Scholar]
- 25.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 27.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. Epub 009 Mar 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Burstin J, Eser S, Paul MC, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 2009. 2009 Jul;137:361–371. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. Epub 2009 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klymkowsky MW, Savagner P. Epithelial-mesenchymal transition: a cancer researcher's conceptual friend and foe. Am J Pathol. 2009;174:1588–1593. doi: 10.2353/ajpath.2009.080545. Epub 2009 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter JM, Ting AH, Vilardell F, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res. 2008;14:412–418. doi: 10.1158/1078-0432.CCR-07-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell I, Polyak K, Haviv I. Clonal mutations in the cancer-associated fibroblasts: the case against genetic coevolution. Cancer Res. 2009;69:6765–6768. doi: 10.1158/0008-5472.CAN-08-4253. discussion 9. [DOI] [PubMed] [Google Scholar]
- 34.Walter K, Omura N, Hong SM, Griffith M, Goggins M. Pancreatic cancer associated fibroblasts display normal allelotypes. Cancer Biol Ther. 2008;7:1146–1156. doi: 10.4161/cbt.7.6.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brune K, Hong SM, Li A, et al. Genetic and epigenetic alterations of familial pancreatic cancers. Cancer Epidemiol Biomarkers Prev. 2008;17:3536–3542. doi: 10.1158/1055-9965.EPI-08-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter K, Omura N, Hong SM, et al. Overexpression of Smoothened activates the Sonic Hedgehog signaling pathway in pancreatic cancer associated fibroblasts. Clin Cancer Res. 2010;16:1781–1789. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davoren PA, McNeill RE, Lowery AJ, Kerin MJ, Miller N. Identification of suitable endogenous control genes for microRNA gene expression analysis in human breast cancer. BMC Mol Biol. 2008;9:76. doi: 10.1186/1471-2199-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Chen J, Chang P, et al. MicroRNAs in Plasma of Pancreatic Ductal Adenocarcinoma Patients as Novel Blood-Based Biomarkers of Disease. Cancer Prev Res (Phila Pa) 2009 doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 40.Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–2462. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elloul S, Elstrand MB, Nesland JM, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 42.Imamichi Y, Konig A, Gress T, Menke A. Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene. 2007;26:2381–2385. doi: 10.1038/sj.onc.1210012. Epub 006 Oct 9. [DOI] [PubMed] [Google Scholar]
- 43.Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 44.Adam L, Zhong M, Choi W, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin Cancer Res. 2009;15:5060–5072. doi: 10.1158/1078-0432.CCR-08-2245. Epub 2009 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber B, Stresemann C, Brueckner B, Lyko F. Methylation of human microRNA genes in normal and neoplastic cells. Cell Cycle. 2007;6:1001–1005. doi: 10.4161/cc.6.9.4209. [DOI] [PubMed] [Google Scholar]
- 46.Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 47.Lujambio A, Ropero S, Ballestar E, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 48.Strobel O, Rosow DE, Rakhlin EY, et al. Pancreatic Duct Glands Are Distinct Ductal Compartments That React to Chronic Injury and Mediate Shh-Induced Metaplasia. 2009 doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. Epub 2008 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, VandenBoom TG, 2nd, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. Epub 2009 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.