Abstract
Stem cells reside in specialized microenvironments or “niches” which regulate their function. In vitro studies employing hypoxic culture conditions (≤ 5% O2) have revealed strong regulatory links between O2 availability and stem/precursor cell functions1–6. Therefore, while some stem cells are perivascular, others may occupy hypoxic niches and be regulated by O2 gradients. However, the underlying mechanisms remain unclear. Here, we show that Hypoxia Inducible Factor-1α (HIF-1α), a principal mediator of hypoxic adaptations, modulates Wnt/β-catenin signalling in hypoxic embryonic stem (ES) cells by enhancing β-catenin activation and expression of downstream effectors LEF-1 and TCF-1. This regulation extends to primary cells, including isolated neural stem cells (NSCs), and is not observed in differentiated cells. In vivo, Wnt/β-catenin activity is closely associated with low O2 regions in the subgranular zone (SGZ) of the hippocampus, a key NSC niche7. Hif-1α deletion impairs hippocampal Wnt-dependent processes, including NSC proliferation, differentiation and neuronal maturation. This decline correlates with reduced Wnt/β-catenin signalling in the SGZ. Therefore, O2 availability may have a direct role in stem cell regulation via HIF-1α modulation of Wnt/β-catenin signalling.
Keywords: Embryonic stem cells, hypoxic niche, HIF-1α/ARNT, Wnt/β-catenin signalling, neurogenesis
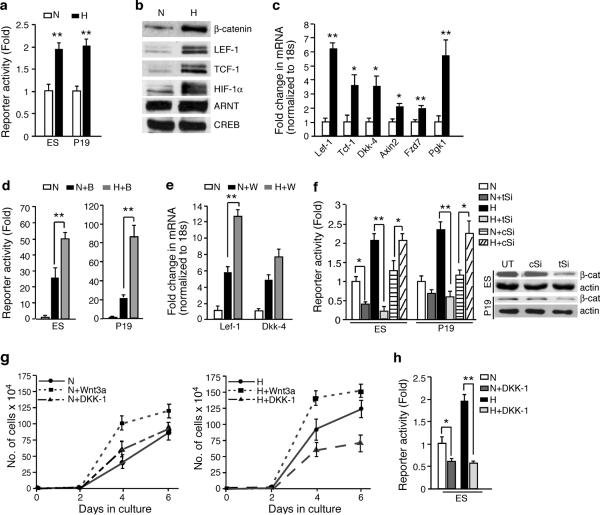
Wnt/β-catenin pathway activation is characterized by (i) stabilization of cytoplasmic β-catenin upon receptor engagement by Wnt ligands, (ii) β-catenin nuclear translocalion, (iii) β-catenin interaction with lymphoid enhancer-binding factor-1/T-cell factor-1 (LEF/TCF) transcription factors, and (iv) stimulation of target genes8,9. To determine if this pathway is modulated by O2 availability, we transiently transfected murine ES cells and P19 embryonal carcinoma (EC) cells with a luciferase-based TOP-Flash (TCF optimal promoter) Wnt reporter plasmid, and compared reporter activity under “normoxia” (21% O2), and “hypoxia” (1.5% O2). Exposure to hypoxia significantly enhanced reporter activity (2 fold) in both embryonic cell lines (Fig. 1a and see Supplementary Information, Fig. S1a, b). Hypoxic exposure also increased levels of nuclear β-catenin, LEF-1 and TCF-1 proteins (Fig. 1b). Quantitative real time PCR (qRT-PCR) analyses of hypoxic cells showed increased expression (2–6 fold) of Wnt target genes, such as Axin2 and Dkk-4 (Dickkopf-4), and β-catenin activators, Lef-1 and Tcf-1 (Fig. 1c). This is consistent with our previous data showing increased Lef-1 expression in hypoxic murine ES cells10. HIF-1α protein stabilization and upregulation of the HIF-1α target Pgk-1 confirmed the induction of a hypoxic response (Fig. 1b, c).
Figure 1. Hypoxia activates Wnt/β-catenin signalling in mouse embryonic cells.
a, ES and P19 EC cells transiently transfected with TOP-Flash and pRL-SV40 plasmids were grown either in normoxia (N, 21% O2) or hypoxia (H, 1.5% O2 for 16h) (n=9). b, β-catenin and LEF-1/TCF-1 analysis in ES cells under indicated O2 conditions by western blot analysis of nuclear extracts. CREB served as a loading control, c, qRT-PCR analysis of Wnt target gene levels in hypoxic ES cells relative to 18S rRNA levels, normalized to normoxic cells (n=9). d, TOP-Flash activity in ES and P19 EC cells treated with 200 nM Broraoindirubin-3' oxime (B) and cultured either in hypoxia or normoxia (n=9). e, qRT-PCR analysis of Wnt-3a CM (W) treated ES cells show increased induction of Wnt target genes under hypoxia compared to normoxia (n=6). f, Severe reduction of reporter activity in ES and P19 EC cells transfected with pools of siRNA against β-catenin at indicated O2 levels (UT: untransfected, tSi: target siRNA, cSi: control siRNA) (n=6) (left), and western blot analysis of silenced β-catenin 48 h post transfection (right), g, Growth rate of ES cells treated with Wnt-3a CM, or Wnt inhibitor DKK-1 (300 ng ml−1) under indicated O2 conditions (n=3). h, TOP-Flash activity in ES cells treated with DKK-1 (300 ng ml−1) (n=3). *= P <0.05, **= P <0.005., Student's t-test. Error bars represent S.D..
Hypoxia exerted a similar effect in stimulated cells. While Wnt pathway stimulators, including 6-Bromoindirubin-3' oxime (BIO), Lithium Chloride (LiCl) or Wnt-3a condilioned medium (Wnt-3a CM), enhanced reporter activity ~20 fold, exposure to hypoxia increased TOP-Flash activity 50–80 fold in stimulated cells relative to untreated controls (Fig. 1d and Supplementary Information, Fig. S2a, b). Hypoxic exposure also further increased expression of Wnt target genes Lef-1 and Dkk-4 in stimulated cells (Fig. 1e). TOP-Flash assays in RNAi-mediated β-catenin depleted cells confirmed the involvement of β-catenin in hypoxia induced luciferase activity (Fig. 1f). We excluded the possible involvement of other signalling pathways proposed to promote β-catenin stabilization (e.g. Akt/PDK) by inhibiting glycogen synthase kinase-3β (GSK-3β)11, by assessing GSK3β phosphorylation levels, which remained unchanged under hypoxia (Supplementary Information, Fig. S1c). Collectively, these data indicate that ES and P19 EC cells maintain constitutively active Wnt signalling that is β-catenin dependent, and markedly enhanced by hypoxia.
Hypoxic induction of Wnt signalling was also evident in cell proliferation assays. Hypoxic ES cells displayed increased numbers (based on cell counts) compared to normoxic cells (Fig. 1g). This reflected increased cell survival, as hypoxic exposure had modest effects on ES cell cycle, but significantly reduced apoptotic cell death (Supplementary information, Fig. S2c, d). Addition of Wnt-3a CM, which stimulates cell expansion/self-renewal12, increased the numbers of both normoxic and hypoxic cells relative to untreated controls. In contrast, treatment with Dickkopf-1 (DKK-1), an extracellular Wnt pathway inhibitor, exclusively decreased cell numbers under hypoxia (Fig. 1g). Of note, DKK-1 treatment downregulated TOP-Flash activity in both normoxic and hypoxic ES cells (Fig. 1h), suggesting that hypoxia sensitizes ES cells to the growth effects of Wnt/β-catenin signalling.
One of the primary mediators of hypoxic responses is HIF-1, a heterodimeric transcription factor containing an O2 sensitive α subunit (HIF-1α) and a constitutively expressed β subunit (HIF-1β, also known as ARNT). To determine whether hypoxia activates Wnt signalling via HIF-1, we analyzed TOP-Flash activity in Hif-1α−/− ES cells. In contrast to a report showing HIF-1α mediated repression of Wnt signalling in colon cancer cells13, Hif-1α deletion significantly downregulated TOP-Flash activity in hypoxic ES cells, but had minimal effect on basal activity (Fig. 2a). Combined treatment of Wnt-3a CM and hypoxia did not superinduce TOP-Flash activity in Hif-1α−/− cells (Fig. 2b), and loss of Hif-1α had no effect on normoxic responses to Wnt stimulation (Fig. 2b). However, normoxic HIF-1α stabilization in WT ES cells by the hypoxia mimetic deferoxamine (DFX) significantly restored reporter activity (> 1.5 fold) (Fig. 2a). We observed a similar regulatory link between hypoxic ARNT activity and Wnt/β-catenin signalling (Fig. 2c). Deletion of Hif-1α and Arnt also diminished expression of Wnt target genes including Dkk-4, Lef-1 and Tcf-1 under hypoxia (Fig. 2d, e and Supplementary Information, Fig. S3a). Thus, hypoxic induction of Wnt/β-catenin signalling is mediated by HIF-1α/ARNT complexes. Moreover, as expected from increased levels of both β-catenin and LEF-1 in hypoxic cells (Fig. 1b), we detected increased association of nuclear β-catenin extracted from hypoxic ES cells with immunoprecipitated LEF-1 (Fig. 2f). Intriguingly, we noted reduced levels of β-catenin in whole cell extracts of hypoxic Hif-1α−/− cells as compared to hypoxic Hif-1α+/+ (Fig. 2e).
Figure 2. HIF-1 (HIF-1α/ARNT complex) mediates hypoxia induced Wnt signalling in embryonic cells.
a, Hif-1α−/− ES cells (middle) show attenuated TOP-Flash activity under hypoxia as compared with hypoxic Hif-1α+/+ cells (left). Increased reporter activity in normoxic ES cells treated with DFX (16 h) (right) (n=9). b, TOP-Flash activity in Hif-1α+/+ and Hrf-1α−/− cells stimulated with Wnt-3a CM (W) and cultured under indicated O2 conditions (n=9). c, TOP-Flash activity is attenuated in hypoxic Arnt−/− ES cells, and rescued in hypoxic AmtRes cells (Arnf−/− cells with restored Arnt expression) compared to hypoxic Arnt+/+ cells (n=9). d, qRT-PCR analysis of Hif-1α−/− cell extracts for Wnt target gene expression (n=6). e, Western blot analysis of β-catenin, LEF-1 and TCF-1 proteins in whole cell extracts of null cells (indicated by the absence of specific protein) or corresponding wild type cells cultured either under normoxia or hypoxia. Actin served as the loading control. f, Immunoprccipitation with LEF-1 antibody was performed on nuclear extracts of normoxic or hypoxic ES cells. CREB served as the loading control. g, ES cells were cultured under 21% or 1.5% O2 for 16 h and then assayed by ChlP. Following IP with antibody against HlF-1α or isotype control, DNA extracts were assessed by qRT-PCR. Results of each genomic region tested (upper) are expressed as fold difference between HIF-1α IP and mouse IgG control (lower). Lef-1 coding region (I.C) served as a control (n=4). *= P <O.05, **= P <0.005., Student's t-test and one-way ANOVA (b). Error bars represent S.D..
Since hypoxic induction of Lef-1 and Tcf-1 mRNA and corresponding proteins strongly correlated with HIF-1α protein accumulation, we examined whether HIF-1α directly contributes to increased transcription of Lef/Tcf genes. Analysis of murine Lef-1 and Tcf-1 gene sequences revealed multiple putative HREs (hypoxia response elements) spanning exon 1 and the upstream promoter and enhancer regions (+3000 bp) (Fig. 2g upper). Subsequently, in chromatin IP (ChIP) assays, compared to normoxia, hypoxic ES cells exhibited increased (4–10 fold) HIF-1α association at each genomic region tested (Fig. 2g lower). Therefore, HIF-1α regulates LEF-1/TCF-1 protein abundance and function in embryonic cells. In proliferation assays, neither Hif-1α−/− nor Arnt−/− cells displayed growth advantage under hypoxia compared to the corresponding wild type Hif-1α+/+ and Arnt+/+ cells (Supplementary Information, Fig. S3b, c).
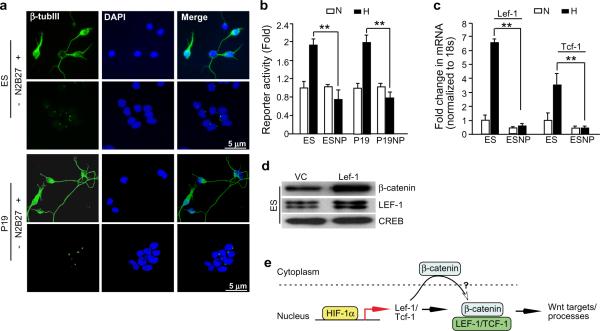
Stem and committed progenitor cells can respond differently to low O2 signals5. Therefore, we asked if hypoxic effects on Wnt/β-catenin signalling are a function of differentiation stage. Embryonic cells were stimulated with neuronal supplements (N2B27) to form neuronal precursors14. Thereafter, we subjected ES and P19 EC cell-derived neurons expressing the neuronal markers β-tubulin III β-tubIII) and doublecortin (DCX) (Fig. 3a and Supplementary Information, Fig. S4a) to TOP-Flash assays. The transfection efficiency was monitored using GFP as a reporter (data not shown). Whereas hypoxia enhanced TOP-Flash activity in undifferentiated ES and P19 cells (Fig. 3b), it did not have any effect on the embryonic cell- derived neurons. This observation implied that hypoxia selectively activates Wnt/β-catenin signalling in undifferentiated cells. We also observed hypoxic induction of Lef-1 and Tcf-1 genes exclusively in undifferentiated cells (Fig. 3c). Furthermore, neuronal differentiation coincided with a significant loss of baseline Lef-1/Tcf-1 levels (3–5 fold) (Fig. 3c). We suggest that the Lef-1 and Tcf-1 loci become epigenetically modified and therefore inaccessible to HIF-1 in differentiated cells.
Figure 3. O2 regulation of Wnt/β-catenin signalling is differentiation stage specific.
a, ES and P19 EC cells were treated with (+) or without (−) N2B27 neuronal growth and differentiation supplements, and monitored for the formation of neuronal progenitors (NP) through the expression of neuronal marker β-tub III. b, ES and P19 EC cell-derived NPs demonstrate attenuated TOP-Flash reporter activity under hypoxia compared to hypoxic undifferentiated controls (n=3) c, ES cell dcrived-NPs were cultured under normoxia or hypoxia for 16 h and assessed for Lef/Tcf gene expression by qRT-PCR. (n=3).**= P <0.005., Student's t-test. Error bars (b–c) represent S.D.. d, Western blot analysis of nuclear extracts of ES-Lef-1 and the corresponding control virus (VC) transduced ES cells cullured under normoxia. CREB served as the loading control. e, Schematic representation of a model wherein hypoxia (via H1F-1α) induces β-catenin transcriptional effectors, Lef-1 and Tcf-1, resulting in increased nuclear translocation of β-catenin (? indicates mechanism is unknown), subsequent interaction between β-catenin and LEF-1/TCF-1 and activation of Wnt target genes.
It has been shown that LEF/TCF over-expression increases β-catenin nuclear translocation, and activates transcription in multiple embryonic cell types15,16. To investigate if β-catcnin accumulation in hypoxic ES cells involved Lef-1 induction, we engineered LEF-1 overexpressing ES cells (ES-Lef-1). LEF-1 overexpression resulted in increased levels of nuclear β-catenin under normoxia relative to vector controls (VC) (Fig. 3d). Also, ESL-Lef-1 cells displayed enhanced cell proliferation, and were sensitive to DKK-1 inhibition (Supplementary Information, Fig. S4c). This observation recapitulated Wnt/β-catenin dependent hypoxic effects on ES cell proliferation (Fig. 1g). Moreover, we reproduced nuclear β-catcnin-HIF-1α interaction in hypoxic ES cells (see Supplementary Information, Fig. S4b), similar to previous observations in differentiated neoplastic cells13, further highlighting LEF-1's involvement in differentiation-stage specific responses of the Wnt/β-catenin pathway to hypoxia. Taken together, we propose a model where HIF-1α regulates Lef-1 and Tcf-1 in undifferentiated cells, leading to increased nuclear β-catenin-LEF/TCF interaction, and activation of Wnt/β-catenin targets (Fig. 3e).
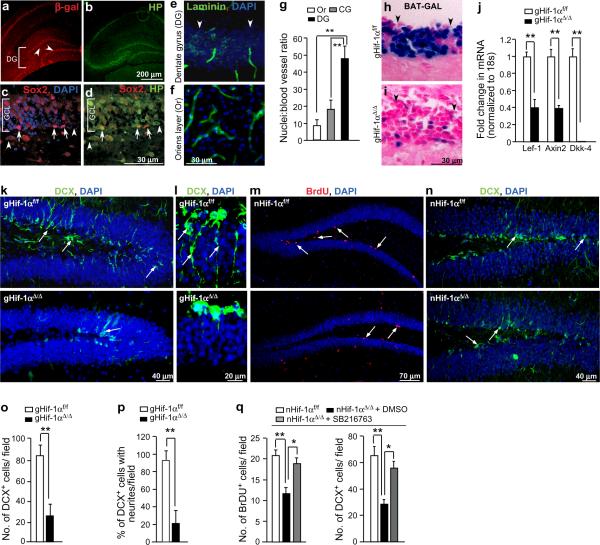
To determine the physiological relevance of the above model, we next investigated whether decreased O2 levels correlate with Wnt/β-catenin activity in vivo. We screened for areas of Wnt/β-catenin activity in a Wnt reporter (BAT-GAL) mouse strain with β-galactosidase immunostaining17, and compared its distribution with that of low O2 regions detected by immunostaining with the hypoxia marker pimonidazolc hydrochloride. Pimonidazole detects O2 partial pressures of less than 1Onim Hg (~1.3% O2)18. Active Wnt/β-catenin signalling was detected in close proximity to low O2 regions in both embryonic and the adult brain (Fig. 4a, b and Supplementary Information, Fig. S5 a–d). One such region positive for Wnt/β-catcnin activity was the subgranular zone (SGZ) of the dentate gyrus (DG) (Fig. 4a, and Supplementary Information, Fig. S5 e–g), a primary neurogenic niche in which adult hippocampal progenitors (AHPs) proliferate and differentiate into dentate granule neurons.7 Pimonidazole clearly marked the SGZ, as identified by its colocalization with the NSC marker Sox2 (Fig. 4c, d). Importantly, enumeration of blood vessels marked by laminin immunostaining revealed a much higher nuclei:blood vessel ratio within the granule cell layer of the DG in comparison to other areas in the brain including the adjacent hippocampal Oriens (Or) layer, and the cerebellar granule cell (CG) layer (Fig. 4e–g). Other vascular markers such as CD31 and India ink showed a similar trend (Supplementary Information, Fig. S5h–k). This suggests that hypoxia within the dentate gyrus, as independently confirmed by HIF-1α stabilization and the expression of hypoxia responsive genes carbonic anhydrase IX (CAIX) and vascular endothelial growth factor (VEGF), (Supplementary Information, Fig. S5l–q), is likely a consequence of O2 limitation due to fewer blood vessels vessels.
Figure 4. Deletion of Hif-1α in vivo suppresses adult hippocampal neurogenesis.
a, Wnt activity in the adult dentate gyrus (DG) is marked by β-galactosidase (β-gal) immunostaining (red) in BAT-GAL reporter mice. Arrowheads indicate the subgranular zone (SGZ) in the DG. b, Hypoxic regions within the adult hippocampus marked by pimonidazole staining (HP, green) include the adult DG. c, The SGZ of the DG is marked by the stem cell marker Sox2. d, Colocalization of pimonidzole with Sox2+ cells indicates a hypoxic SGZ. Arrowheads (c–d) indicate the orientation of the SGZ of the granule cell layer (GCL). e, Laminin staining marks blood vessels in the DG, and in the adjacent hippocampal Oriens layer (Or) (f). g, Quantification of the nuclei: blood vessel ratio in the DG, Or and cerebcllar granule layer (CG) (n = 3; 5 sections per animal, **=P <O.005., Student's t test). Error bars represent S.E.M.. h–i, X-gal staining of the DG of Hif-1αf/f, BAT-GALTg (h) and gHif-1αΔ/Δ, BAT-GALTg (i) animals (n=3; 5 sections per animal). j, qRT-PCR analysis of hippocampal extracts for Wnt target genes (Lef-1, Axin2 and Dkk4) (n=3–5 per group). k, o, Significant reduction of DCX+ cells (arrows) in gHif-1αΔ/Δ SGZ and GCL compared to control Hif-1αf/f aniirials (n=7). l, p, DCX+ cells with neurtees are significantly fewer in gHif-1αΔ/Δ DG as compared to gHif-1αf/f DG. DCX+ cells with processes > 20 μM were counted positive for neurites (n=7). m, n, q, Lack of neuronal Hif-1α in the SGZ reduces NSC proliferation as indicated by BrdU+ cells (arrows) (m, q left panel), and NSC differentiation as indicated by DCX+ cells (arrows indicate DCX+ cells with neuritis, arrowheads indicate the direction of SGZ) (n, q right panel) (n=6). q, Quantification of BrdU+ and DCX+ cells in nHif-1αΔ/Δ DG treated with SB216763 (2mg Kg−1) every other day for 2 weeks. Vehicle treated nHif-1αΔ/Δ and untreated nHif-1αf/f mice served as controls (n=6). *= P <0.05, **= P <O.005., Student's t-tost (j, o, p) and one-way ANOVA (q). Error bars (j, o–q) represent S.E.M..
To further examine the potential link between HIF-1α and Wnt/β-catenin signalling in vivo, we engineered a BAT-GAL reporter line in the background of conditional global HTF-1α mutants (gHif-1αΔ/Δ, BAT-GALTg). Because global Hif-1α deletion results in embryonic lethality19, we crossed mice homozygous for the loxP-flanked (floxcd) Hif-1α allelc (Hif-1αf/f) with Hif-1αf/f mice expressing tamoxifen inducible Cre driven by a Ubiquitin C (UbC) promoter, such that tamoxifen administration will result in global postnatal Hif-1α (gHtf-1αΔ/Δ) deletion (Supplementary Information, Fig. S6a, b). Hif-1α deletion reduced hippocampal Wnt signalling, based on loss of BAT-GAL reporter activity in mice expressing the BAT-GAL transgene (n-3) (Fig. 4h, i and Supplementary Information, Fig. S6c, d). Hif-1α deletion also decreased expression of Wnt target genes such as Dkk-4 (undetected), Lef-1 and Axin2 (2.5 fold) (n=5) in gHif-1αΔ/Δ animals (Fig. 4j).
Because Wnt/β-catenin signalling has been identified as a positive regulator of neurogenesis occurring in the SGZ of adult hippocampus7, we employed the SGZ as a model stem cell niche to evaluate the role of HIF-1α in stem cell functions. Based on the SGZ HIF-1α-Wnt /β-catenin connection, we hypothesized that loss of HIF-1α may result in aberrant neurogenesis in vivo. We analyzed the dentate gyrus of young adult (7–8 weeks) gHif-1αΔ/Δ or control mice lacking Cre (gHif-1αf/f) for cells expressing the early neuronal marker doublecortin (DCX). gHif-1αΔ/Δ dentate gyrus displayed a 3.4 fold reduction in DCX+ cells as compared to gHif-1αf/f control animals (n=7) (Fig. 4k, o). Moreover, DCX+ neurons in the gHif-1αΔ/Δ mice were severely diminished (4 fold) in their capacity to form neurites (n=1) (Fig. 4l, p), a phenotype critical for integration of the newborn neurons into the existing neuronal circuitry.
To distinguish between cell-intrinsic versus -extrinsic effects underlying HIF-1α's impact on hippocampal neurogenesis, we further analyzed adult neurogenesis in a neuron specific Hif-1α deletion model. To selectively delete HIF-1α from murine neurons, we crossed Hif-1αf/f mice with R1ag#5 (R1) mice expressing Cre driven by the calcium/catmodulin-dependent Kinase (αCamKII) promoter20,21. Cre expression in the R1 animals results in postnatal neuronal Hif-1α(nHif-1αΔ/Δ) deletion in the forebrain21, including Sox2+ cells of the SGZ of the DG (Supplementary Information, Fig. S5r–t). Similar to global Hif-1α deletion, neuronal Hif-1α loss significantly reduced BrdU+ (which labels proliferating NSCs and progenitors) cell numbers (2.2 fold) and DCX+ newborn neurons (2 fold) in the SGZ of nHif-1αΔ/Δ mice as compared to nHif-1αf/f control animals (n=6) (Fig. 4m,n and q). That the DCX+ cells were derived from NSCs and AHPs was confirmed by double-immunostaining with BrdU and DCX (Supplementary Information, Fig. S6e). Moreover, similar to gHif-1αΔ/ΔDCX+ cells, the DCX+ cells of nHif-1αΔ/Δ mice were severely diminished in their capacity to form neurites (3 fold) (n=3) (Supplementary Information, Fig. S6f, g). Importantly, the neurogenesis defects were rescued pharmacologically in nHif-1αΔ/Δ mice treated with GSK-3 inhibitor SB 216763 (2mg Kg−1) as compared to vehicle-treated nHif-1αΔ/Δ mice (n=6) (Fig. 4q). We also made similar observations in nHif-1αΔ/Δ mice transduced with lentiviruses expressing a constitutively active form of β-catenin (Δ-GSK-β-catenin)22 (n = 3–5 per group) (Supplementary Information, Fig. S6h, i). These models cannot exclude a potential role for HIF-1α in regulating trophic signals provided by the postmitotic mature granule neurons of the DG in the survival and integration of NSCs into hippocampal circuitry. Nevertheless, HIF-1α clearly regulates adult neurogenesis by promoting NSC/progenitor cell proliferation and differentiation via Wnt/β-catenin signalling.
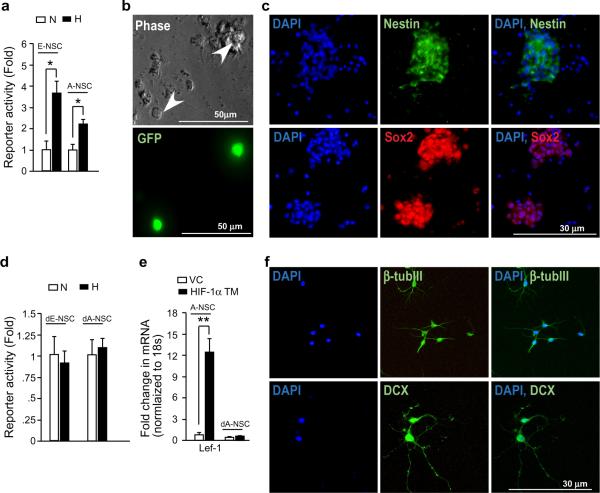
We also observed that the hypoxic regulation of Wnt/β-catenin activity exhibited by ES cells is shared by primary cells of the nervous system. Isolated embryonic and adult neural stem cells (E-NSC and A-NSC) transfected with TOP-Flash plasmid displayed significantly increased reporter activity under hypoxia (Fig. 5a, b and c), and this effect was lost in differentiated neurospheres (Fig. 5d and f). Of note, transient transfection with a normoxia-active HIF-1α triple mutant (HTF-1α TM[P402A/P577A/N815A]) plasmid23 increased Lef-1 levels ~ 12 fold exclusively in the undifferentiated neurospheres (Fig. 5e). Moreover, differentiated neursopheres exhibited significantly reduced baseline Lef-1 levels as compared to undifferentiated control neurospheres (Fig. 5e), reminiscent of reduced basal Lef-1 expression observed in ES cell-derived neurons (Fig. 3c). We conclude that the regulation of Wnt/β-catenin by HIF-1α through Lef-1 induction represents an important mechanism of stem cell homeostasis, and may be applicable to stem cell microcnvironments beyond the hippocampal neurogenic niche.
Figure 5. Neuronal Hif-1α regulates neurogenesis through Wnt/β-catenin signalling.
a, E14 NSCs (E-NSC) or adult NSCs (A-NSC) isolated from 4–6 week old hippocampus were cultured as neurospheres, and employed for TOP-Flash assay under normoxia or hypoxia (n=6). b, Transfection efficiency for NSC TOP-Flash assays was monitored with GFP in 2-day post disassociation neurospheres (white arrows in phase). c, Neurospheres were assayed for progenitor markers including Nestin (a upper panel) and Sox2 (a lower panel). d, TOP-Flash assay in differentiated E14 embryonic (dE-NSC), or adult NSCs (dA-NSC) cultured under normoxia or hypoxia (n=6). e, qRT-PCR analysis of Lef-1 levels in A-NSCs or differentiated A-NSCs (dA-NSC) transfected with HIF-1α TM plasmid or corresponding vector control (VC) (n=3). f, Differentiation of neursopheres was assessed by the expression of differentiation markers including β-tublll (f upper panel) and DCX (f lower panel). *= P <0.05, **= P <0.005., Student's t-test (a, d–e). Error bars (a, d–e) represent S.D..
Numerous adult stem cells have been found closely associated with the vasculature24–27. Interestingly, we now demonstrate that low O2 levels may also contribute to stem cell microenvironments. This suggests that whereas some adult stem cells occupy well-oxygenated perivascular domains, others reside within hypoxic niches and are regulated by O2 deprivation. The induction of Wnt/β-catenin signalling by HIF-1α represents a molecular mechanism underlying this regulation. These studies extend the positive effects of HIFs previously shown for other stem cell pathways, including Oct-4 and Notch-128,29. The opposite result was reported for HCT116 colon cancer cells, in which HIF-1α inhibited Wnt/β-catcnin activity13, suggesting that the interaction between HIF-1α and Wnt/β-catenin in undifferentiated stem/progenitor cells and more differentiated cell types (including neoplastic cells) is functionally distinct. In summary, the present findings confirm the emerging concept that HIFs not only act in metabolic adaptations, but can also regulate critical stem cell phenotypes.
METHODS
Cell culture and treatments
Arnt−/−, ArntRes and corresponding WT (Arnt+/+) ES cells have been described previously30, 31. ES cells were cultured as described4. P19 EC cells (ATCC) were cultured as per manufacturer's protocol. Hypoxia (0.5%, 1.5% and 3% O2) was achieved using a HERAcell 240 hypoxic workstation with O2 control. Deferoxamine (DFX) (Calbiochem) was used as a hypoxia mimetic at a final concentration of 200 μM for 16 h. Wnt-3a conditioned medium (Wnt-3a CM) and corresponding control medium were harvested from L Wnt-3A and L Cells respectively (ATCC). LiCl and BIO were purchased (Sigma) and used in final concentration as follows; LiCl (20 mM) and BIO (200 nM).
Reporter assay
Transient transfections of cells with TOP-Flash or FOP-Flash plasmids were performed with Lipofectamine 2000 (Invitrogen) in 24-well plates. Luciferase assays were performed using the dual luciferase protocol (Promega) with the following modification for siRNA: Cells were first transfected with siRNA against β-catenin (ON-TARGET plus SMART pool L-040628-00-0005, Dharmacon), and after 24 h was followed by a second co-transfection with firefly (200 ng) and Renilla luciferase (20 ng) reporter gene expression plasmids. β-catenin knockdown was verified 48–72 h post transfection by standard western blot procedure.
Apoptosis and cell proliferation
For apoptosis studies, undifferentiated ES monolayers were grown on gelatin coated coverslips, and cultured under hypoxia for indicated time period. TUNEL+ cells were visualized using an ApopTag kit (Millipore). TUNEL+ cells were enumerated as percentage of total cells in the field detected by DAPI nuclear staining. 3 randomly chosen fields per plate (n=3) were analyzed and averaged per condition. For proliferation analysis, 104 cells were seeded on 6 cm2 plates, and two plates were counted per time point in a hemocytometer over 6 days. Recombinant mouse DKK-1 (R&D systems) was used at a final concentration of 300 ng ml−1. For cell cycle analysis, ES monolayers were cultured either under normoxia or hypoxia, briefly pulsed with 5′-bromo-2′ 3′-deoxyuridine (BrdU; 10 μm for 20 min), stained with propidium iodide (PI) and analyzed by flow cytometry.
qRT-PCR analyses
Total RNA (2 μg of each sample isolated using RNeasy [Qiagen]) was reverse transcribed using High Capacity cDNA Reverse Transcription kit (ABI) and assayed for gene expression by SYBR-GREEN technology (ABI). The following primer sequences were used: Lef-IF: 5′ TCCTGAAATCCCCACCTTCT 3′, Lef-IR: 5′ TGGGATAAACAGGCTGACCT 3′; Tcf-1/7F: 5′ CAGCTCCCCCATACTGTGAG 3′, Tcf-1/7R: 5′TGCTGTCTATATCCGCAGGAA 3′; Dkk-4F: 5′ ACGAAGAAATCACAAAGCAGTAAG 3′, Dkk-4R: 5′ AAAAATGGCGAGCACAGC 3′; Axin2F: 5′ GAGAGTGAGCGGCAGAGC 3′, Axin2R: 5′ CGGCTGACTCGTTCTCCT 3′; Fzd7F: 5′ GGGTATCTCTGTGTAGCCCTGA 3′, Fzd7R: 5′ AGAGGCAGGTGGATGTCTGT 3′, Pgk1F: 5′ TACCTGCTGGCTGGATGG 3′, Pgk1R: 5′ CACAGCCTCGGCATATTTCT 3′; HIF-1α KOF: 5′ GCACTAGACAAAGTTCACCTGAGA 3′, HIF-1α KOR: 5′ CGCTATCCACATCAAAGCAA 3′. ChIP primers used were as follows: Lef-1 P1F: 5′ TTCCCAGCGCTCATCATCA 3′, Lef-1 P1R: 5′ CCTTTCGCTTCGGTTTTCCT 3′; Lef-1 P2F: 5′ AAAACAAAACCCCAAATCACC 3′, Lef-1 P2R: 5′ TCACCGTGCAAAACCTCTC 3′; Lef-1 P3F: 5′ CGGCGTAGACGCTCTCAG 3′, Lef-1 P3R: 5′ CGCTTTCCCACTTAGAAGGAC 3′; Tcf-1 P1F: 5′ ACACCGAAACGTTCTTGAGGC 3′, Tcf-1 P1R: 5′ TCACCACGACCGATCACTGTT 3′, Tcf-1 P2F: 5′ GGATGCAACTTCCCAGACTGAG 3′, Tcf-1 P2R: 5′ GCTTAGAACCTGCTGTCCAGGA 3′
Immunoprecipitation assay
For Chromatin immunoprecipitation (ChIP) experiments, the sonicated nuclear extracts of hypoxic cells were immunoprecipitated with anti-HIF-1α monoclonal antibody (Novus Biologicals), reverse cross-linked and analyzed by qRT-PCR. For immunoprecipitation experiments, 500 μg of hypoxic ES nuclear protein extracts were cleared with either 10 μl of anti-HTF-1α polyclonal antibody (Novus Biologicals) pre-coupled to protein-G-sepharose (Roche) or 5–10 μl of anti-LEF-1 polyclonal antibody (Cell Signaling) pre-coupled to prolein-A sepharose (Roche).
Generation and Analysis of Mice
Generation and analysis of Hif-1αf/f 19, CamKIIalpha-Cre20, and Ubc-Cre-ERT2 32 has been described previously. BAT-GAL reporter mice were obtained from the Jackson laboratory and genotyped as previously described17. To generate global Hif-1αΔ/Δ knockout mice (gHif-1αΔ/Δ) Hif-1αf/f mice were crossed with Hif-1αf/f mice expressing Ubc-Cre-ERT2 and offsprings were screened for the presence of 1lox P using the genotyping primers P1: 5′ GCAGTTAAGAGCACTAGTTG 3′, P2: 5′ GGAGCTATCTCTCTAGACC and P3: TTGGGGATGAAAACATCTGC 3′. Cre mediated recombination between the loxP sites in the 2loxP allele produces the 1loxP allele, which lacks exon 2 and results in a mutant mRNA transcript containing multiple in frame stop codon downstream of exon 1 sequences. Tamoxifen free base (MP Biomedicals) was administered to nursing mothers at a concentration of 200 mg Kg−1 as previously described33 to induce recombination. Neuronal deletion of Hif-1αf/f (nHif-1αΔ/Δ) was achieved by crossing mice carrying Hif-1αf/f alleles with Hif-1αf/f mice expressing Cre recombinase under the control of the calcium/calmodulin-dependent kinase Cam KIIalpha promoter (CamKII-Cre) as described previously21. All mice were maintained in microisolator cages and treated in accordance with NIH and American Association of Laboratory Animal Care Standards, and consistent with the animal care and use regulations of the University of Pennsylvania, Philadelphia.
LacZ and Hypoxyprobe Detection
Dissected embryos, or sections from adult organs were washed in PBS, fixed for 30 minutes in 4% paraformaldehyde, and incubated in the 5-bromo-4-chloro-3-indolyl-β-d-galactosidase (X-Gal) staining solution for 30 minutes −16 h. Hypoxic regions in developing mouse embryos and adults were detected using pimonidazole hydrochloride detection kit (Chemicon), per manufacturer's protocol. Briefly, pimonidazole in water was administered intraperitoneally at a dosage of 60 mg Kg−1. To minimize Hypoxyprobe background, a washout period of 1 h was allowed. Animals were sacrificed and brains were harvested following cardiac perfusion.
Antibodies for Immunoblotting, Imnunofluorescence and Quantitation
Primary antibodies were obtained as mentioned: Lef-1, Tcf-1, HIF-1α, ARNT, CREB, actin, GSK-3β and pGSK-3β (Cell signal), β-tubulin III and Doublecortin (Abeam), BrdU (USBiologicals), HIF-1 α (Cayman for IHC), β-catenin (BD Transduction Laboratories), Cre recombinase monoclonal (Covance), Sox2 (Chemicon and R&D Systems) and β-galactosidase polyclonal (Fitzgerald).
To examine the effects of Hif-1 α deletion, young adult (7–8 weeks) neuronal or global Hif-1 αΔ/Δ or control mice lacking Cre (Hif-1 αf/f) were injected wilh BrdU (5-Bromo-2′-deoxyuridine; 100 mg Kg−1 for four consecutive days) (Roche) to label proliferating AHPs and their progeny. Mice were perfused after the final BrdU injection. For rescue analysis of neuronal defects, animals were injected with either GSK3 inhibitor SB216763 (2mg Kg−1), or vehicle (DMSO) every alternate day for 7 doses as previously described34. For tissue staining, organs were harvested from perfused experimental animals and embedded in Tissue Tek Optimal Cutting Temperature (OCT) media (Fisher), then frozen to −80 °C for storage. 14 μm frozen sections were cut using a Microm HM 550 Cryostat. Sections were fixed with 5% buffered formalin for 2 minutes, washed three times in PBS and incubated in prolein blocking agent for thirty minutes at room temperature. Primary antibody cocktails of BrdU and DCX diluted in 2% BSA in PBS, or per manufacturer's protocol were then added on the tissue sections and incubated at 4 °C overnight. Slides were washed three times in PBS and then incubated in secondary antibody cocktail in 3% BSA in PBS for one hour. Slides were mounted in Vectashield mounting medium (Vector Laboratories) and visualized on a Leica Leitz 500 microscope. Images were acquired and processed using Photoshop CS3 (Adobe).
For quantitation of BrdU+, DCX+ and β-galactosidase+ cells, and for the enumeration of laminin positive vessels, 5–7 tissue sections offering an anterior to posterior coverage of the DG from each animal were stained. Positive cells were counted per field (at 10 × magnifications) and correlated per mice. The results were pooled to generate average values. For neurite quantification DCX+ cells with processes longer than 20 μm extensions were counted as positive.
Neural stem cell Isolation, Culture, Differentiation and Transfection
Mouse Ventral Mesencephalon E14 neurospheres were purchased from STEMCELL Technologies. Isolated neural stem cells and embryonic neurospheres (STEMCELL Technologies) were cultured using STEMCELL Technologies' kit (05715). Briefly, 5–6 hippocampus dissected from 4–6 week young adult mice were collected in a dish containing neurocult tissue collection solution. The tissue was chopped into small pieces using a razor, subjected to brief enzymatic disassociation in Neurocult disassociation solution at 37 °C, mechanically disassociated by gentle pipetting, and resuspended in Neurocult resuspension solution. Primary cells were seeded at a density of 1 × 105 cells in T-25cm2 flasks, and growth of neursopheres monitored under the microscope. For transfection, 1:3 diluted culture of 3–4 day old neurospheres, generated from a seeding density of 2 × 106 cells 10 ml−1 were plated into 24 or 6-well dish (500 μl-3ml), and transfected with 500 ng-2 μg plasmid using Lipofectamine 2000 (Invitrogen), and samples treated formRNA or reporter activity analysis 48 h post-transfection. For differentiation assay, neurospheres were seeded on Poly-ornithine coated glass coverslips and cultured in Neurocult Differentiation media (STEMCELL Technologies). For immunostaining of neurospheres, the floating spheres were first attached to glass coverslips using Cell-Tak cell tissue adhesive (BD) and then processed similar to adherent cells.
Generation of ES-lef-1 cells
Lef-1 cDNA (Open Biosystems: MMM1013-9334706) was subcloned into pLKO.1 neo (Addgene: Plasmid 13425, PI: Shiela Stewart) with Age-1 and Eco-R1. LEF-1 overexpressing lentivirus was prepared in HEK293T cells using the pMDLg/pRRE, pRSV-Rev and Envelope system. ES cells were transduced with virus supernatant and selected with 0.4 mg ml−1 Geneticin. ES cells transduced with empty pLKO.1 neo virus (VC) served as control.
Generation of SA-β-catenin lentivirus and stereotactic injection
The control vector was pHIV-IRES-Zsgreen (Addgene: Plasmid 18983, PI: Zena Warb, Bryan Welm). ΔGSK β-catenin insert has been described before22. The insert was subcloned into pHIV-IRES-Zsgreen. 1 μ1 of high titer lentivirus (~2×109 transducing units ml−1) was injected into the DG of 10–12 week old mice bilaterally (AP −2, ML ±1.5, DV −1.8 from Bregma, n=3–5 per group) as previously described34. Viral spread was assessed by visualizing the presence of Zsgreen positive cells along the anterior–posterior axis of the DG (about 1.2–1.6 mm). Following 2 weeks of recovery, mice were injected with BrdU (100 mg Kg−1) i.p daily for 4 days. The mice were then perfused with 4% paraformaldehyde and the hippocampal sections processed for BrdU and DCX staining.
Statistical analyses
Statistical significance was computed using Student's t test or one-way ANOVA, and significance was set at P <0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank K. Kinzler and B. Vogelstein for the Wnt (TOP/FOP) reporter constructs, P. Carmeliet for Hif-1α−/− ES cells, and E. Brown for Ubc-Cre-ERT2 mice. We thank X. Sun, Case Western Reserve University, and H. Yu, University of Pennsylvania for technical assistance with tissue harvesting and sectioning. We thank B. Keith and the Simon lab for discussion and critical review of the manuscript. This work was supported by funds from the Howard Hughes Medical Institute, the Abramson Family Cancer Research Institute, and the National Institutes of Health (Grant No. MH58324 to P.S.K). M.C.S is an investigator of the HHMI.
Footnotes
AUTHOR CONTRIBUTIONS: J.M. and W.T.O. designed, performed and analyzed the experiments, P.S.K and M.C.S assisted in the interpretation of results, J.C.C and J.C.L. provided the αCamKII-Cre- Hif-1αf/f mice, R.S.J. provided the Hif-1αf/f mice, J.M. made the figures and wrote the paper, M.C.S. edited the paper.
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS The authors declare that they have no competing financial interests.
REFERENCES
- 1.Studer L, et al. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20(19):7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14(24):3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genbacev O, Zhou Y, Ludlow JW, Fisher SJ. Regulation of human placental development by oxygen tension. Science. 1997;277(5332):1669–1672. doi: 10.1126/science.277.5332.1669. [DOI] [PubMed] [Google Scholar]
- 4.Adelman DM, Maltepe E, Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13(19):2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112(1):126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SJ, et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20(19):7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 8.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434(7035):843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 9.van de Wetering M, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88(6):789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 10.Hu CJ, et al. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol CellBiol. 2006;26(9):3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naito AT, et al. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97(2):144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- 12.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 13.Kaidi A, Williams AC, Paraskeva C. Interaction between beta-catenin and HIF-1 promotes cellular adaptation to hypoxia. Nat Cell Biol. 2007;9(2):210–217. doi: 10.1038/ncb1534. [DOI] [PubMed] [Google Scholar]
- 14.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21(2):183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 15.Huber O, et al. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59(1):3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86(3):391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 17.Maretto S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100(6):3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raleigh JA, et al. Hypoxia and vascular endothelial growth factor expression in human squamous cell carcinomas using pimonidazole as a hypoxia marker. Cancer Res. 1998;58(17):3765–3768. [PubMed] [Google Scholar]
- 19.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. Embo J. 1998;17(11):3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dragatsis I, Zeitlin S. CaMKIIalpha-Cre transgene expression and recombination patterns in the mouse brain. Genesis. 2000;26(2):133–135. doi: 10.1002/(sici)1526-968x(200002)26:2<133::aid-gene10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Baranova O, et al. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27(23):6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barth AI, Stewart DB, Nelson WJ. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant beta-catenin. Proc Natl Acad Sci U S A. 1999;96(9):4947–4952. doi: 10.1073/pnas.96.9.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu CJ, Sataur A, Wang L, Chen H, Simon MC. The N-terminal transactivation domain confers target gene specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha. Mol Biol Cell. 2007;18(11):4528–4542. doi: 10.1091/mbc.E06-05-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317(5845):1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 26.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3(3):279-–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen Q, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3(3):289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Covello KL, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20(5):557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson MV, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9(5):617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386(6623):403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 31.Maltepe E, Keith B, Arsham AM, Brorson JR, Simon MC. The role of ARNT2 in tumor angiogenesis and the neural response to hypoxia. Biochem Biophys Res Commun. 2000;273(1):231–238. doi: 10.1006/bbrc.2000.2928. [DOI] [PubMed] [Google Scholar]
- 32.Ruzankina Y, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gruber M, et al. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci U S A. 2007;104(7):2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Y, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.