Abstract
Changes in emotional state are known to alter neuronal excitability and can modify learning and memory formation. Such experience–dependent neuronal plasticity can be long-lasting and is thought to involve the regulation of gene transcription. Here we show that a single fear-inducing stimulus increases GluR2 mRNA abundance and promotes synaptic incorporation of GluR2-containing AMPA receptors (AMPARs) in mouse cerebellar stellate cells. The switch in synaptic AMPAR phenotype is mediated by noradrenaline and action potential prolongation. The subsequent rise in intracellular Ca2+ and activation of Ca2+-sensitive ERK /MAPK signaling trigger new GluR2 gene transcription and a switch in the synaptic AMPAR phenotype from GluR2-lacking, Ca2+-permeable, to GluR2-containing Ca2+-impermeable receptors on the order of hours. The change in glutamate receptor phenotype alters synaptic efficacy in cerebellar stellate cells. Thus, a single fear-inducing stimulus can induce a long-term change in synaptic receptor phenotype and may alter the activity of an inhibitory neural network.
INTRODUCTION
The ability of the nervous system to respond to a transient stimulus with a persistent change in the efficacy of synaptic transmission depends upon regulation of gene transcription1,2. The best understood postsynaptic modification involves a change in the phosphorylation state and number of AMPARs3–6, but may also involve long-lasting changes in AMPAR subunit composition and Ca2+ permeability4,7,8. These changes arise not only due to regulated receptor trafficking, but also local dendritic protein synthesis3–12. Given the importance of experience-dependent gene transcription in learning/memory, an alteration in AMPAR transcription represents a powerful means to produce a long-lasting change in synaptic AMPARs and activity of entire neuronal circuits. However whether experience can regulate AMPAR transcription is, as yet, unclear.
The stress hormone noradrenaline mediates memory consolidation by emotion13. During emotional arousal, noradrenaline is released from neurons arising in the locus coeruleus and lateral brain stem tegmentum and produces its effects at synapses throughout the central nervous system14,15. In the cerebellum, these fibers terminate primarily in the molecular and Purkinje/granule cell layers, where noradrenaline acts via β-adrenergic receptors to increase the action potential firing rate of inhibitory stellate cells16,17 and alter the spontaneous firing of Purkinje neurons14,15. Noradrenaline plays a central role in motor learning and fear-related memories, which affect synaptic transmission in the cerebellum18–21. Noradrenaline also produces powerful effects on synaptic plasticity, widely believed to be a cellular substrate for learning and memory. In the hippocampus and visual cortex, noradrenaline lowers the threshold for induction of long-term potentiation by facilitating phosphorylation and synaptic delivery of GluR1-containing AMPARs22,23. However, the ability of noradrenaline to alter synaptic AMPAR phenotype is unclear.
Cerebellar stellate cells spontaneously fire APs of brief duration and express GluR2-lacking AMPARs, a characteristic of inhibitory interneurons24,25. Synaptic AMPARs lacking the edited GluR2 subunit exhibit high Ca2+ permeability, rapid decay kinetics and are blocked by intracellular polyamines26. These properties allowed us to follow directly the synaptic incorporation of GluR2 subunits in cerebellar stellate cells. Targeted expression of GluR2 in inhibitory interneurons disrupts long-range synchrony of gamma oscillations in the hippocampus27. Thus, an alteration in GluR2 gene expression could have profound consequences on neuronal function and activity of neuronal circuits. The present study was undertaken to examine the impact of emotional arousal in the intact animal on AMPAR GluR2 transcription, and synaptic AMPAR phenotype. Here we show that a single fear-inducing stimulus acts via β-adrenergic receptors to increase GluR2 mRNA abundance and alter synaptic AMPAR phenotype in cerebellar stellate cells. We further elucidate the mechanism by which the switch in AMPAR phenotype occurs. Noradrenaline prolongs the action potential duration. The subsequent rise in intracellular Ca2+ activates the Ca2+-sensitive ERK/MAPK pathway, which drives transcription of GluR2 and synaptic incorporation of GluR2-containing AMPARs at parallel fiber to stellate cell synapses. This novel, transcription-dependent form of synaptic plasticity may underlie brain processing of fear-inducing stimuli.
RESULTS
Emotional stress alters AMPAR subtype and elevates GluR2 mRNA
Exposure of mice to fox urine, a natural olfactory stimulus, causes innate fear and promotes noradrenaline release in the brain22,28. To examine whether emotional stress can induce an alteration synaptic AMPAR properties, we exposed mice to fox urine for 5 min and monitored locomoter activity. A single exposure to fox urine induced a freezing behavior, indicative of fear (Fig. 1A). We next monitored excitatory postsynaptic currents (EPSCs) at parallel fibre to stellate cell synapses in cerebellar slices from mice at indicated times after exposure to the fear-inducing stimulus. To examine synaptic AMPAR properties, we first used IEM-1460, a subunit-selective blocker of Ca2+-permeable AMPARs. In control cells, application of IEM-1460 (100 µM) reduced the EPSC amplitude at −60 mV by ~50% (n = 3; Fig. 1B). At 3 h after exposure to fox urine, the block by IEM-1460 was greatly diminished (~20%; P < 0.05; Fig. 1B), consistent with a stress-induced switch in AMPAR phenotype from GluR2-lacking to GluR2-containing.
Figure 1.
An olfactory stimulus altered synaptic AMPA receptor subtype and expression of GluR2 mRNA in stellate cells. A. A natural olfactory stimulus, fox urine caused fear (measured as a freezing response). % freezing was calculated during the 3 min control and 5 min fox urine exposure period (n = 5; *, P < 0.01). B. Inhibition of EPSCs at −60 mV by IEM-1460 (100 µM), a selective Ca2+-permeable AMPAR blocker (n = 3; P < 0.05). C. Cumulative distribution of decay time constant of EPSC at −60 mV of individual synaptic events from 5 cells under each condition (Kolmogorov-Smirnov test, P < 0.0001, control vs. 3 hr). D. Synaptic currents and I–V relationship when spermine was included in the pipette in stellate cells. Control cells displayed an I–V relationship with pronounced inward rectification, suggesting the presence of GluR2 lacking receptors. Mice were exposed to fox urine, a fear-inducing olfactory stimulus, for 5 min. Cerebellar slices were prepared 15 min after olfactory stimulus, synaptic currents were recorded within 2 hour after fox urine exposure and exhibited an inwardly rectifying I–V relation (n = 4). Three hours following fox urine exposure the synaptic current in stellate cells showed a near linear I–V relationship (n = 5, P < 0.01), indicating that it was mediated mainly by GluR2-containing AMPARs. When slices were prepared 15 hours after fox urine exposure the synaptic current still showed a near linear I–V relationship (n = 4). E. Rectification index (*, P < 0.01; **, P < 0.005). F. The GluR1, 2 and 3 mRNA level in individual stellate cells was determined using real time single cell RT-PCR. GFP positive neurons (~8 µm in diameter) were isolated from the cerebellar cortex of GAD-65 GFP mice (control, 23 cells from 4 animals; 3 hr following fox urine exposure, 18 cells; 15 hr following fox urine exposure, 15 cells; *, P < 0.05). Scale bars, 200 µM (top) and 20 µM (bottom). Error bars show ± s.e.m.
Second, we measured spontaneous EPSCs (sEPSCs) as a function of holding potential in the presence of the polyamine spermine. Inclusion of spermine in the pipette solution confers voltage-dependent block of AMPARs lacking GluR2 and produces a characteristic inwardly rectifying I–V relationship4,7,8. Synaptic incorporation of GluR2-containing AMPARs is detected as decreased rectification of AMPAR-mediated EPSCs. The sEPSC provides a measure of intrinsic activity of all synapses onto stellate cells and enables comparison of the mean synaptic current over the prolonged time during which the fear-inducing stimulus alters synaptic strength.
In stellate cells from control mice, the sEPSC amplitude was reduced at positive membrane potentials. This inwardly rectifying I–V relation implies that the sEPSCs are mediated mainly by GluR2-lacking, Ca2+-permeable AMPARs (Fig. 1D, control). In contrast, in stellate cells from mice exposed to fox urine, synaptic currents recorded at 3 h after exposure showed a near linear I–V relation (Fig. 1D), indicating that they are mediated mainly by GluR2-containing AMPARs. Whereas fox urine increased the sEPSC amplitude from 10.7 ± 0.9 pA (control) to 18.2 ± 0.8 pA at +40 mV (n = 5, P < 0.001), there was little or no change in the sEPSC amplitude at −60 mV. Fox urine increased the rectification index from predominantly inwardly rectifying (0.34 ± 0.03; n = 5) to nearly linear (0.82 ± 0.11; n = 5; P < 0.01; Fig. 1E). Fox urine also prolonged the decay time constant of the EPSC at −60 mV (Fig. 1C), consistent with the slower time course of EPSCs mediated by GluR2-containing AMPARs vs. GluR2-lacking AMPARs27,29.
Whereas activity-dependent regulation of AMPAR trafficking occurs within 15–30 min after parallel fiber stimulation29,30, the stress-induced switch in synaptic AMPAR phenotype was delayed. Synaptic currents exhibited an inwardly rectifying I–V relation as late as 2 h after fox urine exposure (0.39 ± 0.03, n = 4; Fig 1D,E). Thus, the appearance of GluR2-containing AMPARs at stellate cell synapses does not occur until 2–3 h after a fear-inducing stimulus. The switch in synaptic AMPAR phenotype was long lasting in that synaptic currents exhibited a near linear I–V relationship (0.78 ± 0.08, n = 4; P < 0.01 vs. control, Figs. 1D,E), elevated amplitude at +40 mV (16.6 ±1.7 pA; P < 0.01) and characteristically long decay time at −60 mV (Fig. 2C) as late as 15 h after the fear-inducing stimulus.
Figure 2.
β-adrenergic receptors mediated the olfactory stimulus-induced change in synaptic AMPA receptor subtype. Mice were injected with propranolol or saline (as control) 15–30 min prior to the fox urine exposure. A. A natural olfactory stimulus, fox urine caused fear (measured as a freezing response). % freezing was calculated during the 3 min control and 5 min fox urine exposure period (n = 5; *, P < 0.01). B. Slices were prepared 15 hours after fox urine exposure. Synaptic currents and I–V relationship of EPSCs in stellate cells from the mice pre-injected with saline (n = 6) and propranolol (n = 6). C. Cumulative distribution of EPSC amplitude at +40 mV and decay time constant of EPSC at −60 mV of individual synaptic events from 6 cells under each condition (Kolmogorov-Smirnov test, P < 0.0001, 15 h vs. propranolol). D. Rectification index (***, P < 0.001). Error bars show ± s.e.m.
The long delay between the fear-inducing stimulus and the increase in synaptic GluR2 is consistent with a requirement for transcription-dependent alterations. To examine whether fox urine exposure alters GluR2 mRNA expression, we measured GluR2 mRNA abundance in individual GFP-positive stellate cells from glutamic acid decarboxylase (GAD)-65- GFP knock-in mice by quantitative single cell RT-PCR. Fox urine exposure increased GluR2 mRNA in stellate cells by 63 ± 19% (P < 0.05) at 3 h and 103 ± 36% (P < 0.05) at 15 h (Fig. 1F). The stress-induced change in GluR2 mRNA was subunit-specific in that GluR1 and GluR3 mRNA were unaltered (Fig. 1F). Thus, incorporation of functional GluR2-containing AMPARs at synapses is associated with an increase in GluR2 mRNA content of individual stellate cells. These results do not, however, distinguish between transcription of new mRNA vs. enhanced stability of existing mRNA.
We reasoned that during emotional arousal noradrenaline released onto cerebellar stellate cells might activate β-adrenergic receptors, which are expressed by stellate cells and mediate fear-induced freezing behavior (Fig. 2A)22,31. The β-adrenergic receptor blocker propranolol injected 15–30 min prior to fox urine exposure largely abolished stress-induced increase in GluR2-containing AMPARs at stellate cell synapses (stress+propranolol, 0.37 ± 0.03; n = 6, P < 0.01 vs. stress alone; stress+saline, 0.80 ± 0.07, n = 6; P < 0.0005 vs. stress+propranolol; Fig 2B–D). Thus, noradrenaline and β-adrenergic receptors likely mediate the stress-induced change in synaptic AMPAR phenotype.
Noradrenaline alters AMPARs and membrane excitability
We next examined whether application of noradrenaline directly to brain slices can mimic the fear-induced switch in AMPAR phenotype at stellate cell synapses. Application of noradrenaline (10 µM) via the bath perfusate (at 36°C) produced a modest increase in sEPSC frequency (control, 0.37± 0.16; noradrenaline, 0.58 ± 0.34 Hz; n = 6; P = 0.24). To avoid a contribution of altered glutamate or GABA release, we incubated slices in the presence of kynurenic acid (1 mM, to block AMPARs and NMDARs) and picrotoxin (100 µM, to block GABAARs; Fig. S1). Kynurenic acid and picrotoxin (3h) did not detectably alter the sEPSC rectification index (0.25 ± 0.07, n = 4; P = 0.23 vs. no treatment). Application of noradrenaline (3 h) in the presence of kynurenic acid and picrotoxin caused a switch in the I–V relation of the sEPSC (Fig. 3A) and evoked EPSC (Fig. 3C) from inwardly rectifying to near linear. Whereas noradrenaline increased the sEPSC amplitude from 7.8 ±1.7 (control) to 16.3 ±1.1 pA at +40 mV (n = 8, P < 0.001), it produced little or no change in EPSC amplitude at −60 mV (control, −46.9 ± 6.7, n = 4; noradrenaline, −39.0 ±1.6 pA; n = 8). Noradrenaline increased the rectification index from inwardly rectifying (control, 0.25 ± 0.07) to nearly linear (noradrenaline, 0.74 ± 0.10; P< 0.01; Fig. 3D). Noradrenaline also prolonged the decay time constant of sEPSCs at −60 mV (control, 0.95 ± 0.04; noradrenaline, 1.29 ± 0.09 ms; P < 0.02; (Fig. 3B). Together, these results strongly suggest that noradrenaline promotes synaptic incorporation of GluR2-containing receptors. Noradrenaline treatment for 0.5 h, followed by 2.5 h incubation, increased the rectification index of sEPSCs (0.57 ± 0.12; n = 5; P < 0.05; Fig. 3D). These findings indicate that noradrenaline treatment for 0.5 h) is sufficient to induce the switch in synaptic AMPAR phenotype in stellate cells, although the switch is delayed relative to its induction.
Figure 3.
Noradrenaline induced a change in synaptic AMPA receptor phenotype. A. Average sEPSCs displayed an inwardly rectifying I–V relationship in control, and became more linear following noradrenaline treatment (control, n = 4; noradrenaline treatment, n = 8). Cerebellar slices were incubated with kynurenic acid (1 mM) and picrotoxin (100 µM) in the absence (control) or presence of noradrenaline (10 µM, 3 h). Following each treatment noradrenaline and kynurenic acid were washed out prior to recordings of sEPSCs. B. The decay time of sEPSCs at −60 mV increased following noradrenaline treatment (Kolmogorov-Smirnov test, P < 0.0001). Cumulative distribution of decay time constant of EPSC at −60 mV of individual synaptic events from 4 control cells and 8 noradrenaline treated cells. C. Noradrenaline also induced a change in the I–V relationship of evoked EPSCs at the parallel fibre to stellate cell synapse (control, n = 4; noradrenaline treatment, n = 5). D. Summary of rectification index of EPSCs. Cerebellar slices were incubated with 10 µM noradrenaline for 3 h, 0.5 h (+2.5 h in picrotoxin and kynurenic acid control, n = 5; picrotoxin and kynurenic acid control, n = 4). E. CNQX (10 µM) and cyclothiazide (100 µM) evoked inward currents of comparable amplitude in control (n = 4) and noradrenaline treated cells (n = 5; two way ANOVA test, P = 0.39). (*, P < 0.05; **, P < 0.005). F. Noradrenaline (10 µM) increased the frequency (left panel) and duration (right panel) of spontaneous action potentials in stellate cells at 36°C (frequency, n = 5, P < 0.05; duration, n = 5, P < 0.005). Error bars show ± s.e.m.
Association of Transmembrane AMPAR regulatory proteins (TARPs) such as stargazin with GluR2-lacking AMPARs can relieve block of synaptic AMPA currents by intracellular spermine and increase the EPSC rectification index32. Two factors indicate that the noradrenaline-induced increase in rectification index is unlikely to reflect increased association of synaptic AMPARs with stargazin. First, we included 100 µM spermine in the patch pipette, a concentration sufficient to block Ca2+-permeable AMPARs at +40 mV, even in the presence of stargazin32. Second, in the presence of stargazin, CNQX acts as a partial agonist at AMPARs and elicits an inward current which is diagnostic for association of AMPARs with TARPs33. In control cells, CNQX (10 µM with 100 µM cyclothiazide, to reduce AMPAR desensitization) produced a modest inward current (–32.1± 5.3 pA, n = 4), which was not altered by noradrenaline (–33.9 ± 2.9 pA; n = 5, Fig. 3E).
To examine mechanisms by which noradrenaline alters AMPA EPSCs in stellate cells, we examined the impact of noradrenaline on frequency and duration of spontaneous action potentials. Application of noradrenaline increased the frequency of spontaneous action potentials in cerebellar stellate cells by 146 ± 33% (from 12.1 ± 3.7 to 29.6 ± 9.8 Hz; n = 5; P < 0.05; Figs. 3F,S2), consistent with previous findings16,17,31. Noradrenaline also prolonged the duration of action potentials, defined as the action potential half-width (control, 0.97 ± 0.06; noradrenaline, 1.27 ± 0.06 ms; n = 5; P < 0.005), and reduced the amplitude of the after-hyperpolarization (from −26.2 ± 5.4 to −22.7 ± 5.5 mV; P < 0.05; Figs. 3F,S2). Noradrenaline acts via β-adrenergic receptors to promote formation of cAMP, which activates the hyperpolarization-activated inward current, I(h) and increases spike frequency in cerebellar stellate cells31. We reasoned that the noradrenaline-induced changes in action potential waveform might arise due to an increase in I(h). Consistent with this, the I(h) inhibitor ZD7288 (10 µM) did not alter action potential duration, but abolished the noradrenaline-induced spike broadening (Table S1; Fig. S2C). Thus, the increase in spike frequency and spike broadening arise, at least in part, due to an increase in I(h) current.
Spike broadening increases synaptic GluR2 receptors
The results thus far indicate that noradrenaline increases action potential firing frequency and prolongs action potential duration in stellate cells. To examine whether either change alone is sufficient to trigger the switch in AMPAR phenotype, we selectively altered action potential frequency or duration. First, we increased spike frequency while maintaining constant spike duration. Block of inhibitory transmission by application of picrotoxin (100 µM) increased action potential firing frequency by 203 ± 72% (n = 5; P < 0.05; Fig. S3), with no change in duration or after-hyperpolarization (Table S2). Picrotoxin (3h) did not alter the rectification index or sEPSC amplitude at stellate cell synapses (Figs. 4C,S3). Hence, an increase in action potential frequency but not duration does not alter synaptic AMPAR phenotype.
Figure 4.
Increasing the action potential duration in stellate cells induces a change in rectification of the I–V relationship. A. Duration of spontaneous action potentials in cerebellar stellate cells increased during bath application of TEA at room temperature. B. sEPSCs displayed a nearly linear I–V relationship following TEA treatment (control, n = 5; TEA treatment, n = 6). Cerebellar slices were incubated with kynurenic acid (1 mM) and picrotoxin (100 µM) in the absence (control) or presence of TEA (1 mM, 3 h). C. Summary of rectification index of EPSCs. Cerebellar slices were incubated with 100 µM picrotoxin (n = 5; control, n = 4) or with 1 mM TEA for 3 h (at 37°C, n = 3; at room temperature, n = 6). D. The decay time of sEPSCs increased following TEA treatment (Kolmogorov-Smirnov test, P < 0.0001). Error bars show ± s.e.m.
Next, we increased spike duration while maintaining constant spike frequency. The potassium channel blocker tetraethylammonium (TEA, 1 mM) increased action potential duration (control, 1.3 ± 0.07; TEA, 1.8 ± 0.14 ms; n = 9; P < 0.0005; Fig. 4A) and reduced the amplitude of the after-hyperpolarization (from −30.2 ± 3.3 to −14.4 ± 3.5 mV; P < 0.0001), but not the frequency of spontaneous action potentials (Table S3) in postsynaptic stellate cells. We next examined the impact of TEA on the synaptic AMPAR phenotype in stellate cells. Application of TEA (1 mM with kynurenic acid and picrotoxin, 3 h) altered the I–V relation of the sEPSC from inwardly rectifying to nearly linear and increased the rectification index (control, 0.29 ± 0.03; n = 5; TEA, 0.88 ± 0.09; n = 6; P < 0.005; Fig. 4B,C), indicative of a switch from GluR2-lacking to GluR2-containing AMPARs. Whereas TEA increased the sEPSC amplitude at +40 mV (control, 7.9 ± 0.5; TEA, 18.5 ±1.6 pA; n = 6, P < 0.005), there was little or no change at −60 mV. However, TEA increased the decay time of sEPSCs at −60 mV (Fig 4D). These results suggest that TEA promotes synaptic incorporation of GluR2-containing receptors. Whereas TEA treatment for 1 h did not alter the sEPSC rectification index (0.31± 0.03, n = 3; Fig 4C), TEA (1 h), followed by 2 h incubation in control solution significantly increased the rectification index of synaptic currents (0.60 ± 0.09; n = 3; P < 0.05 vs. control). Thus, whereas brief (1h) application of TEA is sufficient to trigger the switch in AMPAR phenotype, expression of GluR2-containing AMPARs at stellate cell synapses does not occur until >1h.
To examine a possible contribution of mGluRs and GABABRs to the switch in AMPAR phenotype, we applied TEA in the presence of the group I/II mGluR inhibitor MCPG (1 mM) and GABABR inhibitor SCH50911 (10 µM) for 3 h. Neither mGluR nor GABABR blockade altered the TEA-induced switch in AMPAR phenotype (TEA+MCPG+SCH50911, 0.77 ± 0.04; n = 4, P < 0.0001 vs. control). Moreover, TEA did not alter the frequency of sEPSCs (Table S3), indicating that TEA does not affect transmitter release from presynaptic granule cells. During TEA treatment (3h) the action potential duration remained prolonged and spontaneous action potential frequency was unaltered relative to that in the absence of TEA (Table S3). These findings suggest that action potential broadening contributes to the noradrenaline-induced increase in synaptic GluR2 expression.
Enhanced Ca2+ entry during action potentials is required
Spike broadening could increase Ca2+ entry via voltage-gated Ca2+ channels during action potentials. To examine whether the noradrenaline-induced increase in action potential duration is associated with enhanced Ca2+ entry, we measured Ca2+ currents in stellate cells under voltage-clamp using action potential waveforms that mimicked either action potential in control (control-AP) or in the presence of noradrenaline (NA-AP). Ca2+ entry during a NA-AP (defined as current integrated over action potential duration) was ~40% greater than that of a control-AP waveform (n = 5; control-AP: 176 ± 32; NA-AP: 229 ± 34 pA.ms; P < 0.05; Fig. 5A). The L-type Cav1 blocker, nifedipine (20 µM), blocked 62.0 ± 4.6% (n = 5) of the Ca2+ current during a control-AP waveform (Fig. 5A), indicating that a substantial fraction of the Ca2+ current associated with an action potential is mediated by L-type channels. Application of nifedipine alone increased the rectification index of the EPSC (nifedipine, 0.49 ± 0.06; n = 5; P < 0.05 vs. control; Fig. 5C) to an extent less than that produced by noradrenaline (noradrenaline, 0.89 ± 0.05, n = 8; P < 0.05 nifedipine vs. noradrenaline). However, nifedipine blocked the noradrenaline-induced switch in AMPAR phenotype, assessed by the sEPSC amplitude at +40 mV (noradrenaline+nifedipine, 11.3 ± 0.6 pA; n = 6; P < 0.005 vs. noradrenaline) and rectification index (noradrenaline+nifedipine, 0.35 ± 0.03; P < 0.005 vs. noradrenaline; P < 0.05 vs. nifedipine alone; Fig. 5B, C). These findings are consistent with nifedipine-induced block rather than occlusion of the noradrenaline effect and indicate that Ca2+ entry via L-type Ca2+ channels is required for the noradrenaline-induced switch in AMPAR phenotype at stellate cell synapses.
Figure 5.
Noradrenaline increases Ca influx during the action potential and Ca entry via L-type Ca channels is required for NA-induced change in AMPAR phenotype. A. Duration of Ca2+ currents was enhanced using NA-AP as the voltage command, compared to control (n = 5). Nifedipine (20 µM) blocked most of the Ca2+ current using control-AP and NA-AP as the voltage command (n = 5). B. Following 3 hour incubation with noradrenaline and nifedipine sEPSCs displayed an inwardly rectifying I–V relationship (n = 6). C. Summary of rectification index of EPSCs (nifedipine alone, n = 5). (*, P < 0.05; **, P < 0.005). Error bars show ± s.e.m.
Activation of ERK and gene transcription is required
Ca2+ entry via L-type Ca2+ channels can activate Ca2+-sensitive ERK/MAPK signaling, leading to activation of gene transcription34,35. To determine whether ERK/MAPK signaling is required for the increase in synaptic GluR2, we applied noradrenaline in the presence of the selective MEK1/2 inhibitor, U0126. U0126 did not itself alter the rectification index of sEPSCs, but abolished the noradrenaline-induced switch in sEPSC I–V relation from inwardly rectifying to near linearity (Fig. 6A,E) and the increase in EPSC amplitude observed at +40 mV (P < 0.0005; n = 8). U0126 also blocked the TEA-induced change in the EPSC rectification (Fig. 6B,F). The ERK inhibitor PD98059 also prevented the TEA-induced change in the sEPSC properties (Fig. 6F). These findings implicate ERK/MAPK signaling in the switch from GluR2-lacking to GluR2-containing AMPARs.
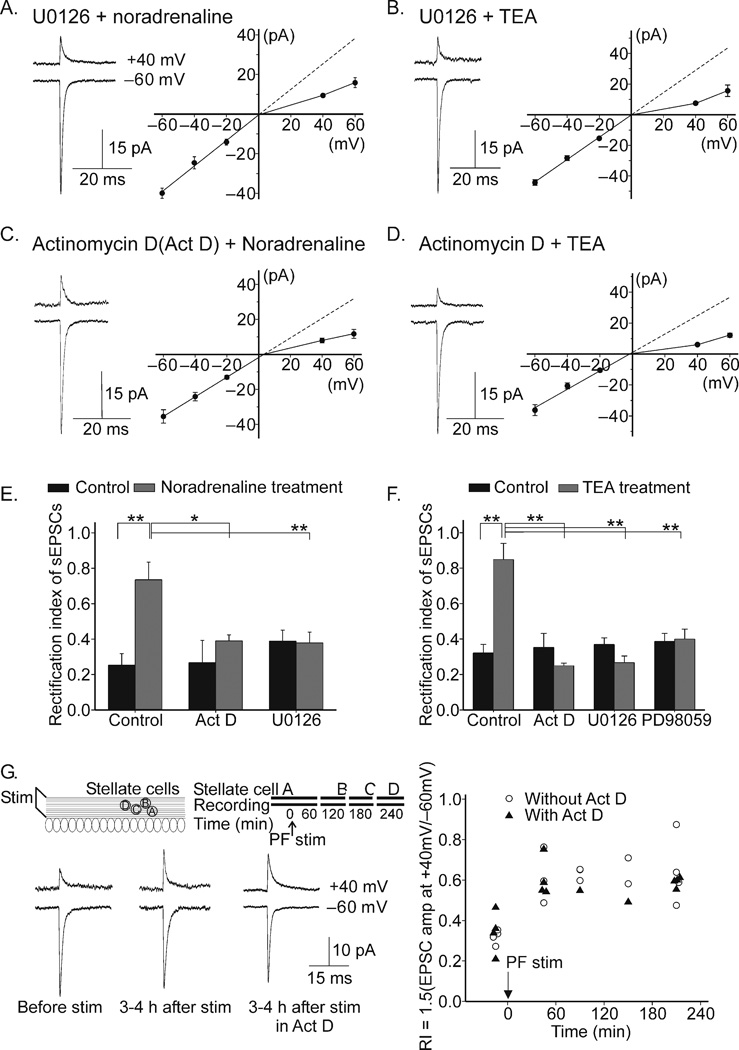
Figure 6.
Activation of ERK-dependent pathways and gene transcription are required for the noradrenaline and action potential broadening-induced change in sEPSC rectification. A. I–V relationship of sEPSCs remained inwardly rectifying in a MEK1/2 inhibitor, U0126 (2 µM, n = 5) which was included during the noradrenaline treatment. B. I–V relationship was unaltered if U0126 (2 µM, n = 3; 20 µM, n = 5) was present during TEA treatment. C. The presence of actinomycin D (25 µM) during noradrenaline treatment prevented noradrenaline-induced change in sEPSC rectification (n = 5). D. Inclusion of actinomycin D (25 µM) during TEA treatment blocked TEA-induced change in sEPSC rectification (actinomycin D + TEA, n = 5). E. Summary of rectification index (actinomycin D alone, n = 3; U0126 control, n = 4). F. Summary of rectification index (actinomycin D alone, n = 5; DMSO control, n = 4; DMSO + TEA, n = 5; U0126 control, n = 5; PD98059 control, n = 3; PD98059 + TEA, 10 µM, n = 2; 25 µM, n = 5). No difference in sEPSC amplitude was observed between the two different concentrations of each inhibitor and therefore the data was pooled. G. Burst stimulation of parallel fibres induced a change in sEPSC rectification that lasted for 4 hours (before stimulation, RI = 0.32 ± 0.02; 30–60 min after stimulation, RI = 0.62 ± 0.1; 3–4 hours after stimulation, RI = 0.64 ± 0.08; n = 4, P < 0.03). Actinomycin D did not prevent this change (before stimulation, RI = 0.34 ± 0.03; 30–60 min after stimulation, RI = 0.61 ± 0.05; 3–4 h after stimulation; RI= 0.59 ± 0.01; n = 4; P < 0.02). Recordings were made sequentially from a group of stellate cells located in the same region of the molecular layer. (*, P < 0.05; **, P < 0.005). Error bars show ± s.e.m.
To determine whether new gene transcription is required for the noradrenaline-induced increase in GluR2-containing AMPARs at synapses, we applied noradrenaline in the presence of the transcriptional inhibitor actinomycin D (25 µM). Actinomycin D alone (3h) did not significantly alter the rectification index of synaptic currents (Fig. 6E), but abolished the noradrenaline-induced switch in I–V relation from inwardly rectifying to nearly linear (noradrenaline, 0.74 ± 0.10; noradrenaline+Act D, 0.39 ± 0.03, n = 5; P < 0.05; Figs. 6C,E). Actinomycin D also blocked the noradrenaline-dependent increase in sEPSC amplitude at +40 mV (8.0 ±1.4 pA; n = 5; P < 0.001 vs. noradrenaline). Thus the noradrenaline-induced increase in GluR2-containing AMPARs at stellate cell synapses, assessed electrophysiologically, is transcription-dependent. If noradrenaline enhances GluR2 expression via increasing action potential duration, we would expect actinomycin D would also abolish the TEA-induced switch in AMPAR phenotype. Indeed, actinomycin D blocked the TEA-induced increase in synaptic GluR2-containing receptors (TEA, 0.85 ± 0.09; n = 5; TEA+Act D; 0.25 ± 0.02; n = 5; P < 0.05; Figs. 6D,F). Thus, action potential broadening alone is sufficient to increase GluR2 transcription.
PF stimulation induces a transcription-independent switch
High frequency stimulation of presynaptic parallel fibres (PFs) can induce a rapid change in synaptic AMPARs from Ca2+-permeable to Ca2+-impermeable receptors in cerebellar stellate cells. This effect is triggered by activation of NMDARs and AMPARs and requires interaction between GRIP and GluR2 subunits29,30,36,37. To examine whether the switch in AMPAR phenotype induced by synaptic stimulation requires new gene transcription, we examined the impact of actinomycin D on this phenomenon. We stimulated PFs with 100 bursts of 4 depolarizations (at 37°C) over a period of 10 min and measured the synaptic currents at −60 and +40 mV. High frequency stimulation induced an increase in EPSC amplitude ratio that was detectable by 30–60 min and persisted for at least 4 h (n = 4, P < 0.03; Fig. 6G). PF stimulation increased the amplitude of the synaptic current at +40 mV from 8.6 ±1.8 pA (control) to 17.8 ± 3.5 pA (3–4 h after stimulation; n = 4, P < 0.05), without a change in the current amplitude at −60 mV. Thus, the PF stimulation triggered a rapid increase in synaptic GluR2-containing AMPARs that persisted for several hours. Continuous perfusion of actinomycin D throughout the experiment, however, did not affect the stimulation-induced change in rectification of sEPSCs (Fig. 6G). Thus, unlike the noradrenaline- or spike broadening-induced switch in AMPAR subunit composition which occurs on the order of hours in a transcription-dependent manner, the PF stimulation-induced change in synaptic AMPAR phenotype occurs on the order of minutes29,30 and is transcription-independent.
Noradrenaline increases the level of GluR2 mRNA expression
We next examined whether noradrenaline increases the expression of GluR2 mRNA in stellate cells. Toward this end, we incubated acute rat cerebellar slices in noradrenaline (3 h, with kynurenic acid and picrotoxin) and then processed cerebellar sections for in situ hybridization. Noradrenaline markedly increased the number of cells expressing GluR2 mRNA and intensity of GluR2 mRNA expression in individual cells within the molecular layer (Fig. 7A,B,I,L). Actinomycin D prevented the noradrenaline-induced increase in GluR2 mRNA (Fig. 7C,I,L). At this age (P18–21), the vast majority of neurons in the molecular layer of the cerebellar cortex are inhibitory basket/stellate cells, which express parvalbumin (Fig. S4). These data indicate that noradrenaline acts via transcription of new GluR2 mRNA to increase the number of GluR2-containing AMPARs at parallel fibre-stellate cell synapses.
Figure 7.
Noradrenaline and TEA treatment increased the level of GluR2, but not GluR1 mRNA expression (n = 5). A–H. DIG-labeled RNA antisense probes for GluR1 and GluR2 mRNA (A–C; E–H) or the sense probes (D) were used. A and E. Control. B and C. noradrenaline treatment increased the level of GluR2 mRNA expression and actinomycin D (Act D) blocked the NA-induced increase in GluR2 expression. F. TEA treatment increased the level of GluR2, but not GluR1 mRNA expression. G and H. Actinomycin D (Act D) and U0126 prevented the TEA-induced increase in GluR2 mRNA expression in stellate cells. ML, molecular layer; PL, Purkinje cell layer; GL, granule cell layer; KYNA, kynurenic acid; picrotoxin, picrotoxin. The labeling that has intensity higher than background within an outline of typical stellate cells (~ 8 µm) was selected as regions of interest. Images are typical of n = 5 in each group. I, G and K. The number of labeled stellate cells that express high level of GluR2 mRNA under each condition relative to control. We used the mean of background intensity plus two standard deviations as threshold for positive labeling. (*, P < 0.05, by unpaired Student’s t-test). L, M and N. Cumulative distribution of the labeling intensity of selected regions of interest after background subtraction illustrated the changes in staining intensities of positive stained cells under each treatment condition (the number of ROIs ranged from 104 to 365 under each condition; noradrenaline vs. control or noradrenaline + Act D, TEA vs. control, or TEA + Act D, or TEA + U0126, Kolmogorov-Smirnov test, P < 0.0001). Scale bars, 200 µM (left panels), and 50 µM (right panels).
We next investigated whether lengthening the duration of postsynaptic action potential is sufficient to selectively enhance the expression of GluR2 mRNA. TEA (3 h) induced a marked increase in GluR2 mRNA expression in individual stellate cells and in the number of cells expressing GluR2 mRNA (Fig. 7E,F,J,M). The change in GluR2 expression was subunit-specific in that GluR1 mRNA expression was unaltered (Fig. 7E,F,K,N). As predicted actinomycin D abolished the TEA-induced increase in GluR2 mRNA expression (Fig. 7G,J,M). Moreover, the ERK/MAPK inhibitor U0126 also blocked the TEA-elicited increase in GluR2 mRNA expression in stellate cells (Fig. 7H,J,M). These findings indicate that lengthening the action potential duration, which enhances Ca2+ entry, stimulates the Ca2+-sensitive ERK/MAPK pathway, which in turn elevates GluR2 mRNA and promotes a switch in AMPAR phenotype at stellate cell synapses.
DISCUSSION
Long-lasting, activity-dependent alterations in glutamatergic synaptic transmission require activation of transcription factors, such as CREB1,2,38. However, whether an experience directly regulates glutamate receptor gene expression is, as yet, unclear. We found that noradrenaline released during emotional arousal and action potential broadening, which enhance Ca2+ entry, selectively upregulate GluR2 mRNA expression and provide direct evidence for an activity-dependent, subunit-specific increase in GluR2 gene transcription. Moreover, enhanced GluR2 transcription leads to synaptic incorporation of GluR2-containing AMPARs and a switch in AMPAR phenotype at stellate cell synapses. This switch is initiated by enhanced Ca2+ entry via L-type voltage-gated Ca2+ channels and requires activation of ERK/MAPK signaling. The net result is an increase in GluR2-containing receptors and a reduction in Ca2+ entry through AMPARs at synaptic sites. Although changing action potential frequency alone did not alter GluR2 expression, enhanced frequency in combination with action potential broadening during noradrenaline treatment may contribute to the alteration in synaptic AMPAR phenotype.
That Ca2+ entry through L-type Ca2+ channels activates ERK/MAPK signaling and promotes phosphorylation and activation of the transcription factor CREB is well established34,35. Both CREB and RE1-element silencing transcription factor (REST), a transcriptional repressor that binds the proximal promoter of the GluR2 gene and silences GluR2 expression, are implicated in regulation of GluR2 expression in response to neuronal activity and neuronal insults39,40,41. It is therefore possible that upregulation of GluR2 mRNA expression by noradrenaline could be mediated by one or both of these transcription factors. Our findings do not, however, rule out the possibility that noradrenaline also regulates stability of the GluR2 transcript, which further enhances mRNA abundance.
An important mechanism responsible for changes in synaptic strength involves regulation of AMPAR trafficking3–8,12. Arc mRNA, which is induced by neuronal activity and targeted to stimulated synaptic area, plays a critical role in AMPAR trafficking42,43. Repetitive activation of synaptic glutamate receptors can also regulate local dendritic protein synthesis of AMPARs in a synapse specific manner9,10,11. In contrast to these spatially localized changes, synaptic modification resulting from AMPAR gene transcription would produce a long lasting change in synaptic AMPARs throughout an entire neuron. Regulation of AMPAR gene transcription therefore represents a powerful means to alter activity of neuronal circuits.
A possibility suggested by our work and work of others is that neurons that display brief action potentials are likely to have low levels of GluR2 mRNA expression26,44,45. Activation of Kv3 and Ca2+-activated large-conductance potassium channels can shorten action potential duration, and both channels are inhibited by 1 mM TEA25,46. Intriguingly, a recent study involving gene expression profile analysis of populations of CNS neurons reveals an inverse relationship between the levels of Kv3 channel expression and that of GluR245. This is precisely what one would predict given the findings of the present study. Generally, fast spiking GABAergic interneurons (such as cerebellar stellate cells) express low levels of GluR2 mRNA and high levels of potassium channels that impose a brief action potential duration26,44. However release of noradrenaline suppresses the normal regulatory pathway of AMPAR subunits by prolonging the action potential and promoting synaptic incorporation of GluR2-containing AMPARs and a switch in AMPAR phenotype.
Neuronal activity can regulate the synaptic incorporation of GluR2-containing receptors by facilitating their targeting to the parallel fiber-stellate synapse without an alteration in GluR2 gene transcription. This mechanism requires activation of synaptic glutamate receptors and involves interactions with PICK1, NSF and GRIP29,30,36,37. Synaptic activity also selectively suppresses the synthesis of GluR1 (but not GluR2) subunits in hippocampal neurons10 and enhances the synthesis of GluR2 in the dopamine neurons in the ventral tegmental area9. Our results demonstrate that noradrenaline increases action potential duration and Ca2+ entry, which regulates GluR2 gene transcription. In cerebellar stellate cells, elevated Ca2+ influx through L-type channels during a postsynaptic action potential elevates GluR2 mRNA and thereby increases the number of synaptic GluR2-containing receptors. Thus, newly synthesized AMPARs are ultimately incorporated via receptor trafficking at stellate cell synapses, a mechanism may involve interactions with PICK1 and GRIP36,37. This modification would be expected to reduce the Ca2+ permeability of synaptic AMPARs and alter short-term synaptic plasticity26,47, producing a qualitative change in synaptic transmission. Dynamic regulation of AMPAR transcription may provide a mechanism for homeostatic control of synaptic transmission48. Such a transcription-dependent mechanism reveals a significant role for neuromodulators and postsynaptic action potentials acting over hours to determine synaptic AMPAR phenotype.
Methods
The fox-urine exposure experiments were carried out as described previously by Kopec et al (2007)49. 18– to 23–day-old C57BL/6J mice were placed in a cage (13 × 9 × 6 in) for 3 minutes as control. A paper towel soaked with fox urine (5 ml) was then inserted below the raised floor containing small holes at regular intervals that allow the odor to permeate into the chamber. The activities of mice were monitored with an infrared CCD camera for the entire 8 min period at a 5 Hz frame rate and stored in a computer. The movement of the experimental animal was characterized by the amount of motion that occurs between two successive frames, using a custom written program. To determine the threshold in significant motion pixels, the freezing responses were scored by a lab member blind to the experimental conditions. If “movement” within a 1 second period remains below the threshold it will be considered as a freezing episode49. The duration of freezing was determined and the total cumulative freezing time during each period will be used to calculate % freezing for both the control and stress periods. In some experiments mice were injected with propranolol (20 mg/kg) or saline (IP) 15–30 min before entering the cage. Care was taken to minimize the handling stress of the mice.
Cerebellar slices (250 µm) were obtained from 18- to 23-day-old C57BL/6J mice as described previously50. In some experiments, slices were incubated in ACSF (in mM: 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 25 glucose, saturated with 95% O2-5% CO2, pH 7.3) that contained 1 mM (or 5 mM) kynurenic acid (KYNA), 100 µM picrotoxin and 10 µM noradrenaline or other inhibitors (as noted) for 3 hours, unless otherwise indicated. Noradrenaline (NA) and picrotoxin treatments were performed at 36°C, and TEA treatment at 36°C (Fig 4C) and at room temperature (Fig 4). In several experiments 1 mM MCPG and 10 µM SCH50911 were also included during TEA treatments to block mGluRs and GABABRs.
Electrophysiology
Whole cell voltage and current clamp recordings were made with Multiclamp 700A and Axopatch 200B amplifiers (Axon instruments, Foster city, CA). Synaptic currents, Ca2+ currents and action potentials were filtered at 2–5 kHz and digitized at 20 kHz.
Spontaneous excitatory postsynaptic currents were recorded from stellate cells using a Cs-based pipette solution (in mM: 135 CsCl, 10 HEPES, 10 EGTA, 2 NaCl, 4 ATP-Mg, 5 TEA, 1 QX314, 0.1 spermine, pH 7.3) in ACSF containing 100 µM picrotoxin at room temperature, unless otherwise noted. Inclusion of spermine in the whole cell pipette solution blocks AMPARs lacking GluR2 subunits (not GluR2-containing receptors) at positive potentials, producing a characteristic inwardly rectifying I–V relationship, and therefore was used to determine the subunit composition of synaptic receptors. To obtain an I–V relationship of synaptic currents, spontaneous EPSCs were recorded at several potentials. Average sEPSCs at each holding potential (typically average of 50–100 events) were measured using N version 4.0 (written by Steve Traynelis, Emory University) as described previously29. The reversal potential was extrapolated from linear fitting of the currents at negative potentials and was about 0 pA (1.2 ± 0.4 pA, n = 78). We also monitored sEPSCs at 0 mV and did not detected synaptic currents. The rectification index of the I–V relationship was defined as the ratio of the current amplitude at +40 mV to the predicted linear value at +40 mV (extrapolated from linear fitting of the currents at negative potentials), unless otherwise noted. When the currents at positive potentials show strong inward rectification, solid lines were drawn to connect the data points at positive potentials. In a few experiments the ratio of EPSCs at +40 mV to −60 mV (R+40mV/−60mV) was measured and the rectification index was calculated as 1.5*R+40mV/−60mV, assuming that the reversal potential is 0 mV. In some experiment EPSCs were minimally evoked with a patch-electrode containing external solution, by stimulating in the molecular layer (100–140 µs pulses of 2–13 V at 0.33 Hz). CNQX-induced currents were recorded in ACSF that contained picrotoxin (100 µM), D-AP5 (50 µM), strychnine (1 µM) and TTX (0.5 µM) to block GABAA, NMDA and glycine receptors and Na+ channels.
Spontaneous action potentials were recorded using a perforated or whole cell patch configuration in ACSF that contained 1 mM KYNA, 100 µM picrotoxin (to block ionotropic glutamatergic and inhibitory transmission, respectively). The pipette solution contained (in mM) 115 KMeSO3, 10 NaCl, 2 MgCl2, 0.16 CaCl2, 0.5 EGTA, 10 HEPES, 4 ATP-Na, 0.4 GTP-Na, 14 Tris2-creatine phosphate, (0.66 mg/ml amphotericin B for perforated patch recordings), pH 7.3. The frequency of spontaneous action potentials were recorded using a cell-attached configuration with a glass electrode filled with ACSF. The effect of noradrenaline and picrotoxin was determined at 36°C and that of TEA at room temperature.
Ca2+ currents evoked by single action potentials were determined using a voltage clamp protocol that mimicked the action potential waveform at 36°C. The waveforms of both the control action potential (control–AP) and the action potential in the presence of 10 µM noradrenaline (NA–AP) were recorded in current clamp from a stellate cell. The waveforms displayed an action potential half width of 0.8 ms and 1.3 ms and after-hyperpolarization of −40 mV and −35 mV; average control–AP and NA–AP, respectively. They were therefore used as voltage commands. The pipette solution contained (in mM): 119 CsCl, 9 EGTA, 10 HEPES, 1.8 MgCl2, 14 Tris-creatine phosphate, 4 ATP-Mg, 0.4 GTP-Na, 10 TEA, 1 QX314, pH 7.3. The external solution included 10 mM TEA, 300 nM TTX, 10 µM ZD7288, 1 mM KA, 100 µM picrotoxin to block potassium, sodium and h-currents and synaptic currents, respectively. Cd2+ (100 µM), a general Ca channel blocker, was used to block Ca2+ channels. The Ca2+ current was monitored as the difference current (I – ICd). Ca2+ currents were measured in stellate cells prior to any treatments.
To stimulate parallel fiber inputs a parallel bipolar electrode (150 µm spacing) was placed across the molecular layer about 200 µm from the recording electrode. The stimulus intensity ranged from 3 – 13 V with a stimulus duration of 120–200 µs. The stimulation protocol contained 100 sweeps of 4 depolarizations at 50 Hz, with a 2 sec interval between two sweeps and 20 sec between every 10 sweeps. sEPSCs were recorded from a stellate cell before and after the simulation, followed by recordings from neighboring stellate cells. To test the effects of actinomycin D on the parallel fibre stimulation-induced change, application of ACSF that contained 25 µM actinomycin D began 30 minutes before the recording of the first cell and PF stimulation, and continued throughout the experiment. The experiments were performed at 36°C.
Real time single cell PCR
horizontal cerebellar slices (350 µm) were obtained from 18- to 23-day-old GAD-65 GAP mice. The cerebellar cortex that were isolated from slices were incubated in extracellular solution (in mM: 135 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, 11 Glucose; pH adjusted to 7.3 with 1M NaOH) containing 32 units/ml papain at 37°C for 40–50 min, washed and then triturated through a glass pipette. Individual GFP positive cells with a diameter of 6–10 µm were picked up using a fire-polished glass electrode (that was filled with DEPC treated water containing 0.5 u RNase inhibitor) under visual guidance. The cell content was then injected into the RT solution (10 µl) prepared for reverse transcription (high capacity cDNA RT kit, Applied Biosystems). The probes for GluR1, 2 and 3 (TagMan gene expression assays) were obtained from Applied Biosystem. Two house keeping protein genes, GAPDH and cyclophilin, were tested and showed no detectable change following fox urine exposure, and therefore were used in our experiments. Cerebellar total RNA was used as positive control. Negative control samples include bath solution without cells and omission of reverse transcription, and both showed no detectable signals. Real time PCR was performed using the ABI 7300 Sequence Detection System (Applied Biosystems) at Penn State Nucleic acid facility.
In situ hybridization
transverse cerebellar slices (400 µm) from P18–21 Sprague Dawley rats were incubated with noradrenaline or TEA and inhibitors in ACSF for 3 h as described above. After incubation, slices were chilled at 4°C, embedded, and 20 µm slices were cut by cryostat (Shandon) and fixed with paraformaldehyde (4% for 30 min). A digoxigenin (DIG)-labeled RNA probe was prepared by in vitro transcription of GluR1 and GluR2 cDNAs with T3 and T7 RNA polymerases in the presence of dig-labeled UTP (Roche Molecular Biochemicals). In situ hybridization was performed as described40. Signals were detected by immunocytochemistry with an alkaline phosphatase-conjugated anti-DIG antibody, according to the DIG RNA Detection kit (Roche Molecular Biochemicals). For each treatment condition, several areas (5–10) of the molecular layer (100 µm × 160 µm area) of the vermis (lobules IV to VIII) from multiple slides were sampled. The labeling that has intensity higher than background within an outline of the soma of typical stellate cells (a circle of 10 µm diameter) was selected using ImageJ (version 1.38×, NIH) by the person blind to the experimental condition. The background was measured by selecting 14–32 (average, 24 ± 4) cell-free areas from each image. Since GluR1 mRNA in cerebellar stellate cells using single cell RT-PCR (Fig. 1F), GluR1 was used as a control. Both the labeling and subsequent quantification was done in a blind and unbiased manner.
Immunocytochemistry
Transverse cerebellar slices (400 µm thick) were prepared and incubated in the absence or presence of noradrenaline or TEA and inhibitors as described above. After incubation, slices were fixed with 4% paraformaldehyde and rinsed in phosphate-buffered-saline (PBS, 0.03 M phosphate buffer with 0.9% NaCl). After fixation, slices were cut into sections (20 µm) with a cryostat (Shandon). The sections were washed thoroughly in PBS, then blocked for 2 h in PBS containing 10% normal goat serum, 0.5% bovine serum albumin (BSA) and 0.2% saponin (20 mg/10 ml PBS). Sections were incubated overnight at 4°C with a monoclonal antibody against parvalbumin (1:200). After washing 3 × with PBS containing 0.01% saponin, slices were incubated for 1 hr in secondary antibody, Alexa Fluor 488 goat anti-mouse IgG (1:200) in PBS containing 0.01% saponin. After washing in PBS, slices were mounted with Prolong-Gold and images acquired with a FluoView 1000 confocal microscope (Olympus).
All values are expressed as mean ± SEM. Statistical significance was assessed by a two-tailed Student’s t-test or ANOVA test.
Supplementary Material
Acknowledgements
This work was supported by National Science Foundation Grant IBN-0344559 and National Institutes of Health Grant NS58867. (S.Q.J.L.) and by National Institutes of Health Grant NS46742 (R.S.Z.). R.S.Z. is the F.M. Kirby Professor in Neural Repair and Protection. We thank Drs. Deb Grove and Shaona Acharjee for scientific input, and Drs. Michael Bennett, Bernhard Luscher, Pablo Castillo, Leonard Kaczmarek and Matthew Whim for helpful discussions and comments on the manuscript.
Footnotes
Author contributions
Y.L. designed and conducted the behavioral, electrophysiological and real time single cell RT-PCR experiments, prepared figures and participated in writing the manuscript. L.F. designed and carried out the in situ hybridization assays. I.S. performed the behavioral, real time single cell RT-PCR and some electrophysiology experiments, analyzed in situ hybridization data and prepared figures. Y.T. helped to perform the in situ hybridization assays and participated in data collection and analysis. G.S. provided GAD-65 GFP mice. R.S.Z. supervised the in situ hybridization experiments and contributed to writing and editing of the manuscript. S.J.L. conceived and designed the study and wrote the manuscript.
References
- 1.Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci. Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- 2.West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat. Rev. Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- 3.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 4.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr. Opin. Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr. Opin. Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 6.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Isaac JT, Ashby M, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 10.Sutton MA, et al. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 11.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat. Neurosci. 2004;7:244–253. doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- 12.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 13.Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 14.Siggins GR, Hoffer BJ, Oliver AP, Bloom FE. Activation of a central noradrenergic projection to cerebellum. Nature. 1971;233:481–483. doi: 10.1038/233481a0. [DOI] [PubMed] [Google Scholar]
- 15.Bickford-Wimer P, Pang K, Rose GM, Gerhardt GA. Electrically-evoked release of norepinephrine in the rat cerebellum: an in vivo electrochemical and electrophysiological study. Brain. Res. 1991;558:305–311. doi: 10.1016/0006-8993(91)90782-q. [DOI] [PubMed] [Google Scholar]
- 16.Saitow F, Satake S, Yamada J, Konishi S. beta-adrenergic receptor-mediated presynaptic facilitation of inhibitory GABAergic transmission at cerebellar interneuron-Purkinje cell synapses. J. Neurophysiol. 2000;84:2016–2025. doi: 10.1152/jn.2000.84.4.2016. [DOI] [PubMed] [Google Scholar]
- 17.Kondo S, Marty A. Differential effects of noradrenaline on evoked, spontaneous and miniature IPSCs in rat cerebellar stellate cells. J. Physiol. 1998;509(Pt 1):233–243. doi: 10.1111/j.1469-7793.1998.233bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartford MC, Samec A, Fister M, Bickford PC. Cerebellar norepinephrine modulates learning of delay classical eyeblink conditioning: evidence for post-synaptic signaling via PKA. Learn. Mem. 2004;11:732–737. doi: 10.1101/lm.83104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacchetti B, Baldi E, Lorenzini CA, Bucherelli C. Cerebellar role in fear-conditioning consolidation. Proc. Natl. Acad. Sci. USA. 2002;99:8406–8411. doi: 10.1073/pnas.112660399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sacchetti B, Scelfo B, Tempia F, Strata P. Long-term synaptic changes induced in the cerebellar cortex by fear conditioning. Neuron. 2004;42:973–982. doi: 10.1016/j.neuron.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Scelfo B, Sacchetti B, Strata P. Learning-related long-term potentiation of inhibitory synapses in the cerebellar cortex. Proc. Natl. Acad. Sci. USA. 2008;105:769–774. doi: 10.1073/pnas.0706342105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu H, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Seol GH, et al. Neuromodulators control the polarity of spike-timing-dependent synaptic plasticity. Neuron. 2007;55:919–929. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonas P, Bischofberger J, Fricker D, Miles R. Interneuron Diversity series: Fast in, fast out--temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 2004;27:30–40. doi: 10.1016/j.tins.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 26.Geiger JR, et al. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs EC, et al. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc. Natl. Acad. Sci. USA. 2001;98:3571–3576. doi: 10.1073/pnas.051631898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayley S, Borowski T, Merali Z, Anisman H. Central monoamine activity in genetically distinct strains of mice following a psychogenic stressor: effects of predator exposure. Brain. Res. 2001;892:293–300. doi: 10.1016/s0006-8993(00)03262-5. [DOI] [PubMed] [Google Scholar]
- 29.Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- 30.Sun L, Liu SJ. Activation of extrasynaptic NMDA receptors induces a PKC-dependent switch in AMPA receptor subtypes in mouse cerebellar stellate cells. J. Physiol. 2007;583(Pt 2):537–553. doi: 10.1113/jphysiol.2007.136788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saitow F, Konishi S. Excitability increase induced by beta-adrenergic receptor-mediated activation of hyperpolarization-activated cation channels in rat cerebellar basket cells. J. Neurophysiol. 2000;84:2026–2034. doi: 10.1152/jn.2000.84.4.2026. [DOI] [PubMed] [Google Scholar]
- 32.Soto D, Coombs ID, Kelly L, Farrant M, Cull-Candy SG. Stargazin attenuates intracellular polyamine block of calcium-permeable AMPA receptors. Nat. Neurosci. 2007;10:1260–1267. doi: 10.1038/nn1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menuz K, Stroud RM, Nicoll RA, Hays FA. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- 34.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 35.Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- 36.Liu SJ, Cull-Candy SG. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat. Neurosci. 2005;8:768–775. doi: 10.1038/nn1468. [DOI] [PubMed] [Google Scholar]
- 37.Gardner SM, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 39.Myers SJ, et al. Transcriptional regulation of the GluR2 gene: neural-specific expression, multiple promoters, and regulatory elements. J. Neurosci. 1998;18:6723–6739. doi: 10.1523/JNEUROSCI.18-17-06723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderone A, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J. Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, et al. Expression of Ca(2+)-permeable AMPA receptor channels primes cell death in transient forebrain ischemia. Neuron. 2004;43:43–55. doi: 10.1016/j.neuron.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chowdhury S, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J. Neurophysiol. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- 45.Sugino K, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat. Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 46.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 47.Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature. 1999;401:594–598. doi: 10.1038/44151. [DOI] [PubMed] [Google Scholar]
- 48.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Kopec CD, et al. A robust automated method to analyze rodent motion during fear conditioning. Neuropharmacology. 2007;52:228–233. doi: 10.1016/j.neuropharm.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Liu SJ, Lachamp P. The activation of excitatory glutamate receptors evokes a long-lasting increase in the release of GABA from cerebellar stellate cells. J. Neurosci. 2006;26:9332–9339. doi: 10.1523/JNEUROSCI.2929-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.