Abstract
Intraluminal valves are required for the proper function of lymphatic collecting vessels and large lymphatic trunks like the thoracic duct. Despite recent progress in the study of lymphvasculogenesis and lymphangiogenesis, the molecular mechanisms controlling the morphogenesis of lymphatic valves remains poorly understood. Here, we report that gap junction proteins, or connexins (Cxs), are required for lymphatic valvulogenesis. Cx37 and Cx43 are expressed early in mouse lymphatic development in the jugular lymph sacs, and later in development these Cxs become enriched and differentially expressed by lymphatic endothelial cells on the upstream and downstream sides of the valves. Specific deficiencies of Cx37 and Cx43 alone or in combination result in defective valve formation in lymphatic collecting vessels, lymphedema, and chylothorax. We also show that Cx37 regulates jugular lymph sac size and that both Cx37 and Cx43 are required for normal thoracic duct development, including valve formation. Another Cx family member, Cx47, whose human analog is mutated in some families with lymphedema, is also highly enriched in a subset of endothelial cells in lymphatic valves. Mechanistically, we present data from Foxc2−/− embryos suggesting that Cx37 may be a target of regulation by Foxc2, a transcription factor that is mutated in human lymphedema-distichiasis syndrome. These results show that at least three Cxs are expressed in the developing lymphatic vasculature and, when defective, are associated with clinically manifest lymphatic disorders in mice and man.
Keywords: connexin, gap junction, lymphatic development, valvulogenesis, lymphedema, chylothorax
Introduction
Lymphatic (Ly) vessels are essential for tissue fluid balance, immune function, and the absorption and transport of dietary fat. Excess interstitial fluid is taken up by Ly capillaries and transported as lymph through valved collecting vessels, filtered by lymph nodes, and then carried through major Ly trunks which empty into the venous system. Valves are a crucial feature of the Ly vasculature because they ensure that lymph movement continues anterograde, propelled in part by Ly intrinsic contractions (Zawieja, 2009). Defects of the Ly system lead to a number of congenital and acquired disorders and syndromes including lymphedema, chylothorax, metabolic disorders, inflammation, and immune dysfunction. In addition, the Ly vasculature is a major route for tumor metastasis. Understanding the molecular mechanisms of lymphangiogenesis and lymphvasculogenesis both in normal contexts and during tumor growth will be important in efforts to develop novel molecular therapeutics (Jurisic and Detmar, 2009; Witte et al., 2001).
In recent years substantial progress has been made in identifying key genes and proteins involved in Ly development. Knockout and transgenic mouse models as well as other approaches have established the importance of key transcription factors (Prox1, Foxc2, Sox18, NFATc1, Coup-TFII, Net); signaling proteins (VEGF-C/D, Ang1 and Ang2, FIAF, EphrinB2, podoplanin, Syk, Akt/PKB (Zhou et al., 2010), PI3K); receptors (VEGFR-3, Np2); and cell-matrix interactions (integrin-α9, FnEIIIA, Emilin1) in Ly development (for reviews see (Mäkinen et al., 2007; Tammela and Alitalo, 2010; Oliver and Srinivasan, 2010; Oliver and Srinivasan, 2008)). In addition, several congenital Ly diseases have been linked to gene mutations, including Milroy’s disease (VEGFR3) (Ferrell et al., 1998), lymphedema-distichiasis syndrome (FOXC2) (Fang et al., 2000), hypotrichosis-lymphedema-telangiectasia (SOX18) (Irrthum et al., 2003), congenital chylothorax (ITGA9) (Ma et al., 2008), and generalized Ly dysplasia (CCBE1) (Connell et al., 2010). Despite these advances, the overall process of Ly development is still incompletely understood. The current view is that the process begins at E9.5-E10.5 in the mouse when a subgroup of committed Prox1-expressing endothelial cells in the anterior cardinal veins migrate laterally and form the Ly primordia (lymph sacs) (Oliver and Srinivasan, 2010). Primitive Ly capillary networks assemble by a process of centrifugal sprouting from the lymph sacs, and then subsequently these networks combine and remodel into a hierarchal network of initial and collecting Ly vessels (Tammela and Alitalo, 2010). However, detailed knowledge about the signaling mechanisms that govern these processes, and in particular, those that control Ly valve morphogenesis is at an early stage.
One area that has not been investigated in any detail is the role of gap junction (GJ) proteins, or connexins (Cxs), in Ly vascular development and function. Cxs are a family of 21 proteins in humans which assemble into GJ channels, structures that allow for the direct transfer of small molecules between adjacent cells (Goodenough and Paul, 2009). GJ channels are dynamically regulated, rapidly at the single channel level, and on a slower timescale at the level of Cx synthesis, assembly, post-translational modification, and degradation (Laird, 2006; Solan and Lampe, 2009). Besides intercellular channels, Cxs can also form hemichannels (undocked channels) which act as release sites for extracellular signaling molecules (Stout et al., 2004). Some Cxs bind other proteins within the cell, contributing to signaling that may be unrelated to channel function (Jiang and Gu, 2005; Laird, 2010; Dbouk et al., 2009). It is well established that endothelial cells and smooth muscle cells of many blood vessels are coupled by Cx-comprised intercellular channels (Yeh et al., 1997; Gabriels and Paul, 1998; Simon and McWhorter, 2003). Furthermore, Cxs have been shown to be necessary for various aspects of blood vessel development, propagation of conducted arteriolar vasomotor responses, and for communicating antiinflammatory signals between blood vessel endothelial cells (Simon and McWhorter, 2002; Walker et al., 2005; Figueroa and Duling, 2009; Brisset et al., 2009). Regarding Ly vessels, it has been suggested that GJs could provide a pathway for conduction of spontaneously evoked contractions in Ly vessels (Zawieja et al., 1993; McHale and Meharg, 1992). To date, however, the argument for GJs in Ly vessels is based mainly on GJ inhibitor studies. Because the inhibitors used were not specific for GJ channels, the evidence for GJs in Ly vessels has not been conclusive. While recent microarray studies of human dermal Ly endothelial cells (LECs) have provided evidence of expression of Cx mRNA in cultured LECs, the expression and localization of Cx proteins in developing and mature Ly vessels, as well as their potential functions in these vessels, has not been investigated (Shin et al., 2008; Wick et al., 2007). In this study, we examine the expression of Cxs in both the developing and mature Ly vasculature and use Cx deficient mice to investigate their functions in the Ly system.
Materials and Methods
Mice and genotyping
Cx37−/− (Gja4−/−) (Simon et al., 1997), Cx40−/− (Gja5−/−) (Simon et al., 1998), Cx43−/− (Gja1−/−) (Reaume et al., 1995), and Foxc2−/− (Iida et al., 1997) mice have been described previously. Cx37−/− and Cx43+/− mice were interbred to generate mice deficient in both Cxs. Mice were maintained on a C57BL/6 background and genotyped by PCR using previously published protocols for Cx40−/− (Simon and McWhorter, 2002), Cx43−/− (Bobbie et al., 2010) and Foxc2−/− lines (Kriederman et al., 2003). Primers for Cx37−/− genotyping were: Primer 1: 5’-GATCTCTCGTGGGATCATTG-3’; Primer 2: 5’-TGCTAGACCAGGTCCAGGAAC-3’; and Primer 3: 5’-GTCCCTTCGTGCCTTTATCTC-3’. Animal protocols were approved by the IACUC Committee at the University of Arizona (Tucson, AZ).
Antibodies
Primary antibodies used for immunostaining were as follows: rabbit antibodies to Cx37 (Simon et al., 2006), Cx37 (40–4200, Invitrogen), Cx40 (Gabriels and Paul, 1998), Cx43 (C6219, Sigma), Cx47 (36– 4700, Invitrogen), LYVE-1 (ab14917, Abcam), NG2 condroitin sulfate (AB5320, Millipore), Prox1 (ab11941, Abcam); mouse antibodies to Cx26 (a gift from Paul Lampe), Cx43 (35–5000, Invitrogen), smooth muscle actin (C6198, Sigma), NFATc1 (sc-7294, Santa Cruz Biotechnology); rat antibodies to CD31 (MON1149, Cell Sciences), CD45 (550539, BD Biosciences), F4/80 (MF48000, Invitrogen), LYVE-1 (53–0443, eBioscience); goat antibodies to ephrin-B2 (AF496, R&D Systems), Foxc2 (ab5060, Abcam), integrin-α9 (AF3827, R&D Systems), Prox1 (ab11941, Abcam), VEGFR-3 (AF743, R&D Systems); hamster antibodies to CD3e (550275, BD Biosciences). AffiniPure minimal cross reactivity secondary antibodies (conjugated to Cy3, Cy5, or Dylight649) and unlabeled Fab fragments were from Jackson ImmunoResearch, AlexaFluor 488 goat anti-rat IgG from Invitrogen, and Vectastain Elite ABC kit (Rabbit IgG) from Vector Laboratories.
Section immunostaining
Tissues were frozen unfixed in Tissue-tek O.C.T. and sectioned at 10 µm. Sections were fixed in acetone at −20°C for 10 minutes, blocked in a solution containing PBS, 4% fish skin gelatin, either 1% goat serum or 1% donkey serum, 0.25% Triton X-100, and incubated with primary antibodies for 2 hours. Sections were washed with PBS containing 0.25% Triton X-100 and then incubated with secondary antibodies for 30 minutes. After washing, sections were mounted in Mowiol 40–88 (Aldrich) containing 1,4-diazobicyclo-(2,2,2)-octane and viewed with an Olympus BX51 fluorescence microscope fitted with a Photometrics CoolSnap ES2 camera or viewed with a Zeiss LSM 510 confocal microscope. Ly vessels were identified by staining with antibodies against Prox1, VEGFR-3, or LYVE-1.
Whole-mount immunostaining
Mesentery was fixed in 1% paraformaldehyde overnight at 4°C, washed in PBS, permeabilized with PBS containing 0.3% Triton X-100, and then blocked overnight in PBS containing 3% goat serum and 0.3% Triton X-100. Primary antibodies diluted in PBS containing 0.3% Triton X-100 were applied to the tissue overnight at 4°C. After washing, fluorescently labeled secondary antibodies were incubated overnight at 4°C. Following final washes, the mesenteries were mounted on slides in Citifluor mountant (Electron Microscopy Sciences). Ear tissue was treated similarly except fixation was for 1 hour at room temperature. For whole-mount Prox1 immunostaining of the TD and diaphragm muscle from embryos, the procedure was the same as with mesentery except Vectastain Elite ABC kit secondary and tertiary reagents and DAB substrate were used.
Lymphangiography with Evans blue dye
Mice were anesthetized with an intraperitoneal injection of sodium pentobarbital and kept warm. Evans blue dye (EBD) (1% w/v) was injected intradermally into the hindpaws and a dissecting microscope was used to examine EBD transport. Evidence of abnormal dye reflux into hindlimb skin or mesenteric lymph nodes was noted if present. The thoracic cavity was then opened and the presence of EBD in the TD was noted along with any abnormal dye reflux into intercostal Ly vessels. In some mice, EBD was also serially injected into forepaws, snout, or ear to examine EBD movement in axillary, jugular, and ear regions, respectively.
Quantification of thoracic duct valves
Following EBD lymphangiography, the TD was dissected out, still attached to the aorta, from just above the diaphragm muscle to the top of the heart. The tissue was frozen unfixed in Tissue-tek O.C.T. and 10 µm serial sections (~750 sections/sample) were collected along the length of the TD. The number of valves per TD was determined from the serial sections. The position of the TD on the sections was determined by the location of EBD in the cryoblock face. Selected sections from the series were haematoxylin and eosin (H&E) stained or immunostained for Prox1 to further confirm the identification of the TD.
Chyle analysis
Chyle samples were submitted to the University of Arizona Animal Care Pathology Services Laboratory for determination of total protein, cholesterol, triglycerides and white cell counts. Triglyceride measurements were performed at Antech Diagnostics.
Quantification of mesenteric valves
Mesenteries from E18.5 embryos, cut into 3–4 segments, were whole-mount immunostained for Prox1 and CD31 as described above. The number of Ly valves per mesentery was determined by counting regions of locally elevated Prox1 staining, by an observer blind to the genotype. The CD31 co-staining pattern was used to confirm the identification of Ly valves.
Quantification of jugular lymph sac (JLS) cross-sectional area and 3D volume reconstructions
Embryos were collected at E13.5, embedded in paraffin, serially sectioned transversely (6 µm sections), and stained with H&E. The lumenal areas of the right and left JLS were measured at the point where the cervical nerve crosses the JLS. Images were taken using an Olympus microscope (BX51) equipped with a CCD camera and analyzed using Image-Pro Plus software. The number of animals analyzed for each genotype was: WT (7); Cx43−/− (4); Cx37−/− (6); Cx37−/−Cx43−/− (6). The Student’s t-test (unpaired, unequal variance) was used for statistics. For 3D volume reconstructions, coronal sections were imaged at low magnification and stitched together with Photoshop CS4. Serial images were manually aligned and imported into BioImageXD for volume rendering. Volume estimates were determined using the Cavalieri method as described by Howard and Reed (Howard, 2005).
Results
Cx37, Cx43, and Cx47 are expressed in the developing lymphatic vasculature and become progressively enriched at lymphatic valves
We looked for expression of vascular Cxs in Ly vessels during mouse embryonic development by immunofluorescence, starting with the jugular lymph sac (JLS), an early Ly primordium. At E12.5, Cx43 was already evident in the endothelium of the JLS but Cx37 expression was sparse. By E13.5, Cx37 and Cx43 were both clearly present in the JLS (Fig. 1A,C), whereas two other Cxs, Cx40 and Cx45, were not detected. Within the JLS, Cx37 and Cx43 were typically differentially expressed in distinct domains, with regions of high Cx37/low Cx43 expression next to regions of high Cx43/low Cx37 expression. Segregation of Cx37 or Cx43 expression was sometimes associated with extensions or sprouting regions of the JLS (Fig. 1D). Cx26, which is associated with Ly vessel invasion in breast cancer (Naoi et al., 2007), and Cx47, which is mutated in some families with primary lymphedema (Ferrell et al., 2010), were not detected in the E13.5 JLS.
Figure 1. Expression of Cx37, Cx43, and Cx47 in developing lymphatic vessels of the wild-type mouse embryo.
(A–E) Immunolabeling of jugular lymph sac (jls) of E13.5 WT mouse embryos. Cx37 (green) and Cx43 (red) are differentially expressed in distinct domains in the JLS. The Cx37 antibody crossreacts with some muscle fiber types (upper right and lower right in panel A). (C) A higher magnification view of the JLS in (A). (D) Cx37 expression in this area is associated with a sprouting region of the JLS. (B,E) Prox1 labeling of sections adjacent to those in (A,D), respectively. (F–K) Immunolabeling of dermis and subdermis of E16.5 WT embryos. Cx37 and Cx43 (both green) is present in dermal lymphatics (ly) (F,H) as well as in deeper subcutaneous lymphatics (G,I). Cx37 is also highly expressed in arteries (a). Cx47 is weakly detected (arrows) in some E16.5 dermal (J) and subcutaneous lymphatics (K). (L–Q) Immunolabeling of thoracic duct (td) cross sections of E18.5 WT embryos. Cx37 and Cx43 are both present in the E18.5 TD (the TD lumen is collapsed in these sections) and there is significant colocalization of the Cx immunosignals (N). (O) Cx47 is weakly detected (arrow) in the E18.5 TD. (P,Q) VEGFR-3 and Prox1 labeling of adjacent sections to those in (L–O). Scale bars: (A,B) 50 µm; (C) 50 µm; (D, E) 50 µm; (F–K) 50 µm; (L–Q) 25 µm.
At E16.5, Cx37 and Cx43 were present in dermal lymphatics of the skin as well as in deeper subcutaneous lymphatics (Fig. 1F–I), which tended to show higher Cx expression than superficial lymphatics strongly positive for VEGFR3 or LYVE-1. Cx47 was weakly detected in some of the E16.5 dermal and subcutaneous lymphatics (Fig. 1J,K) whereas Cx26 was not detected. At E18.5, Cx37 and Cx43 were both present in the Ly endothelium of the TD (Fig. 1L–N), and Cx47 was weakly detected there as well (Fig. 1O). Cx37 and Cx43 were also detected in other central Ly trunks at E18.5.
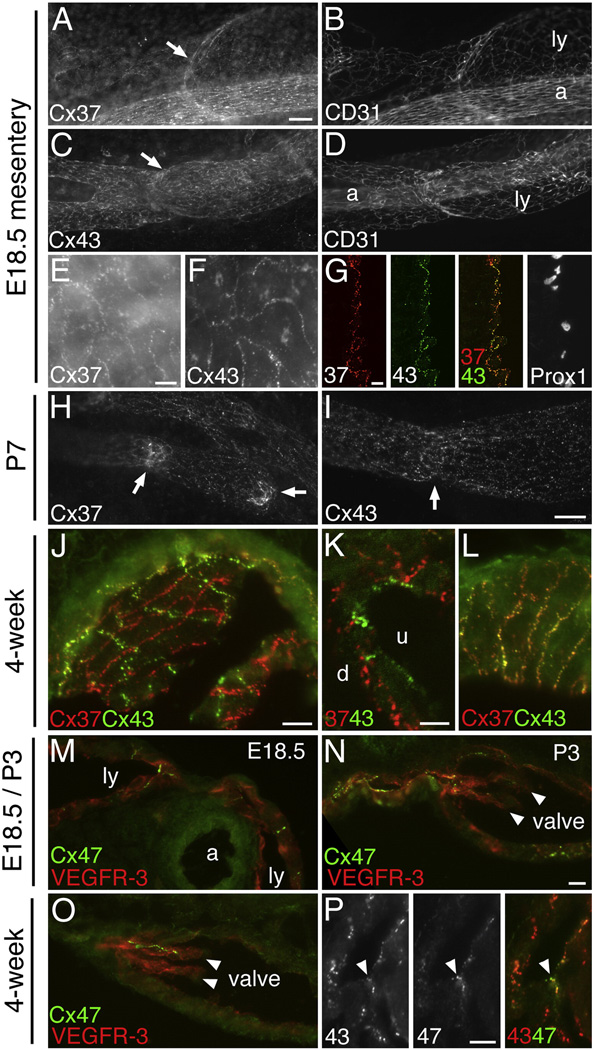
Cx37 and Cx43 were also prominently expressed in the developing mesenteric collecting lymphatics at E18.5. Ly vessels in the small intestine and its associated mesentery are essential for the absorption and transport of dietary fat from the intestine to the TD. Cx37 was also expressed in the endothelium of mesenteric arteries but not mesenteric veins. Cx43 was not detected in the endothelium of either mesenteric arteries or veins. Whole-mount immunostaining revealed that Cx37 (Fig. 2A,E) and Cx43 (Fig. 2C,F) were concentrated at cell-cell interfaces throughout the endothelium of the collecting lymphatics, including at the intraluminal valves. Double labeling showed that Cx37 and Cx43 were colocalized in the endothelium of the collecting lymphatics (Fig. 2G) (except in valves, as discussed below). In the intestinal wall, Cx43 but not Cx37 was detected in the submucosal lymphatics and lacteals (see Fig. S6 in the supplemental material). Cx37 and Cx43 were also detected in E16.5 mesenteric collecting lymphatics and trace amounts of Cx37 were observed as early as E15.5, but not earlier (not shown). Postnatally, at P3-P7, Cx37 expression (Fig. 2H) became progressively enriched in the valves of the collecting lymphatics whereas Cx43 (Fig. 2I) remained uniformly expressed in the Ly endothelium, including valves. In the collecting lymphatics of 3–4 week old mice, Cx37 was highly enriched in the valves (Fig. 2J). Cx43 was also concentrated in valves at this age, although the degree of enrichment was more variable (Fig. 2J). Surprisingly, double labeling showed that Cx37 and Cx43 were remarkably differentially localized in the mesenteric valve leaflets (Fig. 2J,K) but not in the non-valve endothelium (Fig. 2L) (discussed further below).
Figure 2. Cx37, Cx43, and Cx47 become progressively enriched at mesenteric lymphatic valves and are differentially expressed in mesenteric valve leaflets.
(A–G) Immunolabeling of mesenteric collecting lymphatics (ly) of E18.5 WT embryos. Whole-mount immunolabeling shows Cx37 and Cx43 labeling throughout the Ly endothelium, including at valves (arrows). (B,D) CD31 labeling of the same sections shown in (A,C) respectively. Arteries are labeled (a). (E,F) Higher magnification of Cx37 and Cx43 labeling shows that the Cx immunosignals outline individual endothelial cells. (G) Cx37 (red) and Cx43 (green) colocalize in this transverse section through a mesenteric Ly vessel (non-valve region). The far-right panel shows Prox1 labeling of an adjacent section. (H,I) At P7, Cx37 is enriched at mesenteric Ly valves (arrows) whereas Cx43 is more uniformly expressed in the Ly endothelium. (J,K) At postnatal 4-weeks, Cx37 (red) and Cx43 (green) is highly enriched at mesenteric Ly valves and is differentially localized in the valve leaflets. A flat, en face view of a valve leaflet is shown in (J) and a transverse section of a leaflet is shown in (K). Cx43 is enriched in the upstream side of the leaflet (u) and Cx37 is enriched in the downstream side (d). (L) Labeling of an oblique section through the wall of a 4-week postnatal mesenteric Ly shows that Cx37 and Cx43 colocalize in non-valve Ly endothelium. (M–P) Cx47 expression in mesenteric lymphatics at different stages of development. (M) At E18.5, Cx47 is found in a subset of LECs in the mesenteric lymphatics. (N) At P3, Cx47 is found associated with valve leaflets (arrowheads). (O) At 4 weeks, Cx47 is highly restricted to a subset of cells within valve leaflets (arrowheads). In valve LECs expressing Cx47, Cx47 and Cx43 colocalize (arrowheads in panel P). In (M–O) VEGFR-3 labeling identifies the lymphatics. Scale bars: (A–D) 50 µm; (E, F) 10 µm; (G) 10 µm; (H, I) 50 µm; (J, L) 10 µm; (K) 5 µm; (M–O) 10 µm; (P) 10 µm.
In addition to Cx37 and Cx43, we also detected Cx47 in a subset of LECs in E18.5 mesenteric collecting lymphatics (Fig. 2M). At P3, Cx47 expression remained patchy in mesenteric lymphatics and was associated with valve leaflets (Fig. 2N). In the 3–4 week old mouse, Cx47 expression was highly restricted to a subset of cells within the valve leaflets of larger mesenteric collecting lymphatics and was often found near the base of the leaflet (Fig. 2O). Cx47 colocalized with Cx43 in those areas (Fig. 2P).
Cx37, Cx43, and Cx47 are present in lymphatic vessels of the adult mouse and are differentially expressed in valves of the thoracic duct
Cx expression in adult mouse Ly vessels was also examined, starting with the TD, the largest Ly vessel of the body. The TD originates with the confluence of the lumbar and intestinal Ly trunks of the abdomen and extends anteriorly through the chest and into the neck, where it delivers lymph to the venous system in the area of the left jugular and subclavian veins. We detected Cx37 (Fig. 3A) and Cx43 (Fig. 3B), but not Cx26, Cx40, Cx45, or Cx47 in the general Ly endothelium (non-valve regions) of the vessel. Levels of Cx37 were comparatively much lower in the TD than in arterial endothelium (Fig. 3C). Cx43 expression in the TD was more variable than Cx37, and detection required a high affinity antibody. When both Cx37 and Cx43 were detected in the same sections of TD, the staining colocalized (Fig. 3D) (except in valves, as discussed below).
Figure 3. Cx37, Cx43, and Cx47 are highly enriched in lymphatic valves in the adult mouse and are differentially expressed in upstream and downstream sides of thoracic duct valves.
(A–H) Immunolabeling of thoracic duct (td) transverse sections from adult WT mice for Cx37 and Cx43. Cx37 and Cx43 are both detected in the endothelium of the TD general wall (non-valve region) and colocalize there (D). (C) Cx37 expression is comparatively much lower in TD than in arteries (a). (E,F) Cx37 and Cx43 are highly enriched in valves of the TD. (G) Epifluorescent imaging of a flat, en face TD valve section colabeled for Cx37 (green) and Cx43 (red) shows that the two Cxs are differentially localized. (H) Confocal z-stack imaging of another flat, en face TD valve section colabeled for Cx37 and Cx43 shows that Cx43 is enriched in the upstream side of the leaflet and Cx37 is enriched in the downstream side. In the center is the x-y projection through the z-stack. At the top is the x-z projection and at the right is the y-z projection, both showing separation of the Cx37 and Cx43 signals. (I,J) Cx47 is found exclusively in a subset of cells of the valve in the TD (a flat, en face portion of the valve is shown), where it colocalizes with Cx43 (arrowheads in J). (K–N) Immunolabeling of Cx37 and Cx43 in the ear of adult mice. Cx37 and Cx43 (both green) are detected in valved collecting lymphatics of the ear (arrows in K and L) but not in the Ly capillaries highly expressing LYVE-1 (red) (arrows in M and N). In (I,K,L) VEGFR-3 labeling highlights the lymphatics. Scale bars: (A, B) 10 µm; (C) 10 µm; (D) 10 µm; (E, F) 20 µm; (G–I) 10 µm; (J) 5 µm; (K, L) 10 µm; (M, N) 10 µm.
Cx37, Cx43, and Cx47 were found to be strikingly enriched in the valves of the TD (Fig. 3E,F,I). As in mesenteric Ly valves, Cx37 and Cx43 displayed remarkably distinct expression patterns in TD valves (Fig. 3G,H). Since valve leaflets are comprised of two endothelial layers separated by a thin extracellular matrix (Takada, 1971) (see Fig. S1 in the supplemental material for electron microscopy), we hypothesized that Cx37 and Cx43 might be differentially expressed in the two endothelial layers. To address this possibility, we used confocal microscopy to examine sections of TD and mesenteric lymphatics where the valve leaflet was relatively flat and intact. Analysis of the z-stack series of these en face sections showed that Cx37 was highly expressed on the downstream side of the valve leaflet, whereas Cx43 was enriched on the upstream side (Fig. 3H and see also Fig. S2 in the supplemental material for schematic summary). Endothelial cells on the downstream side of the leaflet tended to be more elongated and densely spaced than cells on the upstream side. Consistent with the mesentery data, Cx47 was found exclusively in the valve leaflets of the TD (Fig. 3I), typically in a subset of LECs that tended towards the base of the valve, where it colocalized with Cx43 (Fig. 3J). We also sampled a few other Ly vessels in the adult for Cx37 and Cx43 expression, including deep lymphatics of the submaxillary and axillary regions, and confirmed expression of both Cxs (not shown). In the peripheral lymphatics of the ear, Cx37 and Cx43 were detected in collecting lymphatics (Fig. 3K,L) but not in the LYVE-1 positive Ly capillaries (Fig. 3M,N).
Cx37−/− embryos have enlarged jugular lymph sacs at E13.5
We next examined Cx37−/− and Cx43−/− mice, as well as mice deficient in both Cxs during embryogenesis to investigate the role Cx37 and Cx43 play in Ly development, starting with the JLS. Morphometric analysis revealed that the JLS cross-sectional area at E13.5 was greatly enlarged in Cx37−/−(1.7-fold) and Cx37−/−Cx43−/− (2.6-fold) embryos compared to WT embryos but was not significantly altered in Cx43−/− embryos (Fig. 4A,B). 3D volume reconstructions from serial sections showed that the total JLS volume in Cx37−/−Cx43−/− embryos (n=2) was also much larger (~6-fold) than in a WT control (Fig. 4C).
Figure 4. Cx37−/− and Cx37−/−Cx43−/− embryos have enlarged jugular lymph sacs at E13.5.
(A) H&E stained transverse sections of E13.5 mouse embryos. Sections were taken just above the point where the cervical nerve crosses the JLS. The paired right and left JLS lumen is outlined in red for each genotype.. (B) Cross-sectional area measures of the JLS for the various genotypes show that the JLS is significantly enlarged in Cx37−/− and Cx37−/−Cx43−/− embryos compared to WT (asterisk indicates p < 0.05). Error bars indicate s.e.m. (C) 3D volume reconstruction of JLS (green) and cardinal vein (blue) from serially sectioned WT and Cx37−/−Cx43−/− E13.5 embryos. The volume of the JLS in the Cx37−/−Cx43−/− embryo was much larger (~6-fold) than in the WT control. Axes: z (blue), dorsal; y (green), caudal; x (red), lateral. Scale bar: (A) 100 µm.
Cx37−/−Cx43−/− embryos display lymphedema, abnormal thoracic duct development, and blood-filled lymphatics
At E18.5 or P0, Cx37−/−Cx43−/− mice exhibited severe edema, particularly encircling the neck (nuchal edema) (Fig. 5A). Cx37+/−Cx43−/− mice also showed edema but at a lower frequency. In contrast, edema was seldom observed in Cx37−/− (Fig. 5B) or Cx43−/− (Fig. 5C) embryos or newborns. Sections of E16.5 Cx37−/−Cx43−/− embryos revealed widely dilated superficial lymphatics in the skin as well as a brawny thickening of the subcutaneous tissue characteristic of lymphedema (Fig. 5D,F). Dilated superficial lymphatics were not observed in E16.5 Cx37−/− or Cx43−/− embryos. We also looked at TD morphology in E18.5 embryos by whole-mount Prox1 immunostaining. WT TDs (Fig. 6A) exhibited relatively uniform caliber at this stage whereas TDs of Cx43−/− (Fig. 6C) and Cx37−/−Cx43−/− (Fig. 6D) embryos displayed extremely erratic caliber as well as blind-ended outcroppings and bifurcated segments. In addition, the morphology of the left and right intercostal Ly trunks was abnormal (Fig. 6F). In contrast, the TD and intercostal trunks looked normal in Cx37−/− embryos (Fig. 6B). Finally, the Ly network on the thoracic surface of the diaphragm muscle, visualized by Prox1 staining, was diminished in Cx43−/− and Cx37−/−Cx43−/− E18.5 embryos (Fig. 6H), suggesting that there was reduced invasion of Ly sprouts into this muscle.
Figure 5. Cx37−/−Cx43−/− embryos exhibit lymphedema and dilated superficial lymphatics.
(A–C) Upper portion of embryos at E18.5. Cx37−/−Cx43−/− embryos exhibit edema whereas Cx37−/− and Cx43−/− embryos do not. (D,E) H&E stained transverse sections of E16.5 embryos show evidence of massive edema (thickening of the subcutaneous tissue is marked by a double arrow) in the Cx37−/−Cx43−/− embryo (D) but not in the WT control (E). (F,G) Transverse sections of E16.5 embryos labeled for LYVE-1 (red) and CD31 (green). The LYVE-1 labeling reveals widely dilated superficial lymphatics (arrows) in the Cx37−/−Cx43−/− embryo (F) but not in the WT control (G). Scale bars: (D,E) 400 µm; (F, G) 100 µm.
Figure 6. Central lymphatic patterning is abnormal in Cx43−/− and Cx37−/−Cx43−/− embryos at E18.5.
Prox1 whole-mount immunostaining of E18.5 thoracic duct (td) (A–D), E18.5 intercostal Ly trunks (E, F), and E18.5 diaphragm muscle (G, H). Cx43−/− and Cx37−/−Cx43−/− TDs have extremely erratic caliber, blind-ended outcroppings, and bifurcated segments compared with WT and Cx37−/− TDs. The intercostal trunk Lys (arrows) in a Cx37−/−Cx43−/− embryo (F) are sac-like compared to the WT control (E). The Ly network (arrows) on the thoracic surface of the diaphragm muscle is diminished in the Cx37−/−Cx43−/−embryo compared to the WT control. Scale bars: (A–D) 200 µm; (E, F) 200 µm; (G, H) 200 µm.
Cx37−/−Cx43−/− embryos and Cx37+/−Cx43−/− embryos often presented with significant amounts of blood in the Ly vasculature (Fig. 7A,B). At E18.5 or P0, Cx37−/−Cx43−/− mice were usually identifiable in litters by the bloody discoloration in superficial skin lymphatics. We confirmed that the large bloody vessels in the skin were lymphatics rather than blood vessels by whole-mount Prox1 staining (Fig. 7C). Blood was also present in the TD (Fig. 7D,E) and intercostal Ly trunks (Fig. 7D,F) of E18.5 Cx37−/−Cx43−/− embryos and could be followed through Ly trunks into a central sac-like area of the mesentery and then into the mesenteric collecting lymphatics and the superficial lymphatics of the intestinal wall (Fig. 7G). Prox1 staining confirmed the Ly identity of the blood-filled vessels in the mesentery (Fig. 7H). Mesenteric blood vessels in E18.5 Cx37−/−Cx43−/−, Cx37+/−Cx43−/−, and Cx43−/−embryos looked normal, and aberrant connections between blood vessels and Ly vessels were not observed. The frequency of Ly defects for E18.5-P0 Cx-deficient mice is summarized in Table S1 in the supplemental material.
Figure 7. Cx37+/−Cx43−/− and Cx37−/−Cx43−/− embryos display blood-filled lymphatics.
(A) Bloody discoloration in superficial skin lymphatics of a Cx37+/−Cx43−/− E18.5 embryo. (B) Higher magnification view of blood in superficial skin lymphatics of a Cx37+/−Cx43−/− E18.5 embryo. (C) Whole-mount skin Prox1 (green) immunostaining of a Cx37−/−Cx43−/− embryo confirms the Ly identity of the bloody vessels. Blood (red) was detected by autofluorescence with UV excitation. (D) Bloody discoloration in the TD (arrow) and intercostal Ly trunks (arrowhead) of a Cx37−/−Cx43−/− E18.5 embryo. Note: the specimen was photographed after whole-mount immunostaining for Prox1 (faint spots) and blood that was in the aorta has been washed away during tissue processing. (E,F) Higher magnification views of bloody TD (arrow) and bloody intercostal Ly trunks (arrowhead) shown in (D). (G) Blood-filled mesenteric collecting lymphatics (arrows) as well as bloody superficial intestinal lymphatics are observed in this Cx37+/−Cx43−/− E18.5 specimen. (H) The same specimen as in (G) stained for Prox1 (green) and CD31 (red) confirms the Ly identity of the large blood-filled vessels (arrows point to the same vessels as in G). (I) A Cx37−/−Cx43+/− specimen shows no blood in mesenteric or intestinal lymphatics. Scale bars: (C) 100 µm; (D) 400 µm; (H) 400 µm.
Cx37 and Cx43 are required for lymphatic valve development in collecting vessels of the mesentery and skin
We considered the possibility that some of the Ly disorders in Cx knockout embryos, including bloody lymphatics, might be due to an abnormality in Ly valves. The number of valves in mesenteric collecting lymphatics of WT, Cx37−/−, Cx43−/−, and Cx37−/−Cx43−/− embryos were compared at E18.5. In WT embryos (Fig. 8A,B,I), valves were abundant in the mesenteric collecting lymphatics (26.3±1.9 valves/mesentery) and could be visualized easily by whole-mount immunostaining for Prox1 and CD31. Prox1 expression was elevated in valve LECs and CD31 staining also highlighted the valve leaflets. However, Ly valves were completely absent in Cx43−/− (Fig. 8E,I) and Cx37−/−Cx43−/− (Fig. 8G,I) mesentery samples. In addition, collecting lymphatics of Cx43−/− and Cx37−/−Cx43−/− embryos often appeared jagged or enlarged, and LEC morphology was somewhat disorganized (see Fig. S5J in the supplemental material, for example). Furthermore, compared to WT, there was a 47% reduction in the number of valves in Cx37−/− mesentery (13.9±1.5) (Fig. 8C,D,I) and a 23% reduction in valves in Cx37+/−Cx43+/− mesentery (20.3±1.8) (Fig. 8I). Finally, whole-mount Prox1 immunostaining of the thoracic cavity showed that valves were also absent from the intercostal Ly trunks of E18.5 Cx43−/− and E18.5 Cx37−/−Cx43−/− embryos (see Fig. S3 in the supplemental material).
Figure 8. Cx37 and Cx43 are required for lymphatic valve development in collecting vessels of the mesentery.
(A–H) Whole-mount immunolabeling of E18.5 mesentery samples for Prox1 (green) and CD31 (red). The intestine is at the bottom of the field. (B,D,F,H) are higher magnification views of (A,C,E,G), respectively. Valves, highlighted by elevated Prox1 expression, are found in WT (A,B) and Cx37−/−(C,D) samples (arrows) but not in Cx43−/− (E, F) or Cx37−/−Cx43−/− (G,H) specimens. (I) Quantification of valves in E18.5 mesenteries showed that valves are completely absent in specimens lacking Cx43. Compared to WT, there was a 47% reduction in the number of valves in Cx37−/− mesentery. Asterisks indicated a statistically significant difference from WT (Cx37+/+Cx43+/+), p < 0.05 for Cx37+/−Cx43+/−; p < 0.01 for the others. Error bars indicate s.e.m. The number of mesenteries analyzed for each genotype is indicated at the bottom. ly, lymphatic. a, artery. v, vein. Scale bars: (A,C,E,G) 200 µm; (B,D,F,H) 200 µm.
Valves were also examined in collecting lymphatics of the skin from E18.5 embryos by whole-mount immunostaining (see Fig. S4 in the supplemental material). In WT embryos, valves could be detected in the dorsal skin at this stage but these were entirely absent in the Cx37−/−Cx43−/− samples, and the lymphatics were often dilated or sac-like. In contrast to the mesentery, however, some valves were still present in Cx43−/− skin samples, although the frequency of valves appeared reduced. Valves were also retained in Cx37−/− skin samples. Thus, although the loss of Cx43 alone is sufficient to completely prevent valve formation in mesenteric collecting lymphatics and intercostal lymphatics, skin lymphatics require the loss of both Cx37 and Cx43 before valves fail to form entirely.
Valves might not form in Cx-deficient lymphatics if these vessels fail to initiate the developmental program for formation of collecting lymphatics. To address this issue, we examined whole-mount mesentery collected at E17.5-P0 for expression of a number of markers whose expression has been characterized during collecting vessel development (Norrmén et al., 2009), including Foxc2, NFATc1, LYVE-1, and VEGFR-3 (see Fig. S5 in the supplemental material). In all cases, marker immunostaining in Cx43−/− or Cx37−/−Cx43−/− mesenteric lymphatics appeared similar to controls, except for the absence of valve staining. NFATc1 was detected in the nucleus in both Cx43−/− and Cx43+/− mesentery, suggesting that there was no difference in its activation state. LYVE-1 showed normal down regulation from relatively high levels at E18.5 to lower levels at E19.5 or P0, as expected during collecting vessel maturation (Norrmén et al., 2009). We also immunostained mesentery for smooth muscle actin and NG2 chondroitin sulfate as markers for mural cell recruitment. At E18.5, there was very little smooth muscle cell actin or NG2 staining of WT mesenteric lymphatics, and this feature was not altered in Cx43−/− mesentery. NG2 staining was also unchanged in Cx43−/− and Cx37−/−Cx43−/− skin lymphatics.
Ly valve defects have also been observed in mice with mutations in EphrinB2 (Mäkinen et al., 2005) or integrin-α9, and one interesting possibility is that Cx37 or Cx43 might interact with EphrinB2 or integrin-α9 in Ly vessels (Bazigou et al., 2009; Huang et al., 2000). As an initial step towards investigating this scenario, we compared the distribution of Cx37 and Cx43 with that of EphrinB2 and integrin-α9 in WT Ly vessels and looked for colocalization (see Fig. S6 in the supplemental material). In whole-mount P4 mesentery, Cx37 and integrin-α9 partially overlapped in the valve leaflets where both were substantially concentrated. Cx37 was often more highly expressed towards the base of the valve. In addition, Cx43 and EphrinB2 colocalized in distinct punctae in some submucosal lymphatics of the E18.5 intestine.
Cx37−/−Cx43+/− mice display retrograde lymph flow and chylothorax
We next turned our attention to Cx37−/−Cx43+/− mice, which show reduced postnatal viability. Only ~50% of the expected frequency of this genotype was obtained at weaning (see Table S2 and Table S3 in the supplemental material). Moreover, many of the Cx37−/−Cx43+/− mice that survived to weaning died suddenly and prematurely in the following weeks, with ~40% of the deaths occurring before 8 weeks (Fig. 9A). Necropsy revealed a milky effusion around the heart and lungs consistent with chylothorax, a disorder resulting from a leak or disruption of the TD or one of its chyle (intestinal lymph) containing tributaries (Fig. 9B). We measured triglycerides, cholesterol, protein, and the cellularity of the fluid and the results confirmed its chylous nature. In particular, it was high in triglycerides (2,740±845 mg/dL; n=5) and cholesterol (196±47 mg/dL; n=5) and the cholesterol/triglycerides ratio (0.11±0.03; n=5) was typical of chyle. Many of the cells (~70%) present in the fluid were lymphocytes. Chylothorax was not observed in Cx43+/− mice and was extremely rare in Cx37−/− mice (one case out of ~300 mice).
Figure 9. Cx37−/−Cx43+/− mice display retrograde lymph flow and frequently die prematurely with chylothorax.
(A) Percentage of deaths of Cx37−/−Cx43+/− mice versus age. Approximately 40% of the deaths occurred before 8 weeks of age. (B) Milky chylous effusion surrounding the heart and lungs of a Cx37−/−Cx43+/− mouse with chylothorax. (C–N) EBD visual lymphangiography was performed to follow lymph drainage patterns. (C,D) Following hindpaw injection of EBD, in WT and Cx43+/− mice the dye is restricted to the TD (arrow) as it moves unidirectionally cephalad. (E–G) In many Cx37−/− and Cx37−/−Cx43+/− mice, EBD shows reflux (retrograde flow) into intercostal lymphatics (asterisks) lateral to the TD (arrow). (G) A higher magnification view of area of intercostal dye reflux marked by asterisk in (F). (H) In some Cx37−/−Cx43+/− mice, blood is observed in the same intercostal Ly vessels that contains EBD due to reflux (arrow). (I) Cx37−/−Cx43+/− mice show a high incidence of EBD reflux into mesenteric lymph nodes (ln). (J) EBD injection into the dermis of the ear of a WT mouse (injection site is marked by a white circle) results in unidirectional drainage and convergence of the dye into Ly collecting vessels. (K) Reflux (marked by an asterisk) and increased lateral spread occurs when EBD is injected into the ear of a Cx37−/−Cx43+/− mouse. (L–N) Hindlimb skin in the area above the EBD-injected hindpaw. The Cx43+/− hindlimb skin (L) shows no dye reflux whereas hindlimb skin from Cx37−/− (M) and Cx37−/−Cx43+/− (N) mice show prominent reflux of EBD into a network of surrounding lymphatics in the skin.
To assess Ly function in Cx37−/−Cx43+/− mice, we performed Evans blue dye (EBD) visual lymphangiography to follow lymph drainage patterns. When EBD is injected into the dermis of the hindpaws, the dye is taken up by Ly capillaries, binds tightly to protein in tissue fluid and lymph, and is transported through the Ly trunks of the leg and abdomen, through the iliac and retroperitoneal lymph nodes, and eventually into the TD. EBD can therefore normally be traced from the injected hindpaw all the way through the TD. With WT mice, we observed the expected unidirectional anterograde transport of EBD, and in the chest wall, the dye was restricted to the TD as it moved cephalad (Fig. 9C). In contrast, with Cx37−/−Cx43+/− mice, we noted reflux (retrograde flow) of EBD into a network of surrounding lymphatics in the hindlimb skin (Fig. 9N), as well as reflux into mesenteric lymph nodes (Fig. 9I) and intercostal lymphatics (Fig. 9F,G). Reflux and increased lateral spread of EBD also occurred when the dye was injected into the dermis of the ear (Fig. 9K). While all the Cx37−/−Cx43+/− mice exhibited EBD reflux into the hindlimb skin lymphatics, intercostal dye reflux varied from severe to mild or, less commonly, was undetectable. When EBD was injected into the hindpaw of a mouse with ongoing chylothorax, the dye leaked into the thoracic cavity and mixed with the chylous effusion, consistent with rupture of the TD or one of its tributaries. In some Cx37−/−Cx43+/− mice, EBD did not fill the TD following hindpaw injection, indicating a severe impairment of lymph transport. Peripheral edema was not observed in Cx37−/−Cx43+/− mice, however, suggesting that the primary defect in mice with this genotype involves central rather than peripheral lymphatics. To investigate this issue further, we looked at peripheral Ly networks in the ear by whole-mount LYVE-1immunostaining, which labels Ly capillaries. Ly patterning looked normal in ears of Cx37−/−Cx43+/− and Cx37−/− mice, and the number of branch points in the Cx37−/−Cx43+/− Ly network was not different from WT (see Fig. S7 in the supplemental material).
EBD injections were also performed with Cx37−/−, Cx43+/−, Cx37+/−Cx43+/− and Cx40−/− mice. Surprisingly, we found that Cx37−/− mice also frequently exhibited dye reflux into hindlimb skin (Fig. 9M) and intercostal lymphatics (Fig. 9E). Intercostal reflux, however, was not usually as severe in Cx37−/− mice as in Cx37−/−Cx43+/− mice, and there were also fewer instances in which the TD did not fill at all with dye. Moreover, reflux into mesenteric lymph nodes was observed in Cx37−/−Cx43+/− mice but not in Cx37−/− mice. These results indicate that although chylothorax is very rare in Cx37−/− mice, Ly function is nevertheless significantly impaired in these mice. EBD transport was normal in Cx43+/− (Fig. 9D) and Cx40−/− mice, and reflux occurred only very rarely in Cx37+/−Cx43+/− mice. The frequency of Ly defects for adult Cx-deficient mice is summarized in Table S4 in the supplemental material.
Cx37 and Cx43 are required for thoracic duct valve development
The phenotype of Cx37−/−Cx43+/− mice and Cx37−/− mice suggested either a defect in the ability of truncal lymphatics to move lymph effectively in an anterograde fashion or, alternatively, an obstruction in the truncal lymphatics. Given the valve data from Cx-deficient embryos, we hypothesized that Cxs are also necessary for normal development of the TD valves. To test this idea, an extensive histological analysis of excised TDs from adult Cx37−/−Cx43+/−, Cx37−/−, and WT mice was performed (TDs from Cx43−/− and Cx37−/−Cx43−/− mice could not be examined as those genotypes are not viable postnatally). TDs were serially sectioned (transversely) from just above the diaphragm all the way to the top of the heart, and sections were either stained with H&E or immunostained with Prox1 to identity the TD and to visualize valves. In WT mice, valves (typically bicuspid) were present only in the rostral half of the TD and were more closely spaced towards the top (Fig. 10A and see Fig. S8 in the supplemental material for a schematic of valve distribution). We observed up to four valves (3.20±0.37 valves/TD) in the WT TDs (Fig. 10E). In contrast, the number of valves in TDs from Cx37−/−Cx43+/− mice was reduced by ~10-fold (0.30±0.15 valves/TD) (Fig. 10E). In most of the Cx37−/−Cx43+/− mice, no TD valves were found while others had only one valve. Cx37−/− mice also had a substantial reduction in TD valves (1.20±0.37 valves/TD) (Fig. 10E), but there were clearly more valves in Cx37−/− mice than in Cx37−/−Cx43+/− mice. These results support the hypothesis that Cx37 and Cx43 are required for TD valve development and provide a plausible explanation for the Ly functional defects observed in Cx37−/−Cx43+/− and Cx37−/−mice. We also considered the possibility that the TD was compromised in Cx-deficient mice by a deficiency in associated smooth muscle cells. Sections of Cx37−/−Cx43+/− and WT TD were immunostained for smooth muscle cell actin but no differences in staining were observed (see Fig. S9 in the supplemental material).
Figure 10. Cx37 and Cx43 are required for thoracic duct valve development.
(A–D) H&E stained transverse sections of TD (td) from adult mice. (A) Representative example of a TD valve from WT mouse, showing typical bicuspid morphology. (B) TD valves are observed in Cx37−/− mice but the frequency of valves is reduced. (C,D) Rare examples of TD valves present in Cx37−/− Cx43+/− mice. In most Cx37−/−Cx43+/− mice, however, no TD valves were found. Based on its appearance in serial sections, the valve shown in (D) was likely functionally insufficient. (E) Quantification of the number of valves per TD for WT, Cx37−/−, and Cx37−/−Cx43+/− mice. Asterisks indicate statistically significant differences from WT (p < 0.01). There was also a statistically significant difference (denoted by #) between Cx37−/− and Cx37−/−Cx43+/− mice (p < 0.05). Error bars indicate s.e.m. The number of TDs analyzed for each genotype is indicated within the bar. Scale bars: (A–C) 20 µm; (D) 40 µm.
A number of additional Ly phenotypes were noted sporadically in the analysis of Cx37−/−Cx43+/− mice (see Fig. S10 in the supplemental material). These included blood in the lumen of the TD, large aggregates of immune cells obstructing the duct, atresia of the duct, and wide dilation of the duct. The immune cell aggregates were CD45+ leukocytes and contained a mixture of F4/80+ macrophages/monocytes, CD3+ T cells, and CD19+ B cells. In addition, we observed cases of abnormal sharp turns in the TD and instances in which the duct was on the opposite side of the midline. Anatomical aberrations of this type were also noted with some frequency in Cx37−/− mice. In one instance, a Cx37−/− mouse was found to have a grossly overgrown and convoluted TD valve. Finally, significant abnormal fat deposition in the thoracic cavity was noted in some Cx37−/−Cx43+/− mice, particularly along the aorta and TD and around the heart.
Cx37 expression in jugular lymph sac and mesenteric collecting lymphatics is reduced in the absence of the transcription factor Foxc2
Because Foxc2 has been implicated in Ly valvulogenesis (Petrova et al., 2004; Kriederman et al., 2003; Norrmén et al., 2009), we hypothesized that Cx37 and Cx43 might be downstream of this forkhead family transcription factor in the collecting vessel developmental pathway. To test this idea, we compared Cx37 and Cx43 expression in WT and Foxc2−/− embryos, which fail to initiate the collecting vessel developmental program (Norrmén et al., 2009). Foxc2−/− embryos die variably between E12.5 and P0, so embryos were initially collected at E13.5, and Cx expression was examined in the JLS. Cx43 expression was unaffected by the loss of Foxc2 (Fig. 11B). Cx37 expression, however, was dramatically reduced in the JLS of Foxc2−/− embryos (Fig. 11B). In a few sections, residual expression of Cx37 was observed. In most sections, however, Cx37 was undetectable in the Foxc2−/− JLS whereas Cx37 expression in nearby arteries was unaffected. Interestingly, similar to Cx37−/− embryos, we noted that the JLS of Foxc2−/− embryos were greatly enlarged compared to WT controls. We also examined Cx37 expression in E17.5 mesenteric lymphatics and found that Cx37 expression was reduced but not eliminated in the Foxc2−/− embryos (Fig. 11F) compared to WT embryos (Fig. 11E).
Figure 11. Cx37 expression in jugular lymph sac and mesenteric collecting lymphatics is reduced in the absence of the transcription factor Foxc2.
(A–D) Immunolabeling of JLS (jls) of E13.5 WT and Foxc2−/− embryos. (A) Cx37 (green) and Cx43 (red) expression in the JLS (arrow) of a E13.5 WT embryo. The Cx37 antibody crossreacts with some muscle fiber types (upper right and middle left). (B) In Foxc2−/− embryos, Cx37 expression is greatly reduced in the JLS whereas Cx43 is still present. Cx37 expression in arteries (a) is unaffected by the absence of Foxc2. (C, D) Prox1 labeling of sections adjacent to those in (A,B) respectively. (E,F) Cx37 immunolabeling of transverse sections of mesentery from E17.5 WT and Foxc2−/− embryos. (E) In WT mesentery, Cx37 is expressed by collecting lymphatics (ly) and by arteries (a). (F) Foxc2−/− mesentery shows reduced expression of Cx37 in the lymphatics but not in the arteries. Scale bars: (A–D) 50 µm; (E, F) 20 µm.
Discussion
In this study, we report for the first time that the GJ proteins Cx37, Cx43, and Cx47 are expressed in developing and mature Ly vessels of the mouse and that Cx37 and Cx43 are necessary for the formation of valves in Ly collecting vessels. Mice deficient in these Cxs either lack valves entirely in collecting vessels or have reduced numbers of valves, depending on the genotype. Doubly deficient Cx37−/−Cx43−/− mice also develop severe lymphedema and exhibit bloody lymphatics. Additionally, we find that Cx43 is required for normal patterning of the TD and that both Cx37 and Cx43 are important for TD valve development. Cx37−/−Cx43+/− mice are initially viable but are severely deficient in TD valves and develop lethal chylothorax. Furthermore, we show that another Cx family member, Cx47, is highly enriched in valve leaflets postnatally. Finally, we demonstrate that Cx37 expression in developing lymphatics depends, at least in part, upon Foxc2 expression, suggesting that Cx37 may be a target of Foxc2 regulation during Ly development. These data indicate that multiple Cxs are essential for normal development and function of the Ly vasculature.
Considering the marker expression data, Cx37 and Cx43 seem to be required specifically for Ly valve development rather than more generally required for collecting vessel development. In comparison, other mouse models like Foxc2−/− (Norrmén et al., 2009) and Ang2−/− (Gale et al., 2002; Dellinger et al., 2008) mice fail to initiate the collecting vessel developmental program. Thus, Cx37 and Cx43 appear to be downstream factors in the pathway and may play a direct role in coordinating LECs during valvulogenesis. The signals that locally instruct LECs to proliferate, migrate, and organize into valve leaflets are not known. Since Cx37 and Cx43 are initially expressed throughout the Ly endothelium of the developing collecting lymphatics, they could function to locally communicate second messengers at sites where a signal for valve formation is received or initiated by the Ly endothelium. A mechanism of this sort has been suggested to occur during atrioventricular valve development in the heart, where expression of Cx45 in the cardiac endothelium is required for normal endocardial cushion formation (Kumai et al., 2000; Nishii et al., 2001). In the absence of Cx45, signaling through NFATc1 was blocked in the cardiac endothelium, and a critical epithelial-mesenchymal transformation of the endothelium was impaired. Another feature of Foxc2−/− mice and Ang2−/− mice is that both models exhibit premature recruitment of smooth muscle cells to developing lymphatics (Petrova et al., 2004; Dellinger et al., 2008). It has been proposed that early association of smooth muscle cells might prematurely stabilize immature lymphatics and lead to impaired valvulogenesis (Petrova et al., 2004). In the case of Cx-deficient mice, however, we did not observe premature recruitment of smooth muscle cells to lymphatics. Thus, abnormal association of mural cells during Ly development is not a prerequisite event for valve agenesis to occur.
Since Cx43−/− mice die perinatally with cardiac developmental defects (Reaume et al., 1995), LEC-specific ablation of Cx43 will ultimately be required to determine if the cardiovascular anomalies in Cx43−/− mice contribute at all to the Ly defects in those mice. Ly defects also occur in Cx37−/− and Cx37−/−Cx43+/− mice, however, and these genotypes do not exhibit the cardiac defects associated with Cx43−/−mice, arguing that Cxs do have LEC-specific roles consistent with their expression in Ly vessels. Moreover, Ly defects were not reported for mice with a cardiac-restricted knockout of Cx43 (Gutstein et al., 2001) nor were they reported for mice with a conditional knockout of Cx43 from thoracic neural tube and cardiac neural crest, a model which reproduces the cardiac defects (infundibular bulging and abnormal coronary vascular development) seen in global Cx43 knockout mice (Liu et al., 2006).
Although lymph-blood mixing can occur if there is a failure of separation between Ly vessels and blood vessels (Abtahian et al., 2003; Uhrin et al., 2010; Bertozzi et al., 2010; Sebzda et al., 2006; Bäckhed et al., 2007), we saw no evidence of inappropriate connections between Ly vessels and blood vessels of Cx37−/−Cx43−/− or Cx43−/− mice at E18.5. The bloody lymphatics in the intestinal wall and mesentery of Cx-deficient mice did not resemble the tortuous bloody vessels observed in mouse models where a nonseparation phenotype has been described (Abtahian et al., 2003). In addition, blood vessels of the mesentery looked normal in the Cx deficient mice, and no aberrant connections between blood vessels and Ly vessels within the mesentery were observed. Moreover, tissue hemorrhage was not a source of red blood cells in the lymphatics. Rather, we think that valve deficiencies in the Cx-deficient mice allow venous blood to inappropriately, and perhaps intermittently, based on differential pressures, enter the Ly vasculature. Consistent with this idea, blood was observed in the TD of Cx-deficient embryos and could be traced into the mesentery. Moreover, even in adult Cx37−/−Cx43+/− mice, blood was sometimes found within the TD.
In adult animals, Ly collecting vessels undergo spontaneous contractions which are propagated along the vessel, and it has been suggested that GJs in the Ly wall could provide a pathway for conduction of contractile activity, as in arterioles (Zawieja et al., 1993; McHale and Meharg, 1992). Since the Ly developmental defects in Cx-deficient mice occur before the recruitment of mural cells, however, the defects cannot be entirely a consequence of an inability to actively pump lymph. Nevertheless, the question of whether or not functional GJs are actually present between LECs in embryos or adult animals deserves more attention. It will be important to investigate this issue in intact vessels by electron microscopy to look for morphologically identifiable GJs and to use dye transfer assays and electrophysiological methods to determine if LECs are functionally coupled by intercellular channels. If technical issues associated with using mouse lymphatics can be overcome, it will also be essential to directly test whether propagation of Ly contraction is altered in Cx-deficient mice. Finally, given the restricted distribution of Cxs in mature collecting lymphatics, it is tempting to speculate that Cxs could play some role in controlling pacemaker activity associated with spontaneous Ly contractions (Ohhashi et al., 2005; Zawieja, 2009).
The Cx-deficient mice in this study share some features with other mouse models displaying Ly disorders. Mutations in Ita9 (integrin-α9) (Bazigou et al., 2009; Huang et al., 2000) and EfnB2 (ephrinB2) (Mäkinen et al., 2005) have been shown to result in Ly collecting vessel valve deficiency and to cause chylothorax, and in humans, an Integrin-α9 missense mutation has been associated with congenital chylothorax (Ma et al., 2008). Integrin-α9−/− mice have only rudimentary valve leaflets in mesenteric collecting lymphatics and the leaflets contain a disorganized fibronectin matrix core (Bazigou et al., 2009). Integrin−α9β1 has also been linked to accelerated migration of cells in some settings (Gupta and Vlahakis, 2009) and, similarly, Cx43 is important for directed cell migration, for example, in neural crest cells (Xu et al., 2001) and during coronary blood vessel development (Rhee et al., 2009). EphrinB2ΔV mice, engineered with a C-terminal PDZ interaction site deleted, exhibit blood in their Ly vessels as do Cx37−/−Cx43−/− and Cx43−/− mice (Mäkinen et al., 2005). Because of these similarities and our preliminary colocalization studies, we speculate that Cx37 or Cx43 might interact with EphrinB2 or integrin-α9 in Ly vessels and contribute to differential cell migration, sorting, and growth control during Ly development. Along with forming GJ channels and hemichannels, Cxs can contribute to signaling via protein-protein interactions. Indeed, a different Ephrin family member, EphrinB1, has been shown previously to co-immunoprecipitate with Cx43, and interaction between the two proteins was important for normal Cx43 distribution (Davy et al., 2006). Moreover, the PDZ binding domain of EphrinB1 was required for it to interact with the phosphorylated form of Cx43 (Davy et al., 2006).
Our data suggest that the primary defect in Cx37−/−Cx43+/− mice involves central lymphatics rather than peripheral lymphatics. Patterning and function of peripheral lymphatics was normal in these mice, and peripheral edema was not observed. However, the forward movement of lymph through the central lymphatics of Cx37−/−Cx43+/− mice was compromised, and this phenomenon can be explained by a severe deficiency in TD valves. EBD lymphangiography revealed retrograde reflux of the dye into collateral lymphatics along the route of lymph transport, including reflux into intercostal lymphatics. The lack of TD valves, which normally promote unidirectional fluid movement, likely results in a backup of lymph in the central lymphatics. Reflux into collateral lymphatics then occurs to accommodate the excess lymph. Eventually, the intercostal lymphatics or the TD itself become overburdened and rupture occurs, resulting in chylothorax. Consistent with this model, there was a good correlation between the number of TD valves in Cx37−/− and Cx37−/−Cx43+/− mice and the degree of Ly impairment.
To our knowledge, this study is the first to quantify valves in the mouse TD and to identify genes that are required for development of TD valves. We found that TDs from WT mice typically contain 3–4 valves. By comparison, adult rats and humans have an average of 11.9 and 14.7 valves per TD, respectively (Petrenko and Kruglov, 2004). Our data are consistent with those from adult rat and human showing that TD valves are usually found in the upper portion of the duct, although human fetuses may transiently have more numerous and distributed TD valves during development (Petrenko and Kruglov, 2004; Kampmeier, 1928). It is not known if the pathways critical for valve development in collecting lymphatics (of the mesentery, for example) are the same as for TD valve development. Kampmeier described a process of valvulogenesis in the human TD that was qualitatively distinct from valvulogenesis occurring in the periphery (Kampmeier, 1928). At present, we do not know if abnormal JLS development at E13.5 contributes to later TD valve deficiencies in Cx37−/−Cx43+/− mice.
A number of sporadic phenotypes occurred in Cx37−/−Cx43+/− mice, including abnormal fat deposition, a characteristic feature of some Ly disorders in humans, including chronic lymphedema (Harvey, 2008). In Prox1 haploinsufficient mice, adult-onset obesity occurs associated with abnormal lymph leakage from mispatterned and ruptured Ly vessels (Harvey et al., 2005). In addition, chyle was found to have adipogenic activity when added to 3T3-L1 preadipocytes in culture (Harvey et al., 2005). Our observations are consistent with this model, as abnormal fat in Cx37−/−Cx43+/− animals accumulated only in the thoracic cavity, where leakage of chyle occurred.
Differential expression of Cx37 and Cx43 occurs in the JLS at E13.5, where there is an inverse relationship between Cx37 and Cx43 expression. Cx37 and Cx43 were previously shown to have a reciprocal relationship in specific regions of the rat aorta, such as at the aortic bifurcation (Gabriels and Paul, 1998). To our knowledge, however, the present data are a unique example of such distinct heterogeneity in gene expression amongst JLS LECs. It will be important to determine what role Cxs play in the proliferation, sprouting, and remodeling that occurs in the JLS during this period. In the absence of Cx37, the JLS is clearly enlarged, suggesting that Cx37 may play an important role in regulating the proliferation of LECs of the JLS. Consistent with this model, Cx37 has been shown to have growth suppressive properties in some settings (Burt et al., 2008; Morel et al., 2010). Quantitative differences in the relative expression levels of Cx37 and Cx43 might also explain why, in skin lymphatics, the loss of both Cx37 and Cx43 is required before valves are entirely absent whereas, in mesenteric lymphatics, only the loss of Cx43 is required.
Cx37 and Cx43 were also found to be exquisitely differentially expressed in the upstream versus downstream endothelial layers of mature Ly valve leaflets which are separated in places by less than 200 nm. In the rat heart, 70- to 200-fold greater expression of Cx43 was found in the upstream versus downstream surfaces of cardiac valves, consistent with our finding of Cx43 on the upstream side of Ly valves (Inai et al., 2004). Differential expression of endothelial Cxs has also been observed in blood vessels at ostia and flow dividers (Gabriels and Paul, 1998). In these settings, shear stress and disturbed flow were implicated in the differential expression of Cxs. The upstream and downstream surfaces of Ly valves are also likely to experience distinct flow conditions and shear stress, and the differential expression of Cx37 and Cx43 in Ly valves may therefore be a response to unequal exposure to mechanical stress. Interestingly, morphological and biochemical differences between upstream and downstream sides of Ly valve leaflets have been previously noted (Bannykh et al., 1995; Ji and Kato, 2001). Thus, Cx37 and Cx43 may have adaptive physiological functions in mature lymphatics that are distinct from their developmental roles. Alternatively, we speculate that differential Cx expression in developing collecting lymphatics may play a role in establishing functional boundaries between lymphangions, the functional units of a lymph vessel that lie between contiguous valves, much like Cx43 expression and localization regulates joint location in zebrafish fins (Sims et al., 2009).
Our data on Cx expression in Foxc2−/− mice raise the possibility that the Foxc2 transcription factor directly regulates Cx37 gene (Gja4) expression. Interestingly, ablation of a different forkhead box gene, Foxo1, resulted in a large decrease (90%) in Cx37 mRNA in the E9.5 yolk sac vasculature (Furuyama et al., 2004). Moreover, another family member, Foxo3a, is implicated in the regulation of Cx37 and Cx43 expression in mouse oocytes (Liu et al., 2007). Other observations also suggest a relationship between Foxc2 and Cx37 in the Ly system. First, the JLS is enlarged in both Foxc2−/− and Cx37−/− embryos. Second, Cx37 expression is elevated in valves in postnatal mesenteric lymphatics, as is Foxc2 expression (Petrova et al., 2004). Norrmén et al. used a Chip-chip assay to compile a human genome-wide map of Foxc2 binding sites in LECs and pulled out two Foxc2 sites where the nearest gene was (GJA1) Cx43, although these sites were rather distant (~380 kb and ~128 kb away) from the GJA1 locus (Norrmén et al., 2009). We directly searched for potential binding sites for Foxc2 and NFATc1 in the flanking regions of the mouse Cx37 gene (Gja4) using a position frequency matrix for each transcription factor consensus binding site (Norrmén et al., 2009). Candidate sites which closely match the position frequency matrix for Foxc2 and NFATc1 binding were identified within an 11 Kb region immediately downstream of the mouse Cx37 gene (Gja4). Future studies will aim to determine if these potential sites are functional binding sites for Foxc2 and NFATc1 and if they are used to regulate expression of Cx37 in LECs.
Recently, missense mutations in GJC2, encoding Cx47, were found to cause dominantly inherited lymphedema in humans (Ferrell et al., 2010). Cx47 has previously been documented in oligodendrocytes of the central nervous system but its expression in Ly vessels has not been studied (Odermatt et al., 2003; Menichella et al., 2003). We report here that Cx47 is expressed in the valves of the TD, as well as in valves of mesenteric collecting lymphatics, in a subset of LECs. We also detected Cx47 expression in embryonic Ly vessels, although it was earlier absent from the E13.5 JLS. Some of the identified human Cx47 mutations are predicted to be dominant-negative, and therefore, Cx37 or Cx43 function could be affected in LECs coexpressing these Cxs (Ferrell et al., 2010).
In conclusion, the results of this study, along with the Cx47 human lymphedema mutations described by Ferrell et al. (2010), demonstrate that at least three Cxs have critical roles in Ly development and function. Our data show that Cx37 and Cx43 participate in lymphatic valve development as well as morphogenesis of the JLS and TD. Furthermore, Gja4 (Cx37) and Gja1 (Cx43) are potential candidate genes for congenital chylothorax, lymphedema, and other Ly disorders caused by valve defects as well as potential targets for development of novel molecular therapeutics.
Highlights.
Cx37, Cx43, and Cx47 are expressed in lymphatic endothelial cells
Cxs differentially expressed in lymphatic valves and required for valvulogenesis
Cxs contribute to development of jugular lymph sac and thoracic duct
Cx deficiencies in mice result in lymph reflux, lymphedema and chylothorax
Cx37 may be a target of regulation by the transcription factor Foxc2
Supplementary Material
Acknowledgements
We thank Gerald Kidder for Cx43+/− mice, Naoyuki Miura for Foxc2+/− mice, Paul Lampe for Cx26 antibody, David Paul for Cx40 antibody, and Robert Erickson for comments on the manuscript. We thank Caterina Sellito for assistance with the Cx37−/−Cx43+/− mice in the early stages of this work. This work was supported by NIH grant HL64232 to A.M. Simon. MT Dellinger was supported by Arizona Disease Control Research Commission Contract 9002 (Robert P. Erickson).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John D. Kanady, Email: jkanady@email.arizona.edu.
Michael T. Dellinger, Email: Michael.Dellinger@UTSouthwestern.edu.
Stephanie J. Munger, Email: sjmunger@email.arizona.edu.
Marlys H. Witte, Email: lymph@email.arizona.edu.
Alexander M. Simon, Email: amsimon@u.arizona.edu.
References
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannykh S, Mironov A, Bannykh G, Mironov A. The morphology of valves and valve-like structures in the canine and feline thoracic duct. Anat. Embryol. (Berl) 1995;192:265–274. doi: 10.1007/BF00184751. [DOI] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Crawford PA, O'Donnell D, Gordon JI. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc. Natl. Acad. Sci. U. S. A. 2007;104:606–611. doi: 10.1073/pnas.0605957104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobbie MW, Roy S, Trudeau KM, Munger SJ, Simon A, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest. Ophthalmol. Vis. Sci. 2010;51:3758–3763. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 2009;11:267–282. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JM, Nelson TK, Simon AM, Fang JS. Connexin 37 profoundly slows cell cycle progression in rat insulinoma cells. Am. J. Physiol. Cell Physiol. 2008;295:C1103–C1112. doi: 10.1152/ajpcell.299.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell F, Kalidas K, Ostergaard P, Brice G, Homfray T, Roberts L, Bunyan DJ, Mitton S, Mansour S, Mortimer P, Jeffery S Lymphoedema Consortium. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum. Genet. 2010;127:231–241. doi: 10.1007/s00439-009-0766-y. [DOI] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS. Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell. Comm. Signal. 2009;7:4. doi: 10.1186/1478-811X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger M, Hunter R, Bernas M, Gale N, Yancopoulos G, Erickson R, Witte M. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Dev. Biol. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell RE, Baty CJ, Kimak MA, Karlsson JM, Lawrence EC, Franke-Snyder M, Meriney SD, Feingold E, Finegold DN. GJC2 Missense Mutations Cause Human Lymphedema. Am. J. Hum. Genet. 2010;86:943–948. doi: 10.1016/j.ajhg.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell RE, Levinson KL, Esman JH, Kimak MA, Lawrence EC, Barmada MM, Finegold DN. Hereditary lymphedema: evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 1998;7:2073–2078. doi: 10.1093/hmg/7.13.2073. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Duling BR. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 2009;11:251–266. doi: 10.1089/ars.2008.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Kitayama K, Shimoda Y, Ogawa M, Sone K, Yoshida-Araki K, Hisatsune H, Nishikawa S, Nakayama K, Nakayama K, Ikeda K, Motoyama N, Mori N. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J. Biol. Chem. 2004;279:34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ. Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Gap junctions. Cold. Spring. Harb. Perspect. Biol. 2009;1:a002576. doi: 10.1101/cshperspect.a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Vlahakis NE. Integrin alpha9beta1 mediates enhanced cell migration through nitric oxide synthase activity regulated by Src tyrosine kinase. J. Cell. Sci. 2009;122:2043–2054. doi: 10.1242/jcs.041632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 2001;88:333–339. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NL. The link between lymphatic function and adipose biology. Ann. N. Y. Acad. Sci. 2008;1131:82–88. doi: 10.1196/annals.1413.007. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Howard V. Unbiased stereology. New York: Garland Science; 2005. [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol. Cell. Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Koseki H, Kakinuma H, Kato N, Mizutani-Koseki Y, Ohuchi H, Yoshioka H, Noji S, Kawamura K, Kataoka Y, Ueno F, Taniguchi M, Yoshida N, Sugiyama T, Miura N. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development. 1997;124:4627–4638. doi: 10.1242/dev.124.22.4627. [DOI] [PubMed] [Google Scholar]
- Inai T, Mancuso MR, McDonald DM, Kobayashi J, Nakamura K, Shibata Y. Shear stress-induced upregulation of connexin 43 expression in endothelial cells on upstream surfaces of rat cardiac valves. Histochem. Cell Biol. 2004;122:477–483. doi: 10.1007/s00418-004-0717-6. [DOI] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns JP, Van Steensel MA, Vikkula M. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am. J. Hum. Genet. 2003;72:1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RC, Kato S. Histochemical analysis of lymphatic endothelial cells in lymphostasis. Microsc. Res. Tech. 2001;55:70–80. doi: 10.1002/jemt.1158. [DOI] [PubMed] [Google Scholar]
- Jiang JX, Gu S. Gap junction- and hemichannel-independent actions of connexins. Biochim. Biophys. Acta. 2005;1711:208–214. doi: 10.1016/j.bbamem.2004.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, Detmar M. Lymphatic endothelium in health and disease. Cell. Tissue. Res. 2009;335:97–108. doi: 10.1007/s00441-008-0644-2. [DOI] [PubMed] [Google Scholar]
- Kampmeier OF. Further observations on the numerical variability, position, function, and fate of the valves in the human thoracic duct. The Anatomical Record. 1928;38:225–231. [Google Scholar]
- Kampmeier OF. The genetic history of the valves in the lymphatic system of man. American. Journal of Anatomy. 1928;40:413–457. [Google Scholar]
- Kriederman BM, Myloyde TL, Witte MH, Dagenais SL, Witte CL, Rennels M, Bernas MJ, Lynch MT, Erickson RP, Caulder MS, Miura N, Jackson D, Brooks BP, Glover TW. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum. Mol. Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Laird DW. Life cycle of connexins in health and disease. Biochemical Journal. 2006;394:527. doi: 10.1042/BJ20051922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. The gap junction proteome and its relationship to disease. Trends Cell Biol. 2010;20:92–101. doi: 10.1016/j.tcb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu F, Schneider AE, St Amand T, Epstein JA, Gutstein DE. Distinct cardiac malformations caused by absence of connexin 43 in the neural crest and in the non-crest neural tube. Development. 2006;133:2063–2073. doi: 10.1242/dev.02374. [DOI] [PubMed] [Google Scholar]
- Ma GC, Liu CS, Chang SP, Yeh KT, Ke YY, Chen TH, Wang BB, Kuo SJ, Shih JC, Chen M. A recurrent ITGA9 missense mutation in human fetuses with severe chylothorax: possible correlation with poor response to fetal therapy. Prenat. Diagn. 2008;28:1057–1063. doi: 10.1002/pd.2130. [DOI] [PubMed] [Google Scholar]
- Mäkinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes. Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen T, Norrmén C, Petrova TV. Molecular mechanisms of lymphatic vascular development. Cell. Mol. Life Sci. 2007;64:1915–1929. doi: 10.1007/s00018-007-7040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale NG, Meharg MK. Co-ordination of pumping in isolated bovine lymphatic vessels. J. Physiol. 1992;450:503–512. doi: 10.1113/jphysiol.1992.sp019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. J. Neurosci. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel S, Burnier L, Roatti A, Chassot A, Roth I, Sutter E, Galan K, Pfenniger A, Chanson M, Kwak BR. Unexpected role for the human Cx37 C1019T polymorphism in tumour cell proliferation. Carcinogenesis. 2010;11:1922–1931. doi: 10.1093/carcin/bgq170. [DOI] [PubMed] [Google Scholar]
- Naoi Y, Miyoshi Y, Taguchi T, Kim SJ, Arai T, Tamaki Y, Noguchi S. Connexin26 expression is associated with lymphatic vessel invasion and poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2007;106:11–17. doi: 10.1007/s10549-006-9465-8. [DOI] [PubMed] [Google Scholar]
- Nishii K, Kumai M, Shibata Y. Regulation of the epithelial-mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc. Med. 2001;11:213–218. doi: 10.1016/s1050-1738(01)00103-7. [DOI] [PubMed] [Google Scholar]
- Norrmén C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, Augustin HG, Ylä-Herttuala S, Alitalo K, Petrova TV. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell. Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt B, Wellershaus K, Wallraff A, Seifert G, Degen J, Euwens C, Fuss B, Büssow H, Schilling K, Steinhäuser C, Willecke K. Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 2003;23:4549–4559. doi: 10.1523/JNEUROSCI.23-11-04549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohhashi T, Mizuno R, Ikomi F, Kawai Y. Current topics of physiology and pharmacology in the lymphatic system. Pharmacol. Ther. 2005;105:165–188. doi: 10.1016/j.pharmthera.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Oliver G, Srinivasan RS. Lymphatic vasculature development: current concepts. Ann. N. Y. Acad. Sci. 2008;1131:75–81. doi: 10.1196/annals.1413.006. [DOI] [PubMed] [Google Scholar]
- Oliver G, Srinivasan RS. Endothelial cell plasticity: how to become and remain a lymphatic endothelial cell. Development. 2010;137:363–372. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrenko VM, Kruglov SV. Thoracic duct valves in man and albino rat. Morfologiia. 2004;126:40–42. [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmén C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Ylä-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat. Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Rhee DY, Zhao XQ, Francis RJ, Huang GY, Mably JD, Lo CW. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development. 2009;136:3185–3193. doi: 10.1242/dev.032334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebzda E, Hibbard C, Sweeney S, Abtahian F, Bezman N, Clemens G, Maltzman JS, Cheng L, Liu F, Turner M, Tybulewicz V, Koretzky GA, Kahn ML. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Dev. Cell. 2006;11:349–361. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Shin JW, Huggenberger R, Detmar M. Transcriptional profiling of VEGF-A and VEGF-C target genes in lymphatic endothelium reveals endothelial-specific molecule-1 as a novel mediator of lymphangiogenesis. Blood. 2008;112:2318–2326. doi: 10.1182/blood-2008-05-156331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AM, Chen H, Jackson CL. Cx37 and Cx43 localize to zona pellucida in mouse ovarian follicles. Cell. Commun. Adhes. 2006;13:61–77. doi: 10.1080/15419060600631748. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Li E, Paul DL. Female infertility in mice lacking connexin 37. Nature. 1997;385:525–529. doi: 10.1038/385525a0. [DOI] [PubMed] [Google Scholar]
- Simon AM, Goodenough DA, Paul DL. Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 1998;8:295–298. doi: 10.1016/s0960-9822(98)70113-7. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 2002;251:206–220. doi: 10.1006/dbio.2002.0826. [DOI] [PubMed] [Google Scholar]
- Simon AM, McWhorter AR. Decreased intercellular dye-transfer and downregulation of non-ablated connexins in aortic endothelium deficient in connexin37 or connexin40. J. Cell. Sci. 2003;116:2223–2236. doi: 10.1242/jcs.00429. [DOI] [PubMed] [Google Scholar]
- Sims K, Eble DM, Iovine MK. Connexin43 regulates joint location in zebrafish fins. Dev. Biol. 2009;327:410–418. doi: 10.1016/j.ydbio.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr. Opin. Cell Biol. 2004;16:507–512. doi: 10.1016/j.ceb.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Takada M. The ultrastructure of lymphatic valves in rabbits and mice. Am. J. Anat. 1971;132:207–217. doi: 10.1002/aja.1001320207. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fässler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- Walker DL, Vacha SJ, Kirby ML, Lo CW. Connexin43 deficiency causes dysregulation of coronary vasculogenesis. Dev. Biol. 2005;284:479–498. doi: 10.1016/j.ydbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Wick N, Saharinen P, Saharinen J, Gurnhofer E, Steiner CW, Raab I, Stokic D, Giovanoli P, Buchsbaum S, Burchard A, Thurner S, Alitalo K, Kerjaschki D. Transcriptomal comparison of human dermal lymphatic endothelial cells ex vivo and in vitro. Physiol. Genomics. 2007;28:179–192. doi: 10.1152/physiolgenomics.00037.2006. [DOI] [PubMed] [Google Scholar]
- Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc. Res. Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW. Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J. Cell. Biol. 2001;154:217–230. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HI, Dupont E, Coppen S, Rothery S, Severs NJ. Gap junction localization and connexin expression in cytochemically identified endothelial cells of arterial tissue. J. Histochem. Cytochem. 1997;45:539–550. doi: 10.1177/002215549704500406. [DOI] [PubMed] [Google Scholar]
- Zawieja DC. Contractile physiology of lymphatics. Lymphat. Res. Biol. 2009;7:87–96. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ. Distribution, propagation, and coordination of contractile activity in lymphatics. Am. J. Physiol. 1993;264:H1283–H1291. doi: 10.1152/ajpheart.1993.264.4.H1283. [DOI] [PubMed] [Google Scholar]
- Zhou F, Chang Z, Zhang L, Hong YK, Shen B, Wang B, Zhang F, Lu G, Tvorogov D, Alitalo K, Hemmings BA, Yang Z, He Y. Akt/Protein Kinase B Is Required for Lymphatic Network Formation, Remodeling, and Valve Development. Am. J. Pathol. 2010;177:2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.