Abstract
The switch of tumor cells from an epithelial to a mesenchymal-like phenotype (designated as epithelial-to-mesenchymal transition, EMT) is known to induce tumor cell motility and invasiveness, therefore promoting metastasis of solid carcinomas. While multiple studies have focused on elucidating the signaling events that initiate this phenotypic switch, there has been so far no characterization of the pattern of soluble mediators released by tumor cells undergoing EMT, and the potential impact that this phenotypic switch could have on the remodeling of the tumor microenvironment. Here we demonstrate that induction of EMT in human carcinoma cells via overexpression of the transcription factor Brachyury is associated with enhanced secretion of multiple cytokines, chemokines, and angiogenic factors and, in particular, with the induction of the IL-8/IL-8R axis. Our results also indicate the essential role of IL-8 signaling for the acquisition and/or maintenance of the mesenchymal and invasive features of Brachyury-overexpressing tumor cells, and demonstrate that IL-8 secreted by tumor cells undergoing EMT could potentiate tumor progression by inducing adjacent epithelial tumor cells into EMT. Altogether, our results emphasize the potential role of EMT in the modulation of the tumor microenvironment via secretion of multiple soluble mediators and suggest that IL-8 signaling blockade may provide a means of targeting mesenchymal-like, invasive tumor cells.
Keywords: metastasis, Brachyury, EMT, IL-8, tumor microenvironment
Introduction
Multiple cytokines, chemokines, and growth factors play a critical role as mediators of paracrine signals between the tumor and various components of the tumor microenvironment, which will ultimately lead to tumor growth, survival, and progression (1-3). These soluble mediators could be secreted either by the tumor cells themselves or by any of the various cellular components of the tumor microenvironment, including cancer-associated fibroblasts (CAFs), immune cells, and endothelial cells, among others (4, 5). In particular, tumor-derived soluble factors have been shown to function in a paracrine fashion to reprogram the normal stroma to a tumorigenic stroma (6), as well as to work in an autocrine way promoting tumor growth, survival, and acquisition of metastatic potential (7). Interleukin 8 (IL-8, CXCL8), a proinflammatory CXC chemokine, constitutes an example of a soluble mediator released by tumor cells that simultaneously functions in an autocrine and paracrine mode within the tumor microenvironment. In melanoma, tumor-derived IL-8 has been demonstrated to promote tumor cell proliferation, survival, and migration via its autocrine activity, while inducing an angiogenic response in endothelial cells and the recruitment of neutrophils to the tumor site via its paracrine activity (8, 9).
In recent years, the importance of an epithelial-mesenchymal phenotypic switch of tumor cells (EMT) has been demonstrated during the progression of carcinomas (10, 11). The role of multiple growth factors, cytokines, and components of the extracellular matrix (ECM) in inducing EMT in epithelial tumor cells has been well documented and, for example, transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), and tumor necrosis factor-alpha (TNF-α) have all been implicated in the induction of EMT (10, 12-14). To date, however, there has been no comprehensive analysis of the pattern of soluble factors released by tumor cells undergoing a switch from an epithelial to a mesenchymal-like phenotype along the course of carcinoma progression. The characterization of the “secretory phenotype” of tumor cells undergoing EMT might help to understand the potential impact that this phenotypic switch could have on the remodeling of the tumor microenvironment, therefore favoring tumor progression.
In a previous study, we have demonstrated the role of the T-box transcription factor Brachyury in the induction of EMT and metastatic potential in various human cancer cell lines (15). We have also demonstrated that Brachyury mRNA expression is predominant among high stage lung tumor tissues, with lower expression among lung tumors of stage I (15). Here we have investigated whether Brachyury-induced EMT drives the secretion of a distinctive set of soluble factors by the tumor cells, and the potential role that these secreted mediators could play in the context of tumor progression. Our results have demonstrated that overexpression of Brachyury in epithelial tumor cells is associated with enhanced secretion of multiple cytokines, chemokines, and angiogenic factors. In particular, we have shown that the phenotypic switch of epithelial tumor cells in response to Brachyury is accompanied by the induction of the IL-8/IL-8R axis which, in turn, appears to be essential for the acquisition and/or maintenance of the mesenchymal and invasive features of Brachyury-overexpressing tumor cells. Moreover, our results also demonstrate that Brachyury-induced tumor secreted factors promote EMT in epithelial tumor cells, an effect that is, at least in part, mediated by IL-8.
Altogether, our results emphasize the potential role of EMT in the modulation of the tumor microenvironment via secretion of multiple soluble mediators and suggest that IL-8 signaling blockade may provide a means of targeting mesenchymal-like, invasive tumor cells.
Materials and Methods
Cell Culture
The human carcinoma cell lines used in this study, luminal breast MCF7, T47D, and BT474; basal breast MDA-MB-231 and MDA-MB-436; pancreatic PANC-1, and the lung cancer cell line H460 were obtained from the American Type Culture Collection (ATCC, Gaithersburg, MD) and propagated in RPMI 1640 medium with 2mM glutamine, 1X solution of penicillin/streptomycin (Mediatech, Inc., Herndon, VA) and 10% fetal bovine serum (Gibco, Invitrogen, Carlsbad, CA). MCF7 and PANC-1 cells were stably transfected with a control pcDNA4/TO or a vector encoding for the full length human Brachyury protein (pBrachyury); the lung H460 and the breast MDA-MB-436 tumor cells were stably transfected with a control or a shRNA specific for Brachyury, as previously described (15).
Cytokine and chemokine array
The level of secreted cytokines and chemokines was analyzed in the supernatants of MCF7 and PANC-1 tumor cell pairs by using a protein cytokine array for 79 human cytokines (RayBio Human G Series Array V, RayBiotech, Inc., Norcross GA). Cells were plated in serum free RPMI 1640 medium (Mediatech, Inc.) for 24 hours; supernatants were collected and analyzed following the manufacturer's recommendations. Cytokine and chemokine levels in each membrane were normalized by comparison to the intensity of positive control spots.
Treatment of tumor cells with culture supernatants or IL-8
Culture supernatants (designated here as conditioned media, CM) from MCF7-pcDNA and MCF7-pBrachyury cultures were collected as described above and subsequently used for the treatment of adherent tumor cells for 72 hours. For IL-8 receptor blocking studies, antibodies specific for the IL-8RA (ab8041, Abcam, Cambridge, MA) IL-8RB (ab10401, Abcam) or control IgG (AbD Serotec, Raleigh, NC) were added to the cultures at a final concentration of 1μg/ml, and incubated for 72 hours. For treatment with IL-8, adherent tumor cells were cultured in the presence of recombinant IL-8 (Peprotech, Rocky Hill, NJ) at the indicated concentrations for 72 hours in serum-free medium.
RNA interference
ON-TARGET plus SMART pool siRNA for Brachyury and a non-targeting control siRNA (Dharmacon, Chicago, IL) were used following the manufacturer's instructions. Briefly, tumor cells were incubated with 100 nM of each siRNA prepared in DharmaFECT reagent in antibiotic-free RPMI 1640 medium for 48 hours.
Real Time PCR
Real time PCR was conducted as previously described (15) using 10 ng of cDNA and the following TaqMan gene expression assays (Applied Biosystems, Foster City, CA): Brachyury (Hs00610080), Snail (Hs00195591), Slug (Hs00161904), Fibronectin (Hs00415006), human IL-8 (Hs01567913), human IL-8RA (Hs00174146), human IL-8RB (Hs01011557), and GAPDH (4326317E). Mean Ct values for target genes were normalized to mean Ct values for the endogenous control GAPDH [-ΔCt=Ct(GAPDH)-Ct(target gene)]. The ratio of mRNA expression of target gene vs. GAPDH was defined as 2(-ΔCt).
IL-8 ELISA
IL-8 in 100 μl of serum-free supernatants from tumor cell pairs was measured using a human IL-8 ELISA Kit (RayBiotech, Inc.), as directed by the manufacturer.
Promoter assay
Tumor cells were transfected with 50 ng of a Brachyury promoter- or a control promoter-luciferase vector (SwitchGear Genomics, Menlo Park, CA) using Fugene-6 (Roche, San Francisco, CA), and subsequently treated with recombinant IL-8 in triplicate wells. Forty-eight hours later, tumor cells were incubated with 100 μl of ONE-Glo Luciferase substrate (Promega, Madison, WI) and Luciferase activity was measured using a 1450 Betaplate reader (Perkin-Elmer, Waltham, MA).
Immunofluorescence
Immunofluorescent analysis of tumor cells cultured on glass cover slips was performed as previously described (15). For inhibition of IL-8 signaling, cells were cultured for 72 hours in medium containing 1μg/ml of blocking antibodies specific for the IL-8 receptors or a neutralizing IL-8 antibody (clone 6217, R&D Systems, Minneapolis, MN).
Migration and invasion assays
Invasion assay was performed as previously described (15). For IL-8 receptor blocking experiments, cells were incubated with 1μg/ml of anti-IL-8RA, anti-IL-8RB or control IgG for 1 hour at 37°C. Antibody was washed off and tumor cells were resuspended in serum free RPMI-1640 medium and subsequently analyzed for invasiveness. The assay duration for each tumor cell line was as follows: 48 hours for MCF7; 24 hours for MDA-MB-231 and MDA-MB-436. For IL-8 signaling blockade in the presence of culture supernatant (CM), cells were incubated for 72 hours with MCF7-pBrachyury CM in the presence of antibodies and subsequently analyzed for invasiveness.
Statistical methods
Data were analyzed using GraphPad Prism (version 4) (GraphPad Software, La Jolla, CA). Data points in graphs represent the mean ± SEM and p< 0.05 is considered significant.
Results
Secretory phenotype of epithelial tumor cells undergoing Brachyury-induced EMT
To investigate whether overexpression of Brachyury could modulate the pattern of soluble mediators secreted by epithelial tumor cells, we analyzed by using protein arrays the levels of multiple cytokines, chemokines, and growth factors released by the human breast MCF7 and pancreatic PANC-1 carcinoma cells that were transfected with an empty vector (pcDNA) versus a vector encoding for human Brachyury (pBrachyury) (Fig. 1A and B). Serum-free conditioned media (CM) was obtained from cell-normalized cultures of each cell line; as shown in Figure 1C and D, overexpression of Brachyury in both tumor cell lines resulted in enhanced secretion of multiple soluble factors. In particular, the levels of osteoprotegerin (OPG, TNFRSF11B), IL-8, Regulated upon Activation Normal T-cell Expressed and Secreted (RANTES, CCL5), growth-regulated oncogene (GRO), IL-6, and MIP-1δ (CCL15) were significantly increased in the supernatants from MCF7-pBrachyury versus control cells. In PANC-1 cells, overexpression of Brachyury resulted in enhanced secretion of IL-8, insulin-like growth factor binding proteins 1 and 2 (IGFBP-1, -2), OPG, granulocyte-macrophage colony stimulating factor (GM-CSF), and several angiogenic factors, including VEGF, Angiogenin, and placenta growth factor (PlGF).
Figure 1. Brachyury-induced tumor secreted factors.

Brachyury mRNA expression in MCF7 (A) and PANC-1 (B) cells transfected with a control vector (pcDNA) or a vector encoding for human Brachyury (pBrachyury). (C, D) Serum-free culture supernatants from both tumor cell pairs were analyzed by cytokine arrays. Shown in the graphs is the expression of soluble factors that demonstrated a significant change as a result of Brachyury overexpression.
Brachyury overexpression induces expression of IL-8 and its cognate receptors
As IL-8 is a chemokine with a proven role in tumor progression (16), here we have further evaluated the levels of secreted IL-8 protein by ELISA assay and IL-8 mRNA in various tumor pairs (low vs. high Brachyury). As shown in Figure 2A, a 4- and a 5-fold increase in the levels of secreted IL-8 were observed in the supernatants of Brachyury-overexpressing MCF7 and PANC-1 cells, respectively, compared to the control cells. Moreover, stable inhibition of Brachyury expression in the basal breast MDA-MB-436 and the lung H460 cancer cell lines that exhibit a mesenchymal phenotype and express higher levels of Brachyury resulted in a 8- and 2-fold inhibition of IL-8 secretion, respectively (Fig. 2A). These changes were also observed at the transcriptional level, as the expression of IL-8 mRNA was markedly enhanced in Brachyury-overexpressing MCF7 and PANC-1 cells, or significantly reduced in MDA-MB-436 and H460 carcinoma cells in response to Brachyury inhibition (Fig. 2B), thus reinforcing the positive correlation between Brachyury and IL-8 expression in human tumor cells.
Figure 2. Brachyury induces IL-8 and IL-8R expression in epithelial tumor cells.
(A) IL-8 protein levels in culture supernatants from various tumor cell pairs. Real-time RT-PCR analysis of expression of IL-8 (B) or IL8-R (C) in various tumor cell pairs. (D) Expression of IL-8RB protein in the MDA-MB-436 tumor cell pair.
The biological effects of IL-8 are mediated by two different receptors, designated as IL-8R-alpha (IL-8RA, CXCR1) and IL-8R-beta (IL-8RB, CXCR2) (17). In order to determine whether Brachyury may also have an impact on the expression of the IL-8 receptors, we used real time RT-PCR to evaluate IL-8RA and IL-8RB mRNA levels in the MCF7, PANC-1, and H460 tumor cell pairs (low vs. high Brachyury expression). A 3-fold increase in the expression of IL-8RA and no change in the levels of IL-8RB were observed in MCF7-pBrachyury cells, compared with the control cells (Fig. 2C). Unlike with the MCF7 cells, overexpression of Brachyury in PANC-1 cells induced the expression of IL-8RB by several fold (Fig 2C) and had no effect on the expression of IL-8RA, which remained undetectable in the PANC-1 cells regardless of the status of Brachyury expression. Similarly, the lung carcinoma line H460 showed expression of IL-8RB that was significantly decreased after Brachyury knockdown (Fig. 2C), while IL-8RA was undetectable regardless of the expression of Brachyury. MDA-MB-436 cells showed no expression of mRNA encoding for both receptors; however, western blot analysis revealed expression of IL-8RB protein that was significantly reduced in cells stably inhibited for the expression of Brachyury (Fig. 2D). Altogether, these results indicated that the Brachyury-induced phenotypic switch in epithelial tumor cells is accompanied by the induction of the IL-8/IL-8R axis. As the analysis of the promoter region of these genes showed no putative Brachyury binding sites, the upregulation of IL-8 and IL-8R expression might be indirect and not a consequence of direct transcriptional regulation by Brachyury.
Autocrine role of the IL-8/IL-8R axis: IL-8 signaling is essential for EMT
We next determined if the IL-8/IL-8R axis has any role in the acquisition of a metastatic phenotype as a result of Brachyury overexpression. To this end, we assessed the expression of epithelial E-cadherin and mesenchymal Fibronectin in MCF7-pBrachyury cells that were previously treated with a control IgG or a blocking antibody directed against the IL-8RA or IL-8RB. As shown in Figure 3A, blockade of IL-8 receptors did not affect E-cadherin expression but markedly reduced Fibronectin expression and the invasiveness of MCF7-pBrachyury cells. The breast cancer cell lines MDA-MB-231 and MDA-MB-436, classified as Basal B type, have been previously shown to exhibit mesenchymal and invasive characteristics (18, 19). Both cell lines were selected to evaluate the role of the IL-8/IL-8R axis on tumor cells that naturally exhibit a mesenchymal phenotype. Blockade of IL8 signaling resulted in a significant reduction of Fibronectin expression at the mRNA and protein levels (Fig. 3B), a reduction on the expression of Brachyury mRNA in both cell lines (Fig. 3C) and, more importantly, a marked reduction of the invasive capacity of both tumor cell lines (Fig. 3D). Therefore, a functional IL-8/IL-8R axis appears to be essential for the acquisition and/or maintenance of the mesenchymal and invasive features of breast cancer tumor cells.
Figure 3. Role of IL-8 signaling in EMT.

(A) Immunofluorescent analysis of E-cadherin and Fibronectin in MCF7-pcDNA cells versus MCF7-pBrachyury cells that were incubated with various antibodies. The green signal represents the staining of the corresponding protein and the blue signal represents the DAPI-stained nuclei. Right panel: tumor cells treated as above were analyzed for invasiveness. (B) Real time RT-PCR analysis and immunofluorescent analysis of Fibronectin expression in MDA-MB-231 and MDA-MB-436 cells treated with anti-IL-8RB or a neutralizing anti-IL-8 antibody. (C) Expression of Brachyury mRNA and (D) ECM invasion assay of MDA-MB-231 and MDA-MB-436 cells treated as indicated.
Brachyury-induced tumor-secreted factors promote EMT in breast cancer cells
Tumor cells undergoing EMT might constitute a small fraction of the total primary tumor mass. We have hypothesized that, via secretion of multiple soluble factors, a few EMT-induced tumor cells could have a profound impact on the phenotype of adjacent cells, including other epithelial tumor cells that did not undergo the phenotypic switch, as well as the various components of the tumor stroma. To test this hypothesis, we incubated parental MCF7 and T47D luminal breast cancer cells in the presence of conditioned media (CM) derived from MCF7-pBrachyury versus MCF7-pcDNA cells. Incubation of both tumor cells in the presence of MCF7-pBrachyury CM resulted in a significant decrease of epithelial E-cadherin and enhancement of the mesenchymal protein Fibronectin (Fig. 4A), changes that are characteristic of a switch from an epithelial to a mesenchymal-like phenotype (i.e., EMT). Interestingly, similar treatment of MDA-MB-231 cells that normally exhibit a mesenchymal phenotype further enhanced their expression of Fibronectin (Fig. 4A). Accordingly, the expression of mRNA encoding for the EMT transcriptional regulators Brachyury, Snail, and Slug was significantly enhanced in all three tumor cell lines treated with culture supernatants obtained from MCF7-pBrachyury versus MCF7-pcDNA cells (Fig. 4B).
Figure 4. Brachyury-induced tumor-secreted factors mediate EMT in human breast tumor cells.

(A) Immunofluorescent analysis of E-cadherin and Fibronectin in tumor cells that were incubated with conditioned media (CM) obtained from cultures of MCF7-pcDNA or MCF7-pBrachyury cells. (B) Real-time RT-PCR analysis of Brachyury, Slug, and Snail mRNA expression.
IL-8 induces a mesenchymal-like phenotype in breast cancer cells
To investigate whether IL-8 is critical for the EMT-inducing activity of culture supernatants from MCF7-pBrachyury cells, parental MCF7 and MDA-MB-231 cells were incubated with MCF7-pBrachyury CM in the presence of IL8-R blocking antibodies or control IgG, and subsequently tested for their invasive properties in vitro. Incubation in the presence of conditioned medium obtained from MCF7-pBrachyury vs. MCF7-pcDNA cultures significantly increased the ability of parental MCF7 and MDA-MB-231 cells to invade the extracellular matrix in vitro (Fig 5A). Furthermore, tumor invasiveness observed in both cell lines treated with MCF7-pBrachyury supernatants was significantly inhibited by IL-8 signaling blockade, thus suggesting that IL-8 is, at least in part, responsible for the EMT-promoting activity of Brachyury-induced secreted factors (Fig. 5B and 5C).
Figure 5. Role of the IL-8/IL-8R axis in the ECM invasion of tumor cells.
(A) In vitro ECM invasion assay for MCF7 and MDA-MB-231 cells incubated with conditioned media (CM) obtained from cultures of MCF7-pcDNA or MCF7-pBrachyury cells. (B) MCF7 and (C) MDA-MB-231 tumor cells were incubated with MCF7-pBrachyury CM in the presence of indicated antibodies for 72 hours, and subsequently analyzed for invasion of ECM in vitro.
We further evaluated the role of IL-8 in the induction of tumor EMT by directly exposing MCF7, T47D, and MDA-MB-231 cells to purified, recombinant human IL-8. As shown in Figure 6A, MCF7 and T47D breast cancer cells treated with IL-8 showed a significant reduction of E-cadherin expression, an increase of Fibronectin expression, and upregulation of Brachyury expression (Fig. 6B). MDA-MB-231 showed enhanced expression of Fibronectin (Fig. 6A) and simultaneous upregulation of Brachyury as a result of IL-8 treatment (Fig. 6B). To further evaluate the mechanism by which IL-8 might induce the expression of Brachyury, MCF7 cells were transiently transfected with a reporter vector encoding for the luciferase gene under the control of the Brachyury promoter versus a control promoter. Treatment of MCF7 cells with recombinant IL-8 resulted in approximately a 4-fold increase in luciferase activity, therefore indicating that IL-8 treatment of tumor cells results in the enhanced transcriptional activity of the Brachyury promoter in MCF7 cells (Fig. 6C).
Figure 6. IL-8 induces mesenchymal characteristics in tumor cells.
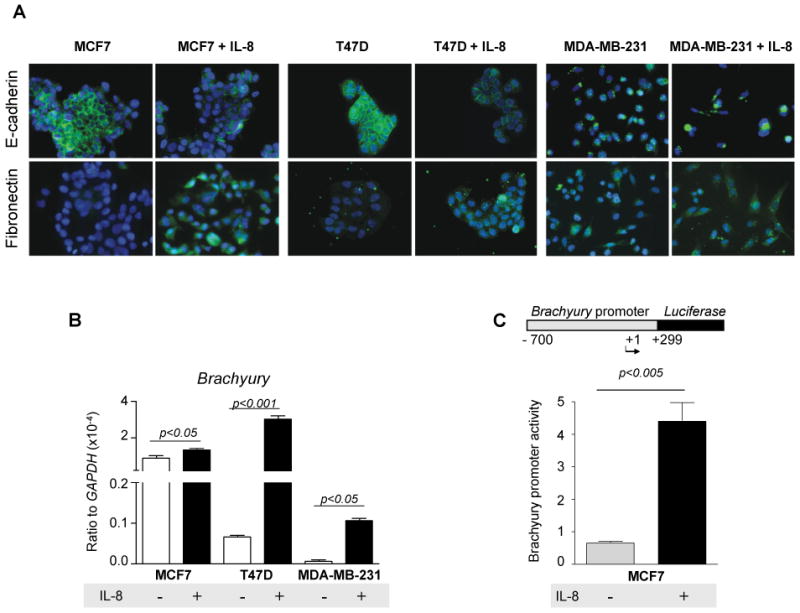
(A) Tumor cells were left untreated or incubated with recombinant IL-8 (10 ng/ml) for 72 hours and analyzed for expression of E-cadherin and Fibronectin by immunofluorescence. (B) Expression of Brachyury mRNA by real time RT-PCR. (C) Brachyury promoter reporter assay in MCF7 cells left untreated or treated with IL-8 as above. Shown is also a schematic of the Brachyury promoter fragment used in the assay.
Brachyury is essential for the induction of invasiveness and Fibronectin expression mediated by IL-8
Treatment of MCF7 cells with recombinant IL-8 significantly improved their ability to invade the extracellular matrix in vitro (Fig. 7A). IL-8 was also able to enhance the invasive abilities of the MDA-MB-231 and MDA-MB-436 tumor cells, which naturally exhibit higher invasive capacity than MCF7 cells (Fig. 7A). To investigate whether Brachyury upregulation in response to IL-8 treatment might be essential for the induction of tumor invasiveness, MDA-MB-436 cells were transiently transfected with a control siRNA or a siRNA specific for Brachyury. While tumor cells transfected with control siRNA demonstrated a marked induction of invasiveness in response to IL-8 treatment, cells inhibited for Brachyury expression were unable to invade in response to IL-8 (Fig. 7B). A similar approach was used with MCF7 and BT474 cells. Both tumor cell lines upregulated Fibronectin following IL-8 treatment when transfected with control siRNA but not with Brachyury siRNA (Supplemental Fig. 1). These results thus indicated that Brachyury upregulation is essential for the IL-8-mediated epithelial-mesenchymal switch of tumor cells, including the acquisition of mesenchymal Fibronectin and enhanced tumor invasiveness.
Figure 7. IL-8 induces invasiveness of breast cancer cells in a Brachyury-dependent manner.
(A) Tumor cells were left untreated or treated with recombinant IL-8 and analyzed for invasion of ECM in vitro. (B) Invasiveness of MDA-MB-436 cells in response to IL8. Tumor cells were transiently transfected with a control siRNA or siRNA specific for Brachyury.
Discussion
Multiple studies have indicated a critical role of the epithelial-mesenchymal phenotypic switch of tumor cells (EMT) during cancer progression by demonstrating a positive correlation between poor prognosis or advanced disease and the activation of an EMT program in various types of human carcinomas (15, 20-22). In recent years, it is becoming clear that inflammation plays a role in promoting tumor progression via the activity of soluble factors that mediate bidirectional, paracrine signals between tumor cells and the various components of the tumor stroma (23). To our knowledge, the secretory phenotype of tumor cells undergoing an epithelial-mesenchymal phenotypic switch and, therefore, the contribution of EMT to interactions between tumor cells and their microenvironment have not yet been characterized.
The T-box transcription factor Brachyury, a molecule highly expressed in a variety of human tumors with low or undetectable expression among most human normal tissues, has been shown to induce EMT in various human tumor cells (15, 24). Here we have further demonstrated that the transition of human tumor cells from an epithelial to a mesenchymal-like phenotype as a result of Brachyury overexpression is associated with the secretion of multiple cytokines, chemokines, and growth factors, including IL-8, IL-6, GRO, RANTES, MIP-1δ, GM-CSF, and the angiogenic factors VEGF, Angiogenin, and placenta growth factor (PlGF). Many of these factors have been previously associated with tumor progression in various types of carcinomas (25-28).
Tumor cells undergoing a switch from an epithelial to a mesenchymal-like phenotype may constitute only a small fraction of the total primary tumor mass. By secreting a variety of soluble factors, we hypothesized that a few mesenchymal-like tumor cells might markedly influence other cellular components of the tumor stroma as well as other tumor cells that remained in the epithelial state. In this study, we have been able to demonstrate that various breast cancer cell lines incubated with culture supernatants from MCF7-pBrachyury, but not MCF7-pcDNA control cells, were induced to undergo a phenotypic switch characterized by the overexpression of Fibronectin, induction of tumor cell invasiveness, and upregulation of mRNA encoding for the EMT transcriptional regulators Brachyury, Snail, and Slug. Therefore, in the context of a tumor, the soluble factors secreted by a few tumor cells undergoing Brachyury-mediated EMT could potentially function in a paracrine mode to induce adjacent epithelial tumor cells to undergo the phenotypic switch and to acquire metastatic potential.
In particular, expression of the chemokine IL-8 in cancer has been associated with tumor growth and survival, increased tumor cell migration and invasion, and increased neovascularization (29-31). Elevated IL-8 serum levels have been reported in patients with pancreatic, breast, and prostate cancer, and a positive correlation has been established between IL-8 serum levels, advanced disease, and diminished survival in breast cancer patients (32-36). The cellular origin of the elevated IL-8 levels, however, remains unclear. The positive association observed in our studies between Brachyury expression and the expression of IL-8 would suggest that tumor cells undergoing Brachyury-mediated EMT could be one potential source of IL-8 found in the tumor environment of progressive tumors. Experiments with blocking antibodies demonstrated that IL-8 is at least one of the factors responsible for the EMT-promoting properties of MCF7-pBrachyury-derived culture supernatants. Moreover, the EMT-promoting activity of IL-8 was also demonstrated here by directly incubating various breast cancer cell lines with recombinant IL-8, which resulted in upregulated expression of Brachyury, enhanced mesenchymal phenotype, and improved tumor invasiveness. Previous studies have demonstrated that IL-8 is a transcriptional target of Ras signaling and that Ras-induced IL-8 expression is critical for the initiation of tumor-associated inflammation and neovascularization during Ras oncogene-dependent tumor progression (37). During the development of vertebrate mesoderm, Ras signaling plays an essential role in the fibroblast growth factor (FGF)-induced Brachyury expression (38). A preliminary analysis of several human lung carcinoma cell lines showed no obvious correlation between the levels of Brachyury mRNA and the mutational status of Ras. However, given the relevant role of Ras signaling during the embryonic induction of Brachyury, further experiments aimed at elucidating a potential link between Ras activation and Brachyury expression in epithelial tumor cells are planned.
In addition to the induction of IL-8 expression and secretion, our studies also demonstrated that the levels of IL-8R are enhanced in tumor cells undergoing EMT in response to Brachyury. This observation prompted us to determine whether Brachyury-induced IL-8 and IL-8 receptors could also play a role in the acquisition and/or maintenance of a mesenchymal-like phenotype in tumor cells. Antibody blocking experiments indicated that the IL-8/IL-8R axis, functioning in autocrine positive loop, is critical to promote the acquisition and/or maintenance of the metastatic phenotype in various breast tumor cell lines. Additionally, reinforcing the idea of a positive feedback loop between Brachyury and IL-8 is our observation that Brachyury upregulation in response to IL-8 treatment of tumor cells is a necessary step for the induction of invasiveness as well as Fibronectin expression, a typical marker of mesenchymal phenotype.
The induction of EMT in human mammary epithelial cells has been shown to enrich a population of cells with stem cell-like properties, characterized by a CD44high/CD24low phenotype (39, 40). This association between the acquisition of a mesenchymal-like phenotype and tumor stemness has also been recently proposed by the demonstration that residual breast tumor cell populations surviving post-conventional treatments are enriched in CD44+/CD24−/low cells that also exhibit mesenchymal features (41). In recent reports, it has been demonstrated that the IL-8/IL-8RA axis plays a critical role in cancer stem cell function; the expression of IL-8RA has been shown to be elevated in populations of breast cancer stem cells (CSCs), characterized by a high activity of aldehyde dehydrogenase (ALDH) (42). Moreover, the addition of IL-8 to breast cancer cell lines has been shown to enhance the percentage of ALDH positive cells, as well as to promote the invasion and chemotaxis of CSCs (42). The concepts emerging from these studies are brought together by the results from our studies, as the induction of Brachyury expression in epithelial tumor cells not only promotes EMT, which has been associated with tumor stemness, but also induces the IL-8/IL-8R axis, which, in turn, has been shown to promote the tumor initiating phenotype.
The data in this study further strengthen the role of the epithelial-mesenchymal phenotypic switch of tumor cells along the course of tumor progression by demonstrating that tumor cells undergoing this phenotypic switch are also endowed with the capacity to secrete a milieu of cytokines, chemokines, and growth factors that could potentiate metastatic dissemination by the modulation of the stroma and/or tumor compartments. The development of strategies aimed at interfering with cytokine and growth factor regulatory loops appears then as a rational approach for improving therapeutic efficacy against tumor progression. In particular, from our results, blockade of the IL-8/IL-8R axis seems an attractive strategy that could result in the reversion of the metastatic phenotype of Brachyury-positive tumor cells by interfering with the autocrine positive loop between Brachyury and IL-8 signaling. Additionally, blockade of the IL-8/IL-8R axis could also lessen the paracrine signals that Brachyury-positive tumor cells could exert on other epithelial, non-metastatic tumor cells, as well as on the various cellular components of the stroma.
Neutralizing antibodies to IL-8RA and IL-8RB, a humanized antibody against IL-8, and a small-molecule IL-8R inhibitor (Repertaxin) have all been used in several preclinical studies demonstrating their ability to inhibit angiogenesis, tumor growth and metastasis of various xenograft tumor models (37, 43, 44). The results here provide a further mechanism by which agents that target IL-8 signaling in solid tumors might favor clinical outcome by suppressing the EMT-promoting activity of the IL-8/IL-8R axis, thus preventing the switch of tumor cells from an epithelial to a mesenchymal metastatic phenotype.
Supplementary Material
Acknowledgments
The authors thank Dr. Jeffrey Schlom for helpful discussions on this manuscript, Margie Duberstein for technical assistance, and Debra Weingarten for editorial assistance.
Grant support: Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH
References
- 1.Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R. The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev. 21:27–39. doi: 10.1016/j.cytogfr.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nannuru KC, Singh RK. Tumor-stromal interactions in bone metastasis. Curr Osteoporos Rep. 8:105–13. doi: 10.1007/s11914-010-0011-6. [DOI] [PubMed] [Google Scholar]
- 7.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117:3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 9.Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A, et al. Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression. Eur J Cancer. 2009;45:2618–27. doi: 10.1016/j.ejca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–8. doi: 10.172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 13.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–65. doi: 10.1038/nrm1175. [DOI] [PubMed] [Google Scholar]
- 15.Fernando RI, Litzinger M, Trono P, Hamilton DH, Schlom J, Palena C. The T-box transcription factor Brachyury promotes epithelial-mesenchymal transition in human tumor cells. J Clin Invest. 2010;120:533–44. doi: 10.1172/JCI38379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, et al. A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells. Am J Pathol. 2001;158:639–46. doi: 10.1016/S0002-9440(10)64005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–80. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- 18.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–42. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 20.Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21:3241–6. doi: 10.1038/sj.onc.1205416. [DOI] [PubMed] [Google Scholar]
- 21.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005;65:5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 22.Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ, et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res. 2005;11:8070–8. doi: 10.1158/1078-0432.CCR-05-0687. [DOI] [PubMed] [Google Scholar]
- 23.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–9. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Palena C, Polev DE, Tsang KY, Fernando RI, Litzinger M, Krukovskaya LL, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy. Clin Cancer Res. 2007;13:2471–8. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 25.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luboshits G, Shina S, Kaplan O, Engelberg S, Nass D, Lifshitz-Mercer B, et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999;59:4681–7. [PubMed] [Google Scholar]
- 27.Dannenmann C, Shabani N, Friese K, Jeschke U, Mylonas I, Bruning A. The metastasis-associated gene MTA1 is upregulated in advanced ovarian cancer, represses ERbeta, and enhances expression of oncogenic cytokine GRO. Cancer Biol Ther. 2008;7:1460–7. doi: 10.4161/cbt.7.9.6427. [DOI] [PubMed] [Google Scholar]
- 28.Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–9. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–65. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 30.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J Immunol. 1993;151:2667–75. [PubMed] [Google Scholar]
- 31.Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin-8 (CXCL-8)-mediated modulation of metastatic phenotype. Clin Exp Metastasis. 2003;20:723–31. doi: 10.1023/b:clin.0000006814.48627.bd. [DOI] [PubMed] [Google Scholar]
- 32.Wigmore SJ, Fearon KC, Sangster K, Maingay JP, Garden OJ, Ross JA. Cytokine regulation of constitutive production of interleukin-8 and -6 by human pancreatic cancer cell lines and serum cytokine concentrations in patients with pancreatic cancer. Int J Oncol. 2002;21:881–6. doi: 10.3892/ijo.21.4.881. [DOI] [PubMed] [Google Scholar]
- 33.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin Cancer Res. 2004;10:7157–62. doi: 10.1158/1078-0432.CCR-04-0812. [DOI] [PubMed] [Google Scholar]
- 34.Zakrzewska I, Kozlowski L, Wojtukiewicz M. Value of interleukin-8 determination in diagnosis of benign and malignant breast tumor. Pol Merkur Lekarski. 2002;13:302–4. [PubMed] [Google Scholar]
- 35.Kozlowski L, Zakrzewska I, Tokajuk P, Wojtukiewicz MZ. Concentration of interleukin-6 (IL-6), interleukin-8 (IL-8) and interleukin-10 (IL-10) in blood serum of breast cancer patients. Rocz Akad Med Bialymst. 2003;48:82–4. [PubMed] [Google Scholar]
- 36.Lehrer S, Diamond EJ, Mamkine B, Stone NN, Stock RG. Serum interleukin-8 is elevated in men with prostate cancer and bone metastases. Technol Cancer Res Treat. 2004;3:411. doi: 10.1177/153303460400300501. [DOI] [PubMed] [Google Scholar]
- 37.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Umbhauer M, Marshall CJ, Mason CS, Old RW, Smith JC. Mesoderm induction in Xenopus caused by activation of MAP kinase. Nature. 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- 39.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. Epub 2009 Mar 5. [DOI] [PubMed] [Google Scholar]
- 41.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106:13820–5. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, et al. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9:3167–75. [PubMed] [Google Scholar]
- 44.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–97. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





