Abstract
Meiotic cells undergo two successive divisions without an intervening S phase. However, the mechanism of S-phase omission between the two meiotic divisions is largely unknown. Here we show that Wee1, a universal mitotic inhibitor, is absent in immature (but not mature) Xenopus oocytes, being down-regulated specifically during oogenesis; this down-regulation is most likely due to a translational repression. Even the modest ectopic expression of Wee1 in immature (meiosis I) oocytes can induce interphase nucleus reformation and DNA replication just after meiosis I. Thus, the presence of Wee1 during meiosis I converts the meiotic cell cycle into a mitotic-like cell cycle having S phase. In contrast, Myt1, a Wee1-related kinase, is present and directly involved in G2 arrest of immature oocytes, but its ectopic expression has little effect on the meiotic cell cycle. These results strongly indicate that the absence of Wee1 in meiosis I ensures the meiotic cell cycle in Xenopus oocytes. Based on these results and the data published previously in other organisms, we suggest that absence of Wee1 may be a well-conserved mechanism for omitting interphase or S phase between the two meiotic divisions.
Keywords: Cell cycle, meiosis, Mos, S phase, Wee1, Xenopus oocyte
The mitotic cell cycle in all eukaryotes consists of two alternating S and M phases with intervening G1 and G2 phases (Murray and Hunt 1993). The G2/M transitions are brought about by activation of Cdc2 kinase (Nurse 1990). In interphase (mainly S and G2 phases), Cdc2 associates with cyclin B but undergoes immediate, dominant inhibitory phosphorylations on Thr-14 and Tyr-15 (Norbury and Nurse 1992; King et al. 1994). Tyr-15 phosphorylation is catalyzed mainly by the universal Wee1 kinase, whereas Thr-14 phosphorylation is catalyzed exclusively by Myt1 kinase (at least in animal cells) (Coleman and Dunphy 1994; Fattaey and Booher 1997). On entry into M phase, Cdc25, a dual-specificity phosphatase, dephosphorylates Cdc2 on both Thr-14 and Tyr-15, thus causing its activation (Strausfeld et al. 1991; Millar and Russell 1992). G2 checkpoint control, which is activated by the presence of damaged or unreplicated DNA (Hartwell and Weinert 1989), inhibits Cdc25 and requires Wee1 activity to delay mitosis until DNA repair/replication is completed (Nurse 1997; Russell 1998).
Compared to mitosis, meiosis has a specialized cell cycle in which two successive divisions, reductional meiosis I and equational meiosis II, occur after a single round of S phase or the pre-meiotic S phase (Murray and Hunt 1993). The interval between meiosis I and meiosis II (called interkinesis) differs greatly from mitotic interphase in that it is very short and does not accompany S phase (or DNA replication); this S-phase omission is essential for the generation of haploid cells, a central objective of meiosis (John 1990). Despite its obvious importance, however, the mechanism(s) of S-phase omission between the two meiotic divisions is poorly understood (Sagata 1996). In principle, the mechanism could involve some meiosis-specific factor(s) that actively suppresses S phase, as exemplified by Mos in Xenopus oocytes (Furuno et al. 1994). However, because meiosis is most likely evolved from mitosis, a simple lack of some universal mitotic regulator(s) (required for interphase) might also be involved in the S-phase omission.
In immature Xenopus oocytes arrested at prophase I, Cdc2 kinase (already complexed with cyclin B) exists in an inactive Thr-14/Tyr-15-phosphorylated form (Ferrell et al. 1991; Gautier and Maller 1991). In these oocytes, the Thr-14/Tyr-15 kinase Myt1 is present (Palmer et al. 1998), but curiously, the universal Tyr-15 kinase Wee1 is not present. Wee1 is detected only after meiosis I or during meiosis II and early embryonic cell cycles (Murakami and Vande Woude 1998). Interestingly, in starfish oocytes, Wee1 is also not present during meiosis I but is present during meiosis II (Kishimoto 1998). In mice, the concentration of Wee1 decreases substantially during the growth of prophase I oocytes (Mitra and Schultz 1996). Moreover, in the fission yeast Schizosaccharomyces pombe, Wee1, which is present during the premeiotic S phase, disappears around entry into meiosis I and is not detectable thereafter (Daya-Makin et al. 1992). Thus, although little noticed so far, the absence of Wee1 in meiosis I may be common to meioses in many organisms and systems (see Discussion). Because Wee1 is generally required, at least in part, for interphase in the mitotic cell cycle (Coleman and Dunphy 1994; Nurse 1997; Russell 1998), its absence in meiosis I might have crucial implications in the omission of interphase or S phase during the transition to meiosis II.
In this study we have tested the above possibility by using the Xenopus oocyte system. We show that Wee1 expression is down-regulated specifically late during oogenesis, primarily at the translational level, and that ectopic expression of Wee1 during meiosis I converts the meiotic cell cycle into a mitotic-like cell cycle having S phase. Moreover, we demonstrate that although Myt1 is directly involved in prophase I arrest of immature oocytes, its ectopic expression has little effect on the meiotic cell cycle. These results, together with the data published previously in other organisms, suggest that the absence of Wee1 in meiosis I may be a well-conserved mechanism for omitting interphase or S phase between the two meiotic divisions. We also discuss the possibility that the absence of Wee1 might cancel the DNA replication checkpoint that could otherwise occur between the two meiotic divisions.
Results
Specific down-regulation of Wee1 expression during oogenesis
By Western blot analysis, we first examined the expression pattern of Xenopus Wee1 (XeWee1) during progesterone-induced oocyte maturation. XeWee1 protein was not detected in full-grown stage VI immature oocytes (arrested at prophase I) and in oocytes undergoing germinal vesicle breakdown (GVBD) or meiosis I but was detected in oocytes from 1–1.5 hr after GVBD (Fig. 1A, top), or from the onset of meiosis II, which was determined by the second increase in histone H1 kinase activity of Cdc2 (Fig. 1A, bottom). Thus, during oocyte maturation, XeWee1 protein was expressed only after meiosis I, essentially as reported previously (Murakami and Vande Woude 1998).
Figure 1.

Expression of XeWee1 during oogenesis and oocyte maturation. (A) Western blot analysis of XeWee1 during oocyte maturation. Immature stage VI oocytes were treated with progesterone (PG), collected at the indicated times, and subjected to either Western blot analysis with anti-XeWee1 antibody (Wee1) or histone H1 kinase assays of Cdc2 (H1). The time of GVBD and periods of meiosis I (MI) and meiosis II (MII) are indicated. (B) Western blot analysis of XeWee1 during oogenesis. Twenty micrograms of proteins each from stage I to VI oocytes and mature meiosis II oocytes (MO) was subjected to Western blot analysis of XeWee1, Cdc25C, or Cdc2. In MO, Cdc25C and Cdc2 showed prominent mobility shifts due to phosphorylation. (C) RT–PCR analysis of XeWee1 transcripts during oogenesis. Total RNA from four oocytes at each stage was subjected to RT–PCR analysis using XeWee1, Cdc25C, or Cdc2 oligonucleotides as primers. (D) Stability of (ectopic) XeWee1 in prophase I arrest. Immature stage VI oocytes injected with XeWee1 mRNA (0.7 ng/oocyte) were cultured, treated with cycloheximide (CHX) at 12 hr, and collected at the indicated times (after the mRNA injection) for Western blot analysis of XeWee1. At 10 hr, (ectopic) XeWee1 protein was expressed in three- to fourfold excess over endogenous XeWee1 in mature oocytes.
We next examined XeWee1 protein expression during oogenesis or from small previtellogenic stage I oocytes to full-grown stage VI oocytes (Dumont 1972). XeWee1 protein was not detected in stage IV and V oocytes (late-diplotene stages), as in full-grown stage VI oocytes but was detected in stage I, II, and III oocytes (zygotene to mid-diplotene stages), albeit at much lower levels than in mature (meiosis II) oocytes (Fig. 1B). For comparison, we also examined the expression pattern of Cdc2 kinase and Wee1-antagonizing Cdc25 phosphatase. Unlike Wee1, both of these proteins showed a progressive increase in levels during oogenesis, particularly between stage III and IV oocytes (Fig. 1B). Thus, interestingly, Wee1 protein expression was specifically down-regulated late during oogenesis. To test whether this down-regulation occurred at the transcriptional level, we further examined the levels of XeWee1 mRNA during oogenesis by RT-PCR analysis. Results revealed that XeWee1 transcripts, like those of Cdc2 and Cdc25, were present at approximately constant levels (per oocyte) throughout oogenesis (and oocyte maturation) (Fig. 1C). Thus, the down-regulation of XeWee1 protein expression was clearly not due to a transcriptional regulation.
Our inability to detect XeWee1 protein in immature stage IV–VI oocytes might have been due to its intrinsic instability during these periods. To test this possibility, we injected immature stage VI oocytes with XeWee1 mRNA and cultured them for 12 hr, by which time steady-state levels of XeWee1 protein were produced (Fig. 1D, cf. lanes 1 and 2). Treatment of these oocytes with the protein synthesis inhibitor cycloheximide did not affect appreciably the levels of XeWee1 protein for at least 5 hr (Fig. 1D). Thus, (ectopic) XeWee1 was rather stable in immature stage VI oocytes. These results suggest that the expression of Wee1 protein in Xenopus oocytes is specifically down-regulated, primarily at the translational level (see Discussion), so that the protein will be absent late during oogenesis (and early during oocyte maturation).
Absence of Wee1 is required for initiation of maturation
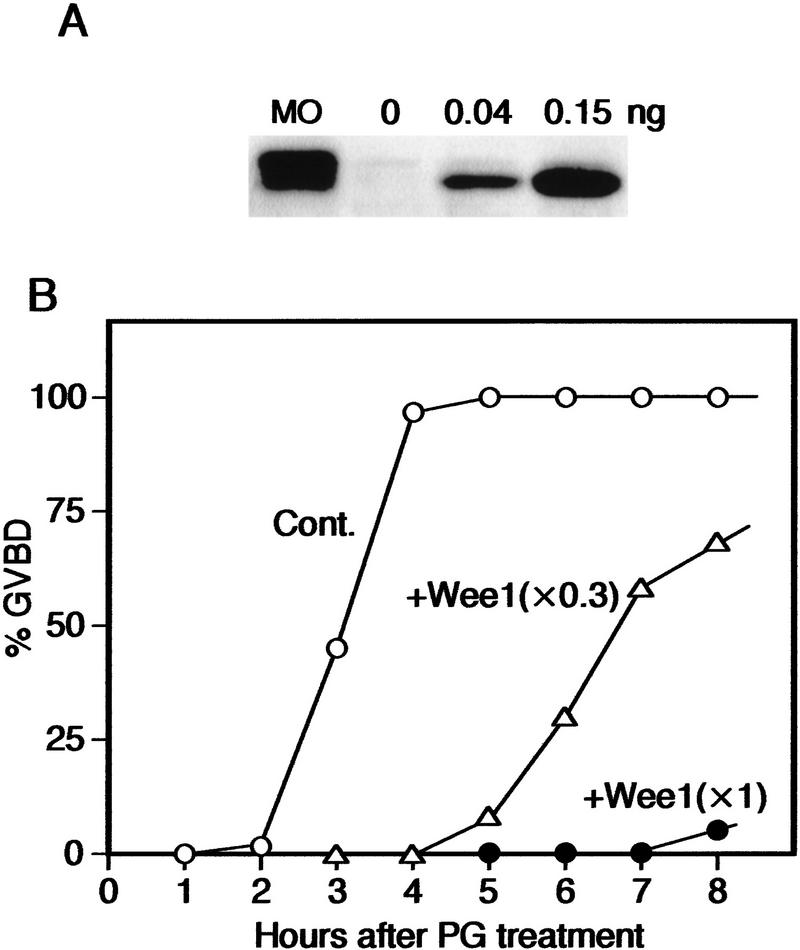
To examine the biological significance of the absence of Wee1 in full-grown immature oocytes (arrested at prophase I), we tested whether ectopic expression of Wee1 at the physiological levels (i. e., the levels comparable to endogenous Wee1 in mature meiosis II oocytes) could have any effect on the initiation of oocyte maturation. Immature stage VI oocytes that had been injected with XeWee1 mRNA (0.15 ng/oocyte) and then left for 10 hr expressed XeWee1 protein at approximately the same level as that of endogenous Wee1 in mature oocytes (Fig. 2A). After progesterone treatment, these oocytes underwent maturation very poorly, only 5%–10% of them showing GVBD 8 hr after the treatment, whereas uninjected control oocytes and those injected with unrelated control mRNA (not shown) underwent 100% GVBD as early as 4 hr (Fig. 2B). In some experiments, none of the XeWee1-expressed oocytes showed GVBD even after 12 hr of progesterone treatment, Cdc2 Tyr-15 phosphorylation being maintained at high levels due to the ectopic Wee1 expression (N. Nakajo et al., unpubl.). Even the oocytes injected with 0.04 ng of XeWee1 mRNA (and hence expressing a threefold less amount of Wee1 protein; Fig. 2A) underwent maturation very slowly, 50% of them showing GVBD as late as 7 hr after the progesterone treatment (Fig. 2B). Thus, these results show clearly that Wee1 protein, even at the physiological or lower levels, must not be present in immature prophase-I-arrested oocytes for these cells to initiate maturation normally.
Figure 2.
Inhibition of oocyte maturation by ectopic XeWee1 expression. (A) Quantification of ectopically expressed XeWee1. Immature stage VI oocytes were left uninjected or injected with either 0.04 or 0.15 ng of XeWee1 mRNA and cultured for 10 hr; the levels of ectopically expressed XeWee1 were compared with that of endogenous XeWee1 in mature oocytes (MO; 4 hr after GVBD) by Western blotting. (B) Kinetics of GVBD. Thirty immature oocytes uninjected (Cont.) or injected with XeWee1 mRNA (+Wee1) as above were treated with progesterone 10 hr after the injection, cultured, and scored for the percentage GVBD. In parentheses are shown the amounts of ectopically expressed XeWee1 relative to that of endogenous XeWee1, which were determined from the data in A.
Absence of Wee1 is required for entry into meiosis II
We next examined whether ectopic expression of Wee1 (at the physiological levels) in maturing (meiosis I) oocytes could influence entry into meiosis II. For this, we injected oocytes with XeWee1 mRNA 1.5 hr after progesterone treatment or 1–1.5 hr before GVBD, shortly before activation of endogenous Cdc2 kinase (cf. Fig. 1A). Injection of 0.35 ng of XeWee1 mRNA per oocyte yielded, during the next 2–4 hr, ectopic Wee1 protein in amounts comparable to endogenous Wee1 in mature oocytes (Fig. 3A). Under these conditions, the oocytes showed essentially the same GVBD kinetics as buffer-injected control oocytes; but from ∼2.5 hr after GVBD, they exhibited prominent cortical changes in pigmentation and even a cleavage furrow-like streak on the animal half (Fig. 3B,C). These surface changes resembled those of maturing or mature oocytes that were mitotically activated by pricking with a glass needle (Schuetz 1985), suggesting that the ectopic Wee1-expressed oocytes might have failed to enter meiosis II. To test this possibility, we monitored both the Cdc2 kinase activity and Cdc2 Tyr-15 phosphorylation status during maturation. Cdc2 activity in the buffer-injected control oocytes peaked at GVBD (∼3 hr after progesterone treatment), decreased 1 hr later, and then increased again at 2 hr after GVBD (Fig. 3D), indicating normal entry into meiosis II by 2 hr after GVBD (Furuno et al. 1994; Fig. 1A). In the Wee1-expressed oocytes, Cdc2 activity also peaked at GVBD (albeit with a slightly lower activity than in control oocytes) but decreased 1 hr later to much lower levels than in control oocytes, remained so for the next 1 hr, and then increased again appreciably at 3 hr after GVBD (suggesting reentry into another M phase). In these oocytes, Cdc2 Tyr-15 phosphorylation did occur at 1 hr, peaked at 2 hr, and then decreased appreciably at 3 hr after GVBD, whereas in control oocytes it was undetectably low throughout post-GVBD maturation, as described previously (Ferrell et al. 1991; Furuno et al. 1994) (Fig. 3E). These effects were canceled by coexpression of a Wee1-nonphosphorylatable Phe-15 Cdc2 mutant (data not shown), indicating that they were due to specific action of ectopic Wee1 on endogenous Cdc2. Thus, judging from both Cdc2 activity and Tyr-15 phosphorylation status, ectopic Wee1 expression specifically induced a mitotic-like prolonged interphase, instead of a very short interkinesis (lacking Tyr-15 phosphorylation), after meiosis I. These results suggest that the absence of Wee1 during meiosis I is required for normal entry into meiosis II.
Figure 3.
Effects of ectopic XeWee1 expression on entry into meiosis II. (A) Quantification of ectopically expressed XeWee1. Immature stage VI oocytes were injected with 0.35 ng of XeWee1 mRNA 1.5 hr after progesterone treatment, and then collected at the indicated times (after the progesterone treatment) for Western blot analysis. As a standard, endogenous XeWee1 in mature oocytes (MO; 4 hr after GVBD) was used. (B) Kinetics of GVBD and activation phenotypes. Thirty oocytes were treated with progesterone, injected at the indicated time (or at 1.5 hr; Inj.) with either buffer (Cont.) or 0.35 ng of XeWee1 mRNA (+Wee1) as described above, and then scored for the percentages GVBD and activation phenotypes (or cortical changes in pigmentation; see below). (C) Typical external morphology. The oocytes described above were photographed at 4 hr after GVBD. Note that XeWee1 mRNA-injected oocytes show distorted pigmentation and a cleavage furrow-like streak on the animal half, while control oocytes show a regular white spot (indicative of GVBD). (D) Kinetics of Cdc2 activity. Cdc2 kinase activities in the oocytes prepared as in B were measured by histone H1 kinase assays and are shown in an arbitrary unit. (E) Kinetics of Cdc2 Tyr-15 phosphorylation. The oocytes prepared as in B were analyzed by Western blotting using anti-phospho Cdc2 (Y15) antibody.
Absence of Wee1 is required for suppression of DNA replication after meiosis I
During interkinesis in normally maturing Xenopus oocytes, nuclear membranes do not reform, chromosomes remain condensed, and DNA replication does not occur (Gerhart et al. 1984; Furuno et al. 1994). We examined whether the ectopically expressed Wee1 affected the nuclear morphology and activity (in terms of DNA synthesis) after meiosis I. Upon cytological examination, the buffer-injected control oocytes showed an anaphase I/telophase I spindle at 2 hr, a prometaphase II spindle at 2.5 hr, and a typical metaphase II spindle at 3 hr after GVBD (Fig. 4A), as reported previously for normally maturing oocytes (Gard 1992; Furuno et al. 1994). (In normally maturing Xenopus oocytes, the chromosome/spindle cycle lags ∼1 hr behind the Cdc2 activity cycle, Fig. 3D; Furuno et al. 1994; Ohsumi et al. 1994). The Wee1-expressed oocytes also showed a normal-looking anaphase I/telophase I spindle at 2 hr after GVBD but then formed nuclear membranes (with decondensed chromosomes) at 2.5 hr and a spindle-like structure at 3 hr (Fig. 4A), indicating that they did enter interphase after meiosis I and then reentered an M phase-like stage (consistent with the changes in Cdc2 activity; Fig. 3D). To determine whether these oocytes synthesized DNA during the interphasic stage, we injected oocytes with [α-32P]dCTP (before injection with XeWee1 mRNA) and, after GVBD, analyzed their DNA by agarose gel electrophoresis (Furuno et al. 1994). In control oocytes, the nuclear DNA showed virtually no radioactive signals throughout post-GVBD maturation (Fig. 4B). In contrast, Wee1-expressed oocytes exhibited very strong radioactive signals of the nuclear DNA between 2 and 3 hr after GVBD, or when they formed an interphase nucleus (Fig. 4A). This DNA synthesis probably occurred only once, judging from the same intensity of the radioactive signals between 3 and 4 hr after GVBD (Fig. 4B) and from the radioactive counts that were similar to those in Mos-inhibited oocytes, in which a single round of DNA synthesis occurs (Furuno et al. 1994). Thus, maturing (meiosis I) oocytes ectopically expressing Wee1 at the physiological level clearly failed to enter meiosis II by reforming an interphase nucleus and replicating DNA after meiosis I. Similar results were obtained with ectopic Wee1 expression at half the physiological level (data not shown). These results show unequivocally that Wee1, even at the physiological or lower levels, must not be present during meiosis I for the oocytes to enter meiosis II normally. All together, the present results (Figs. 2–4) suggest strongly that the absence of Wee1 during prophase I arrest and meiosis I is to ensure the meiotic cell cycle in oocytes.
Figure 4.

Induction of nucleus reformation and DNA replication by ectopic XeWee1 expression. (A) Cytology. Oocytes were prepared as in Fig. 3 and cytologically examined at the indicated times after GVBD. Bar, 10 μm. (B) Analysis of DNA synthesis. Immature oocytes were injected with [α-32P]dCTP, treated with progesterone, and 1.5 hr later, injected with either buffer or XeWee1 mRNA as described in Fig. 3. DNA was extracted at the indicated times (after progesterone treatment) and analyzed by agarose gel electrophoresis. Only the nuclear DNA, but not the mitochondrial DNA (which migrates more slowly; Furuno et al. 1994), is shown.
Myt1 is directly involved in prophase I arrest but its ectopic expression has little effect on oocyte maturation
Cdc2 is phosphorylated on both Thr-14 and Tyr-15 in immature prophase-I-arrested oocytes, in which the Wee1-related Thr-14/Tyr-15 kinase Myt1(but not Wee1) is present (Mueller et al. 1995a; Palmer et al. 1998). We first tested whether Cdc2 Thr-14/Tyr-15 phosphorylation was involved directly in prophase I arrest of immature oocytes. Immature stage VI oocytes injected with mRNA (10 ng) encoding a Thr-14/Tyr-15-nonphosphorylatable A14/F15 Cdc2 mutant readily underwent GVBD (without progesterone treatment), whereas control oocytes injected with wild-type Cdc2 mRNA did not (Fig. 5A). Thus, Cdc2 Thr-14/Tyr-15 phosphorylation was probably involved in prophase I arrest. We then tested whether Myt1 was responsible for the Cdc2 Thr-14/Tyr-15 phosphorylation and prophase I arrest in immature oocytes. For this, we took advantage of neutralizing anti-Xenopus Myt1 antibody (see Materials and methods). Injection of immature oocytes with the anti-Myt1 antibody (200 ng/oocyte) alone caused full Tyr-15 dephosphorylation at 1.5–2 hr (see Fig. 5B, inset) and then GVBD at 2–2.5 hr, whereas injection with preimmune control antibody did not (Fig. 5B). Importantly, these effects were abolished by overexpression of Myt1 (Fig. 5B) and were also observed with another independently raised anti-Myt1 antibody (not shown). Thus, strikingly, Myt1 was probably the (only) kinase that was responsible for Cdc2 Thr-14/Tyr-15 phosphorylation and involved directly in prophase I arrest of immature oocytes.
Figure 5.
Direct involvement of Myt1 in prophase I arrest and the effect of its overexpression on the initiation of maturation. (A) Induction of maturation by ectopic expression of A14/F15 Cdc2. Thirty immature stage VI oocytes were injected with mRNA (10 ng/oocyte) encoding either wild-type Cdc2 or a Thr-14/Tyr-15-nonphosphorylatable A14/F15 Cdc2 mutant, and then scored for the percentage GVBD. (B) Induction of maturation by injection of anti-Myt1 antibody. Thirty immature oocytes were left uninjected or injected with 3 ng of Myt1 mRNA (+Myt1); 10 hr later, they were injected with either anti-Myt1 antibody (α-Myt1) or preimmune antibody (IgG) (each at 200 ng/oocyte) and then scored for the percentage GVBD. The kinetics of Cdc2 Tyr-15 dephosphorylation after injection of α-Myt1 alone (see inset) was determined by Western blotting with anti-phospho Cdc2 (Y15) antibody. (C) Effects of overexpression of Myt1 on the initiation of maturation. Thirty immature oocytes were injected with buffer (Cont.), 0.1 or 1.5 ng of Myt1 mRNA (+Myt1), or 0.15 ng of Wee1 mRNA (+Wee1); 10 hr later, they were treated with progesterone and scored for the percentage GVBD. The levels of ectopically expressed Myt1 (10 hr after the mRNA injection) are shown in the inset and its relative amounts to that of endogenous Myt1 are shown in parentheses. (For Wee1, cf. Fig. 2).
We next tested whether ectopic Myt1 expression had any effect on oocyte maturation similar to ectopic Wee1 expression. Immature stage VI oocytes injected with 0.1 ng of Myt1 mRNA expressed Myt1 protein in fourfold excess over endogenous Myt1 10 hr after the injection (see Fig. 5C, inset). After progesterone treatment, however, these oocytes underwent GVBD with the same kinetics as uninjected control oocytes (Fig. 5C). Even the oocytes injected with as much as 1.5 ng of Myt1 mRNA (and hence expressing 20-fold more Myt1 protein than endogenous Myt1) underwent GVBD nearly normally or only 30 min later than control oocytes (Fig. 5C). These oocytes showed essentially normal morphology and no sign of DNA replication even long after GVBD (data not shown). Thus, although Myt1 was directly involved in prophase I arrest, its ectopic expression (even up to 20-fold excess over endogenous Myt1) had little effect on maturation. This contrasts sharply with ectopic Wee1 expression, which, even at the physiological or lower levels, strongly affected maturation (Figs. 2 and 5C). This sharp contrast suggests that Myt1 activity is either simply much weaker or more strongly down-regulated by progesterone signaling (and during maturation) than Wee1 activity (see Discussion). In either case, however, the present results highlight the importance of the absence of Wee1 alone in immature (and meiosis I) oocytes, and further support the idea that the absence of Wee1 is to ensure the meiotic cell cycle in oocytes.
Discussion
Omission of S phase by the absence of Wee1
In principle, the omission of S phase between the two meiotic divisions could be achieved by generating a meiosis-specific factor(s) that suppresses S phase. We have shown previously that Mos, an oocyte meiosis-specific kinase functioning upstream of MAPK (Sagata 1997), acts to rapidly reactivate Cdc2 after meiosis I and suppresses S phase in maturing Xenopus oocytes (Furuno et al. 1994). Because M phase is generally dominant over S phase (Johnson and Rao 1970; Nurse 1994), such a rapid reactivation of Cdc2 would surely suppress S phase after meiosis I (Fig. 6A).
Figure 6.
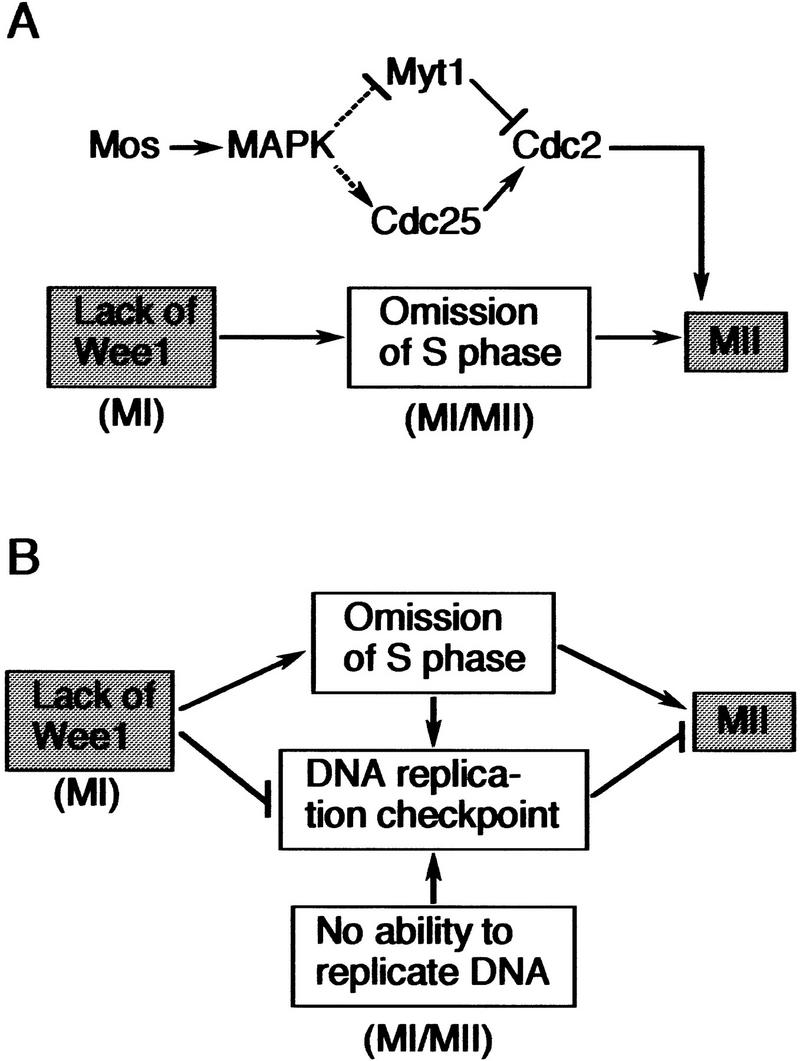
Model for interphase/S phase omission between the two meiotic divisions. (A) Model for meiosis in Xenopus oocytes. Both the Mos/MAPK pathway and the absence of Wee1 are involved in the omission of interphase/S phase between meiosis I (MI) and meiosis II (MII). The Mos/MAPK pathway may function to rapidly reactivate Cdc2 after MI, thereby suppressing interphase/S phase, whereas the absence of Wee1 in MI directly omits interphase/S phase. Either pathway would act to suppress inhibitory Tyr-15 phosphorylation of Cdc2 during the MI/MII transition, thereby ensuring direct entry into MII. See text for details. (B) Model for meiosis in general. Absence of Wee1 is assumed to be the primary mechanism of interphase/S-phase omission between MI and MII. The S-phase omission may in turn activate the DNA replication checkpoint pathway, which, however, would be cancelled immediately also by the absence of Wee1. Large animal oocytes such as Xenopus oocytes may not activate such a replication checkpoint pathway due to constraints specific to them. In the other cases, however, the pathway may be actually activated, and its cancellation by the absence of Wee1 may be important for entry into MII. Particularly in cases where the differentiated meiotic cells no longer have the ability to replicate DNA, cancellation of the replication checkpoint by the absence of Wee1 may be essential for normal entry into MII. (See text for details.)
Using the same Xenopus oocyte system, we have tested here the alternative (but not mutually exclusive) idea that a simple lack of some universal mitotic regulator(s) (required for interphase) might be involved in S-phase omission during meiosis. First, we find that the expression of Wee1 protein, a universal mitotic inhibitor (Nurse 1990; Coleman and Dunphy 1994; Russell 1998), is specifically down-regulated, primarily at the translational level, so that the protein will be absent late during oogenesis and early during oocyte maturation (or during meiosis I) (Fig. 1). Second, we show that full-grown immature oocytes ectopically expressing Wee1 (even at the physiological or lower levels) cannot initiate maturation in response to progesterone (Fig. 2), as reported previously for oocytes highly overexpressing Wee1 (Murakami and Vande Woude 1998). Third and more interestingly, we demonstrate that maturing (meiosis I) oocytes ectopically expressing Wee1 (even at the physiological or lower levels) fail to rapidly reactivate Cdc2 after meiosis I, do enter interphase, and even replicate DNA (and thus enter a mitotic-like cell cycle) after meiosis I (Figs. 3 and 4). These results show clearly that absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes and strongly support the idea that a lack of some universal mitotic regulator(s) might be involved in S-phase omission in meiosis. At present, it is not known whether any interplay exists between the Mos/MAPK pathway (including cyclin B synthesis; O'Keefe et al. 1991) and the Wee1 pathway in suppressing S phase. However, it seems clear that either pathway is needed for reactivation of Cdc2, which has been shown to be an absolute requirement for S-phase omission in oocyte meiosis (Furuno et al. 1994; Picard et al. 1996; Nurse 1994) (Fig. 6A).
Regulation of Wee1 expression
Wee1 protein expression was specifically down-regulated late during oogenesis; the protein was detected only early during oogenesis and late during oocyte maturation (or during meiosis II) (Fig. 1A,B). This down-regulation was probably due primarily to a translational repression, because XeWee1 mRNA was present at constant levels throughout oogenesis (Fig. 1C) and because (ectopically expressed) Wee1 protein was rather stable in immature stage VI oocytes (Fig. 1D). The relatively high stability of ectopic Wee1 in immature G2-arrested oocytes was rather unexpected, because Wee1 or its homolog has been shown to be unstable at G2 or M phase of the mitotic cell cycles in several species (Watanabe et al. 1995; Aligue et al. 1997; Michael and Newport 1998; Sia et al. 1998). How then would XeWee1 mRNA be translationally repressed in immature oocytes (and translationally reactivated during maturation)? In Xenopus, several maternal mRNAs, including those encoding Mos and cyclin B1, are known to be translationally repressed in immature oocytes and reactivated during maturation (Sagata et al. 1988; Kobayashi et al. 1991; Gabrielli et al. 1992). Notably, the 3′-untranslated region of these mRNAs commonly contains, just upstream of the poly(A) signal, the so-called cytoplasmic polyadenylation element (CPE), which is required for both translational repression and reactivation late during oogenesis and during maturation, respectively (Sheets et al. 1995; Stebbins-Boaz et al. 1996; De Moor and Richter 1999). Interestingly, XeWee1 mRNA also harbors a typical CPE sequence or UUUUAU just upstream of the poly(A) signal (Mueller et al. 1995b; Murakami and Vande Woude 1998), and our preliminary results suggest that a region containing the CPE-like sequence is important for the translational repression of XeWee1 mRNA in immature oocytes (N. Furuno and N. Sagata, unpubl.). Thus, it seems likely that XeWee1 mRNA, like several other maternal mRNAs, undergoes translational regulation by the cis-acting CPE during oogenesis and oocyte maturation.
Regulation of Wee1 and Myt1 activities
Unlike Wee1, Myt1, a membrane-associated Wee1-related kinase (Mueller et al. 1995a; Fattaey and Booher 1997), is present in immature prophase I-arrested oocytes (Palmer et al. 1998; Fig. 5C). We have shown strong evidence that Myt1 is responsible for Cdc2 Thr-14/Tyr-15 phosphorylation in immature oocytes and is involved directly in prophase I arrest of the oocytes (Fig. 5A,B). An important question is then why the endogenous Myt1 does not impair entry into meiosis II, whereas (ectopic) Wee1 does (Figs. 3 and 4). By ectopic expression, we have shown evidence that suggests that Myt1 activity is either intrinsically much weaker or more strongly down-regulated during maturation than Wee1 activity (Fig. 5C). [Ectopic Myt1 localized normally to the membrane fraction but this membrane localization was not responsible for the lower activity of Myt1 (M. Iida and N. Sagata, unpubl.)]. A recent study shows that p90rsk, a downstream kinase of the Mos/MAPK pathway (which is active during maturation) (Sturgill et al. 1988), binds to Myt1 but not Wee1, in Xenopus oocytes and inhibits its kinase activity in vitro (Palmer et al. 1998). Thus, Myt1 could be kept inhibited by the Mos/MAPK pathway during the meiosis I/meiosis II transition, whereas Cdc25 might be kept activated also by the MAPK cascade (Izumi et al. 1992; Chau and Shibuya 1998), thereby having no inhibitory effect on entry into meiosis II. If so, suppression of S phase by Mos could be ascribed, in part, to inhibition of Myt1 (Fig. 6A). In contrast, Wee1 (though ectopically expressed at the physiological levels) is not likely to be inhibited greatly during the transition to meiosis II, as evidenced by its pronounced effects on the transition (Figs. 3 and 4). We presume that this can occur primarily because Cdc2 activity is low during the transition (Cdc2 and Wee1 form a negative feedback loop between them; Mueller et al. 1995b). It is also possible that the ectopic Wee1 is activated by the Mos/MAPK pathway during the transition to meiosis II (Murakami et al. 1999). Thus, if present during meiosis I or the transition to meiosis II, only Wee1, but not Myt1, would impair entry into meiosis II. This may be the reason why only Myt1, but not Wee1, is allowed to exist in immature and meiosis I oocytes. Similar regulations of Myt1 and (ectopic) Wee1 activities could occur in the initiation of maturation (Palmer et al. 1998) where progesterone signaling activates the Mos/MAPK pathway to induce Cdc2 activation (Sagata 1997), thus accounting for the results in Figure 5C.
Generality of the absence of Wee1 in meiosis I
Our results clearly show that absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes (Figs. 2–4). Then how general would the absence of Wee1 (and its involvement in the meiotic cell cycle) be in animal oocytes? Intriguingly, in starfish oocytes, Wee1 is absent during prophase I arrest and meiosis I, but is present during meiosis II (Kishimoto 1998) just as in Xenopus oocytes. In mouse oocytes, the concentration of Wee1 protein decreases substantially during the acquisition of meiotic competence, whereas those of Cdc2 and Wee1-antagonizing Cdc25 do increase (Mitra and Schultz 1996), again as in Xenopus oocytes (Fig. 1B). Moreover, in immature goldfish oocytes, Cdc2, which is present only as a monomer (because in these oocytes cyclin B is synthesized only during maturation) (Katsu et al. 1993), does not undergo any transient Tyr-15 (and Thr-14) phosphorylation even upon association with cyclin B both in vivo and in oocyte extracts (containing the Cdc25 inhibitor vanadate) (Yamashita et al. 1995). Hence, most certainly, Wee1 (and even Myt1) is not present in immature goldfish oocytes. Similar situations seem to occur in the oocytes of many other fishes (zebrafish, carp, catfish, and lamprey), amphibians (Rana, Bufo, and newt), and cow (Yamashita 1998). Thus, although little noticed so far, absence of Wee1 in (immature) oocytes seems to be a phylogenetically well-conserved phenomenon. This striking conservation, together with the present results from ectopic Wee1 expression (Figs. 2–4), strongly supports the idea that absence of Wee1 generally ensures the meiotic cell cycle in animal oocytes. In this context, the appearance of Wee1 in mature meiosis II oocytes of both Xenopus (Murakami and Vande Woude 1998; Fig. 1A) and starfish (Kishimoto 1998) would suggest its general requirement for the embryonic cell cycles.
Would the absence of Wee1 protein also be a requirement in meioses of systems other than animal oocytes? Presently, the expression pattern of Wee1 has not been observed in meiosis in animal spermatogenesis or (higher) plant gametogenesis. Our preliminary observations show, however, that XeWee1 is not detectable in Xenopus spermatocytes. In the fission yeast S. pombe, however, it has been shown that the level of Wee1 protein, which increases during commitment to meiosis and peaks at the pre-meiotic S phase, drops to an undetectable level just before M phase (probably in G2 phase of meiosis I) and then remains undetectable until sporulation (Daya-Makin et al. 1992). Thus, even in S. pombe, Wee1 is apparently absent during meiosis I (and also during meiosis II). The disappearance of S. pombe Wee1 protein at the beginning (or G2 phase) of meiosis could be due, at least in part, to its intrinsic instability during this period. As mentioned earlier, Wee1 or its homolog is generally unstable at the corresponding phase of the mitotic cell cycle (Watanabe et al. 1995; Aligue et al. 1997; Michael and Newport 1998; Sia et al. 1998), but not necessarily in G2-arrested Xenopus oocytes (Fig. 1D). Notably, however, unlike the mitotic Wee1 protein [whose abundance generally shows only a moderate oscillation during the cell cycle (Watanabe et al. 1995; Aligue et al. 1997; Sia et al. 1998)], the meiotic Wee1 protein of S. pombe is not detectable throughout meiosis (after the premeiotic S phase) (Daya-Makin et al. 1992). This is also the case with Xenopus Wee1 protein in prophase I and meiosis I oocytes (Fig. 1B). Thus, during meiosis in S. pombe, expression of Wee1 protein might also be down-regulated, perhaps at the translational level, as in Xenopus oocytes. Genetic studies show that Wee1 is not essential for meiotic divisions in S. pombe (Grallert and Sipiczki 1990, 1991). Thus, even in fission yeast, absence of Wee1 might function to ensure the meiotic cell cycle (Fig. 6B).
Absence of Wee1 and the DNA replication checkpoint in meiosis
In the mitotic cell cycle, Wee1 is required for interphase (Nurse 1990; Coleman and Dunphy 1994; Morgan 1995). Therefore, the absence of Wee1 in meiosis I, which now seems to occur generally, would be largely and directly responsible for the omission of interphase or S phase in meiosis (Fig. 6). However, Wee1 activity is also required for the execution of G2 checkpoint control during the mitotic cell cycle (Nurse 1997; Russell 1998). Hence, the absence of Wee1 in meiosis I might also function to cancel the DNA replication checkpoint that could otherwise occur between meiosis I and meiosis II due to the absence of DNA replication (i.e., the presence of unreplicated DNA) during this period. In (large) animal oocytes such as those of Xenopus, such a replication checkpoint pathway may not be activated in practice, as the nucleus (DNA)–cytoplasm ratio of the oocyte is very low (Newport and Dasso 1989; Dasso and Newport 1990) and as Cdc2 is reactivated very rapidly (and chromosomes remain condensed without nuclear membranes) after meiosis I (Figs. 3 and 4; Gerhart et al. 1984; Furuno et al. 1994). In other meioses such as those in spermatocytes and yeast, however, the replication checkpoint pathway might be activated between the two meiotic divisions, because the (small) cells do form interphase nucleus and decondensed chromosomes between the two divisions, as somatic cells do so in interphase (Hotta 1988; John 1990). If so, cancellation of the replication checkpoint pathway (by the absence of Wee1) would be important for these cells to enter meiosis II normally. Cancellation of the replication checkpoint pathway by the absence of Wee1 might be particularly important in cases where the differentiated meiotic cells (e.g., spermatocytes) have no DNA replication ability (irrespective of the absence of Wee1) due to a deficiency in some essential component(s) (e.g., DNA polymerase α) for DNA synthesis (Hecht et al. 1976, 1979; Furuno et al. 1994). If Wee1 were present, these cells would be permanently arrested before entry into meiosis II due to the total absence of DNA replication. Thus, in many cases, absence of Wee1 might also function to cancel the DNA replication checkpoint to ensure entry into meiosis II (Fig. 6B). It will be very interesting to test this model experimentally.
In conclusion, absence of Wee1 ensures the meiotic cell cycle in Xenopus oocytes and may be a well-conserved mechanism for omitting interphase or S phase between the two meiotic divisions. If so, meiosis might have evolved from mitosis, at least in part, by eliminating or down-regulating expression of Wee1, a universal mitotic inhibitor in eukaryotes.
Materials and methods
Preparation, culture, microinjection, and treatment of oocytes
Oocytes were prepared, cultured, and microinjected as described by Furuno et al. (1994) and Nakajo et al. (1999). Staging of the oocytes was done according to Dumont (1972). To induce maturation, stage VI oocytes were treated with progesterone (5 μg/ml); to inhibit protein synthesis, oocytes were treated with cycloheximide (100 μg/ml).
cDNAs, recombinant plasmids, and in vitro transcription
A cDNA encoding XeWee1 (Mueller et al. 1995b) was isolated by PCR of a Xenopus oocyte cDNA library by using 5′-GCCTCTAGAACCATGAGGACGGCCATGTCATGC-3′ as a 5′ primer and 5′-GCGGATCCTTAATACCCTCCGCAGGTGAAG-3′ as a 3′ primer; a Myt1 cDNA (Mueller et al. 1995a) was isolated similarly by using 5′-GCCGCTAGCACCATGCCTGTTCCAGGGGATGAC-3′ as a 5′ primer and 5′-GCCGATATCTCATTGCTCGGTGGCATCGTCAAAG-3′ as a 3′ primer. After subcloning into pT7G(UK+) (Nakajo et al. 1999), nucleotide sequences were determined and confirmed. An A14/F15 Cdc2 mutant was made by Thr-14 → Ala and Tyr-15 → Phe mutagenesis by using the Quick Change Site-Directed Mutagenesis Kit (Stratagene); the primer used was 5′-GATCGGAGAGGGCGCATTTGGGGTTGTGTAC-3′ (for the sense strand). For the Cdc2 cDNA, see Furuno et al. (1994). All of the constructs in pT7G (UK+) were cut singly with NotI and in vitro transcribed by using the MEGA Script T7 Kit (Ambion).
RT–PCR
RNA was extracted from oocytes at various stages using Trizol reagent (GIBCO BRL) and treated with RNase-free DNase I (TAKARA). cDNA was synthesized from the extracted RNA by using random hexamer primers and superscript II RNase H− reverse transcriptase (GIBCO BRL) and was treated with RNase H. Aliquots of the reaction products were amplified with PCR reactions (94°C for 30 sec, 55°C for 1 min, and 72°C for 1 min) for 25 cycles for XeWee1 and Cdc25C or 30 cycles for Cdc2; the 5′ and 3′ primers used were, respectively, 5′-GTGTCCTCTATAAGATCGGGGACCTTGGTCATGTGAC-3′ and 5′-CAACTCCCTCTCAAGCATGGCCGTCTTGAACTTCTCCA-3′ for XeWee1, 5′-CGATACATCACTGGAGAGAC-3′ and 5′-CTTGGTGGTGCATTGGGCAG-3′ for Cdc25C, and 5′-GAAGTGCTGTTGGGGTCAGTC-3′ and 5′-CAGGAAGGCTGGACTTATCC-3′ for Cdc2. Reaction products were fractionated on 2.5% agarose gels, stained with ethidium bromide, and photographed.
Antibodies and Western blot analysis
Polyclonal antibodies were raised in rabbits against bacterially produced XeWee1 protein or Myt1 protein by standard methods and then affinity-purified by using Affigel 15 or 10 (Bio-Rad). The affinity-purified anti-Myt1 antibody was found to inhibit Myt1 kinase activity in vitro in a dose-dependent manner, indicating that it acted as a neutralizing antibody. Routinely, protein equivalent to one oocyte was subjected to Western blot analysis with anti-XeWee1 antibody (1 μg/ml), anti-XeCdc25C antibody (0.5 μg/ml; Nakajo et al. 1999), anti-PSTAIRE antibody (Furuno et al. 1994), or anti-phospho Cdc2 (Y15) antibody (1:500; New England Biolabs). The secondary antibody, either a donkey anti-rabbit IgG antibody (1:1000; Amersham) or a sheep anti-mouse IgG antibody (1:1000; Amersham), was detected by using either the ECL system (Amersham) or the Super Signal West Dura Chemiluminescent Substrate system (Pierce).
H1 kinase assays
Cdc2 kinase activity in oocyte extracts was measured by histone H1 kinase assays in the presence of the protein kinase A inhibitor PKI, essentially as described by Furuno et al. (1994). Under these conditions, most (>85%) of the H1 kinase activities in the extracts were due to the activities of Cdc2/cycling B complexes. After SDS-PAGE, phosphorylated histone H1 was quantified by BAS1000 (Fuji).
Analysis of DNA synthesis
DNA synthesis in maturing oocytes (injected with [α-32P]dCTP) was analyzed exactly as described by Furuno et al. (1994). Under these conditions, the sheared nuclear DNA ran on the agarose gel as a band of ∼50 kb.
Cytological examination
Oocytes were fixed in Smith's solution, dehydrated, embedded, sectioned, stained with Feulgen's stain, and counterstained with fast green, as described previously (Furuno et al. 1994).
Acknowledgments
We thank Dr. N. Furuno for comments, Dr. M. Yamashita for the anti-PSTAIRE antibody, and M. Egashira for editing the manuscript. This work was supported by Grants-in-aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL nsagascb@mbox.nc.kyushu-u.ac.jp; FAX 81-92-642-2645.
References
- Aligue R, Wu L, Russell P. Regulation of Schizosaccharomyces pombe Wee1 tyrosine kinase. J Biol Chem. 1997;272:13320–13325. doi: 10.1074/jbc.272.20.13320. [DOI] [PubMed] [Google Scholar]
- Chau ASS, Shibuya EK. Mos-induced p42 mitogen-activated protein kinase activation stabilizes M-phase in Xenopus egg extracts after cyclin destruction. Biol Cell. 1998;90:565–572. [PubMed] [Google Scholar]
- Coleman TR, Dunphy WG. Cdc2 regulatory factors. Curr Opin Cell Biol. 1994;6:877–882. doi: 10.1016/0955-0674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Dasso M, Newport JW. Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: Studies in Xenopus. Cell. 1990;61:811–823. doi: 10.1016/0092-8674(90)90191-g. [DOI] [PubMed] [Google Scholar]
- Daya-Makin M, Szankasi P, Tang L, MacRae D, Pelech SL. Regulation of p105wee1 and p34cdc2 during meiosis in Schizosaccharomyces pombe. Biochem Cell Biol. 1992;70:1088–1096. doi: 10.1139/o92-154. [DOI] [PubMed] [Google Scholar]
- De Moor CH, Richter JD. Cytoplasmic polyadenylation elements mediate masking and unmasking of cyclin B1 mRNA. EMBO J. 1999;18:2294–2303. doi: 10.1093/emboj/18.8.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972;136:153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Fattaey A, Booher RN. Myt1: A Wee1-type kinase that phosphorylates Cdc2 on residue Thr-14. Prog Cell Cycle Res. 1997;3:233–240. doi: 10.1007/978-1-4615-5371-7_18. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Wu M, Gerhart JC, Martin GS. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. Suppression of DNA replication via Mos function during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielli BG, Roy LM, Gautier J, Philippe M, Maller JL. A cdc2-related kinase oscillates in the cell cycle independently of cyclins G2/M and cdc2. J Biol Chem. 1992;267:1969–1975. [PubMed] [Google Scholar]
- Gard DL. Microtubule organization during maturation of Xenopus oocytes: Assembly and rotation of the meiotic spindles. Dev Biol. 1992;151:516–530. doi: 10.1016/0012-1606(92)90190-r. [DOI] [PubMed] [Google Scholar]
- Gautier J, Maller JL. Cyclin B in Xenopus oocytes: Implications for the mechanism of pre-MPF activation. EMBO J. 1991;10:177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J, Wu M, Kirschner M. Cell cycle dynamics of an M-phase-specific cytoplasmic factor in Xenopus laevis oocytes and eggs. J Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert B, Sipiczki M. Dissociation of meiotic and mitotic roles of the fission yeast cdc2 gene. Mol Gen Genet. 1990;222:473–475. doi: 10.1007/BF00633860. [DOI] [PubMed] [Google Scholar]
- ————— Common genes and pathways in the regulation of the mitotic and meiotic cell cycles of Schizosaccharomyces pombe. Curr Genet. 1991;20:199–204. doi: 10.1007/BF00326233. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: Controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Hecht NB, Farrell D, Davidson D. Changing DNA polymerase activities during the development of the testis in the mouse. Dev Biol. 1976;48:56–66. doi: 10.1016/0012-1606(76)90045-2. [DOI] [PubMed] [Google Scholar]
- Hecht NB, Farrell D, Williams JL. DNA polymerases in mouse spermatogenic cells separated by sedimentation velocity. Biochim Biophys Acta. 1979;561:358–368. doi: 10.1016/0005-2787(79)90144-8. [DOI] [PubMed] [Google Scholar]
- Hotta Y. Meiosis and genetic recombination. Tokyo, Japan: Tokyo University Press; 1988. [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus Cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B. Meiosis. Cambridge, UK: Cambridge University Press; 1990. [Google Scholar]
- Johnson RT, Rao PN. Mammalian cell fusion: Induction of premature chromosome condensation in interphase nuclei. Nature. 1970;226:717–722. doi: 10.1038/226717a0. [DOI] [PubMed] [Google Scholar]
- Katsu Y, Yamashita M, Kajiura H, Nagahama Y. Behavior of the components of maturation-promoting factor, cdc2 kinase and cyclin B, during oocyte maturation of goldfish. Dev Biol. 1993;160:99–107. doi: 10.1006/dbio.1993.1289. [DOI] [PubMed] [Google Scholar]
- King RW, Jackson PK, Kirschner MW. Mitosis in transition. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. Cell cycle arrest and release in starfish oocytes and eggs. Semin Cell Dev Biol. 1998;9:549–557. doi: 10.1006/scdb.1998.0249. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Minshull J, Ford C, Golsteyn R, Poon R, Hunt T. On the synthesis and destruction of A- and B-type cyclins during oogenesis and meiotic maturation in Xenopus laevis. J Cell Biol. 1991;114:755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael WM, Newport J. Coupling of mitosis to the completion of S phase through Cdc34-mediated degradation of Wee1. Science. 1998;282:1886–1889. doi: 10.1126/science.282.5395.1886. [DOI] [PubMed] [Google Scholar]
- Millar JB, Russell P. The cdc25 M-phase inducer: An unconventional protein phosphatase. Cell. 1992;68:407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- Mitra J, Schultz RM. Regulation of the acquisition of meiotic competence in the mouse: Changes in the subcellular localization of cdc2, cyclin B1, cdc25C and wee1, and in the concentration of these proteins and their transcripts. J Cell Sci. 1996;109:2407–2415. doi: 10.1242/jcs.109.9.2407. [DOI] [PubMed] [Google Scholar]
- Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: A membrane-associated inhibitory kinase that phosphorylates Cdc2 on both Threonine-14 and Tyrosine-15. Science. 1995a;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Dunphy WG. Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell. 1995b;6:119–134. doi: 10.1091/mbc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami MS, Vande Woude GF. Analysis of the early embryonic cell cycles of Xenopus: Regulation of cell cycle length by Xe-wee1 and Mos. Development. 1998;125:237–248. doi: 10.1242/dev.125.2.237. [DOI] [PubMed] [Google Scholar]
- Murakami MS, Copeland TD, Vande Woude GF. Mos positively regulates Xe-Wee1 to lengthen the first mitotic cell cycle of Xenopus. Genes & Dev. 1999;13:620–631. doi: 10.1101/gad.13.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A, Hunt T. The cell cycle: An introduction. New York, NY: W.H. Freeman and Company; 1993. [Google Scholar]
- Nakajo N, Oe T, Uto K, Sagata N. Involvement of Chk1 kinase in prophase I arrest of Xenopus oocytes. Dev Biol. 1999;207:432–444. doi: 10.1006/dbio.1998.9178. [DOI] [PubMed] [Google Scholar]
- Newport JW, Dasso M. On the coupling between DNA replication and mitosis. J Cell Sci Suppl. 1989;12:149–160. doi: 10.1242/jcs.1989.supplement_12.13. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- ————— Ordering S phase and M phase in the cell cycle. Cell. 1994;79:547–550. doi: 10.1016/0092-8674(94)90539-8. [DOI] [PubMed] [Google Scholar]
- ————— Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Ohsumi K, Sawada W, Kishimoto T. Meiosis-specific cell cycle regulation in maturing Xenopus oocytes. J Cell Sci. 1994;107:3005–3013. doi: 10.1242/jcs.107.11.3005. [DOI] [PubMed] [Google Scholar]
- O'Keefe SJ, Kiessling AA, Cooper GM. The c-mos gene product is required for cyclin B accumulation during meiosis of mouse eggs. Proc Natl Acad Sci. 1991;88:7869–7872. doi: 10.1073/pnas.88.17.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer A, Gavin A-C, Nebreda AR. A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard A, Galas S, Peaucellier G, Dorée M. Newly assembled cyclin B-cdc2 kinase is required to suppress DNA replication between meiosis I and meiosis II in starfish oocytes. EMBO J. 1996;15:3590–3598. [PMC free article] [PubMed] [Google Scholar]
- Russell P. Checkpoints on the road to mitosis. Trends Biochem Sci. 1998;23:399–402. doi: 10.1016/s0968-0004(98)01291-2. [DOI] [PubMed] [Google Scholar]
- Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude GF. Function of c-mos proto-oncogene product in meiotic maturation of Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Sagata N. Meiotic metaphase arrest in animal oocytes: Its mechanisms and biological significance. Trends Cell Biol. 1996;6:22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- ————— What does Mos do in oocytes and somatic cells? BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- Schuetz AW. Local control mechanisms during oogenesis and folliculogenesis. In: Browder LW, editor. Developmental biology: A comprehensive synthesis. 1 (Oogenesis) New York, NY: Plenum Press; 1985. pp. 3–83. [DOI] [PubMed] [Google Scholar]
- Sheets MD, Wu M, Wickens M. Polyadenylation of c-mos mRNA as a control point in Xenopus meiotic maturation. Nature. 1995;374:511–516. doi: 10.1038/374511a0. [DOI] [PubMed] [Google Scholar]
- Sia RAL, Bardes ESG, Lew DJ. Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J. 1998;17:6678–6688. doi: 10.1093/emboj/17.22.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins-Boaz B, Hake LE, Richter JD. CPEB controls the cytoplasmic polyadenylation of cyclin, Cdk2 and c-mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J. 1996;15:2582–2592. [PMC free article] [PubMed] [Google Scholar]
- Strausfeld U, Labbe JC, Fesquet D, Cavadore JC, Picard A, Sadhu K, Russell P, Dorée M. Dephosphorylation and activation of a p34cdc2/cyclin B complex in vitro by human CDC25 protein. Nature. 1991;351:242–245. doi: 10.1038/351242a0. [DOI] [PubMed] [Google Scholar]
- Sturgill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Broome M, Hunter T. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 1995;14:1878–1891. doi: 10.1002/j.1460-2075.1995.tb07180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Kajiura H, Tanaka T, Onoe S, Nagahama Y. Molecular mechanisms of the activation of maturation-promoting factor during goldfish oocyte maturation. Dev Biol. 1995;168:62–75. doi: 10.1006/dbio.1995.1061. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Molecular mechanisms of meiotic maturation and arrest in fish and amphibian oocytes. Semin Cell Dev Biol. 1998;9:569–579. doi: 10.1006/scdb.1998.0251. [DOI] [PubMed] [Google Scholar]