Abstract
Previous studies using a mixed β1 and β2 adrenergic antagonist, propanolol, have indicated that β adrenoceptors have little effect on the cognitive functioning of the prefrontal cortex. However, recent studies have suggested that endogenous stimulation of β1 adrenoceptors impairs working memory in both rats and monkeys. Since propanolol has no effect on cognition, we hypothesized that activation of β2 adrenoceptors might improve performance in a working memory task. We tested this hypothesis by observing the effects of the β2 agonist, clenbuterol, on spatial working memory performance. Clenbuterol was either infused directly into the prefrontal cortex (rats) or administered systemically (monkeys). Results demonstrated that clenbuterol improved performance in many young and aged rats and monkeys who performed poorly under control conditions. Actions at β2 adrenoceptors were confirmed by challenging the clenbuterol response with the β2 adrenergic antagonist, ICI 118,551. The effects of clenbuterol were not universal and depended on the cognitive status of the animal: the drug moderately improved only a subset of animals with working memory impairment.
Keywords: Clenbuterol; ICI 118,551; Beta-2; Norepinephrine; Working memory; Aging; Prefrontal cortex
1. Introduction
The prefrontal cortex (PFC) is important for guiding behavior, thought, and affect using working memory (WM). The PFC is engaged during the retrieval and encoding of memories (especially if it is an effortful process), and is critical for inhibition of proactive interference, for protecting memories and thoughts from distraction, and allowing us to plan and organize behavior (Bunge et al., 2001; Kapur et al., 1995; Lepage et al., 2000; Stuss and Knight, 2002). Normal aging consistently impairs many of these cognitive functions of the PFC in humans (Albert, 1997; Chao and Knight, 1997; Nielsen-Bohlman and Knight, 1995; Schacter et al., 1996), monkeys (Bartus et al., 1978; Herndon et al., 1997; Rapp and Amaral, 1989) and rats (Ando and Ohashi, 1991; Bimonte et al., 2003). As the aging population grows progressively larger, treatments for age-related cognitive deficits are increasingly needed.
The PFC is very sensitive to levels of norepinephrine (NE). Moderate levels of NE release bind to α2A adrenoceptors and improve PFC function (Franowicz et al., 2002). In contrast, higher levels of NE release, as with stress, bind to α1 receptors and impair PFC function (Birnbaum et al., 1999). Although we have gained extensive knowledge about NE’s actions at α1 and α2 receptors in the PFC, our understanding of NE’s actions at β receptors in the PFC is scarce. This contrasts with the rest of the peripheral and central nervous systems, where NE actions at β adrenoceptors are better understood and often have powerful physiological functions (Berridge et al., 1996; Cahill et al., 1994; Hopkins and Johnston, 1988; Sessler et al., 1989; Tevaearai and Koch, 2004; Waterhouse et al., 1980).
β adrenergic receptors are differentiated into three subtypes, β1, β2, and β3, and all are found in the nervous system. These subtypes are differentially expressed in various regions of the brain, with β1 receptors in higher concentrations in the adult rat cortex compared to the other receptor subtypes (Rainbow et al., 1984). In contrast, β3 mRNA seems to be more present in the developing brain (Summers et al., 1995). β1 receptor mRNA has also been observed in the PFC of rats, while β2 mRNA was not found in the rat PFC (Nicholas et al., 1993). However, β2 mRNA is present in the intralaminar thalamic nuclei and hippocampal formation, both of which project to the PFC (Nicholas et al., 1993). Thus, β2 receptors are likely localized in axon terminals in the rat PFC. Binding studies in primates have demonstrated high concentrations of β receptors in the PFC (Goldman-Rakic et al., 1990), and β2 receptor binding has been documented in both human (Kalaria et al., 1989) and non-human primate (Flugge et al., 1997) PFC. Electron micrographic studies have also documented β2 receptors in the rhesus monkey PFC within axon terminals and in GABAergic interneurons (Aoki et al., 1998). Interestingly, both β1 and β2 receptors are down-regulated in the PFC by stress exposure (Flugge et al., 1997), consistent with high levels of NE release during stress.
Previous studies suggested that β receptors had no effect on PFC function, as there was no effect on WM when β1 and β2 receptors were blocked with the mixed β1/β2 antagonist, propanolol. Neither microinjection of propanolol into the PFC (Li and Mei, 1994), nor systemic administration of propanolol (Arnsten and Goldman-Rakic, 1985) altered PFC function in monkeys. However, a recent study suggests that endogenous activation of the β1 adrenoceptors impairs WM in both rats and monkeys (Ramos et al., 2005). Thus, it is possible that propanolol had no effect on WM because β1 and β2 adrenoceptors, like α1 and α2 receptors, have opposing functional effects.
The current study set out to determine whether β2 adrenoceptors play a role in regulating PFC function. To test this hypothesis, clenbuterol, a β2 adrenergic agonist, was infused directly into the PFC of rats or systemically administered to monkeys prior to cognitive testing. Rats were tested on the delayed alternation task in a T-maze, a test of spatial WM that is impaired by lesions to the prelimbic/infralimbic PFC in rats (Divac, 1971). A parallel study examined the effects of β2 adrenoceptor stimulation in monkeys performing the spatial delayed response task, a test of spatial WM that depends upon the principal sulcal cortex. In both studies clenbuterol treatment was challenged with a dose of ICI 118,551, a selective β2 adrenergic antagonist, using a dose that had no cognitive effect on its own. Results from both studies suggest that clenbuterol produces a modest improvement in WM in a subset of animals with cognitive impairment, and that this improvement can be reversed by ICI 118,551.
2. Subjects and methods
All procedures were approved by the Yale Institutional Animal Care & Use Committee. Care of the rats and monkeys followed the guidelines in “Guide for the Care and Use of Laboratory Animals”.
2.1. Rat studies
2.1.1. Subjects
Aged (20 months), retired breeder, and young adult (9 months) male Sprague-Dawley rats from Harlan (Indianapolis, IN) were single-housed in filter frame cages. Aged rats were approximately 24 months old, while young rats were ~12 months old upon initiation of pharmacological testing. Animals were kept on a 12 h light/dark cycle, and experiments were conducted during the light phase. Rats were slowly habituated to a restricted diet (16 gm/day per rat) of autoclaved Purina (St. Louis, MO) rat chow during the first 2 weeks. Note: both young and aged rats were at free feeding weight upon arrival. Food was given immediately after behavioral testing and water was available ad libitum. Rats were weighed weekly to confirm that they were not undergoing irregular weight loss due to regulated diet. Only aged rats lost weight during the study (547.33 ± 17.78 g upon arrival, 453.22 ± 12.87 g at the end of the study, p < 0.0001). In contrast, young rats significantly gained weight during the study despite being food restricted (265.12 ± 5.48 g upon arrival, 428.94 ± 12.36 g at the end of the study, p < 0.0001). Food rewards during cognitive testing were highly palatable miniature chocolate chips. Rats were assigned a single experimenter who handled them extensively before behavioral testing.
2.1.2. Delayed alternation in T-maze
Rats were habituated to a T-maze (dimensions, 90 cm × 65 cm) until they were readily eating chocolate chips placed at the end of each arm and were acclimated to handling. After habituation, rats were trained on the delayed alternation task. On the first trial, animals were rewarded for entering either arm. Thereafter, for a total of 10 trials per session, rats were rewarded only if they entered the maze arm, which was not previously chosen. Thus, the correct choice alternates between each trial. Between trials the choice point was wiped with alcohol to remove any olfactory clues. The delay between trials started at “0” sec (i.e. about ~1.5 s, minimum possible for delayed alternation) and was subsequently raised in 5 s intervals as needed to maintain performance at 60–70% correct for low baseline and aged rats and at ~80% for high baseline rats.
2.1.3. Cannulae implantation
After training on the delayed alternation task, animals underwent stereotaxic implantation of chronic guide cannulae as described previously (Taylor et al., 1999). Guide cannulae (Plastics One; 2.8 mm) with stylettes were aimed dorsal to the medial PFC (Fig. 1; prelimbic PFC; stereotaxic coordinates—anterioposterior: +3.2 mm; mediolateral:±0.75 mm; dorsoventral: stylette reaching to −4.2 mm). Surgery was performed under low doses of a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg) injected (i.p.) prior to the start of the procedure. These agents were supplemented with gas anesthesia (isoflurane) administered during surgery via nose-cone. Sterile stylettes were inserted in the cannula to maintain patency. Great care was taken to minimize pain and infection postoperatively to decrease stress to the animal. The region surrounding the cemented guide cannula was treated with triple antibiotic and cleaned daily if needed for a period of about a week. Animals were also acutely treated with Buprenex (0.01 mg/kg) prior to the initial incision and after surgery were given medicated water (Metacam, 1 mg/kg) in their home cage for 2 days to reduce any pain associated with the procedure.
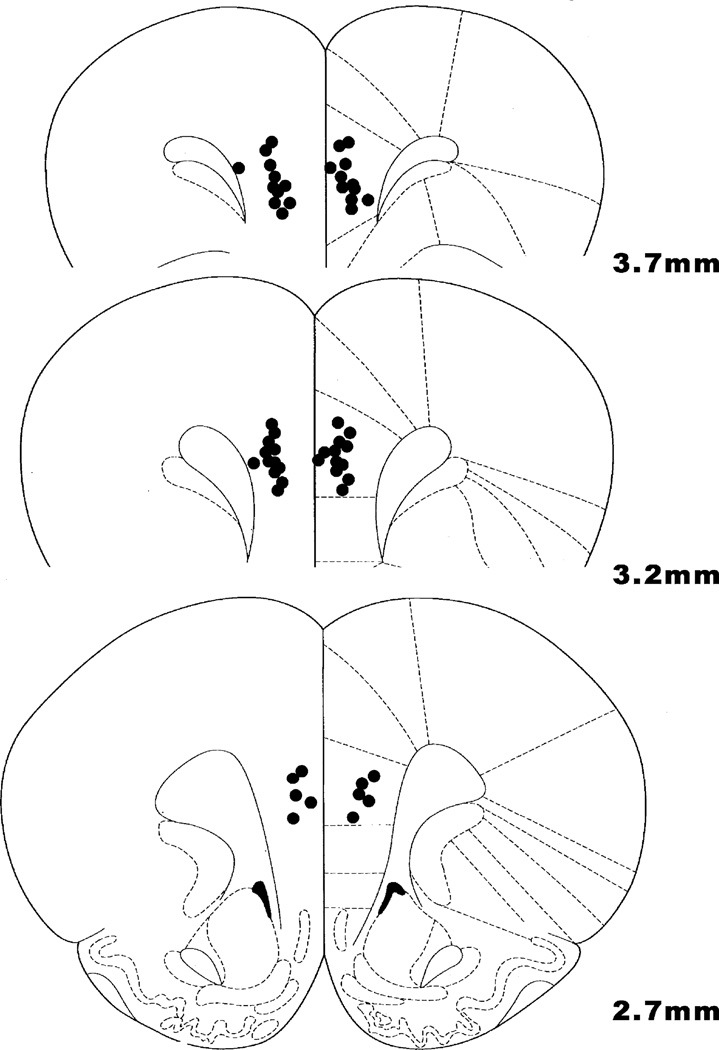
Fig. 1.
Location of cannula tips in the rat medial PFC (prelimbic cortex). All bilateral infusions of 0.5 µL occurred at 4.5 mm DV. Coronal slices indicate distance (mm) anterior from bregma.
2.1.4. Drug infusions
Rats were initially adapted to a mock infusion protocol to minimize any stress associated with the procedure. Rats were gently restrained while the stylettes were removed and replaced with 30 gauge sterile infusion needles that extended to 4.5mm dorsoventral below the skull. Bilateral infusions were driven by a Harvard Apparatus (Hollinston, MA) syringe pump set at a flow rate of 0.25 µL/min using 25µL Hamilton syringes for an infusion time of 2 min. Needles remained inserted in place for 2 min after completion of the infusion. Stylettes were inserted back into the cannulae, and behavioral testing was performed 30 min after the infusion procedure. The testing time post-infusion used in the current study was based on a pilot study that identified this time as the most effective time (variable results were obtained 5 and 15 min after infusion). Drug treatments and vehicle were administered in a counterbalanced order with at least 1 week between each infusion. Counterbalancing ensured that rats received drug infusions both early in the study when delays were short, as well as later in the study when delays were longer. Animals were required to exhibit stable baseline performance (two consecutive test sessions of 60–70% correct for aged and low baseline, young rats and ~80% for high baseline, young rats) prior to drug administration.
Clenbuterol was purchased from Sigma–Aldrich (St. Louis, MO). ICI 118,551 was purchased from Tocris (Ellisville, MO). Both drugs were dissolved in sterile saline solution to the appropriate dose (0.1 µg/µL for clenbuterol; 0.0001µg/µL for ICI 118,551). The dose of clenbuterol presented in the current study was based on a pilot study that identified this as the most effective dose. Higher and lower doses of 1.0 µg/0.5 µL (n = 10, p = 0.54) and 0.01 µg/0.5 µL (n = 8, p = 0.53), respectively, had no significant effect (paired t-test). Thus, the 0.1 µg/0.5 µL dose was the focus of the current research. A dose of ICI 118,551 that had no cognitive effect on its own (0.0001 µg/0.5 µL) was chosen to examine the specificity of clenbuterol’s actions. Due to aged rats fragility and health, the clenbuterol versus ICI experiment was done in young rats in which multiple infusions are easier to achieve. The experimenter testing the animal was unaware of drug treatment conditions.
2.1.5. Histology
Rats were placed in a bell jar that contained a gauze moistened with a large dose of isoflurane. Once the rats were unconscious they were sacrificed by decapitation. Brains were removed, stored in formalin, sectioned, and analyzed for histological verification of cannulae placement. All rats had correctly placed cannulae within the prelimbic or infralimbic regions of the rat cortex (Fig. 1).
2.1.6. Data analysis
The clenbuterol versus ICI 118,551 data were analyzed using a two-way analysis of variance with repeated measures (2-ANOVA-R) with within subject factors of clenbuterol treatment and ICI 118,551 treatment. Planned comparisons (user defined contrasts) were performed to examine whether (1) vehicle/saline significantly differed from clenbuterol/saline; (2) vehicle/saline differed from ICI 118,551/saline; (3) clenbuterol/saline differed from clenbuterol/ICI 118,551, (4) vehicle/saline differed from clenbuterol/ICI 118,551. A paired t-test was also used to analyze the data from young (high and low baseline) and aged rats after clenbuterol infusion.
2.2. Monkey studies
2.2.1. Subjects
The animals used in this study were one male and nine female rhesus monkeys (Macaca mulatta) ranging in age from 13 years (middle aged) to over 30 years (4/5 monkeys were older than 20 years old). Actual birthdates were not available for several of the aged animals who were feral born. Ages were estimated by the veterinarians based on health records, teeth and known history; several had been in the Yale colony for more than 15 years. The monkeys were individually housed and maintained on a diet of Purina monkey chow supplemented with fruit. Animals were always tested at the same time of day immediately prior to feeding. Highly palatable food rewards (e.g. peanuts, raisins or chocolate chips) were utilized during testing to minimize the need for dietary regulation.
2.2.2. Delayed response testing
Cognitive testing occurred in a Wisconsin General Testing Apparatus (WGTA) situated in a sound-attenuating room. Background masking noise (60 dB, wideband) was also used to minimize auditory distractions. Each monkey was assigned to a single experimenter who knew the animal well but was unaware of the drug treatment conditions. The animals were tested twice a week with 3–4 days separating each test session (e.g. Monday and Thursday). The monkeys had been previously trained on the spatial delayed response task as described. During delayed response, the animal watched as the experimenter baited one of several food wells with a food reward. The number of food wells varied from two to four wells depending on the monkey’s performance level and experience with the task. Care was taken by the experimenter to ensure that the animal attended the baiting procedure. The food wells were then covered with identical cardboard plaques, and an opaque screen was lowered between the animal and the food wells for a specified delay. At the end of the delay, the screen was raised and the animal was allowed to choose. Reward was quasi-randomly distributed between the left and right wells over the 30 trials that made up a daily test session. Five different delay lengths (referred to as delays A through E) were quasi-randomly distributed over these 30 trials. The shortest of these delays was less than 1 s (the “0” sec A delay). A transparent screen was lowered for the “0” sec delay condition. The remaining delays were in the range that for each individual monkey yielded baseline performance of about 70% across all delays (i.e. 18–22 trials correct of the possible 30 trials). For example the delays for one animal might be A = 0, B = 5, C = 10, D = 15, and E = 20 s. Delays in the current study varied from B = 2 to B = 12.
2.2.3. Sedation assessment
During each cognitive testing session, the experimenter rated the animal’s level of sedation/agitation and aggression on rating scales. Sedation and agitation were rated using a nine-point scale where −4 = too agitated to test, −3 = agitation which interferes with testing, −2 = slight agitation which does not interfere with testing, −1 = more alert than usual, 0 = normal level of arousal, 1 = quieter than usual, 2 = sedated (drooping eyelids, slowed movements), 3 = intermittent sleeping and 4 = too sedated to test. Aggression was rated using a similar scale where −3 = dramatically more aggressive, −2 = moderately more aggressive, −1 = mildly more aggressive, 0 = normal, 1 = mildly less aggressive, 2 = moderately less aggressive, 3 = dramatically less aggressive. Note: aggressive interactions involved interactions with the experimenter, including aggressive facial expressions or physical attacks directed at the experimenter. In contrast, agitated behaviors were not directed at the experimenter and included behaviors such as circling and pacing.
2.2.4. Drug administration
Clenbuterol and ICI 118,551 were dissolved into saline to the appropriate dose. The wide range of doses of clenbuterol, 0.000001–0.1 mg/kg, were administered intramuscularly to monkeys. However, vomiting was exhibited by some monkeys at the highest dose, and thus this dose was not included in the current study. Even though the monkeys in the study (n = 10) received a wide range of clenbuterol doses, not every animal was improved by clenbuterol treatment. However, a subset of older monkeys (n = 7, 20–30 years old) were improved by clenbuterol. The most effective dose of clenbuterol to have an enhancing effect in this subset of monkeys varied between 0.0001–0.01 mg/kg. Of these seven monkeys, five showed replicable improvement with clenbuterol and were challenged with concomitant ICI 118,551 treatment to test for actions at β2 receptors. These five monkeys were first tested on ICI 118,551 alone (0.0001–0.01 mg/kg) to identify a dose that had no cognitive effect on its own. The remaining two monkeys were unable to be included in this study due to emerging health problems unrelated to drug treatment. Clenbuterol was systemically administered with or without ICI 118,551 2 h prior to cognitive testing. Similar to the rat study, the testing time post-injection presented in the current study was based on a pilot study that identified this time as the most effective time (variable results were obtained at 1 h post-injection). A washout period of at least 10 days occurred between drug treatments. Monkeys were required to return to stable baseline performance for two consecutive testing days prior to new drug treatment. Given these prolonged washout conditions the research took approximately 10 months to complete.
2.2.5. Data analysis
The data were analyzed using either a one-way analysis of variance with repeated measures (1-ANOVA-R) on clenbuterol dose, or a two-way analysis of variance with repeated measures (2-ANOVA-R) using within subjects factors of clenbuterol and ICI 118,551. In this latter analysis, planned comparisons (user defined contrasts) were performed to examine whether: (1) vehicle/saline significantly differed from clenbuterol/saline; (2) vehicle/saline differed from ICI/saline; (3) clenbuterol/saline differed from clenbuterol/ICI 118,551; (4) vehicle/saline differed from clenbuterol/ICI 118,551.
3. Results
3.1. Rats
Aged or young rats were pretrained on the spatial delayed alternation task in a T maze. They were surgically implanted with guide cannula directed above the PFC (Fig. 1), and adapted to infusion procedures. After stable baseline cognitive performance of 60–70% (for low baseline rats) or ~80% (for high baseline rats) was established, they were infused with either saline or clenbuterol (0.1 µg/0.5 µL), 30 min prior to testing. Young rats normally test at ~80%, however, delays were raised for a subset of young rats (low baseline group) to make baseline equivalent to that of the aged rats in the current study. The dose of clenbuterol used was based on a pilot study (see Subjects and methods).
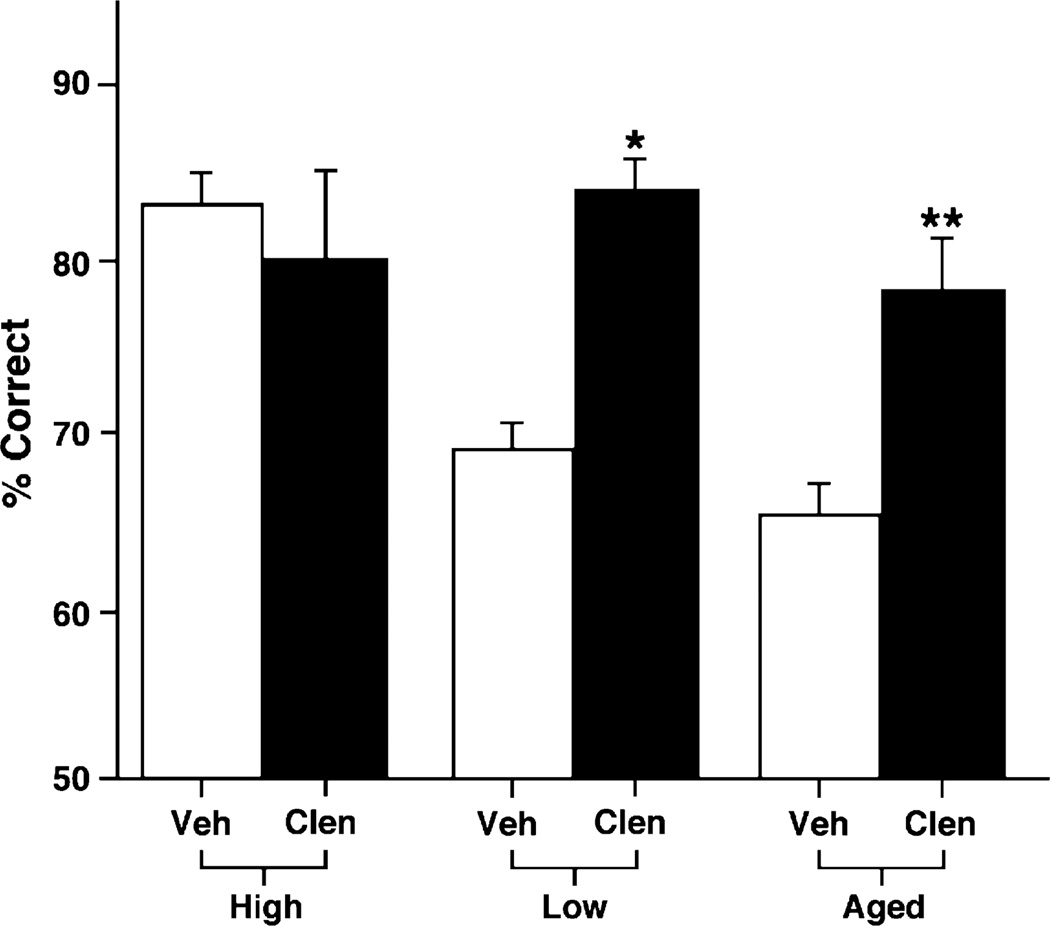
As can be seen in Fig. 2, clenbuterol significantly improved WM performance in both young and aged rats, but only if they were performing at a low baseline (p = 0.0001, paired t-test). Similarly, clenbuterol improved WM performance in aged rats (n = 9) with PFC cognitive impairment (p = 0.004). However, clenbuterol did not improve young rats (n = 6) that were testing at a higher baseline (p = 0.638).
Fig. 2.
Differences in baseline performance contribute to the effects seen with a β2 adrenergic agonist. Clenbuterol (0.1 µg/0.5 µL) improves performance in the delayed alternation task in a T-maze when compared to performance following a vehicle treatment in both young and aged rats with prefrontal cortical deficits (low baseline). However, clenbuterol had no effect when infused into the PFC of high baseline young rats. Results represent mean ± S.E.M. percent correct. *p = 0.0001; **p = 0.004.
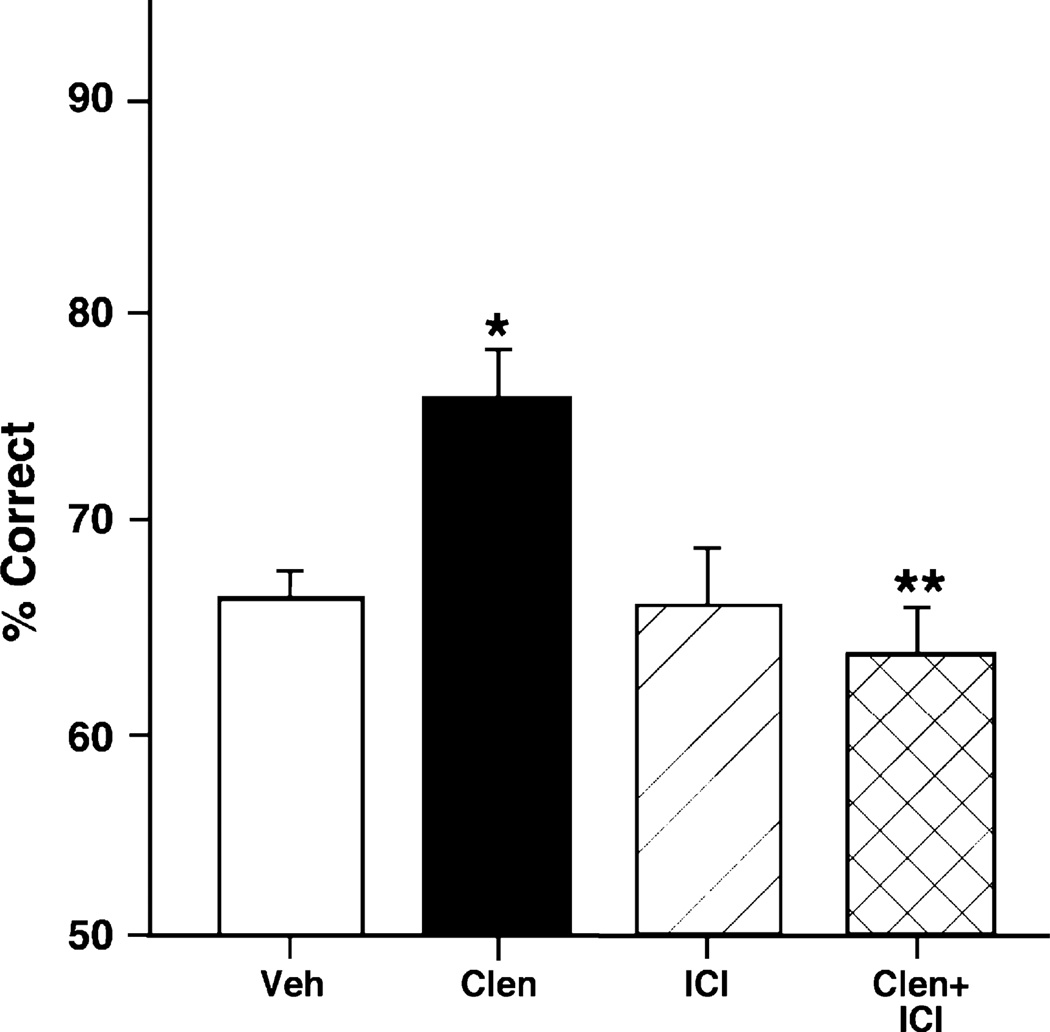
Clenbuterol is not only a β2 adrenergic agonist, but also has some β1 antagonist properties (Ordway et al., 1987). A previous study has shown that the β1 adrenergic antagonist, betaxolol, improves WM performance (Ramos et al., 2005). Thus, it was important to test which receptor is mediating the beneficial effects of clenbuterol. Clenbuterol could be blocking NE’s actions on the β1 adrenoceptor or it could be activating the β2 receptor to improve cognition. To test which receptor is involved, the β2 adrenergic antagonist, ICI 118,551, was used to test whether it could reverse clenbuterol’s beneficial effects on WM performance. As can be seen in Fig. 3, a 0.0001 µg/0.5 µL dose of ICI 118,551 completely reversed the enhancing effects of 0.1 µg/0.5 µL clenbuterol on WM. 2-ANOVA-R analysis demonstrated a significant main effect of clenbuterol infusion {F(1, 4) = 9.85, p = 0.04}; a significant main effect of ICI 118,551 {F(1, 4) = 23.14, p = 0.009}; and a significant interaction between clenbuterol and ICI 118,551 infusions {F(1, 4) = 9.53, p = 0.04}. Planned comparisons (test of effects) showed that infusion of clenbuterol alone significantly improved accuracy of responding compared to saline control {F(1, 4) = 15.21, p = 0.02}. Planned comparisons also showed that ICI 118,551 infusion had no cognitive effect on its own {ICI 118,551 versus saline: F(1, 4) = 0, p = 1}, but significantly reversed the enhancing effects of clenbuterol {clenbuterol treatment significantly different from clenbuterol + ICI 118,551: F(1, 4) = 23.14, p = 0.009; clenbuterol + ICI 118,551 not significantly different than saline: F(1, 4) = 1, p = 0.37}.
Fig. 3.
Clenbuterol’s beneficial effects on working memory performance of young rats are blocked by ICI 118,551. Clenbuterol (0.1 µg/0.5 µL) improves rats’ performance in the delayed alternation task in a T-maze when compared to performance following a vehicle treatment. Blockade of β2 adrenoceptors with ICI 118,551 (0.0001 µg/0.5 µL) reverses the beneficial effects of clenbuterol. Results represent mean ± S.E.M. percent correct, n = 9. *p = 0.02; **p = 0.009.
3.2. Monkeys
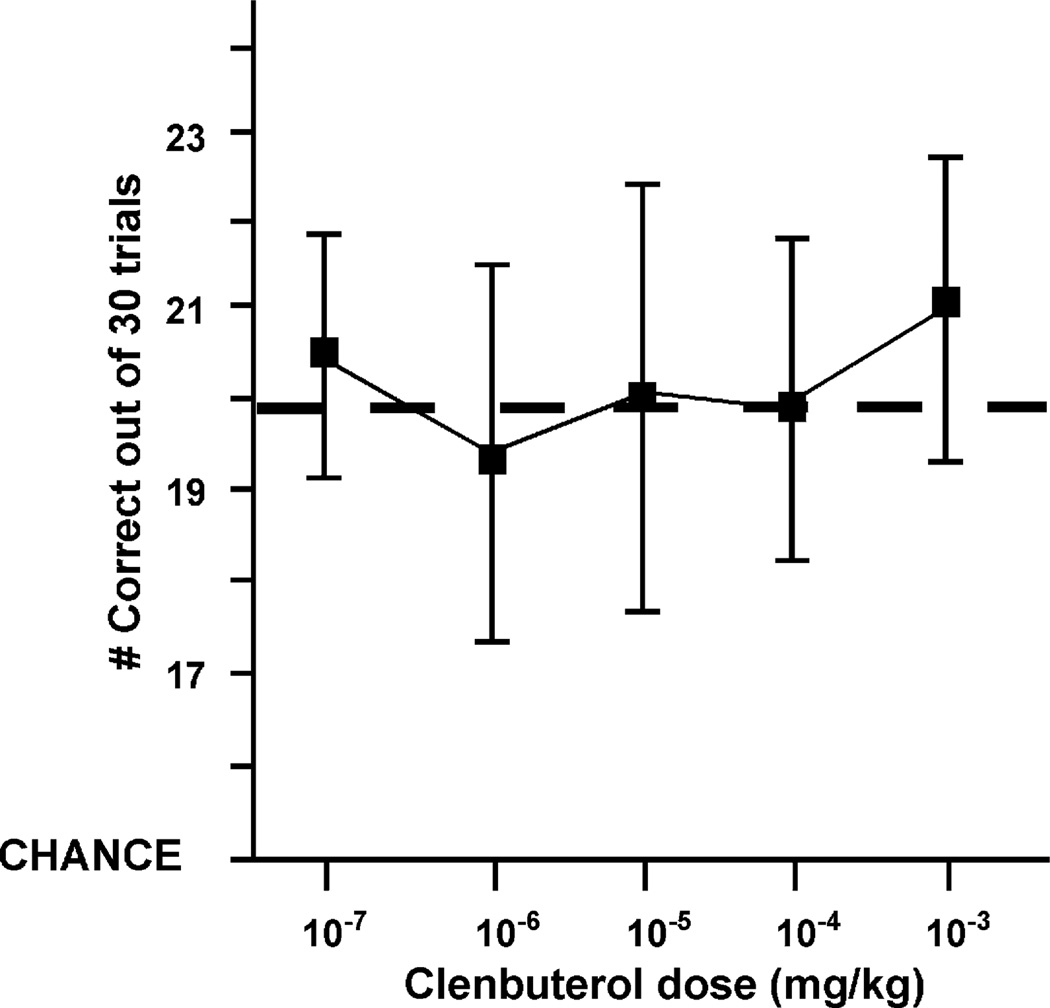
The effects of systemic administration of clenbuterol on spatial WM performance in monkeys were assessed across a wide range of doses (0.000001–0.01 mg/kg, i.m.). Overall, as can be seen in Fig. 4, an analysis of variance with repeated measures showed that clenbuterol had no significant effect on WM performance {F(1, 9) = 2.03, p = 0.19}. The lack of an overall effect was due to the high variability of clenbuterol’s effects at the doses examined. In particular, it is worth mentioning that two of the oldest monkeys showed little or no improvement at most doses, and were even impaired at separate doses (0.00001 or 0.0001 mg/kg) in the WM task. However, a subset of monkeys, particularly older ones (20–30 years old), were reliably improved by clenbuterol treatment (paired t-test for best dose, p = 0.02). For this subset of older monkeys, the effective clenbuterol dose was then re-administered to replicate the improvement, and it was this dose that was used in the clenbuterol versus ICI 118,551 experiment described below.
Fig. 4.
Clenbuterol dose response curve. Systemic administration of clenbuterol does not have a significant effect on working memory performance when all of the monkeys in the study are examined. Dashed line indicates the mean performance after saline treatment (19.93 ± 0.18). Results represent mean ± S.E.M. correct out of 30 trials, n = 10.
For each monkey that showed a reliable and replicable response to clenbuterol, an ICI 118,551 dose (0.0001–0.01 mg/kg) that had no cognitive effect on its own was selected to test whether it could block clenbuterol’s effects on cognition, similar to the rat study above. ICI 118,551 significantly reversed the enhancing effects of clenbuterol in monkeys (Fig. 5). 2-ANOVA-R revealed a trend towards a main effect of clenbuterol injection {F(1, 4) = 5.78, p = 0.07}, a significant main effect of ICI 118,551 {F(1, 4) = 12.12, p = 0.03}, and a significant interaction between clenbuterol and ICI 118,551 injections {F(1, 4) = 8.98, p = 0.04}. Planned comparisons (test of effects) showed that clenbuterol alone significantly improved accuracy of responding compared to saline control {F(1, 4) = 17.61, p = 0.01}, thus confirming clenbuterol’s enhancing effect on prefrontal cortical function. ICI 118,551 had no effect on performance on its own {ICI 118,551 versus saline: F(1, 4) = 0.03, p = 0.86}, but reversed the enhancing effects of clenbuterol {significant difference between clenbuterol treatment and clenbuterol + ICI 118,551: F(1, 4) = 14.07, p = 0.02; no significant difference between saline treatment and clenbuterol + ICI 118,551: F(1, 4) = 6.75, p = 0.06}. Thus, similar to rat studies, clenbuterol’s enhancement of PFC function is consistent with actions at β2 adrenoceptors.
Fig. 5.
Clenbuterol’s beneficial effects on working memory performance of a subset of monkeys are blocked by ICI 118,551. Systemic administration of the best effective dose of clenbuterol (0.0001–0.01 mg/kg) improves the monkey’s performance in the delayed response task when compared to performance following a vehicle treatment. Similar to the rat studies, blockade of β2 adrenoceptors, at a dose that has no effects on its own (0.0001–0.01 mg/kg), reverses clenbuterol’s beneficial effects on working memory performance. Results represent mean ± S.E.M. percent correct, n = 5. *p = 0.01; **p = 0.02.
There was no evidence of side effects with any of the treatments used in these studies (median sedation score of “0”, normal behavior), suggesting that effects seen in the current study arose from altered cognitive ability rather than from non-specific effects on performance. However, generalized improvements in performance cannot be ruled out. An analysis of clenbuterol on performance at each delay length did not show a significant drug by delay interaction. The 2-ANOVA-R found a significant effect of delay {F(4, 16) = 7.17, p = 0.002}, a significant effect of clenbuterol {F(1, 4) = 30.03, p = 0.005}, but no significant interaction between delay and clenbuterol {F(4, 16) = 1.68, p = 0.2}. However, it is worth mentioning that clenbuterol’s effects were more prominent at the longer delays (C–E) where there was the most room for improvement (data not shown).
4. Discussion
The current study is the first to show that β2 adrenoceptor activation can improve PFC spatial WM functioning in both aging rats and monkeys. Results in rats show that clenbuterol infusions into PFC improve WM performance in both young and aged rats with PFC deficits (low baseline), but have no effect in young rats with normal WM performance (high baseline). In monkeys, the results were more modest yet consistent with the rat data: clenbuterol improved performance in a spatial delayed response task that depends on the PFC, but only in a subset of older monkeys. In both rats and monkeys, the cognitive improvement seen with clenbuterol could be reversed by the β2 adrenergic antagonist, ICI 118,551. Thus, activation of β2 adrenoceptors appears to produce a modest enhancement of PFC function in aged animals or in young animals with weaker WM performance.
Neuropharmacological studies suggest that clenbuterol could be one of the few agents to produce global cognitive improvement. While agents such as guanfacine enhance PFC function but impair hippocampal and amygdala function, clenbuterol is one of the few agents that can improve both WM and long-term memory. Indeed, activation of β2 adrenoceptors has been shown to enhance amygdala and, more recently, hippocampal function. For example, McGaugh and co-workers have shown that clenbuterol injected directly into the amygdala immediately after training enhances retention of an inhibitory avoidance task (Introini-Collison et al., 1991). β2 receptors in the amygdala also interact with glucocorticoids to enhance memory consolidation of that same task (Roozendaal et al., 2002). Moreover, clenbuterol may enhance memory storage processes and long-term potentiation in downstream targets of the basolateral amygdala, such as the hippocampus, via an activity regulated cytoskeletal protein (McIntyre et al., 2005).
In contrast to α2 adrenoceptors, β receptors can couple to Gs proteins that lead to an activation of cAMP signaling. Indeed, activation of β2 adrenoceptors in the cortex increases levels of cAMP(Ordway et al., 1987). Moreover, this increase in cAMP is significantly reduced by the β2 antagonist, ICI 118551 (Ordway et al., 1987). Similarly, in the amygdala, β2 adrenoceptors modulate memory storage by a direct coupling to adenylyl cyclase and influencing cAMP formation (Ferry et al., 1999). A recent study by O’Donnell and co-workers suggests that clenbuterol’s antidepressant effects are also via increased cAMP signaling in cortical neurons (Zhang et al., 2005). Thus, future studies should examine whether the effects of clenbuterol on WM performance are via activation of this pathway.
The fact that α2 and β2 adrenoceptors have the same effect even though they have opposite effects on cAMP signaling, suggests that their sites of action are different. Indeed, α2A receptors are highly localized to the dendritic spines (Aoki et al., 1998) and the postsynaptic density (Richman et al., 2001) of PFC pyramidal cells. Conversely, β receptors colocalize mainly with GABA-containing neurons in the monkey PFC (Aoki et al., 1998), suggesting that these receptors may promote inhibitory effects. Thus, clenbuterol may enhance GABAergic neurotransmission (Waterhouse et al., 1981) that has been shown to be important during WM tasks (Constantinidis et al., 2002; Rao et al., 2000).
β receptors are also localized to astrocytes in the rat cortex (Aoki, 1992; Aoki and Pickel, 1992). Therefore, the effects of clenbuterol treatment could result from β adrenoceptors’ ability to enhance the breakdown of glycogen and the export of glucose from astrocytes to increase local cerebral blood flow (Fillenz et al., 1999). For example, blocking β receptors with propanolol prevents delivery of additional glucose directly into the extracellular compartment where it can be taken up by neurons (Fillenz and Lowry, 1998). Interestingly, astrocytic β receptor immunoreactivity frequently contacted blood vessels in the visual cortex of rats (Aoki and Pickel, 1992). The effects of clenbuterol could be even more important in aged individuals with prefrontal cortical deficits, since glucose metabolism have been found to be decreased in the frontal and temporal lobes of aged humans and monkeys (De Santi et al., 1995; Eberling et al., 1997; Noda et al., 2002). Moreover, age-related memory impairment is reversed by administration of glucose or epinephrine, which binds preferentially to β adrenoceptors (Korol and Gold, 1998). Taken together, these findings suggest that β2 adrenoceptor activation may be particularly relevant in the aged population where reduced glucose metabolism may be contributing to prefrontal cortical deficits.
The results from the current study suggest that clenbuterol enhances PFC cognitive function only in those animals that had WM deficits. Certainly, a lack of a clenbuterol effect in high baseline rats could be due to a ceiling effect. However, as was described in the previous paragraph, at least in aged rats or monkeys with PFC cognitive impairment, clenbuterol could be enhancing glucose metabolism, which may be deficient in these animals. Another possibility is that animals with WM deficits have insufficient release of norepinephrine in the PFC. A recent study has shown that excitatory orexin innervation is decreased along with a significant decrease in tyrosine hydroxylase mRNA in the locus coeruleus of aged macaques (Downs et al., 2006), suggesting that there could be decrease in the release of norepinephrine in the aged PFC. Thus, it may be possible that aged animals with PFC deficits benefit from clenbuterol treatment due to low norepinephrine release that does not activate enough β2 adrenoceptors. Low baseline rats could show a similar profile of low norepinephrine release or glucose metabolism, although there is currently no evidence to support that conclusion.
Clenbuterol has been reported to be beneficial for treating a number of non-CNS disorders in humans, including chronic lung disease (Ng et al., 2001) and asthma (Boner et al., 1987) by aiding in bronchodilation. It is also used to treat mild-to-moderate stress incontinence (Noguchi et al., 1997) and allergic inflammation (Yoshimura et al., 1997). However, clenbuterol is also abused by individuals that practice body building because of β2 agonists’ anabolic effects and reduction of subcutaneous fat (Choo et al., 1992; Hesketh et al., 1992; Navegantes et al., 2001; Prather et al., 1995; Ryall et al., 2004; Yimlamai et al., 2005). Thus, clenbuterol would seem like an attractive option for preventing muscle frailty in the elderly as has been suggested from a study in rats (Ryall et al., 2004). However, despite some of the positive data that make clenbuterol an attractive option for clinical use, there are number of other potentially dangerous side effects associated with its use or misuse that should not be ignored. Clenbuterol can cause bone loss which, when couple with muscle growth, can increase the risk of fractures for humans (Bonnet et al., 2005). In addition, clenbuterol ingested directly or via contaminated meat from treated animals can be toxic and can lead to a variety of symptoms in humans, including tachycardia, myocardial infarction, distal tremors, hyperglycemia, among other possible symptoms (Brambilla et al., 2000; Hoffman et al., 2001; Kierzkowska et al., 2005). Clenbuterol treatment has been associated with testicular damage (Blanco et al., 2002) and thymocyte apoptosis, which can suppress immune function (Blanco et al., 2003). Clenbuterol treatment can also impact cardiac function in a negative way at low doses (Burniston et al., 2002; Sleeper et al., 2002). Taken together, the data suggest that great care must be taken when using a β2 agonist, such as clenbuterol.
Interestingly, the effective doses found for some monkeys in the current study (0.0001–0.01 mg/kg) are comparable to those published to be effective for bronchodilation (0.00067–0.00075 mg/kg). In these studies, there were no cardiovascular effects and mild and transient tremors were the only side effects observed at those low doses (Baronti et al., 1980; Boner et al., 1987; Brusasco et al., 1978). Furthermore, a low dose of clenbuterol (0.01 mg/kg), similar to our monkey study, can have anabolic effects in the absence of myocyte death (Burniston et al., 2006) and is somewhat lower than the dose of clenbuterol (0.02–5 mg/kg) shown to have a negative impact on cardiac function in animals (Burniston et al., 2002; Sleeper et al., 2002). However, long-term studies are still needed to determine whether chronic clenbuterol treatment is safe within these dose ranges. Nevertheless, the data suggest that at very low doses, clenbuterol could produce significant global cognitive enhancement with mild side effects.
In conclusion, data suggest that clenbuterol can produce a very modest enhancement of WM performance in both rats and monkeys with WM deficits. The improvement seen after clenbuterol treatment is specific to the β2 adrenoceptor, since a β2 adrenergic antagonist completely reverses the beneficial effects seen during cognitive testing. Even though clenbuterol’s effects were modest in the PFC, it may still be useful as a global enhancer of cognition, as it is one of the few agents that can improve WM as well as long-term memory consolidation. However, a number of different studies add a cautionary note to clenbuterol’s clinical efficacy since there are potentially dangerous side effects associated with its acute or chronic use or misuse. Finally, the efficacy of β2 adrenoceptor activation to improve PFC cognitive function may be particularly important in the elderly population with age-related WM deficits and glucose metabolism reduction, if very low dose clenbuterol treatments are used.
Acknowledgements
We are grateful to Tracy Sadlon, Lisa Ciavarella, Sam Johnson and Jessica Thomas for their technical expertise in carrying out this research. This research was supported by MERIT Award AG06036 to AFTA and by Ford Foundation Predoctoral Fellowship to BPR.
Footnotes
Disclosure
None of the authors have any actual or potential conflicts of interest that could inappropriately influence (bias) this study.
References
- Albert MS. The ageing brain: normal and abnormal memory. Phil. Trans. Roy. Soc. Lond. B. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Ohashi Y. Longitudinal study on age-related changes of working and reference memory in the rat. Neurosci. Lett. 1991;128:17–20. doi: 10.1016/0304-3940(91)90750-n. [DOI] [PubMed] [Google Scholar]
- Aoki C. Beta-adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J. Neurosci. 1992;12(3):781–792. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, Pickel VM. Ultrastructural relations between beta-adrenergic receptors and catecholaminergic neurons. Brain. Res. Bull. 1992;29(3–4):257–263. doi: 10.1016/0361-9230(92)90055-3. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Kurose H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. Adv. Pharmacol. 1998;42:777–780. doi: 10.1016/s1054-3589(08)60862-5. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS. Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged non-human primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Baronti A, Grieco A, Vibelli C. Oral NAB 365 (clenbuterol) and terbutaline in chronic obstructive lung disease: a double-blind, two-week study. Int. J. Clin. Pharmacol. Ther. Toxicol. 1980;18(1):21–25. [PubMed] [Google Scholar]
- Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitating effects on short-term memory. J. Gerontol. 1978;33:858–871. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Bolen SJ, Manley MS, Foote SL. Modulation of forebrain electroencephalographic (EEG) activity in the halothane-anesthetized rat via actions of noradrenergic beta-receptors located within the medial septal region of the basal forebrain. J. Neurosci. 1996;16:7010–7020. doi: 10.1523/JNEUROSCI.16-21-07010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol. Aging. 2003;24(1):37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Gobeske KT, Auerbach J, Taylor JR, Arnsten AFT. A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in prefrontal cortex. Biol. Psychiat. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Blanco A, Flores-Acuna F, Roldan-Villalobos R, Monterde JG. Testicular damage from anabolic treatments with the beta(2)-adrenergic agonist clenbuterol in pigs: a light and electron microscope study. Vet. J. 2002;163(3):292–298. doi: 10.1053/tvjl.2001.0693. [DOI] [PubMed] [Google Scholar]
- Blanco A, Artacho-Perula E, Flores-Acuna R, Moyano R, Monterde JG. Quantitative changes in the normal and apoptotic thymocytes of pigs treated with anabolic doses of the beta2 adrenergic agonist clenbuterol. Vet. Immunol. Immunopathol. 2003;96(1–2):111–115. doi: 10.1016/s0165-2427(03)00162-4. [DOI] [PubMed] [Google Scholar]
- Boner AL, Sette L, Castellani C, Schiassi M. Oral clenbuterol and procaterol. A double-blind comparison of bronchodilator effects in children with chronic asthma. J. Asthma. 1987;24(6):347–353. doi: 10.3109/02770908709070966. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Brunet-Imbault B, Arlettaz A, Horcajada MN, Collomp K, Benhamou CL, Courteix D. Alteration of trabecular bone under chronic beta2 agonists treatment. Med. Sci. Sports Exerc. 2005;37(9):1493–1501. doi: 10.1249/01.mss.0000177592.82507.95. [DOI] [PubMed] [Google Scholar]
- Brambilla G, Cenci T, Franconi F, Galarini R, Macri A, Rondoni F, Strozzi M, Loizzo A. Clinical and pharmacological profile in a clenbuterol epidemic poisoning of contaminated beef meat in Italy. Toxicol. Lett. 2000;114(1–3):47–53. doi: 10.1016/s0378-4274(99)00270-2. [DOI] [PubMed] [Google Scholar]
- Brusasco V, Vibelli C, Chierichetti S, Ramoino R. Dose–response study of a new oral bronchodilator, NAB 365 (clenbuterol) Int. J. Clin. Pharmacol. Biopharm. 1978;16(12):589–593. [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Burniston JG, Ng Y, Clark WA, Colyer J, Tan LB, Goldspink DF. Myotoxic effects of clenbuterol in the rat heart and soleus muscle. J. Appl. Physiol. 2002;93(5):1824–1832. doi: 10.1152/japplphysiol.00139.2002. [DOI] [PubMed] [Google Scholar]
- Burniston JG, Clark WA, Tan LB, Goldspink DF. Dose-dependent separation of the hypertrophic and myotoxic effects of the beta(2)-adrenergic receptor agonist clenbuterol in rat striated muscles. Muscle Nerve. 2006;33(5):655–663. doi: 10.1002/mus.20504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Prins B, Weber M, McGaugh JL. Beta-adrenergic activation and memory for emotional events. Nature. 1994;371:702–704. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Age-related prefrontal alterations during auditory memory. Neurobiol. Aging. 1997;18:87–95. doi: 10.1016/s0197-4580(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Choo JJ, Horan MA, Little RA, Rothwell NJ. Anabolic effects of clenbuterol on skeletal muscle are mediated by beta 2-adrenoceptor activation. Am. J. Physiol. 1992;263:E50–E56. doi: 10.1152/ajpendo.1992.263.1.E50. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nat. Neurosci. 2002;5(2):175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- De Santi S, de Leon MJ, Convit A, Tarshish C, Rusinek H, Tsui WH, Sinaiko E, Wang GJ, Bartlet E, Volkow N. Age-related changes in brain. II. Positron emission tomography of frontal and temporal lobe glucose metabolism in normal subjects. Psychiat. Quart. 1995;66(4):357–370. doi: 10.1007/BF02238755. [DOI] [PubMed] [Google Scholar]
- Divac I. Frontal lobe system and spatial reversal in the rat. Neuropsychologia. 1971;9:175–183. doi: 10.1016/0028-3932(71)90041-8. [DOI] [PubMed] [Google Scholar]
- Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.05.025. (Online) [DOI] [PubMed] [Google Scholar]
- Eberling JL, Roberts JA, Rapp PR, Tuszynski MH, Jagust WJ. Cerebral glucose metabolism and memory in aged rhesus macaques. Neurobiol. Aging. 1997;18(4):437–443. doi: 10.1016/s0197-4580(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha-1-adrenoceptors. J. Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenz M, Lowry JP. Studies of the source of glucose in the extracellular compartment of the rat brain. Dev. Neurosci. 1998;20(4–5):365–368. doi: 10.1159/000017332. [DOI] [PubMed] [Google Scholar]
- Fillenz M, Lowry JP, Boutelle MG, Fray AE. The role of astrocytes and noradrenaline in neuronal glucose metabolism. Acta Physiol. Scand. 1999;167:275–284. doi: 10.1046/j.1365-201x.1999.00578.x. [DOI] [PubMed] [Google Scholar]
- Flugge G, Ahrens O, Fuchs E. Beta-adrenoceptors in the tree shrew brain. II. Time-dependent effects of chronic psychosocial stress on [125I]iodocyanopindolol bindings sites. Cell. Mol. Neurobiol. 1997;17(4):401–415. doi: 10.1023/A:1026387311220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franowicz JS, Kessler L, Dailey-Borja CM, Kobilka BK, Limbird LE, Arnsten AFT. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J. Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Gallager DW. Overlap of dopaminergic, adrenergic, and serotonergic receptors and complementarity of their subtypes in primate prefrontal cortex. J. Neurosci. 1990;10:2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav. Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hesketh JE, Campbell GP, Lobley GE, Maltin CA, Acamovic F, Palmer RM. Stimulation of actin and myosin synthesis in rat gastrocnemius muscle by clenbuterol; evidence for translational control. Comp. Biochem. Physiol. C. 1992;102(1):23–27. doi: 10.1016/0742-8413(92)90037-8. [DOI] [PubMed] [Google Scholar]
- Hoffman RJ, Hoffman RS, Freyberg CL, Poppenga RH, Nelson LS. Clenbuterol ingestion causing prolonged tachycardia, hypokalemia, and hypophosphatemia with confirmation by quantitative levels. J. Toxicol. Clin. Toxicol. 2001;39(4):339–344. doi: 10.1081/clt-100105152. [DOI] [PubMed] [Google Scholar]
- Hopkins WF, Johnston D. Noradrenergic enhancement of long-term potentiation at mossy fiber synapses in the hippocampus. J. Neurophysiol. 1988;59:667–687. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- Introini-Collison IB, Miyazaki B, McGaugh JL. Involvement of the amygdala in the memory-enhancing effects of clenbuterol. Psychopharmacology. 1991;104(4):541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Andorn AC, Tabaton M, Whitehouse PJ, Harik SI, Unnerstall JR. Adrenergic receptors in aging and Alzheimer’s disease: increased beta 2-receptors in prefrontal cortex and hippocampus. J. Neurochem. 1989;53(6):1772–1781. doi: 10.1111/j.1471-4159.1989.tb09242.x. [DOI] [PubMed] [Google Scholar]
- Kapur S, Craik FI, Jones C, Brown GM, Houle S, Tulving E. Functional role of the prefrontal cortex in retrieval of memories: a PET study. Neuroreport. 1995;6:1880–1884. doi: 10.1097/00001756-199510020-00014. [DOI] [PubMed] [Google Scholar]
- Kierzkowska B, Stanczyk J, Kasprzak JD. Myocardial infarction in a 17-year-old body builder using clenbuterol. Circ. J. 2005;69(9):1144–1146. doi: 10.1253/circj.69.1144. [DOI] [PubMed] [Google Scholar]
- Korol DL, Gold PE. Glucose, memory, and aging. Am. J. Clin. Nutr. 1998;67(4):764–771. doi: 10.1093/ajcn/67.4.764S. [DOI] [PubMed] [Google Scholar]
- Lepage M, Ghaffar O, Nyberg L, Tulving E. Prefrontal cortex and episodic memory retrieval mode. Proc. Natl. Acad. Sci. U.S.A. 2000;97:506–511. doi: 10.1073/pnas.97.1.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B-M, Mei Z-T. Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav. Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc. Natl. Acad. Sci. U.S.A. 2005;102(30):10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navegantes LC, Resano NM, Migliorini RH, Kettelhut IC. Cat-echolamines inhibit Ca(2+)-dependent proteolysis in rat skeletal muscle through beta(2)-adrenoceptors and cAMP. Am. J. Physiol. Endocrinol. Metab. 2001;281(3):E449–E454. doi: 10.1152/ajpendo.2001.281.3.E449. [DOI] [PubMed] [Google Scholar]
- Ng GY, da S, Ohlsson A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst. Rev. 2001;3 doi: 10.1002/14651858.CD003214. CD003214. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone VA, Hokfelt T. Cellular localization of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain: an in situ hybridization study. Neuroscience. 1993;56:1023–1039. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cerebral Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Noda A, Ohba H, Kakiuchi T, Futatsubashi M, Tsukada H, Nishimura S. Age-related changes in cerebral blood flow and glucose metabolism in conscious rhesus monkeys. Brain Res. 2002;936(1–2):76–81. doi: 10.1016/s0006-8993(02)02558-1. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Eguchi Y, Ichiki J, Yahara J, Noda S. Therapeutic efficacy of clenbuterol for urinary incontinence after radical prostatectomy. Int. J. Urol. 1997;4(5):480–483. doi: 10.1111/j.1442-2042.1997.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ordway GA, O’Donnell JM, Frazer A. Effects of clenbuterol on beta-1 and beta-2 adrenergic receptors of the rat. J. Pharm. Exp. Ther. 1987;241(1):187–195. [PubMed] [Google Scholar]
- Prather ID, Brown DE, North P, Wilson JR. Clenbuterol: a substitute for anabolic steroids? Med. Sci. Sports Exerc. 1995;27(8):1118–1121. [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB. Quantitative autoradiography of beta1- and beta2-adrenergic receptors in rat brain. Proc. Natl. Acad. Sci. U.S.A. 1984;81:1585–1589. doi: 10.1073/pnas.81.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos B, Colgan L, Nou E, Arnsten AFT. The b1 adrenergic antagonist, betaxolol, improves working memory performance in rats and monkeys. Biol. Psychiat. 2005 doi: 10.1016/j.biopsych.2005.05.022. (Online) [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J. Neurosci. 2000;20(1):485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J. Neurosci. 1989;9:3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated Interaction between alpha2-adrenergic receptors and spinophilin. J. Biol. Chem. 2001;276(18):15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor—cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. Beta 2-agonist administration reverses muscle wasting and improves muscle function in aged rats. J. Physiol. 2004;555:175–188. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Savage CR, Alpert NM, Rauch SL, Albert MS. The role of hippocampus and frontal cortex in age-related memory changes: a PET study. Neuroreport. 1996;7:1165–1169. doi: 10.1097/00001756-199604260-00014. [DOI] [PubMed] [Google Scholar]
- Sessler FM, Mouradian RD, Cheng JT, Yeh HH, Liu WM, Waterhouse BD. Noradrenergic potentiation of cerebellar Purkinje cell responses to GABA: evidence for mediation through the beta-adrenoceptor-coupled cyclic AMP system. Brain. Res. Rev. 1989;499:27–38. doi: 10.1016/0006-8993(89)91132-3. [DOI] [PubMed] [Google Scholar]
- Sleeper MM, Kearns CF, McKeever KH. Chronic clenbuterol administration negatively alters cardiac function. Med. Sci. Sports Exerc. 2002;34(4):643–650. doi: 10.1097/00005768-200204000-00013. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. NY: Oxford University Press; 2002. p. 616. [Google Scholar]
- Summers RJ, Papaioannou M, Harris S, Evans BA. Expression of beta 3-adrenoceptor mRNA in rat brain. Br. J. Pharmacol. 1995;116(6):2547–2548. doi: 10.1111/j.1476-5381.1995.tb17205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Birnbaum SG, Ubriani R, Arnsten AFT. Activation of protein kinase A in prefrontal cortex impairs working memory performance. J. Neurosci. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. (Online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevaearai HT, Koch WJ. Molecular restoration of beta-adrenergic receptor signaling improves contractile function of failing hearts. Trends Cardiovasc. Med. 2004;14:252–256. doi: 10.1016/j.tcm.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Noradrenergic modulation of somatosensory cortical neuronal responses to iontophoretically applied putative transmitters. Exp. Neurol. 1980;69:30–49. doi: 10.1016/0014-4886(80)90141-7. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ. Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology. 1981;20:907–920. doi: 10.1016/0028-3908(81)90020-4. [DOI] [PubMed] [Google Scholar]
- Yimlamai T, Dodd SL, Borst SE, Park S. Clenbuterol induces muscle-specific attenuation of atrophy through effects on the ubiquitin-proteasome pathway. J. Appl. Physiol. 2005;99(1):71–80. doi: 10.1152/japplphysiol.00448.2004. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kurita C, Nagao T, Usami E, Nakao T, Watanabe S, Kobayashi J, Yamazaki F, Tanaka H, Inagaki N, Nagai H. Inhibition of tumor necrosis factor-alpha and interleukin-1-beta production by beta-adrenoceptor agonists from lipopolysaccharide-stimulated human peripheral blood mononuclear cells. Pharmacology. 1997;54(3):144–152. doi: 10.1159/000139481. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Mishler K, Roerig SC, O’Donnell JM. Interaction between the antidepressant-like behavioral effects of beta adrenergic agonists and the cyclic AMP PDE inhibitor rolipram in rats. Psychopharmacology (Berl.) 2005;182(1):104–115. doi: 10.1007/s00213-005-0055-y. [DOI] [PubMed] [Google Scholar]