Abstract
Angiotensin II plays an important role in the development of cardiac hypertrophy and fibrosis, but the underlying cellular and molecular mechanisms are not completely understood. Recent studies have shown that bone marrow-derived fibroblast precursors are involved in the pathogenesis of cardiac fibrosis. Since bone marrow-derived fibroblast precursors express chemokine receptor, CCR2, we tested the hypothesis that CCR2 mediates the recruitment of fibroblast precursors into the heart, causing angiotensin II-induced cardiac fibrosis. Wild-type and CCR2 knockout mice were infused with angiotensin II at 1,500 ng·kg−1·min−1. Angiotensin II treatment resulted in elevated blood pressure and cardiac hypertrophy that were not significantly different between wild-type and CCR2 knockout mice. Angiotensin II treatment of wild-type mice caused prominent cardiac fibrosis and accumulation of bone marrow-derived fibroblast precursors expressing the hematopoietic markers, CD34 and CD45, and the mesenchymal marker, collagen I. However, angiotensin II-induced cardiac fibrosis and accumulation of bone marrow-derived fibroblast precursors in the heart were abrogated in CCR2 knockout mice. Furthermore, angiotensin II treatment of wild-type mice increased the levels of collagen I, fibronectin, and α-smooth muscle actin in the heart, whereas these changes were not observed in the heart of angiotensin II-treated CCR2 knockout mice. Functional studies revealed that the reduction of cardiac fibrosis led to an impairment of cardiac systolic function and left ventricular dilatation in angiotensin II-treated CCR2 knockout mice. Our data demonstrate that CCR2 plays a pivotal role in the pathogenesis of angiotensin II-induced cardiac fibrosis through regulation of bone marrow-derived fibroblast precursors.
Keywords: bone marrow-derived progenitor cells, cardiac fibroblast, myocardial fibrosis, extracellular matrix
left ventricular (lv) remodeling in response to pressure and/or volume overload is associated with myocardial hypertrophy and cardiac fibrosis with abnormal accumulation of extracellular matrix (ECM). Cardiac fibrosis is a pathological feature of many heart diseases, including hypertensive heart disease, myocardial infarction, and cardiomyopathy and is characterized by fibroblast activation and excessive production and deposition of ECM, including multiple collagens and fibronectin (42). However, the origin of fibroblasts that are responsible for cardiac fibrosis remains unclear. They are traditionally thought to arise from resident cardiac fibroblasts. Recent evidence indicates that they may originate from hematopoietic progenitor cells (5, 16, 17, 39, 43).
The bone marrow-derived fibroblast precursor cells, termed fibrocytes, were first identified in the peripheral circulation within the blood mononuclear cell population in 1994 (4). These cells express mesenchymal markers, such as collagen I (Col I), Col III, vimentin, and fibronectin (1, 4, 24, 30). In addition, these cells express the common leukocyte antigen (CD45), the hematopoietic stem cell antigen (CD34), the pan myeloid antigen (CD13), and the adhesion molecules CD11b and CD18 (1, 4, 24, 29, 30, 34). Fibrocytes in culture display an adherent, spindle-shaped morphology and express α-smooth muscle actin (α-SMA) that is enhanced when these cells are treated with transforming growth factor-β1 and consistent with the concept that they can differentiate into myofibroblasts (1, 24, 30, 34). Recent studies from our laboratory and others have shown that bone marrow-derived fibroblast precursors are involved in the pathogenesis of cardiac fibrosis (15–17). However, the molecular mechanisms underlying the recruitment of these cells into the injured heart are not fully understood.
Chemokines play primary roles in mediating the trafficking of circulating cells to sites of inflammation and injury via activation of their seven-transmembrane G protein-coupled receptors (27). The most thoroughly characterized chemokines are monocyte chemoattractant proteins (MCPs). MCPs recruit cells through activation of their cognate receptor, CCR2, which is expressed on monocytes, including circulating fibroblast precursors. MCP-1, a major ligand for CCR2, is upregulated in response to ischemic injury and plays an important role in the pathogenesis of cardiac fibrosis (12).
Our laboratory has recently shown that MCP-1 plays an important role in regulating fibroblast precursors in the pathogenesis of angiotensin II (ANG II)-induced cardiac fibrosis (14). In the present study, we investigated the role of CCR2 in ANG II-induced cardiac remodeling using CCR2 knockout (KO) mice. Our results showed that CCR2 deficiency prevented ANG II-induced cardiac fibrosis through suppression of fibroblast precursor cell infiltration into the heart, while not affecting cardiac hypertrophy.
MATERIALS AND METHODS
Animals.
Animal experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). The CCR2 KO mice on a background of C57BL/6J were purchased from the Jackson Laboratory. Disruption of CCR2 gene was confirmed by Western blot with an anti-CCR2 antibody from Abcam (Fig. 1). Male wild-type (WT) or CCR2 KO mice at 8–12 wk old, weighing 20–30 g, received ANG II (1.5 μg·kg−1·min−1; Sigma) or vehicle (0.9% NaCl) continuously via subcutaneous osmotic minipumps (Alzet) for 7 or 28 days following uninephrectomy. Uninephrectomy was performed to augment blood pressure and organ injury (8, 20). All mice were supplied 1% saline drinking water ad libitum.
Fig. 1.
Targeted disruption of chemokine receptor CCR2 gene in mice. Top: representative Western blots show the levels of CCR2 protein expression in the heart of wild-type (WT) and CCR2-knockout (KO) mice. Bottom: quantitative analysis of CCR2 protein expression in the heart of WT. Values are means ± SE; n = 6 per group. Veh, vehicle; ANG II, angiotensin II. **P < 0.01 vs. WT-Veh.
Blood pressure and heart rate.
Systolic blood pressure (SBP) and heart rate (HR) were measured in conscious mice using a tail-cuff system (Visitech Systems), as reported (14).
Cardiac morphology.
Cardiac sections were stained with hematoxylin and eosin for initial evaluation and picrosirius red or Masson trichrome to identify collagen fiber. The cross-sectional areas of LV myocytes were measured on the mid-free wall of the LV from sections. Suitable cross-sectional areas were defined as having nearly circular capillary profiles and nuclei. A total of 100 cells were counted in each section, and the average area was used for analysis. The picrosirius red-stained sections were scanned using a microscope equipped with a digital camera (Nikon, Melville, NY), and quantitative evaluation was performed using NIS-Elements Br 3.0 software. The area of interstitial fibrosis was calculated as a percentage of the total area, and the area of perivascular fibrosis was determined as the ratio of the area of fibrosis surrounding the vessel wall to the total vessel area.
Immunohistochemistry.
After fixation, cardiac sections were then incubated with rabbit anti-Col I antibody (Rockland), followed by Alexa-488 conjugated donkey anti-rabbit antibody (Invitrogen), rabbit anti-fibronectin antibody (Sigma), followed by Alexa-488 conjugated donkey anti-rabbit antibody (Invitrogen), or rat anti-CD68 antibody (AbD Serotec), followed by Alexa-598 conjugated donkey anti-rat antibody (Invitrogen). For double immunofluorescence, cells were fixed and stained with primary antibodies, followed by appropriate secondary antibodies sequentially. Slides were mounted with VECTASHIELD HardSet mounting medium with 4,6-diamidino-2-phenylindole (DAPI). Fluorescent intensity was visualized using a microscope equipped with a digital camera (Nikon, Melville, NY). For α-SMA staining, paraffin-embedded tissues were cut at 5-μm thickness, deparaffinized, and quenched with 3% hydrogen peroxide in methanol for 10 min. After heat retrieval, slides were incubated with rabbit anti-α-SMA antibody, followed by biotin-conjugated goat anti-rabbit IgG, and then incubated with Vectastain ABC reagent. Bound antibodies were detected using diaminobenzidine as a substrate and counterstained with hematoxylin. Quantitative evaluation of sections was performed using NIS-Elements Br 3.0 software. The positively stained interstitial area was calculated as a percentage of the total area, and the positively stained perivascular area was calculated as the ratio of the area surrounding the vessel to the total vessel area.
Cell isolation and flow cytometry.
Cardiac fibroblasts were isolated as described (17). Cells (5 × 105) were incubated with phycoerythrin (PE)-anti-CD34, FITC-anti-CD45, (all from BD Biosciences, San Jose, CA), PE-anti-α-SMA (R&D Systems, Minneapolis, MN), or biotin-anti-Col I (Rockland, Gilbertsville, PA)/streptavidin-allophycocyanin (APC) (BD Biosciences). Peripheral blood was obtained via cardiac puncture. Samples were lysed with BD PharM Lyse for 15 min, washed in PBS containing DNase, and blocked with PACS buffer for 30 min at 4°C. Cells were stained for CD45, Col I, and CCR2. If necessary, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (PE BD Biosciences), in accordance with the manufacturer's protocol. Cells incubated with irrelevant isotype-matched antibodies and unstained cells were used as controls. The cutoffs were set according to results of controls. FITC/PE/APC fluorescence intensities were measured using a LSR II flow cytometer (BD Biosciences). Data were analyzed using BD FACSDiva software.
Quantitative real-time RT-PCR.
Quantitative analysis of mRNA expression was performed with real-time RT-PCR. Total RNA was extracted from snap-frozen heart tissues with TRIzol Reagent (Invitrogen). Aliquots (1 μg) of total RNA were reverse-transcribed and amplified using IQ SYBR green supermix reagent (Bio-Rad, Hercules, CA) with Opticon real-time PCR machine (MJ Research, Waltham, MA), according to the manufacturer's instructions. The specificity of real-time PCR was confirmed via routine agarose gel electrophoresis and melting-curve analysis. The expression level of the target genes was normalized by the GAPDH level in each sample. The following are the primer sequences: Col I, forward 5′-TGCCGCGACCTCAAGATGTG-3′ and reverse 5′-CACAAGGGTGCTGTAGGTGA-3′; fibronectin, forward 5′-CTTCTCCGTGGAGTTTTACCG-3′ and reverse 5′-GCTGTCAAATTGAATGGTGGTG-3′; α-SMA, forward 5′-ACTGGGACGACATGGAAAAG-3′ and reverse 5′-CATCTCCAGAGTCCAGCACA-3′; brain natriuretic peptide (BNP), forward 5′-AAGTCCTAGCCAGTCTCCAGA-3′ and reverse 5′-GAGCTGTCTCTGGGCCATTTC-3′; and GAPDH, forward 5′-TGCTGAGTATGTCGTGGAGTCTA-3′ and reverse 5′-AGTGGGAGTTGCTGTTGAAATC-3′.
Western blot analysis.
Protein was extracted using the RIPA buffer containing cocktail proteinase inhibitors and quantified with Bio-Rad protein assay. Equal amount of protein was separated on SDS-polyacrylamide gels in a Tris·HCl buffer system, transferred onto nitrocellulose membranes, and blotted with anti-α-SMA antibody, anti-ED-A fibronectin antibody, or anti-CCR2 antibody. The specific bands of target proteins were visualized by chemiluminescence, and band intensities were quantified using National Institutes of Health Image/J. Membranes were then stripped and reblotted with anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). Target signals were normalized to GAPDH signal.
Cardiovascular parameters.
Cardiac function was obtained by two-dimensional-directed M-mode echocardiography (Vevo770; Visual Sonics) and Doppler Ultrasound (model DSPW, Indus Instruments), as previous described (9).
Statistical analysis.
All data were expressed as means ± SE. Multiple-group comparisons were performed by one-way ANOVA, followed by the Bonferroni procedure for comparison of means. P < 0.05 was considered statistically significant.
RESULTS
Blood pressure and HR.
The effects of ANG II treatment on SBP and HR are summarized in Table 1. There were no significant differences among the four groups before ANG II treatment. SBP was similarly elevated in both WT and CCR2-KO mice after ANG II infusion. There was no significant difference in HR among the groups.
Table 1.
Cardiovascular parameters after 4 wk of ANG II infusion
| Parameters | WT-Vehicle | WT-ANG II | KO-Vehicle | KO-ANG II |
|---|---|---|---|---|
| HR, beats/min | 381 ± 15 | 403 ± 13 | 370 ± 16 | 402 ± 18 |
| SBP, mmHg | 99.5 ± 2.8 | 155.8 ± 2.3* | 101.1 ± 1.1 | 149.8 ± 3.6† |
| LVEDV, μl | 72.92 ± 4.46 | 77.9 ± 4.4 | 65.95 ± 3.84 | 89.25 ± 6.81† |
| LVEDD, mm | 4.05 ± 0.10 | 4.16 ± 0.10 | 3.88 ± 0.09 | 4.40 ± 0.15† |
| LVAW-d, mm | 0.97 ± 0.06 | 1.15 ± 0.03* | 1.02 ± 0.04 | 1.21 ± 0.06† |
| LVPW-d, mm | 0.73 ± 0.05 | 1.07 ± 0.06* | 0.77 ± 0.03 | 1.08 ± 0.07† |
| EF, % | 63.68 ± 3.53 | 56.16 ± 2.73 | 68.80 ± 2.86 | 42.86 ± 2.32#† |
| FS, % | 34.82 ± 2.49 | 29.47 ± 1.90 | 38.63 ± 2.32 | 21.07 ± 1.28#† |
| E-peak V, cm/s | 82.36 ± 4.04 | 78.62 ± 5.42 | 75.45 ± 2.36 | 81.90 ± 2.94 |
| IVRT/RR | 0.11 ± 0.00 | 0.17 ± 0.01* | 0.12 ± 0.01 | 0.18 ± 0.01† |
Values are means ± SE; n = 11–14 subjects per group. HR, heart rate; SBP, systolic blood pressure; LVEDV, left ventricular (LV) end-diastolic volume; LVEDD, LV end-diastolic diameter; LVAW-d, LV anterior wall thickness during diastole; LVPW-d, LV posterior wall thickness during diastole; EF, ejection fraction; FS, fractional shortening; E-peak V, early transmitral filling velocity; IVRT/R-R, isovolumic relaxation time corrected by the time per heartbeat; WT, wild type; KO, knockout; ANG II, angiotensin II.
P < 0.05 vs. WT-vehicle.
P < 0.05 vs. WT-ANG II.
P < 0.05 vs. KO-vehicle.
Cardiac fibrosis.
To test whether CCR2 deficiency influenced cardiac fibrosis, WT and CCR2-KO mice were treated with ANG II or vehicle for 4 wk. The staining for both picrosirius red and Masson trichrome was performed on cardiac sections, and the results of the two stainings were similar. Representative photomicrographs are shown in Fig. 2, A and B, and the results of morphometric analysis are shown in Fig. 2, C and D. WT mice treated with ANG II developed marked interstitial and perivascular collagen deposition in the heart. In contrast, CCR2-KO mice exhibited much less increase in collagen accumulation, despite ANG II treatment. These data indicate that CCR2 plays a critical role in the pathogenesis of cardiac fibrosis.
Fig. 2.
Targeted disruption of CCR2 abrogates cardiac fibrosis and extracellular matrix deposition in the heart. A: picrosirius red stain. B: Masson's trichrome stain. Panels show representative photomicrographs of cardiac sections with interstitial (top) and perivascular (bottom) fibrosis. C and D: bar graphs show quantitative analysis of cardiac interstitial (C) and perivascular (D) collagen in different groups, as indicated. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II.
We next investigated the effect of CCR2 deficiency on expression and accumulation of Col I and fibronectin, two major components of ECM. As shown in Figs. 3A and 4A, there was a marked increase in the mRNA levels of Col I and fibronectin in the WT mice treated with ANG II, whereas CCR2-KO mice exhibited much less increase in the mRNA expression of Col I and fibronectin in the heart after ANG II treatment. We then examined the levels of protein expression in the heart in response to ANG II. There was minimal Col I and fibronectin protein accumulation in the normal hearts of both WT and CCR2-KO mice. After ANG II treatment for 4 wk, WT mice developed severe interstitial and perivascular fibrosis, as demonstrated by an accumulation of Col I and fibronectin deposition that was significantly lower in the heart of ANG II-treated CCR2-KO mice (Fig. 3, B–D, and Fig. 4, B–D). These data indicate that targeted deletion of CCR2 attenuates cardiac fibrosis by inhibiting ECM protein production and deposition.
Fig. 3.
Targeted disruption of CCR2 inhibits collagen I (Col I) expression in the heart. A: mRNA levels of Col I in the heart of WT and CCR2-KO mice in response to ANG II, as determined by real-time RT-PCR. Values are means ± SE; n = 3–4 per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II. B: immunostaining for Col I. Panels show representative photomicrographs of interstitial (top) and perivascular (bottom) Col I immunostaining in the heart of WT and CCR2-KO mice after 4 wk of ANG II infusion. C and D: quantitative analysis of interstitial (C) and perivascular (D) Col I protein expression in the heart of WT and CCR2-KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II.
Fig. 4.
Targeted disruption of CCR2 reduces fibronectin (FN) expression in the heart. A: mRNA levels of FN in the heart of WT and CCR2-KO mice in response to ANG II, as determined by real-time RT-PCR. Values are means ± SE; n = 3–4 samples per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II. B: immunostaining for FN. Panels show representative photomicrographs of interstitial (top) and perivascular (bottom) FN immunostaining in the heart of WT and CCR2-KO mice after 4 wk of ANG II. C and D: quantitative analysis of interstitial (C) and perivascular (D) FN protein expression in the heart of WT and CCR2-KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II.
Bone marrow-derived fibroblast precursors.
Bone marrow-derived fibroblast precursors have been shown to play a critical role in the pathogenesis of cardiac fibrosis, and chemokines, through interaction with their cognate receptors, mediate the recruitment and differentiation of bone marrow-derived fibroblast precursors (17, 19). To verify if CCR2, the receptor for MCP-1, is expressed in circulating fibroblast precursors, peripheral blood nucleated cells were stained for CD45, CCR2, and Col I and examined with a deconvolution microscope. Our results showed CD45, CCR2, and Col I triple-positive cells are present in the peripheral circulation (Fig. 5A). These data confirm that circulating fibroblast precursors express CCR2. To determine the percentage of bone marrow-derived fibroblast precursors that express CCR2, peripheral blood nucleated cells were stained for CD45, CCR2, and Col I using flow cytometry. Our results showed that >80% of bone marrow-derived fibroblast precursors express CCR2 (Fig. 5B). To examine the role of CCR2 in the recruitment of bone marrow-derived fibroblast precursors into the heart, WT and CCR2-KO mice were treated with ANG II or vehicle for 7 days. Cardiac fibroblasts were isolated and cultured. These cells were stained for CD45 and Col I, or CD34 and Col I, and examined using a fluorescent microscope. Our results showed that CD45+ and Col I+ cells or CD34+ and Col I+ cells were present in the heart of ANG II-treated WT mice. In contrast, these cells were rarely found in the heart of ANG II-treated CCR2-KO mice (Fig. 6A). To quantify the number of bone marrow-derived fibroblast precursors in the heart, freshly dispersed cardiac cells were stained for CD45, CD34, and Col I and analyzed by flow cytometry. The number of bone marrow-derived fibroblast precursors identified as CD45+ and Col I+ or CD34+ and Col I+ cells was markedly increased in the heart of ANG II-treated WT mice, whereas the number of bone marrow-derived fibroblast precursors was significantly reduced in the heart of ANG II-treated CCR2-KO mice (Fig. 6, B and C). These data indicate that CCR2 mediates the trafficking of bone marrow-derived fibroblast precursors into the heart.
Fig. 5.
Bone marrow-derived fibroblast precursors express CCR2. A: immunofluorescence staining showing bone marrow-derived fibroblast precursors express CCR2. Peripheral blood nucleated cells were stained for CD45 (green), CCR2 (red), and Col I (blue) and identified by a deconvolution fluorescence microscope (original magnification: ×600). CD45, CCR2, and Col I triple-positive fibroblast precursors were detected in the peripheral blood. B: quantitative analysis of CD45+, CCR2+, and Col I+ fibroblast precursors in the peripheral blood, as determined by flow cytometry. Left cytometric diagram shows isotype controls, and right cytometric diagram shows CCR2 and Col I dual-positive cells in CD45 population. n = 4 per group.
Fig. 6.
Bone marrow-derived fibroblast precursors accumulate in the heart in a CCR2-dependent manner. A: immunostaining for bone marrow-derived fibroblast precursors. Panels show representative photomicrographs of cultured cardiac fibroblasts from WT and CCR2-KO mice 1 wk after ANG II infusion stained for CD45 (top) or CD34 (bottom) (red), Col I (green), and 4,6-diamidino-2-phenylindole (DAPI; blue). Small and spindle-shaped cells (arrows) are present in the heart of ANG II-treated mice and stained positive for CD45 (top) or CD34 (bottom) and Col I. Shown are flow cytometric analysis of CD45+ and Col I+ (B), CD34+ and Col I+ (C), and CD45+ and α-smooth muscle actin (α-SMA+) (D) fibroblasts from the heart of WT and CCR2-KO mice 1 wk after ANG II infusion. *P < 0.05 vs. WT-Veh, **P < 0.05 vs. WT-Veh, #P < 0.05 vs. WT-ANG II. Values are means ± SE; n = 3 per group.
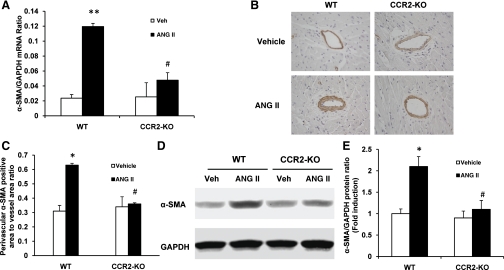
We then determined if CCR2 influences the number of bone marrow-derived myofibroblasts in the heart. WT and CCR2-KO mice were treated with ANG II or vehicle for 1 wk. Freshly dispersed cardiac cells were stained for CD45, a marker of hematopoietic cell, and α-SMA, a marker of myofibroblasts, and then analyzed by flow cytometry. Targeted deletion of CCR2 resulted in a significant reduction of the number of bone marrow-derived myofibroblasts in the heart treated with ANG II (Fig. 6D). Consistent with these findings, real-time RT-PCR showed that the mRNA level of α-SMA was significantly reduced in the heart of ANG II-treated CCR2-KO mice compared with that of WT mice treated with ANG II (Fig. 7A). These findings were confirmed at the protein levels, as demonstrated by immunostaining and Western blot analysis (Fig. 7, B–E). We also examined if CCR2 deficiency affects the expression level of ED-A fibronectin, another marker of myofibroblasts (36, 40). The level of ED-A fibronectin was significantly induced in the heart of ANG II-treated WT mice compared with vehicle-treated WT mice. In contrast, the level of ED-A fibronectin was dramatically reduced in the heart of ANG II-treated CCR2 KO mice compared with ANG II-treated WT mice (Fig. 8). These data indicate that CCR2 plays an important role in the activation and differentiation of bone marrow-derived fibroblasts.
Fig. 7.
Targeted disruption of CCR2 reduces α-SMA expression in ANG II-induced hypertensive heart disease. A: mRNA levels of α-SMA in the heart of WT and CCR2-KO mice in response to ANG II, as determined by real-time RT-PCR. Values are means ± SE; n = 3–4 per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II. B: representative photomicrographs of α-SMA immunostaining in the heart of WT and CCR-KO mice 4 wk after ANG II treatment. C: quantitative measurements of α-SMA protein expression in the heart of WT and CCR2-KO mice. Values are means ± SE; n = 6 per group. *P < 0.05 vs. WT-Veh. #P < 0.05 vs. WT-ANG II. D: representative Western blots show the levels of α-SMA protein expression in the heart of WT and CCR2-KO mice. E: quantitative analysis of α-SMA protein expression in the heart of WT and CCR2-KO mice. Values are means ± SE; n = 6 per group. *P < 0.05 vs. WT-Veh. #P < 0.05 vs. WT-ANG II.
Fig. 8.
Targeted disruption of CCR2 reduces ED-A FN protein expression in ANG II-induced hypertensive heart disease. A: representative Western blots show the levels of ED-A FN protein expression in the heart of WT and CCR2-KO mice. B: quantitative analysis of ED-A FN protein expression in the heart of WT and CCR2-KO mice. Values are means ± SE; n = 6 per group. **P < 0.01 vs. WT-Veh. #P < 0.05 vs. WT-ANG II.
Cardiac hypertrophy.
Hypertrophic responses to sustained hypertension induced by ANG II infusion were assessed in WT and CCR2-KO mice. Before ANG II infusion, WT and CCR2-KO mice did not exhibit significant differences in heart weight. In response to chronic ANG II infusion, both WT and CCR2-KO mice developed comparable cardiac hypertrophy, as indicated by increased heart-to-body weight ratio, heart weight-to-tibia length ratio, and myocyte cross-sectional area (Fig. 9, A–C). Next, we examined the expression levels of BNP, a marker for cardiac hypertrophy, using real-time RT-PCR. Our result showed that mRNA levels of BNP were upregulated in both WT and CCR2-KO mice in response to ANG II, which was not statistically different between the two groups (Fig. 9D). We also evaluated LV hypertrophy (LVH) using echocardiography. After ANG II treatment, both WT and CCR2-KO mice developed comparable cardiac hypertrophy, as judged by anterior and posterior wall thickness (Table 1). These data indicate that CCR2 deficiency does not affect the development of ANG II-induced hypertrophy.
Fig. 9.
Targeted disruption of CCR2 does not affect hypertrophic cardiac remodeling. A: heart weight-to-body weight ratio. *P < 0.05 vs. WT-Veh. +P < 0.05 vs. KO-Veh. n = 11–14 per group. B: Heart weight-to-tibia weight ratio. *P < 0.05 vs. WT-Veh. +P < 0.05 vs. KO-Veh. n = 11–14 per group. C: cardiac myocyte cross-sectional area. *P < 0.05 vs. WT-Veh. +P < 0.05 vs. WT-ANG II. n = 6 per group. D: mRNA levels of brain natriuretic peptide (BNP) in the heart of WT and CCR2-KO mice in response to ANG II, as determined by real-time RT-PCR. *P < 0.05 vs. WT-Veh. +P < 0.05 vs. KO-Veh. n = 3–4 per group. Values are means ± SE.
Cardiac function and dimension.
To investigate the effect of CCR2 deficiency on cardiac function, we performed echocardiographic examination of WT and CCR2-KO mice after 4 wk of ANG II infusion. Both WT and CCR2-KO mice treated with ANG II developed impaired LV diastolic function, as indicated by increased isovolumic relaxation time corrected by the time per heartbeat that was not statistically different between the two groups (Table 1). These data indicate that CCR2 deficiency does not affect ANG II-induced diastolic dysfunction. LV systolic function, as indicated by ejection fraction and fractional shortening, was not statistically different in WT mice treated with ANG II or vehicle. In contrast, the ejection fraction and fractional shortening were decreased in ANG II-treated CCR2-KO mice compared with vehicle-treated CCR2-KO mice, indicating impaired LV systolic function (Table 1). There was no statistical difference in end-diastolic LV diameter between ANG II-treated and vehicle-treated WT mice. However, end-diastolic LV diameter was significantly increased in ANG II-treated CCR2-KO mice compared with vehicle-treated CCR2-KO mice (Table 1).
DISCUSSION
In this study, we demonstrate that 1) WT mice develop hypertension, cardiac hypertrophy, and cardiac fibrosis in response to chronic ANG II infusion, whereas CCR2 deficiency abolishes the development of ANG II-induced cardiac fibrosis but does not affect ANG II-induced cardiac hypertrophy and hypertension; 2) there is significant accumulation of bone marrow-derived fibroblast precursor cells in the heart of WT mice in response to ANG II treatment, and the accumulation of these cells was abrogated in ANG II-treated CCR2-KO mice; and 3) ANG II-treated WT mice exhibit diastolic dysfunction, while the systolic function was preserved in those mice. In contrast, ANG II-treated CCR2-KO mice display significant systolic dysfunction and increased LV dilation without affecting ANG II-induced diastolic dysfunction. These results indicate that CCR2 plays a pivotal role in the development of ANG II-induced cardiac fibrosis through recruiting bone marrow-derived fibroblast precursors into the heart, which prevents systolic dysfunction in ANG II-induced hypertensive heart disease.
Cardiac fibrosis is a hallmark of myocardial remodeling, including hypertensive heart disease. Activated fibroblasts are responsible for the excessive production of ECM. However, the origin of the fibroblasts that are responsible for the excessive production of ECM is still the subject of intense debate. Recent studies have provided evidence that bone marrow-derived fibroblast precursors are recruited into the heart and lead to cardiac fibrosis (15–17). These cells express the hematopoietic markers, such as CD45 and CD34, and the mesenchymal markers, such as Col I. The signaling mechanisms underlying the recruitment of bone marrow-derived fibroblast precursors into the heart are incompletely understood. Chemokines play an important role in the regulation of fibroblast precursor infiltration in response to injury. Our laboratory has previously shown that MCP-1/CCL2 induced by reactive oxygen species plays a critical role in the pathogenesis of cardiac fibrosis in a murine model of ischemic cardiomyopathy induced by daily 15-min ischemia followed by 24-h reperfusion (9). In a subsequent study, our laboratory has demonstrated that targeted disruption of MCP-1 or administration of neutralizing antibodies to MCP-1 markedly reduces the inflammatory and fibrotic response induced by ischemia-reperfusion injury (11). Using the same model, we have shown that bone marrow-derived fibroblast precursors expressing hematopoietic markers (CD34 and CD45) and mesenchymal markers (Col I) accumulate in the heart, causing cardiac fibrosis. Further studies have demonstrated that differentiation of these fibroblast precursors and associated fibrosis are regulated by Fcγ-receptors and Rho-associated kinase-1 (15, 16).
A large body of evidence indicates that activation of renin-angiotensin-aldosterone system (RAAS) plays an important role in the pathogenesis of cardiac fibrosis (3, 13, 23, 41). ANG II is an important profibrotic factor (7, 18, 35). Thus, targeting of RAAS activation with angiotensin-converting enzyme inhibitors or AT1-receptor blockers inhibits cardiac fibrosis (2, 6, 10, 21). However, the underlying cellular and molecular mechanisms involved in ANG II-induced cardiac fibrosis are poorly understood. Bone marrow-derived fibroblast precursors express certain chemokine receptors, such as CCR2, CCR5, CCR7, and CXCR4 (1, 30). This is important because chemokines, through interaction with their receptors, can recruit these cells into area of injury (25, 29, 32). Our laboratory has recently shown that ANG II regulates the uptake of bone marrow-derived fibroblast precursors and cardiac fibrosis through induction of MCP-1 (14). In the present study, we demonstrate that bone marrow-derived fibroblast precursors, identified as CD45 and Col I or CD34 and Col I dual-positive cells, accumulate in the heart in a CCR2-dependent manner after ANG II treatment. These data indicate that CCR2 is a critical mediator for the recruitment of bone marrow-derived fibroblast precursors into the heart in response to ANG II. This effect is most likely related to MCP-1 induction in the heart (14). However, CCR2 is also the receptor for MCP-3 and MCP-5, which have been shown to be pathologically significant (26). Furthermore, our data show that absence of bone marrow-derived fibroblast precursors in the heart of ANG II-treated CCR2-KO mice obviates the development of cardiac fibrosis, indicating that these cells play an obligatory role in the pathogenesis of cardiac fibrosis. Of note, our data reveal that targeted deletion of CCR2 affects more interstitial fibroblasts than perivascular smooth muscle cells (Fig. 1). This may be due to the sensitivity of vascular smooth muscle cells to CCR2 knockdown, which is less than that of the fibroblasts in the surrounding myocardium.
Our data demonstrate that bone marrow-derived myofibroblasts identified as CD45 and α-SMA dual-positive cells accumulate in the heart of ANG II-treated WT mice, and their accumulation is significantly reduced in the hearts of ANG II-treated CCR2-KO mice. This is very important because it is generally accepted that myofibroblasts are the principal effector cells responsible for excessive ECM production in the development of fibrotic heart disease (37, 38). After injury, myofibroblasts are found in the heart, but their origin remains controversial. It was traditionally thought that activated resident fibroblasts transform into myofibroblasts that are responsible for the excessive production and deposition of ECM components. Recently, our laboratory has shown that bone marrow-derived fibroblast precursors are capable of differentiating into myofibroblasts in a murine model of noninfarctive ischemic cardiomyopathy (17). In the present study, our results illustrate that bone marrow-derived fibroblast precursors can be activated and differentiate into myofibroblasts in the heart in response to ANG II.
Our results show that CCR2 deficiency does not block ANG II-induced LVH, indicating that LVH and cardiac fibrosis are two separate pathological processes in the model of ANG II-induced hypertensive heart disease. It has been suggested that activation of AT1 receptor in the cardiomyocytes may be sufficient to trigger cardiac hypertrophy (31). However, recent evidence indicates that the development of cardiac hypertrophy depends on elevated blood pressure (8). On the other hand, studies have shown that the development of cardiac fibrosis is dependent on the activation of RAAS and independent of blood pressure (3, 41).
Our data reveal that CCR2 deficiency led to systolic dysfunction and increased LV dilation after ANG II treatment. These data indicate that ANG II-induced cardiac fibrosis protects against systolic dysfunction and LV dilation in the early stage of ANG II-induced hypertensive heart disease. The precise mechanism by which CCR2 deficiency alters cardiac systolic function after ANG II treatment has yet to be elucidated. We speculate that the loss of an appropriate increase in fibrosis in ANG II-treated CCR2-KO mice led to side-to-side slippage of cardiomyocytes and cardiac systolic dysfunction, as has been reported after myocardial infarction (28). In agreement with our findings, studies have shown that a reduction in cardiac fibrosis causes increased LV dilation and impairs systolic function in mice with osteopontin deficiency following aldosterone or ANG II infusion (22, 33).
In summary, these data define a novel mechanism by which CCR2 participates in cardiac remodeling. In response to ANG II, the activated CCR2 signaling leads to recruitment of bone marrow-derived fibroblast precursors into the heart mediating the development of cardiac fibrosis. Our study indicates that ANG II-induced accumulation of bone marrow-derived fibroblast precursors in the heart via CCR2 protects against cardiac systolic dysfunction and LV dilation by promoting ECM production and plays an important role in cardiac remodeling in hypertensive heart disease.
GRANTS
This work was supported in part by the National Institutes of Health (NIH) Grant K08HL92958; American Heart Association Grant 09SDG2280102; and a grant from Dr. and Mrs. Harold Selzman. J. Xu was supported in part by Science and Technology Commission of Shanghai Municipality Grant 10ZR1438500. M. L. Entman was supported by NIH Grant R01HL89792.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. William E. Mitch for critical reading of the manuscript and helpful discussion. We also thank Jesus Hermosillo-Rodriguez and Thuy Pham for expert technical assistance.
REFERENCES
- 1. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Brilla CG, Funck RC, Rupp H. Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 102: 1388–1393, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 67: 1355–1364, 1990 [DOI] [PubMed] [Google Scholar]
- 4. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994 [PMC free article] [PubMed] [Google Scholar]
- 5. Chu PY, Mariani J, Finch S, McMullen JR, Sadoshima J, Marshall T, Kaye DM. Bone marrow-derived cells contribute to fibrosis in the chronically failing heart. Am J Pathol 176: 1735–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciulla MM, Paliotti R, Esposito A, Diez J, Lopez B, Dahlof B, Nicholls MG, Smith RD, Gilles L, Magrini F, Zanchetti A. Different effects of antihypertensive therapies based on losartan or atenolol on ultrasound and biochemical markers of myocardial fibrosis: results of a randomized trial. Circulation 110: 552–557, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Crawford DC, Chobanian AV, Brecher P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ Res 74: 727–739, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 103: 17985–17990, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A 100: 2700–2705, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Martinez Ubago JL. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation 105: 2512–2517, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation 115: 584–592, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Frangogiannis NG, Entman ML. Chemokines in myocardial ischemia. Trends Cardiovasc Med 15: 163–169, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Gonzalez A, Lopez B, Diez J. Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am 88: 83–97, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, Taffet GE, Entman ML. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol 49: 499–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, Trial J, Entman ML. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res 83: 511–518, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haudek SB, Trial J, Xia Y, Gupta D, Pilling D, Entman ML. Fc receptor engagement mediates differentiation of cardiac fibroblast precursor cells. Proc Natl Acad Sci U S A 105: 10179–10184, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A 103: 18284–18289, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, Hsueh WA. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 101: 1130–1137, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost 101: 613–618, 2009 [PMC free article] [PubMed] [Google Scholar]
- 20. Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lim DS, Lutucuta S, Bachireddy P, Youker K, Evans A, Entman M, Roberts R, Marian AJ. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103: 789–791, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension 43: 1195–1201, 2004 [DOI] [PubMed] [Google Scholar]
- 23. McEwan PE, Gray GA, Sherry L, Webb DJ, Kenyon CJ. Differential effects of angiotensin II on cardiac cell proliferation and intramyocardial perivascular fibrosis in vivo. Circulation 98: 2765–2773, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci 60: 1342–1350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 166: 675–684, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 35: 175–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol 12: 593–633, 1994 [DOI] [PubMed] [Google Scholar]
- 28. Olivetti G, Capasso JM, Sonnenblick EH, Anversa P. Side-to-side slippage of myocytes participates in ventricular wall remodeling acutely after myocardial infarction in rats. Circ Res 67: 23–34, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114: 438–446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 36: 598–606, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res 73: 413–423, 1993 [DOI] [PubMed] [Google Scholar]
- 32. Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 103: 14098–14103, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sam F, Xie Z, Ooi H, Kerstetter DL, Colucci WS, Singh M, Singh K. Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens 17: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 171: 380–389, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res 72: 1245–1254, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol 142: 873–881, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 105: 1164–1176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 39. van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol 214: 377–386, 2008 [DOI] [PubMed] [Google Scholar]
- 40. Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res 257: 180–189, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 83: 1849–1865, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol 26: 279–292, 1994 [DOI] [PubMed] [Google Scholar]
- 43. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007 [DOI] [PubMed] [Google Scholar]