Abstract
Impaired learning and memory are common in epilepsy syndromes of childhood. Clinical investigations suggest that the developing brain may be particularly vulnerable to the effects of intractable seizure disorders. Magnetic resonance imaging (MRI) studies have demonstrated reduced volumes in brain regions involved in learning and memory. The earlier the onset of an epilepsy the larger the effects seem to be on both brain anatomy and cognition. Thus, childhood epilepsy has been proposed to interfere in some unknown way with brain development. Experiments reported here explore these ideas by examining the effects of seizures in infant mice on learning and memory and on the growth of CA1 hippocampal pyramidal cell dendrites. Fifteen brief seizures were induced by flurothyl between postnatal days 7 and 11 in mice that express green fluorescent protein (GFP) in hippocampal pyramidal cells. One to 44 days later, dendritic arbors were reconstructed to measure growth. Spatial learning and memory were also assessed in a water maze. Our results show that recurrent seizures produced marked deficits in learning and memory. Seizures also dramatically slowed the growth of basilar dendrites while neurons in litter-mate control mice continued to add new dendritic branches and lengthen existing branches. When experiments were performed in older mice, seizures had no measureable effects on either dendrite arbor complexity or spatial learning and memory. Our results suggest that the recurring seizures of intractable childhood epilepsy contribute to associated learning and memory deficits by suppressing dendrite growth.
Keywords: Spatial Learning, Hippocampus, CA1 Pyramidal Cells
INTRODUCTION
Cognitive deficits are among the common neurobehavioral comorbidities of epilepsy (Hermann et al., 2008;Berg, 2011;Elger et al., 2004) and while impaired cognition is particularly prominent in the catastrophic epilepsies of childhood, it is not restricted to these syndromes. Studies of a variety of epilepsies have reported intellectual ability to be below that considered normal for age (Nolan et al., 2003;Schoenfeld et al., 1999). While, the basic mechanisms underlying these deficits have been the subject of much speculation (Brooks-Kayal, 2011;Jensen, 2011), it is clear that the nervous system of children appears to be particularly vulnerable to the effects of intractable epilepsy. Numerous studies have compared the risk of comorbidities as a function of age of onset of epilepsy (Dikmen et al., 1975; Dodrill and Matthews, 1992;Hermann et al., 2002;Berg et al., 2008; Bjørnaes al., 2001). Results have shown that the earlier the age of onset the poorer the cognitive abilities. These observations have led some investigators to propose a neurodevelopmental origin for the comorbidities of epilepsy and that childhood seizure disorders negatively impact the normal growth and maturation of the nervous system (Hermann et al., 2008). This concept has been bolstered in recent years by imaging studies. For instance, quantitative MRI studies have reported reductions in regional brain tissue volume in adult (Marsh et al., 1997;Lee et al., 1998;Theodore et al., 2003;Oyegbile et al., 2004) and pediatric (Lawson et al., 2002;Pardoe et al., 2008;Pulsipher et al., 2009) epilepsy patients with a history of early-onset epilepsy. In concert with psychological findings, investigators have also reported greater reductions in whole brain grey-matter and white-matter volumes in patients with early-onset when compared to later-onset epilepsy (Weber et al., 2007;Hermann et al., 2002).
Potential factors that contribute to such changes in brain anatomy and human behavior are likely numerous and not easily identified or understood through clinical observations alone. Recurrent seizures themselves have long been suspected to impact brain development. However co-existing neuropathologies and/or anticonvulsant therapy are confounding factors. In this regard, experiments in animals can be informative since the effects of seizures alone can be evaluated. Indeed, experiments in several animal models of early-life seizures have shown that brief but recurrent seizures can produce deficits in learning and memory(Stafstrom, 2002;Lee et al., 2001;Lynch et al., 2000;Sogawa et al., 2001;Karnam et al., 2009b;Karnam et al., 2009a). How these deficits are produced remains unclear, but previous molecular studies have shown that the expression of postsynaptic markers for glutamatergic synapses is suppressed in a developmentally-dependent manner (Swann et al., 2007b). As animals matured the differences between them and their controls increased. Additional experiments in slice cultures showed that similar biochemical alterations take place in response to seizure-like activity (Swann et al., 2007a) but neuroanatomical experiments revealed that these effects may be best explained by a suppression of dendrite growth (Nishimura et al., 2008).
These results have led us to suspect that the induction of recurrent seizures during a critical period of dendrite maturation could retard their growth. Results from experiments reported here show that seizures can indeed suppress dendrite growth and do not produce similar effects after a critical period of marked dendrite growth.
MATERIAL AND METHODS
Animals
Thy1GFP-M transgenic mice on a C57/BL6 background (Feng et al., 2000) were used in all experiments for morphological analysis of dendrites. Wild-type C57/BL6 (Harlan Sprague Dawley) mice were used for behavioral experiments. The day of birth was designated as postnatal day 0 (P0) and multiple seizures were induced in mice from P7 to P11 or from P30 to P34. Maintenance of animals and surgical procedures were approved by the Baylor College of Medicine institutional animal care committee and were in keeping with guidelines established by the National Institutes of Health.
Flurothyl-induced seizures
Fifteen brief (~ 3 min) seizures were induced in infant mice by flurothyl inhalation. On P7-11, mice (both males and females were studied and randomly assigned treatment groups) were placed in a small (25 × 15 × 15 cm) enclosed Plexiglass chamber and liquid flurothyl (BIS-2, 2, 2-trifluoroethyl ether; Sigma-Aldrich, St. Luis, Mo., USA) was delivered by way of a syringe pump (Harvard Apparatus) at the rate of 50μl/min. Drops of this volatile convulsant were delivered to a piece of filter paper placed on a raised platform in the middle of the chamber. After a 1- to 2- min delay, the first signs of responses to flurothyl were generalized trembling and a straub tail (rigid extension) that was quickly but variably followed by repetitive myoclonic jerks which progressed to clonic forelimb contractions followed by a tonic extension of the forelimbs and then the hind limbs. Timing of seizure duration was initiated at the onset of trembling and straub tail since these were readily identifiable behavioral markers and continued until one of the three following criteria was met:
Full Tonic Extension - If a mouse pup underwent a full tonic extension (simultaneous and prolonged (> 3-5 seconds) extension of both fore limbs and hind limbs), regardless of the time of flurothyl exposure, it was removed from the chamber and the time was recorded.
3 Minutes and Forelimb Tonus - If forelimb tonus was observed (but not hind limb), the mouse was kept in the chamber until 3 minutes had elapsed. The only exception was if the mouse eventually went into full tonic extension before 3 minutes expired, in which case it was removed from the chamber and the time recorded.
4 Minutes without Tonic Limb Extensions - If a mouse did not exhibit a tonic extension; it was left in the chamber for 4 minutes of flurothyl administration from the onset of generalized trembling. During these 4 minutes the mice experienced continuous behavioral seizures that consisted primarily of fore limb and hind limb clonic contractions. EEG recordings have verified that electrographic seizures accompany the behavioral seizures observed (our unpublished observations).
The reason for developing three criteria for terminating seizures was that seizures could be variable not only between mice but in the same mouse from time -to-time. Full tonic extension is a common end point for most laboratories using the flurothyl model in rats. In our experience with mice, prolonged seizures of tonic extension can lead to death. To avoid the loss of animals, mice were removed from the chamber when full tonic extension occurred. But not every animal displayed full tonic extension, most often forelimb tonus was observed but not hindlimb. In these cases, seizures of 3 minute duration were considered adequate to assess the effects brief seizures have on the developing brain. Other animals never displayed either forelimb or hindlimb tonus and instead had continuous clonic seizures. These were judged to be not as severe as tonic seizures and thus a 4 minute end point was used. On average, 13% of seizures reached criteria 1, while 59% and 28% reached criteria 2 and 3 respectively. Based on these criteria, seizure duration was on average 3.13 ± 0.06 min (n =42 mice). Sham-handled littermate control mice were placed in the chamber for 4 minutes but without exposure to flurothyl and thus they did not undergo seizures. Untreated littermate controls remained with their dam throughout these procedures. Results from all experiments reported here showed no significant difference between untreated control and handled control mice – either in terms of dendritic morphology or spatial learning and memory. Thus data from the two control types were combined and results compared to that of the seizure group.
In terms of the seizure group, three seizures were induced daily for 5 days, P7-P11. Mice were allowed to recover for 2 hours before the next seizure was induced. A saline solution (0.1ml) was subcutaneously injected twice a day in both seizure-treated and handled control infant mice to compensate for periods of time when the pups were unable to suckle due to the time required for recovery from seizures. Typically, infant mice were immobile for 10-15 minutes following a seizure, but dams would commonly pick up pups and return them to the nest. Well before the next seizure was induced, mouse pups were suckling. Thus recovery time following seizures was not prolonged. Nonetheless, body weight was routinely monitored before and after seizure induction and our protocol resulted in no significant alterations in body weight. For instance on P12, the day after the last seizure, the weights of control mice and mice that undergone seizures were comparable (Control: 6.23 ± 0.16g; Seizure: 5.50 ± 0.26g; p > 0.05). Similarly, as adults when behavioral testing was undertaken, body weights of control and experimental mice were similar (Control: 23.5 ± 2.02g; Seizure: 21.6 ± 1.50g; p > 0.05).
When seizures were induced in 1 month-old mice (P30-P34, both males and females were assigned to each treatment group), identical procedures were used except flurothyl was delivered at the rate of 33μl/min and mice were removed during rearing and forelimb clonic activity. Tonic extension was avoided since it almost always produced death (Swann et al., 2007b). Seizure duration was on average 2.41 ± 0.05 min (n = 9 mice). Although the average duration of seizures in month-old mice was somewhat less than that in infant mice, the seizures were considered quite severe since they would rapidly evolve to full tonic extension. Nonetheless, the difficulties in directly comparing seizures at different ages are recognized due to differences in behavioral repertoire and maturational difference in the neuronal substrates that generate the seizures. Finally mice were allowed to survive for varying periods (1- 44 days) before the brain was removed for morphological analysis. Behavioral testing was done at 50-60 days of age for mice that experienced seizures as infants or at 1 month of age.
Neuronal tracing and analysis
After perfusion with PBS and 4% paraformaldehyde, brains were fixed overnight in a 4% paraformaldehyde at 4°C. Coronal sections (300μm thick) were cut with a vibratome. Brain sections containing the dorsal hippocampus were rinsed three times in PBS and dried for 3-4 hours (P20 and older) or overnight (P6-15). Finally the sections were dehydrated and mounted on slides with Vectamount mounting media (Vector Laboratories, Burlingame, CA) and glass coverslipped.
Immunohistochemistry for GFP was conducted for P6-P12 brain sections to intensify the endogenous GFP signals. To accomplish this, fixed sections of P6 and P12 brains were rinsed twice in PBS and then once in PBS with 0.3% Triton X-100 (Sigma) at 1 h intervals. The slices were then incubated in a solution containing the primary antibody for 24 hours at 4°C. This solution consisted of PBS, 0.3% Triton X-100 and the primary mouse monoclonal antibody, anti-GFP (1:1000, Sigma). After rinsing the sections three times in PBS, the tissue was then incubated for 2 hours with Alexa488-conjugated goat anti-mouse secondary antibody (1:1000, Invitrogen, Carlsbad, CA) dissolved in PBS. The brain sections were again rinsed three times in PBS and all sections were then dried, dehydrated, mounted on slides and glass coversliped. All immunohistochemical reactions in slices from experimental and control animals were done simultaneously under identical conditions.
Only CA1 neurons with either strong native GFP fluorescence or immunofluorescence (P6-12) throughout their dendritic trees and well isolated from neighboring GFP neurons were selected for analysis. Due to the length of apical dendrites and consequent increased likelihood that portions of these arbors would be lost in adjacent sections as well as numerous GFP-positive processes from other cells crossing them we focused our analysis on basilar dendrites. There was one exception to this. Apical dendrites from P55 mice that had undergone seizures in infancy and their controls were analyzed.
In all experiments, from the large number of neurons selected for possible reconstructions a small number was randomly selected by a blinded researcher for confocal imaging and later reconstructions. Three or four neurons from each mouse were chosen for reconstruction. Confocal imaging was accomplished using a FluoView FV300 confocal laser scanning microscope on a BX50WI fixed stage upright microscope equipped with a FV5-ZM stepper motor and FluoView software (Olympus, Melville, NY). GFP images were acquired via excitation with an argon laser (488 nm line), a 505–525 nm bandpass emission filter set, and a 20XUPlanApo objective (numerical aperture (NA) = 0.8, Olympus) using the appropriate manufacturer-suggested confocal apertures. Steps in the Z-axis were in 2 μm increments. Kalman accumulation averaging of 3 was used. Maximum projection images were generated with FluoView software. Basilar dendritic arbors were manually reconstructed from the image stacks using Neurolucida software (MicroBrightField, Colchester, VT). All Neurolucida reconstructions were conducted in a blinded manner. Quantitative analysis on the traced data was done using Neuroexplorer software (MicroBrightField). The extent of shrinkage due to dehydration at each age was determined by tracing the same dendrite segments (n = 5 neurons and 5 dendrite segments per neuron for each age group) before and after dehydration. Correction for shrinkage in all 3 dimensions at every age was done using Neurolucida subroutines. Sholl and convex hull analysis were also conducted with Neuroexplorer software. Convex hull analysis measures the volume occupied by a dendritic field – and interprets a branched structure as a solid object encompassing a volume of 3-dimensional space (avides-Piccione et al., 2006;McGarry et al., 2010). The analysis computes a convex polygon by connecting the tips of distal dendrites and then calculates the volume of the polygon. Numerical data (total dendritic length and branch points), such as geometric mean and S.E.M. were calculated for each treatment and control group. Each type of experiment was repeated on three separate occasions and results combined for final analysis.
Test for learning and memory
For Morris water maze evaluation, a circular pool (150 cm diameter) of water was used. The water level was approximately 60 cm and maintained at room temperature (21-23°C). Non-toxic white Crayola paint was added to make the water opaque. During hidden-platform training, the pool was conceptually divided into 4 quadrants and the escape platform was located 0.5-1.0 cm below the surface of the water and in the same quadrant for each block of four trials. A trial was started by placing an animal along the edge of the pool facing the wall in one of four start locations. The mouse was allowed 60 seconds to locate the platform. If the mouse did not find the platform it was guided there by the experimenter and allowed to remain on the platform for 30 seconds. This same procedure was repeated until an animal had been given a block of four trials after which it was returned to its holding cage while the next mouse was given a block of trials. Each mouse was given 8 trials a day in 2 blocks of four trials, for 4 consecutive days. To determine if a subject learned the location of the platform a probe trial was given following the last trial of hidden-platform training. During this probe trial, the escape platform was removed and the animal was allowed 60 seconds to search the pool. After the probe trial, a visible platform trial was undertaken to examine both vision and motor performance. A Noldus Videotracking System (Noldus EthoVision XT 6.0: Noldus Information Technology, Sterling, VA) was used to characterize the search pattern of each mouse in all trials. The times to find the platform and swim speeds among other variables were computed for all training and the probe trials.
Statistics
ORIGIN Version 8.0 (OriginLab, Northampton, MA) was employed for construction of histograms and graphs. The data were analyzed statistically using a one-way ANOVA or a two-way ANOVA with a Tukey post hoc test to correct for multiple comparisons (Sigma Stat Version 3.5: Systat Software Inc., San Jose, CA). All data are presented as the mean ± S.E.M.
RESULTS
Recurrent seizures in infant mice produce spatial learning deficits and reduce basilar dendrite length and branching
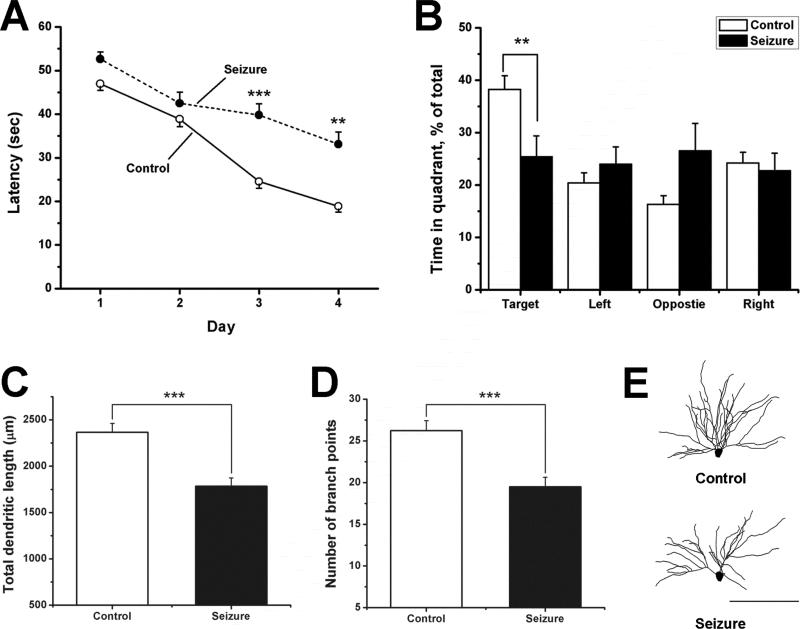
While numerous studies of the effects of recurrent early-life seizures on cognition have been performed in rats, similar studies have not been conducted in mice. Since our aim was to use mice that express GFP in hippocampal pyramidal cells to examine seizure-induced alterations in dendrite anatomy that could be associated with impaired learning and memory, we initially examined the effects recurrent early-life seizures had on learning and memory deficits in mice. To do this, fifteen, brief seizures were induced in C57BL/6 mice between postnatal days 7 and 11. On postnatal day 50-60, their ability to accomplish a spatial learning task in a Morris water maze was evaluated. Results from these experiments (Figure 1A) showed that control mice readily learned this spatial task but while the experimental animals improved their performance over the 4 trial days, it took them significantly longer to find the hidden platform than their littermates. After the final trial, a probe test was performed, which indicated (Figure 1B) that control mice spent a disproportionate amount of time in the quadrant that had previously contained the platform, but mice that had experienced seizures in early life did not. These performance differences could not be explained by disturbances in either visual or motor function since times to reach a visible platform (Control: 10.2 ± 1.1sec versus Seizure: 11.4 ± 1.8 sec) as well as swim speeds (Control: 34.8 ± 2.3 cm/sec versus Seizure: 35.5 ± 2.9 cm/sec) were nearly identical for the control and seizure groups. Activity in an open field was also similar. Thus taken together our results suggest that mice that had experienced seizures not only failed to acquire the spatial learning task as well as their litter mate controls but they were also impaired in their ability to remember the location of the hidden platform during the probe trial.
Figure 1.
Recurrent flurothyl-induced seizures in infant mice produce spatial learning and memory deficits and reduce basilar dendrite length and branching. (A) Performance of mice (50-60 days-old) in a Morris water maze that had experienced 15 brief seizures between P7-11. Average escape latencies for experimental (closed circles) and littermate control (open circles) mice are plotted versus the day of training (2 blocks of 4 trials per day). On the first day, escape latencies were similar between the treatment groups but by day 3 and 4 significant differences in performance occurred where mice that had experienced seizures were slower to learn the task. (B) Following the final block of training on day 4, a probe trial was given to each mouse. Control mice (open bars) showed a strong preference for the target quadrant that had previously contained the submerged platform but mice that had experienced seizures in infancy showed no preference for this quadrant. Time spent in the target quadrant differed significantly between the two groups. Control mice: n = 24, Seizure mice: n = 12. (C-D) Early-life seizures also reduced the total length and number of branches in basilar dendrite arbors in 55 day-old mice. (E) Representative reconstructed dendrites from control and seizure-treated littermates illustrate the reduction in overall dendrite morphology produced by seizures. Control: n = 12, Seizure: n = 12 neurons. All results are presented as mean ± S.E.M. ** p ≤ 0.01, *** p ≤ 0.001. Scale bar = 100μm.
Since dendritic abnormalities are common in human epilepsy (Swann et al., 2000) and early-life seizures have previously been shown to produce persistent alterations in dendrite microanatomy including reductions in branch number and length (Jiang et al., 1998), we next attempted to show that recurrent seizures in mice not only produce learning deficits but also dendritic abnormalities. Flurothyl seizures were induced as before and on P55 (at a time when the behavioral testing was performed), the microanatomy of CA1 pyramidal cells was analyzed. Figure 1C-D show that recurrent seizures resulted in a 25% reduction in both the total length and number of branch points of basilar dendrites. Figure 1E illustrates representative dendrites from the control and experimental group where a simpler dendritic arbor is evident in the seizure group. Surprisingly, when the apical dendrites of CA1 pyramidal cells were similarly analyzed no differences were detected in either total length (Control: 1946 ± 145μm versus Seizure: 2089 ± 183μm: p > 0.05) or number of branch points (Control: 24.6 ± 1.9 versus Seizure: 24.3 ± 1.8: p > 0.05). A similar and apparent unique sensitivity of basilar dendrite length and branch number to recurrent early-life seizures has been reported in a rat tetanus toxin model (Jiang et al., 1998), which also demonstrates spatial learning deficits (Lee et al., 2001). This suggests that these effects on basilar dendrites are not unique to the flurothyl model and may be a more generalized phenomenon.
Hippocampal pyramidal cell dendrites grow rapidly during infancy
Given the dramatic effects early-life seizures have on basilar dendrite anatomy, we next set out to examine how seizures produced these long-term alterations in dendrite morphology. As mentioned above, an earlier study (Jiang et al., 1998) demonstrated dendritic abnormalities in adulthood following recurrent seizures in infancy. Our goal here was to extend these observations by testing the hypothesis that such dendritic abnormalities resulted at least in part from seizures interfering with dendrite growth. However, before examining the effects of seizures, an understanding of the normal patterns of dendrite growth was needed. Figure 2A and 2B show that basilar dendrites undergo dramatic alterations in their morphology from P6 to P30. Not only are dendrites longer by P30 but the branching complexity of these arbors is greater. To characterize the time course of dendrite growth more fully, Sholl analyses were conducted at specific ages between P6 and P30 where virtual concentric spheres – centered on a neuron's cell body were drawn at 10μm intervals (Inset Figure 2C). The number of dendrite branches intersecting each of the spheres was counted. Figure 2C shows that between P6 and P12 a large increase in the number of intersections occurred from 50 to 150μm from the cell body - likely indicating the addition of branches to dendrite arbors. Between P12 and P30, age-dependent additions are evident but occurred at a slower pace – particularly between P20 and P30. At later ages, intersections occurred at greater distances reflecting the growth of the dendrites into the more distant dendritic laminae.
Figure 2.
Age-dependent alterations in CA1 hippocampal pyramidal cell basilar dendritic arbors. (A) Maximum projection confocal images of representative GFP positive pyramidal cells on postnatal days 6 and 30 and (B) reconstructions of the soma and basilar dendrites from these two cells. (C) Patterns of dendrite growth reflected by Sholl analyses. Inset illustrates this type of analysis – where the number of dendritic branches that intersect concentric spheres was counted. In this drawing, cross sections of 2 spheres are shown at 50 and 100μm from the soma. Dendrite growth is rapid between P6 and P12 as indicated by the large increase in number of intersections - thereafter growth progresses but at a slower pace. Scale bar = 100μm.
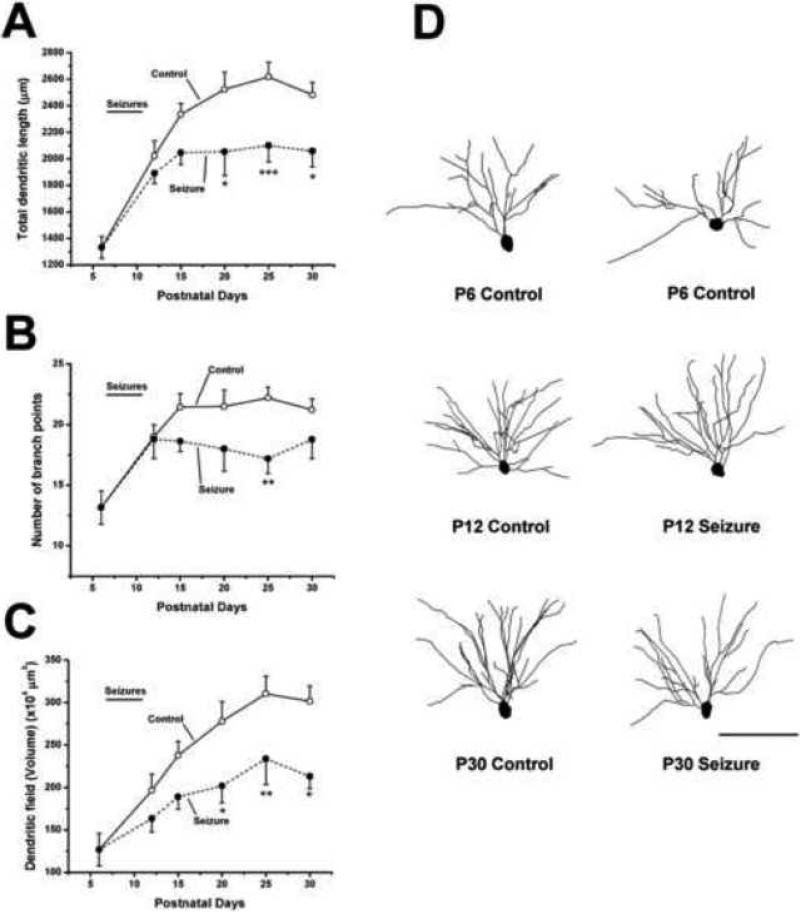
Graphs labeled “Control” in Figure 3A – 3C examine the time course of basilar dendrite growth from these populations of neurons in different ways. In Figure 3A, the total length of dendrites is shown to increase dramatically from P6 through P15 - thereafter growth proceeds but at a slower pace until P25. Paralleling these increases in dendrite length, branches are added to the growing arbors in control mice (Figure 3B) and the volume of neuropile encompassed by the arbor increases as computed by a convex hull analysis. In terms of branch number, there is an increase of nearly 70% from P6 to P15 but afterwards branch number remains rather constant. On the other hand, the volume of tissue encompassed by basilar arbors (Figure 3C), more closely parallels alterations in total dendrite length.
Figure 3.
Growth of hippocampal dendrites is suppressed following the induction of seizures on postnatal days 7 through 11. Graphs summarize results from experiments in which the dendrites from control and flurothyl-treated CA1 hippocampal pyramidal cells were reconstructed and analyzed for differences in (A) total length, (B) numbers of branch points and (C) the volume of the dendritic field. Following 15 seizures between P7 to P11 (demarcated by a line labeled seizures above each graph) a dramatic decrease in the rate of dendrite growth were observed based on measures of the three parameters analyzed. (D) Representative Neurolucida reconstructions of dendrites of GFP positive CA1 pyramidal neurons at selected ages. The soma and basilar dendrites of these cells are shown and clearly illustrate that pyramidal cells from P30 control mice have more elaborate dendritic arbors than those from mice that experienced seizures in infancy. Differences in arbor complexity were not apparent at P12. Two control neurons from P6 mice are shown to illustrate baseline arbor branching and length. Results are presented as mean ± S.E.M. Control: n was between 12 and 29 neurons per age group, Seizure: n was 9–25 per age group.* p ≤ 0.05, ** p 0.01, *** p ≤ 0.001 when comparing seizure groups to controls at the same age. Scale bar = 100 μm
Recurrent seizures suppress dendrite growth
When dendrites from litter-mates that had experienced seizures were analyzed, dramatic differences were observed from that of control mice. Figures 3A-3C show that dendrite growth continues unabated during the time when seizures were being induced. On P12, the day after the last seizure, there were still no significant differences in any of the dendritic measures. However, by day 15, differences between the dendrites of control and experimental animals were seen and these differences became more marked as the animals continued to mature. Results clearly show that the age-dependent differences between experimental animals and their controls were not due to a progressive decrease in dendrite length (Figure 3A) that might have been produced by a retraction of growing arbors. Instead, the differences evolve gradually as the dendrites from control mice continue to grow but those of the experimental mice fail to grow or grow at a much reduced rate. Two-way ANOVA showed that compared to controls the effect of seizures on dendrite development was highly significant for dendrite length (p ≤ 0.001, F(1,214) = 19.0), number of branch points (p ≤ 0.01 F(1,214) = 10.1) and volume of dendritic fields (p ≤ 0.001 F(1,214) = 17.1). Tracings on the right side of Figure 3D illustrate the effects recurrent seizures on dendrite growth. Between P6 and P12, dendrites appear to grow normally. The overall length and branching complexity of the dendrites from the seizure and control groups at P12 appear comparable. However, by P30 differences are apparent. Dendrites in the seizure group have simpler architecture with fewer and shorter branches
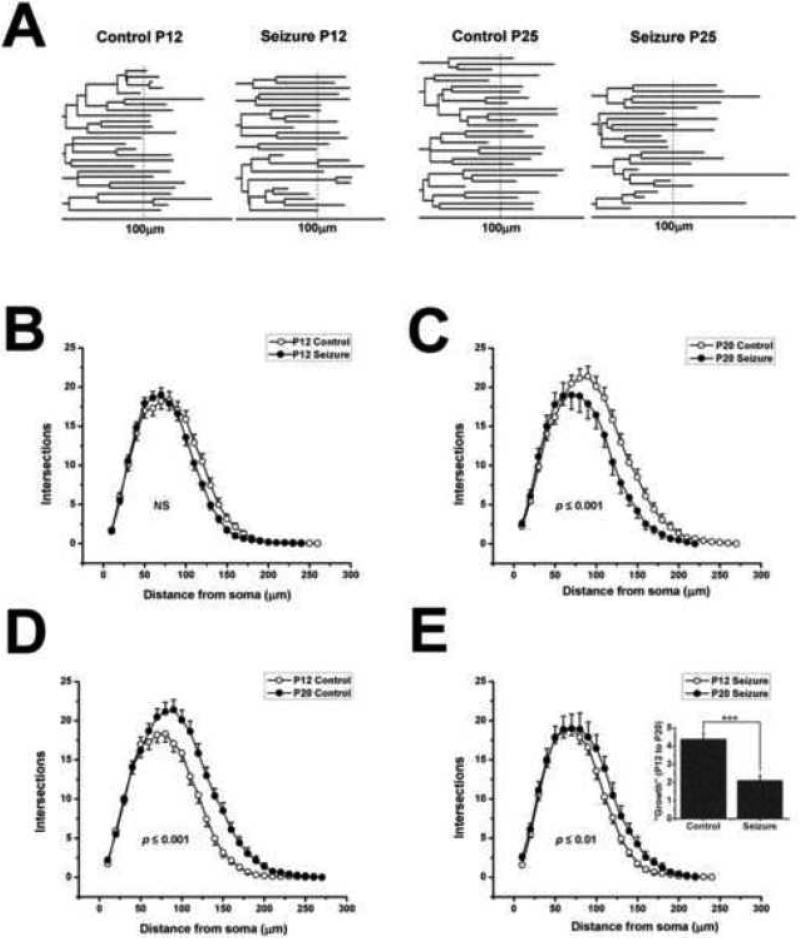
At the level of individual neurons, suppression of dendrite growth could be mediated in different ways via different molecular mechanisms. For instance, retardation of growth could be mediated solely by preventing the addition of new dendritic branches or it could be produced by preventing the growth of existing branches. To address this issue we carried out a detailed age-dependent analysis of alterations in dendritic morphology. Results in Figure 4A shows representative dendrograms and compare basilar dendrites from the control and seizure groups on P12 and P25 – i.e. just before and after the growth suppression illustrated in Figure 3. These computer generated drawings quantitatively trace the branching pattern of the arbors as they emanate from the pyramidal cell body and project into the basilar dendritic layer. The lengths of individual branches are faithfully reproduced. Consistent with the results in Figure 3, the branching complexity of the neuron from the control and seizure group on P12 appear similar. However, at P25 there are noticeably more dendritic branches in the control neuron compared to that of the seizure neuron and the distance many of the dendrites project from the cell body is less in the seizure neuron. These results suggest that seizures act to both limit the addition of dendritic branches and the growth of existing branches.
Figure 4.
Recurrent seizures suppress both the addition of new dendritic branches and growth of existing branches. (A) Representative dendrograms of CA1 hippocampal pyramidal cell basilar dendrites from P12 and P25 control and seizure-treated mice. Dendrite branching patterns were similar on P12 in control and seizure groups. By P25, more and longer branches are present in the control pyramidal cell but this was not the case in the P25 seizure group. For instance in this “Control P25” dendrite, 24 branches project more than 100μm from the cell body, while for the “Seizure P25” dendrite only 14 branches reach this distance. (B) Sholl analysis comparing dendrite branching patterns on P12 in control and seizure group. (C) The same analysis as in B but on P20. On P12 dendritic arbors were similar in the number of intersections at the same disnces from the soma of pyramidal cells. On P20, the greater number of intersections reflects the presence of more dendrite branches in controls. More branches extend greater distances from the cell body in controls. (D) Direct comparison of age-dependent alterations in dendrite arborization in controls. (E) The same analysis as in D but for seizure groups. Between P12 and P20, dendrites in control mice added additional branches that extended greater distances from the soma. Age-dependent alterations of dendrites in seizure groups were far less extensive. (E-Inset) A “growth” comparison of neurons in control and seizure group – differences in the number of intersections between P20 and P12 were averaged (at 80 – 180μm distances) and plotted. Fewer dendrite branches were added by P20 in the seizure group. Number of neurons analyzed was 9 for the P20 seizure group and 25 for all other groups. (B-E) All results are presented as mean ± S.E.M. p values after a two-way ANOVA are indicated within plot areas. NS, not significant, p >0.05. E-inset *** p ≤ 0.001 after a one-way ANOVA.
To support this observation, Sholl analyses were conducted (Figure 4B-4E). Consistent with results in Figure 3 and 4A, this analysis showed that there were only relatively small or no effects of seizures by P12 (Figure 4B: p >0.05: two-way ANOVA comparing P12 control and seizure groups) but by P20 a marked difference in the number of intersections at greater distances from the cell body were observed (Figure 4C: p ≤ 0.001: comparing P20 control and seizure groups) suggesting that early-life seizures impact both the laminar extent and branching complexity of dendritic arbors. To further explore the impact seizures have on dendritic growth we next directly compared Sholl plots from control and experimental mice on P12 and P20. During maturation in control animals there was a substantial growth of dendrites as indicated by the increased number of intersections at greater distances from neuronal cell body (Figure 4D: p ≤ 0.001: two-way ANOVA: Control P12 vs P20) . However, in pyramidal cells from mice that experienced early-life seizures while an increase was still apparent - it was markedly attenuated (Figure 4E: p 0.01: Seizure P12 vs P20). To further evaluate the differences in growth between the control and seizure groups, differences in the mean number of intersections at distances from 80 to180μm for the control group were averaged and compared to the same averages from the seizure group. The inset of Figure 4E shows that the increase in the number of intersections between P12 and P20 (termed “growth” from P12 to P20) was significantly less in the seizure group than in the controls – consistent with our contention that seizures suppress dendrite growth, likely by restricting the number of branches added and limiting the growth of the existing branches.
Recurrent seizures in older mice do not reduce dendrite arbor complexity or impair spatial learning
If the effects of recurrent seizures on dendrites are developmentally dependent, it would follow that if similar seizures were induced in older mice (after the period a marked dendrite growth), they would have less of an effect on dendrite morphology. Furthermore, if dendrite growth suppression plays an important role in learning and memory deficits such as those shown in Figure 1, then cognition may not be as severely impacted in older animals that have experienced recurrent seizures. To explore this possibility, 15 flurothyl-induced seizures were induced from P30-34 using the same protocol as for infant mice. P30 was chosen as a starting age since our developmental studies in control mice (Figure 3) suggested that dendrite growth had plateaued by that age. Dendrite morphology was examined on day 50-55, which is a time post-seizure (16-21 days) comparable to the later time points in the development experiments shown in Figure 3. Dendrite reconstructions failed to detect significant alterations in total dendrite length (Figure 5A), number of branch points (5B) or volume of neuropile occupied by arbors as computed by a convex hull analysis (5C). Representative dendritic reconstructions (Figure 5D) illustrate the similarities in dendrite morphology in control mice and mice that experienced seizures at 1 month of age. Since dendrite morphology – at least branching complexity and length – was not impacted by recurrent seizures in these older mice, we next examined whether seizures could impair spatial learning and memory. Results in Figure 5E and F suggest that mice that had experienced seizures later in life were capable of learning the location of the hidden platform in the Morris water maze to a similar degree to that of their littermate controls. Similarly, deficits in spatial memory were not apparent since during the probe trial the time experimental mice spent in the quadrant that previously contained the platform – the target quadrant – were not significantly different from that of controls.
Figure 5.
Recurrent flurothyl-induced seizures in month-old mice fail to alter dendritic arbors or impact spatial learning and memory. Bar Graphs summarize results from experiments in which CA1 hippocampal pyramidal cell dendrites from control and flurothyl-treated mice were reconstructed. Following 5 days of recurrent seizures from P30 to P34 dendrites were reconstructed from 50-55 days of age. Neither (A) total dendrite lengths, (B) number of branch points nor (C) dendritic field volumes were affected by seizures in older mice. (D) Representative reconstructions of dendrites from control and seizure-treated mice illustrating similar dendritic lengths and branching complexity. Control: n = 18 neurons, Seizure: n = 19 neurons. Scale bar = 100 μm. (E) Performance of mice (50-60 days-old) in a Morris water maze that had experienced 15 seizures beginning at P30. Average escape latencies for experimental (closed circles) and littermate control (open circles) mice are plotted versus the day of training. Mice that had experienced seizures performed equally well as controls. (F) Similarly, the time experimental mice spent in the target quadrant during the probe test did not differ significantly from that of the control group. All results are presented as mean ± S.E.M. Control: n = 10 mice, Seizure: n = 7 mice.
DISCUSSION
Experiments reported here show for the first time that recurrent seizures early in life can suppress the growth of dendrites. This effect does not appear to selectively impact the addition of new branches or the growth of existing branches but simultaneously suppresses both processes. Our analysis of these developmental changes focused on the basilar dendrites of CA1 pyramidal cells. As mentioned in the Methods, there were technical reasons favoring analysis of basilar dendrites. However, this focus was also guided by our observation that recurrent early-life seizures did not produce changes in apical dendrite length or branch number on P55, when these measures of basilar dendrites were significantly reduced. However, it is entirely possible that seizures produce other alterations in apical dendrites – such as spine number, shape etc, but these anatomical features were not examined in this study. Instead, we concentrated our efforts on understanding the robust changes in basilar dendrite morphology.
At this time it is unclear why hippocampal basilar dendrites are impacted in a seemingly selective way by early-life seizure. However, the anatomical separation of basilar and apical dendrites of hippocampal pyramidal cells strongly implies that they have distinct functions. Moreover, during differentiation and growth they are very likely guided by different molecular cues to produce their distinct morphologies. Indeed, previous studies in the developing neocortex have clearly demonstrated that basilar and apical dendrites respond differently to alterations in neuronal activity. For instance, the basilar dendrites of layer 4 pyramidal cell have been reported to respond dramatically to blockade of neuronal activity by either TTX or CNQX by increasing their length and number of branches (McAllister et al., 1996). But the apical dendrites in the same neurons failed to respond to these treatments. Similarly, tetanus toxin has been reported to block basilar dendrite growth without affecting apical dendrites (Groc et al., 2002) Developing apical and basilar dendrites have also been reported to respond differently to a variety of neurotrophins (McAllister et al., 1995). For example, the basilar dendrites of layer 5 pyramidal cells have been reported to double in length in response to NT-4, while apical dendrites do not. Thus it seems likely that early-life seizures alter molecular events of the developing hippocampus that are critical for CA1 basilar dendrite growth.
Our results also show that recurrent seizures produce spatial learning and memory defects in mice. This is an important corollary of the effects observed on dendrite anatomy since CA1 pyramidal cells are thought to play a role in spatial learning in both animals and humans (Bartsch et al., 2010;Okeefe and Nadel, 1978). The Schaffer collaterals of CA3 pyramidal cells to apical dendrites in area CA1 have long been studied for their ability to produce activity-dependent alterations in synaptic efficacy. LTP and LTD are prominent examples of forms of hippocampal synaptic plasticity that are thought to form the cellular basis for hippocampal learning and memory. However, Schaffer collaterals not only contact apical dendrites in area CA1 but also heavily innervate basilar dendrites (Ishizuka et al., 1990;Li et al., 1994) and these synapses undergo robust LTP (e.g. Alarcon et al., 2006). Thus a reduction in basilar dendrite arbor size would be expected to reduce the anatomical and molecular substrates for learning and memory.
We also report that the effects on dendrite anatomy and spatial learning do not occur when seizures are induced in older – month-old rats. Thus there is a period during development- likely a critical period - when the impact of seizures is most pronounced. When seizures occur after this time, dendrites appear largely intact and spatial learning is not impaired. Since basilar dendrite growth appears to have ended by 1 month of age, our results are consistent with the notion that suppression of dendrite growth by recurrent seizures impaires cognition.
Our findings are also consistent with the hypothetical neurodevelopmental origins of cognitive deficits in patients that have a history of early-onset epilepsy (Hermann et al., 2008). As reviewed earlier, research has shown that earlier ages of seizure occurrence are associated with worse cognitive disabilities in adulthood. Imaging studies reporting volume reductions in numerous brain regions including the hippocampus and neocortex also suggest that brain anatomy is impacted by seizures in a similar developmentally-dependent manner. Further support for a neurodevelopmental origin of behavioral comorbidities are recent results from a MRI study of 8-18 year-old children with new-onset epilepsy who were imaged at the time of diagnosis and 2 years later (Hermann et al., 2010). Results showed, that over this time, volumes of neocortical white matter of sibling controls increased but white matter volumes in the children with epilepsy did not – a result consistent with impaired growth. In terms of the results reported here, we have examined dendrite development, but only basilar dendrites of one type of hippocampal pyramidal cell. Given the robust effects we have observed it seems likely that the growth of dendrites and possibly axons of other neuronal populations could be similarly impacted by early-life seizures - especially if they share molecular developmental programs with basilar dendrites that are sensitive to seizure activity. If seizure-induced growth suppression, while not universal, were none-the-less shown to be wide-spread, it could help to explain imaging observations from the clinic.
However, it should be emphasized that seizures are likely to impact the developing brain in a variety of ways and it should be mentioned that flurothyl itself could have a direct effect on developing dendrites – despite the transient exposure to this compound. The effects that we report here on dendrite growth would conceivably impact an animal's ability to learn and remember, but there may be other changes produced by seizures that also contribute to the cognitive deficits observed. For instance, mossy fibers of the dentate gyrus have been reported to sprout in response to neonatal recurrent seizures (Liu et al. 1999). Alterations in hippocampal network activity produced by aberrant excitatory synapses could impair cognition. An understanding of the molecular events underlying growth suppression could allow these effects to be blocked in a selective way. If a rescue of normal dendrite growth resulted in a full or even partial prevention of seizure-induced impaired learning, this would strongly support a role for growth suppression in the cognitive disabilities observed.
Thus, of central importance for future studies is identifying the molecular mechanisms responsible for seizure-induced basilar dendrite growth suppression. Molecular mechanisms that regulate cytoskeletal dynamics in dendrites are important candidate mechanisms for growth suppression. The Rho family of small GTPases, including Rac, RhoA and Cdc42, act on the cytoskeleton and have been shown to participate in the control of dendrite growth (Chiu and Cline, 2010;Sin et al., 2002). As mentioned earlier, signaling through neurotrophins is well known to regulate dendrite growth (McAllister et al., 1996;Marshak et al., 2007;McAllister et al., 1995). Recent studies have also described roles for Wnt signaling (Rosso et al., 2005;Wayman et al., 2006;Yu and Malenka, 2003) and mTOR signaling (Kumar et al., 2005;Wu et al., 2001;Dunah et al., 2005;Chow et al., 2009) in the development of dendrites in the central nervous system. Alterations in one or more of these signaling pathways may mediate the seizure-induced growth suppression reported here.
In this regard, it may be instructive to consider the possibility that recurrent seizures – through hyperactivation of developing glutamatergic synapses - prematurely produce a “stop-growing” signal in hippocampal basilar dendrites. Upon maturation, dendrites normally grow at a much reduced rate. There are likely to be molecular cues produced by neurons that signal the end of the rapid phase of dendrite growth. Early-life seizures may tap into normal homeostatic mechanisms of neural development that slows and stops dendrite growth but just does it earlier than usual. One potential clue to the underlying mechanisms may be the time course of growth suppression. As seen in Figure 3, the growth of basilar dendrites appears relatively unaltered during the 5 days (P7-11) that seizures are induced - even though dendrites are growing very rapidly at this time. Either there is a required cumulative effect of many seizures to produce the molecular signal for growth suppression or the molecular mechanisms are unusually slow in developing and/or expressing themselves.
In terms of growth suppression, neuronal activity is well known to play an important role in dendrite development and activation of glutamate receptors – particularly NMDA receptors – are thought to activate important intracellular signaling cascades that not only stimulate growth but once an adult complement of synapses is formed may actually produce molecular signals that stop growth (Chiu and Cline, 2010;Konur and Ghosh, 2005). In this regard, experiments have shown that calcium/calmodulin-dependent protein kinase II (CaMKII) may serve as a “stop-growing” signal in dendrites (Zou and Cline, 1999). It is interesting to note that CaMKII is a well known downstream signal in the calcium signaling cascade initiated by synaptic activation of NMDA receptors. Experiments in hippocampal slice cultures have shown that chronic pharmacological blockade of NMDA receptors similarly resulted in growth of extensive highly-branched CA1 pyramidal cell dendrites (Luthi et al., 2001). Our previous experiments in a slice culture model suggest that suppression of growth induced by seizure-like discharges is NMDA receptor dependent (Nishimura et al., 2008). Accordingly, studies of the basic mechanisms of activity-dependent growth suppression may provide new insights into the origins of the cognitive comorbidities of childhood epilepsy.
Highlights.
Seizures in infant mice produce deficits in spatial learning and memory
Seizures suppress the growth of hippocampal basilar dendrite
Seizures impact both dendrite branch addition and growth
Seizures in month-old mice do not produce these effects
Acknowledgements
We thank John Le and Kevin Winoske for their assistance in refining the flurothyl model and Scott Baker for genotyping mice. Funding - this work was supported by the National Institutes of Health (NINDS) [NS018309, NS039941and NS062992]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Alarcon JM, Barco A, Kandel ER. Capture of the late phase of long-term potentiation within and across the apical and basilar dendritic compartments of CA1 pyramidal neurons: synaptic tagging is compartment restricted. J Neurosci. 2006;26:256–264. doi: 10.1523/JNEUROSCI.3196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- avides-Piccione R, Hamzei-Sichani F, Ballesteros-Yanez I, DeFelipe J, Yuste R. Dendritic size of pyramidal neurons differs among mouse cortical regions. Cereb Cortex. 2006;16:990–1001. doi: 10.1093/cercor/bhj041. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, Deuschl G, Koch JM. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328:1412–1415. doi: 10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- Berg AT. Epilepsy, cognition, and behavior: The clinical picture. Epilepsia. 2011;52(Suppl 1):7–12. doi: 10.1111/j.1528-1167.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, Kulas J. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Bjørnaes H, Stabell K, Henriksen O, Løyning The effects of refractory epilepsy on intellectual functioning in children and adults. A longitudinal study. Seizure. 2001;10:250–259. doi: 10.1053/seiz.2000.0503. [DOI] [PubMed] [Google Scholar]
- Brooks-Kayal A. Molecular mechanisms of cognitive and behavioral comorbidities of epilepsy in children. Epilepsia. 2011;52(Suppl 1):13–20. doi: 10.1111/j.1528-1167.2010.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SL, Cline HT. Insulin receptor signaling in the development of neuronal structure and function. Neural Dev. 2010;5:7. doi: 10.1186/1749-8104-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X, Trachtenberg JT. Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci. 2009;12:116–118. doi: 10.1038/nn.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen S, Matthews CG, Harley JP. The effect of early versus late onset of major motor epilepsy upon cognitive-intellectual performance. Epilepsia. 1975;16:73–81. doi: 10.1111/j.1528-1157.1975.tb04723.x. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Matthews CG. The role of neuropsychology in the assessment and treatment of persons with epilepsy. Am Psychol. 1992;47:1139–1142. doi: 10.1037//0003-066x.47.9.1139. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Cline HT. Postsynaptic calcium/calmodulin-dependent protein kinase ll is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. The Journal of Neuroscience. 1999;19:8909–8918. doi: 10.1523/JNEUROSCI.19-20-08909.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Hueske E, Wyszynski M, Hoogenraad CC, Jaworski J, Pak DT, Simonetta A, Liu G, Sheng M. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- Elger CE, Helmstaedter C, Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3:663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Groc L, Petanjek Z, Gustafsson B, Ben-Ari Y, Hanse E, Khazipov R. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. Eur J Neurosc. 2002;16:1931–1938. doi: 10.1046/j.1460-9568.2002.02264.x. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Jones J. The neurobehavioural comorbidities of epilepsy: can a natural history be developed? Lancet Neurol. 2008;7:151–160. doi: 10.1016/S1474-4422(08)70018-8. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Dabbs K, Becker T, Jones JE, Gutierrez A, Wendt G, Koehn MA, Sheth R, Seidenberg M. Brain development in children with new onset epilepsy: a prospective controlled cohort investigation. Epilepsia. 2010;51:2038–2046. doi: 10.1111/j.1528-1167.2010.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Bell B. The neurodevelopmental impact of childhood onset temporal lobe epilepsy on brain structure and function and the risk of progressive cognitive effects. In: Sutula T, Pitkänen A, editors. Progress in Brain Research. Vol. 135. 2002. pp. 431–438. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Lee CL, Smith KL, Swann JW. Spine loss and other persistent alterations of hippocampal pyramidal cell dendrites in a model of early-onset epilepsy. J Neurosci. 1998;18:8356–8368. doi: 10.1523/JNEUROSCI.18-20-08356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnam HB, Zhao Q, Shatskikh T, Holmes GL. Effect of age on cognitive sequelae following early life seizures in rats. Epilepsy Res. 2009a;85:221–230. doi: 10.1016/j.eplepsyres.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnam HB, Zhou JL, Huang LT, Zhao Q, Shatskikh T, Holmes GL. Early life seizures cause long-standing impairment of the hippocampal map. Exp Neurol. 2009b;217:378–387. doi: 10.1016/j.expneurol.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Ghosh A. Calcium signaling and the control of dendritic development. Neuron. 2005;46:401–405. doi: 10.1016/j.neuron.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Kumar V, Zhang MX, Swank MW, Kunz J, Wu GY. Regulation of dendritic morphogenesis by Ras-PI3K-AktmTOR and Ras-MAPK signaling pathways. J Neurosci. 2005;25:11288–11299. doi: 10.1523/JNEUROSCI.2284-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson JA, Cook MJ, Vogrin S, Litewka L, Strong D, Bleasel AF, Bye AM. Clinical, EEG, and quantitative MRI differences in pediatric frontal and temporal lobe epilepsy. Neurol. 2002;58:723–729. doi: 10.1212/wnl.58.5.723. [DOI] [PubMed] [Google Scholar]
- Lee CL, Hannay J, Hrachovy R, Rashid S, Antalffy B, Swann JW. Recurrent seizures in infant rats produced spatial learning deficits without a substantial loss of hippocampal pyramidal cells. Neurosci. 2001;107:71–84. doi: 10.1016/s0306-4522(01)00327-x. [DOI] [PubMed] [Google Scholar]
- Lee JW, Andermann F, Dubeau F, Bernasconi A, MacDonald D, Evans A, Reutens DC. Morphometric analysis of the temporal lobe in temporal lobe epilepsy. Epilepsia. 1998;39:727–736. doi: 10.1111/j.1528-1157.1998.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Yang Y, Silveira DC, Sarkisian MR, Tandon P, Huang L-T, Stafstrom CE, Holmes GL. Consequences of recurrent seizures during early brain development. Neurosci. 1999;92:1443–1454. doi: 10.1016/s0306-4522(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Li X-G, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: An in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Luthi A, Schwyzer L, Mateos JM, Gahwiler BH, McKinney RA. NMDA receptor activation limits the number of synaptic connections during hippocampal development. Nat Neurosci. 2001;4:1102–1107. doi: 10.1038/nn744. [DOI] [PubMed] [Google Scholar]
- Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. European Journal of Neuroscience. 2000;12:2252–2264. doi: 10.1046/j.1460-9568.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- Marsh L, Morrell MJ, Shear PK, Sullivan EV, Freeman H, Marie A, Lim KO, Pfefferbaum A. Cortical and hippocampal volume deficits in temporal lobe epilepsy. Epilepsia. 1997;38:576–587. doi: 10.1111/j.1528-1157.1997.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Marshak S, Nikolakopoulou AM, Dirks R, Martens GJ, Cohen-Cory S. Cell-autonomous TrkB signaling in presynaptic retinal ganglion cells mediates axon arbor growth and synapse maturation during the establishment of retinotectal synaptic connectivity. J Neurosci. 2007;27:2444–2456. doi: 10.1523/JNEUROSCI.4434-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- McGarry LM, Packer AM, Fino E, Nikolenko V, Sippy T, Yuste R. Quantitative classification of somatostatin-positive neocortical interneurons identifies three interneuron subtypes. Front Neural Circuits. 2010;4:12. doi: 10.3389/fncir.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M, Owens J, Swann JW. Effects of chronic network hyperexcitability on the growth of hippocampal dendrites. Neurobiol Dis. 2008;29:267–277. doi: 10.1016/j.nbd.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MA, Redoblado MA, Lah S, Sabaz M, Lawson JA, Cunningham AM, Bleasel AF, Bye AM. Intelligence in childhood epilepsy syndromes. Epilepsy Res. 2003;53:139–150. doi: 10.1016/s0920-1211(02)00261-9. [DOI] [PubMed] [Google Scholar]
- Okeefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; Oxford: 1978. [Google Scholar]
- Oyegbile T, Hansen R, Magnotta V, O'Leary D, Bell B, Seidenberg M, Hermann BP. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–737. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- Pardoe H, Pell GS, Abbott DF, Berg AT, Jackson GD. Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy. Neuroimage. 2008;42:611–616. doi: 10.1016/j.neuroimage.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsipher DT, Seidenberg M, Guidotti L, Tuchscherer VN, Morton J, Sheth RD, Hermann B. Thalamofrontal circuitry and executive dysfunction in recent-onset juvenile myoclonic epilepsy. Epilepsia. 2009;50:1210–1219. doi: 10.1111/j.1528-1167.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nature Neuroscience. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Schoenfeld J, Seidenberg M, Woodard A, Hecox K, Inglese C, Mack K, Hermann B. Neuropsychological and behavioral status of children with complex partial seizures. Dev Med Child Neurol. 1999;41:724–731. doi: 10.1017/s0012162299001486. [DOI] [PubMed] [Google Scholar]
- Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature. 2002;419:475–480. doi: 10.1038/nature00987. [DOI] [PubMed] [Google Scholar]
- Sogawa Y, Monokoshi M, Silveira DC, Cha BH, Cilio MR, McCabe BK, Liu X, Hu Y, Holmes GL. Timing of cognitive deficits following neonatal seizures: relationship to histological changes in the hippocampus. Brain Res Dev Brain Res. 2001;131:73–83. doi: 10.1016/s0165-3806(01)00265-6. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE. Sutula T, Pitkänen A, editors. Assessing the behavorial and cognitive effects of seizures on the developing brain. Progress in Brain Research. 2002;135:377–390. doi: 10.1016/S0079-6123(02)35034-9. [DOI] [PubMed] [Google Scholar]
- Swann JW, Al Noori S, Jiang M, Lee CL. Spine loss and other dendritic abnormalities in epilepsy. Hippocampus. 2000;10:617–625. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Swann JW, Le JT, Lam TT, Owens J, Mayer AT. The impact of chronic network hyperexcitability on developing glutamatergic synapses. Eur J Neurosci. 2007a;26:975–991. doi: 10.1111/j.1460-9568.2007.05739.x. [DOI] [PubMed] [Google Scholar]
- Swann JW, Le JT, Lee CL. Recurrent seizures and the molecular maturation of hippocampal and neocortical glutamatergic synapses. Dev Neurosci. 2007b;29:168–178. doi: 10.1159/000096221. [DOI] [PubMed] [Google Scholar]
- Theodore WH, DeCarli C, Gaillard WD. Total cerebral volume is reduced in patients with localization-related epilepsy and a history of complex febrile seizures. Arch Neurol. 2003;60:250–252. doi: 10.1001/archneur.60.2.250. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Weber B, Luders E, Faber J, Richter S, Quesada CM, Urbach H, Thompson PM, Toga AW, Elger CE, Helmstaedter C. Distinct regional atrophy in the corpus callosum of patients with temporal lobe epilepsy. Brain. 2007;130:3149–3154. doi: 10.1093/brain/awm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G-Y, Deisseroth K, Tsien RW. Spaced stimuli stabalize MAPK pathway activiation andd its effects on dendrite morphology. Nature Neuroscience. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]