Abstract
Two distinct p97 membrane fusion pathways are required for Golgi biogenesis: the p97/p47 and p97/p37 pathways. VCIP135 is necessary for both pathways, while its deubiquitinating activity is required only for the p97/p47 pathway. We have now identified a novel VCIP135-binding protein, WAC. WAC localizes to the Golgi as well as the nucleus. In Golgi membranes, WAC is involved in a complex containing VCIP135 and p97. WAC directly binds to VCIP135 and increases its deubiquitinating activity. siRNA experiments revealed that WAC is required for Golgi biogenesis. In an in vitro Golgi reformation assay, WAC was necessary only for p97/p47-mediated Golgi reassembly, but not for p97/p37-mediated reassembly. WAC is hence thought to function in p97/p47-mediated Golgi membrane fusion by activating the deubiquitinating function of VCIP135. We also showed that the two p97 pathways function in ER membrane fusion as well. An in vitro ER reformation assay revealed that both pathways required VCIP135 but not its deubiquitinating activity for their ER membrane fusion. This was consistent with the finding that WAC is unnecessary for p97-mediated ER membrane fusion.
Keywords: ER, Golgi, p37, p47
Introduction
The ER and Golgi apparatus occupy central positions in intracellular membrane traffic. Newly synthesized secretory and membrane proteins are incorporated into the ER and transported to the Golgi during which they receive several post-translational modifications. The Golgi apparatus dispatches all of these proteins towards their correct downstream locations (Mellman and Simons, 1992). Allied to the central roles of the ER and Golgi are their unique and complex architectures: the ER shows a network structure and the Golgi consists of a series of stacked flattened cisternae (Warren, 1995). How the complicated structures of the ER and Golgi are built up and maintained is one of the most important issues in molecular cell biology.
Experiments using an in vitro Golgi reformation assay revealed that Golgi reassembly from membrane fragments requires at least two ATPases; N-ethylmaleimide-sensitive factor (NSF) and p97 (also known as VCP) (Rabouille et al, 1995). The role of NSF has been well studied, while much less is known about the mechanism of action of p97. We previously showed that p97 uses two distinct cofactors for its membrane fusion function: p47 and p37 (Uchiyama et al, 2006). p47 forms a complex with p97 and the complex causes Golgi membrane fusion (Kondo et al, 1997), which requires syntaxin5 as a SNARE (Rabouille et al, 1998). Since p47, which has two localization signals in its peptide sequence, mainly localizes to the nucleus during interphase, the p97/p47 membrane fusion pathway is thought to be specialized for the reassembly of organelles at the end of mitosis (Uchiyama et al, 2003). On the other hand, p37, another cofactor of p97, localizes to the cytoplasm during interphase. The p97/p37 complex also brings about Golgi membrane fusion in which GS15 is utilized as a SNARE instead of syntaxin5 (Uchiyama et al, 2006). p37 is hence thought to be required for organelle maintenance during interphase as well as their reassembly during mitosis.
Both p97 pathways require VCIP135 (p97(VCP)/p47 complex-interacting protein, p135) for their Golgi membrane fusion activities (Uchiyama et al, 2002, 2006). VCIP135 was originally identified as a p97/p47 complex-interacting protein, which binds to the p97/p47/SNARE complex and assists its dissociation via p97-catalysed ATP hydrolysis (Uchiyama et al, 2002, 2006). Wang et al (2004) subsequently reported that VCIP135 possesses deubiquitinating activity, which is essential for p97/p47-mediated Golgi membrane fusion. The deubiquitinating activity of VCIP135 is, however, unnecessary for p97/p37-mediated Golgi membrane fusion, although VCIP135 itself is essential in this pathway (Uchiyama et al, 2006). VCIP135 therefore seems to work in two distinct ways, one via its deubiquitinating activity and the other in a ubiquitin-independent manner, which may be as a p97/p47 (or p37) complex-interacting protein. This leads to the interesting and important question as to how VCIP135 chooses either of its distinct functions.
In contrast to Golgi membrane fusion, the function of the p97 pathway in ER membrane fusion still remains entirely unclear. Originally, the group of Schekman reported that ER membrane fusion was mediated by Cdc48p, a yeast orthologue of p97, using an in vitro system (Latterich et al, 1995). The group of Mattaj later confirmed this by showing that p97 and p47 were required for ER reassembly in vitro (Hetzer et al, 2001). We also reported the in vivo requirement of p47 for ER biogenesis by the microinjection of anti-p47 antibodies into living cells (Uchiyama et al, 2002). However, there has been no detailed in vitro study on the other essential factors, p37 and VCIP135, for which we only showed their in vivo function on ER network structures in living cells (Uchiyama et al, 2002, 2006). Therefore, there remain several important points to be solved, including the question whether VCIP135 and its deubiquitinating activity is required in p97-mediated ER membrane fusion.
In this paper, we have identified a novel VCIP135-binding protein, the WW domain-containing adaptor with coiled coil (WAC). WAC binds to VCIP135 and activates its deubiquitinating activity. An in vitro Golgi reformation assay revealed that WAC is necessary only for p97/p47-mediated Golgi membrane fusion, for which the deubiquitinating activity of VCIP135 is required. We also showed that both p97/p47 and p97/p37 pathways function in ER membrane fusion as well as Golgi membrane fusion. An in vitro ER reformation assay revealed that both pathways require VCIP135 but not its deubiquitinating activity for their ER membrane fusion, which is supported by the finding that WAC is unnecessary for p97-mediated ER membrane fusion.
Results
WAC directly binds to VCIP135 and forms a complex with VCIP135 and p97
Although VCIP135 is required for p97/p47 and p97/p37-mediated Golgi membrane fusion, its deubiquitinating activity is required only in the p97/p47 pathway, and not in the p97/p37 pathway. This indicates that VCIP135 has at least two functions, one ubiquitin-dependent and the other ubiquitin-independent. The question is how VCIP135 chooses either of these distinct functions. One possible answer is that VCIP135 may have its adaptor proteins, which guide it towards one of the functions. Based on this idea, we performed a yeast two-hybrid screen to identify VCIP135-binding proteins, and identified a clone encoding cDNA of WAC. As shown in Figure 1A, WAC was immunoprecipitated from various rat tissues and its amounts were estimated by western blotting. WAC was widely expressed in all the tissues we tested.
Figure 1.
WAC localizes to the Golgi as well as the nucleus. (A) Tissue distribution of WAC. WAC was extracted from 10 mg (wet weight) of rat tissue by immunoprecipitation with an excess amount of anti-WAC antibodies, followed by western blotting using anti-WAC antibodies. (B) Double immunofluorescence staining of WAC and GM130, a Golgi marker. HeLa cells were fixed with 3% PFA for 5 min, permeabilized with 0.1% saponin for 2 min, stained with the antibodies, and observed by confocal microscopy. Bar=10 μm.
The intracellular distribution of WAC was determined by immunofluorescence microscopy with paraformaldehyde fixation, and the results are presented in Figure 1B. Double immunofluorescence staining with polyclonal antibodies to WAC and monoclonal antibodies to GM130, a Golgi marker, showed that WAC mainly localizes to the Golgi as well as the nucleus, although a small amount is present in the cytosol.
We next aimed to confirm the interaction between WAC and VCIP135. Since both WAC and VCIP135 exist in the Golgi (Figure 1B), Golgi membrane extracts were used for immunoprecipitation experiments. Golgi membranes were extracted and WAC and its binding proteins were co-immunoprecipitated with antibodies to WAC. As shown in Figure 2A, both VCIP135 and p97, its binding protein, were co-precipitated with WAC (top and middle panels, lane 2), indicating that WAC in Golgi membranes is involved in a complex containing VCIP135 and p97.
Figure 2.
WAC binds to VCIP135. (A) The complex containing WAC, VCIP135 and p97 in Golgi membranes. Golgi membranes were solubilized and incubated with anti-WAC antibodies. The immunoprecipitates were fractionated by SDS–PAGE, followed by western blotting with antibodies to VCIP135, p97 and WAC. (B) WAC directly binds to VCIP135. GST-tagged WAC (0.6 μg) was incubated with the indicated His-tagged proteins (VCIP135, 0.8 μg; p97, 1.3 μg) in a buffer containing 0.15 M KCl and 1% Triton X-100 on ice, then isolated on glutathione beads, and bound proteins fractionated by SDS–PAGE. The blots were probed with antibodies to VCP135, p97 and WAC. (C) p97 binding to VCIP135 increases the binding affinity between VCIP135 and WAC. GST-tagged VCIP135 (1 μg) was incubated with the indicated His-tagged proteins (p97, 1.8 μg; WAC, 1.5 μg) then isolated on glutathione beads, and bound proteins fractionated by SDS–PAGE. The blots were probed with antibodies to VCP135, p97 and WAC.
We further investigated the binding between WAC and VCIP135 in vitro, using purified recombinant proteins. GST-tagged WAC was incubated with either VCIP135 or p97, as presented in Figure 2B. WAC bound VCIP135 (top panel, lane 2), but not p97 (middle panel, lane 3). When VCIP135 and p97 were added together, WAC bound p97 as well as VCIP135 (lane 4). These results indicate that WAC directly binds to VCIP135 and that p97 is included in the complex via its binding to VCIP135. The amount of VCIP135 bound to WAC increased in the presence of p97 (Figure 2B, top panel, lanes 2 and 4; Supplementary Figure S2A), suggesting that the binding of p97 to VCIP135 increases the binding affinity between WAC and VCIP135. To test this, we performed another binding experiment as presented in Figure 2C. WAC and p97 were incubated with GST-tagged VCIP135 and the amounts bound to VCIP135 were assayed. The amount of WAC bound to VCIP135 was increased in the presence of p97 (Figure 2C, middle panel, lanes 2 and 4; Supplementary Figure S2B), which is consistent with the result in Figure 2B. Regarding the amount of p97 bound to VCIP135, there was no difference between the absence and presence of WAC (Figure 2C, top panel, lanes 3 and 4). Taken together, these results indicate that WAC, VCIP135 and p97 form a complex and the binding affinity between VCIP135 and WAC is enhanced by the binding of p97 to VCIP135.
Isolation of a VCIP135 mutant, VCIP135(Q381A), which lacks binding affinity to WAC
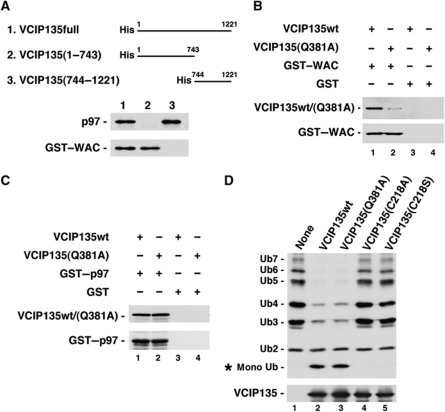
As WAC directly binds to VCIP135, we carried out a rough mapping experiment of VCIP135 to determine the WAC-binding region in VCIP135, as presented in Figure 3A. WAC bound to the N-terminal half of VCIP135, but not to its C-terminal half (bottom panel, lanes 2 and 3). In contrast, p97 bound to the C-terminal half of VCIP135 (top panel, lane 3). These results strongly support our finding that WAC does not compete with p97 for its binding to VCIP135 (Figure 2B and C).
Figure 3.
VCIP135(Q381A) does not bind to WAC. (A) The WAC-binding region in VCIP135. VCIP135 His-tagged VCIP135 fragments were incubated with either p97 or GST–WAC and then precipitated by anti-His-tag antibodies. The blots were probed with antibodies to p97 and WAC. (B) VCIP135(Q381A) does not bind to WAC. GST-tagged WAC (2 μg) was incubated with either His-tagged VCIP135wt or His-tagged VCIP135(Q381A) (1 μg) and then isolated on glutathione beads. The blots were probed with antibodies to His-tag and WAC. (C) VCIP135(Q381A) binds to p97. GST-tagged p97 (2 μg) was incubated with either His-tagged VCIP135wt or His-tagged VCIP135(Q381A) (1 μg) and then isolated on glutathione beads. The blots were probed with antibodies to His-tag and p97. (D) VCIP135(Q381A) still keeps a deubiquitinating activity. A mixture of in vitro-synthesized ubiquitin chains (Ub2–7, 1.25 μg/ml) was incubated alone, with His-tagged VCIP135wt (100 μg/ml), or with His-tagged VCIP135mutant (100 μg/ml), and then analysed by western blotting with antibodies to ubiquitin and His-tag. The amount of substrate was decreased to detect low deubiquitinating activities of VCIP135 and its mutants.
We next aimed to produce VCIP135 mutants with a point mutation in the WAC-binding region. The cDNA of the N-terminal half of VCIP135 was amplified by a PCR reaction with a limiting amount of dATP, resulting in the introduction of random mutations. Full-length cDNAs of VCIP135 including these mutated N-terminal fragments were expressed in yeast cells and their WAC-binding affinities were tested using a yeast two-hybrid system. We screened ∼1 × 103 colonies and successfully obtained one mutant (Q381L) showing low WAC-binding affinity. The Gln-381 is conserved in all VCIP135 orthologues we found (Supplementary Figure S5).
Since full-length VCIP135 protein with Q381L mutation expressed in Escherichia coli became insoluble, VCIP135 with the Q381A mutation (VCIP135(Q381A)) was instead expressed in E. coli and its biochemical characteristics were analysed. Figure 3B shows the binding of VCIP135wt and VCIP135(Q381A) to GST-tagged WAC. VCIP135wt bound to WAC (top panel, lane 1), while VCIP135(Q381A) showed almost no binding to WAC (top panel, lane 2). As to their binding to p97, there was no difference between VCIP135wt and the Q381A mutant (Figure 3C, top panel).
The deubiquitinating activity of VCIP135(Q381A) was also assayed (Figure 3D). Following the method of Wang et al (2004), a mixture of ubiquitin chains (Ub2–7) was used as a substrate, and VCIP135(C218A) and (C218S) were used as negative controls. In order to detect very low deubiquitinating activities of VCIP135wt and its mutants, a smaller amount of substrate was used in this assay compared with that in the other assays. VCIP135(Q381A) generated almost the same amount of monoubiquitin after the reaction as VCIP135wt (top panel, lane 3). Therefore, the Q381A mutation in VCIP135 appears to have no effect on its deubiquitinating activity.
WAC activates VCIP135 deubiquitinating activity and is required for p97/p47-mediated Golgi membrane fusion
VCIP135 is reported to have deubiquitinating activity, which is essential for p97/p47-mediated Golgi reassembly (Wang et al, 2004). We therefore investigated the effect of WAC on the deubiquitinating activity of VCIP135. We first used a mixture of ubiquitin chains as a substrate in the deubiuitinating assay. As presented in Figure 4A, VCIP135 alone showed very low deubiquitinating activity (top panel, lane 1). Addition of WAC significantly increased the deubiquitinating activity of VCIP135 (top panel, lane 2), while the addition of p97 had almost no effect or a very slight effect (top panel, lane 3). When both WAC and p97 were added together, deubiquitinating activity was markedly increased (top panel, lane 4), to greater than 10 times compared with that of VCIP135 alone (Figure 4B). This increase is likely to be caused by the p97-mediated increase in binding affinity between VCIP135 and WAC (Figure 2B and C; Supplementary Figure S2A and B). These results indicate that WAC enhances VCIP135 deubiquitinating activity through its binding to VCIP135. We next used the extract from salt-washed mitotic Golgi fragments as a substrate in the assay (Figure 4C). Although WAC and p97 still remained in salt-washed membranes (third and fourth panels from the top, lane 1), VCIP135 was not detected (second panel from the top, lane 1) and either recombinant VCIP135 or its mutant was added instead. The addition of VCIP135wt increased the generation of mono-Ub (top panel, lane 2), while the addition of VCIP135(Q381A), which lacked binding affinity to WAC (Figure 3), did not (top panel, lane 3). There still remain some possibilities that this might be an indirect effect caused by using crude substrates. However, considering that VCIP135(Q381A) per se has almost the same deubiquitinating activity as VCIP135wt (Figure 3D), this result rather suggests that the binding of VCIP135 to WAC is necessary for the deubiquitinating function of VCIP135. Taking together, it is thought that WAC enhances VCIP135 deubiquitinating activity through its binding to VCIP135.
Figure 4.
WAC activates the deubiquitinating activity of VCIP135. (A) WAC activates VCIP135 deubiqutinating enzyme activity. The deubiquitinating activities of VCIP135 (48 μg/ml) were measured in the presence of WAC (88 μg/ml) and p97 (210 μg/ml). A mixture of ubiquitin chains (Ub2–7, 2.5 μg/ml) was used as a substrate. The blots were probed with antibodies to ubiquitin and VCIP135. (B) The amounts of generated mono-Ub in (A) (asterisk) were quantified using ImageJ software. One-fold represents the deubiquitinating activity of VCIP135 alone. Mean±s.d. (n=4). (C) The deubiquitinating functions of VCIP135 using the extracts from mitotic Golgi fragments as substrates. In all, 1 M KCl-washed mitotic Golgi fragments were solubilized and then incubated together with either VCIP135 or its mutants. The blots were probed with antibodies to ubiquitin, VCIP135, p97, WAC and GM130.
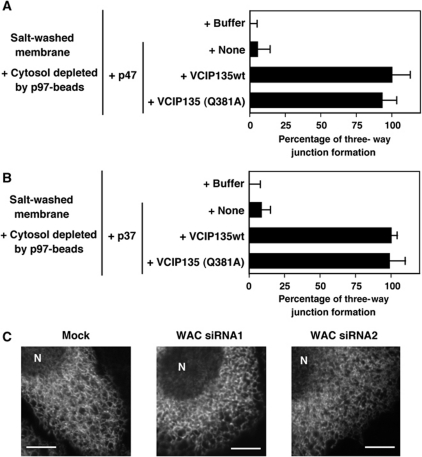
Next, we aimed to clarify the in vivo function of WAC and carried out WAC-knockdown experiments using siRNA (Figure 5A–D). Two distinct WAC siRNA duplexes, specific for the nucleotide sequence of WAC, were introduced in HeLa cells. In WAC siRNA-treated cells, WAC was undetectable by immunofluorescence staining (Figure 5A, panels e and f). Western blotting also showed that both WAC siRNA duplexes decreased WAC levels to <5% of its original level (Supplementary Figure S1B). In WAC-depleted cells, although the Golgi still localized to a perinuclear region, it was slightly dispersed (panels b and c). The ultrastructures of the Golgi apparatus were further investigated by electron microscopy (Figure 5B and C). In WAC-depleted cells, the typical big stacks of Golgi were rarely observed and large-scale vesiculation was present (Figure 5B, top right and bottom panels). Membrane profiles in the Golgi area were calculated and the results are shown in Figure 5C. Both WAC siRNAs decreased the percentage of membranes in Golgi cisternae by ∼40%, while increased the percentage of tubules and vesicles within the Golgi area, compared with the mock control. These results strongly suggest that WAC is important for the biogenesis of the Golgi.
Figure 5.
WAC is essential for the biogenesis of the Golgi in vivo. (A) The Golgi in WAC-depleted cells. HeLa cells were either mock transfected with water or transfected with two distinct siRNA duplexes specific to WAC. The cells were incubated for 32 h and then fixed. Since the transfection efficiency was ∼90%, especially in these images untransfected cells (* in panels e and f) were cocultured 24 h after transfection in order to set a control. Golgi (green, a–c) and WAC (red, d–f) were stained by monoclonal anti-GM130 antibodies and polyclonal anti-WAC antibodies, respectively. Bar=10 μm. (B) Representative EM images of the Golgi in either mock or WAC siRNA-treated HeLa cells. Bar=0.5 μm. (C) Golgi membranes in either mock or WAC siRNA-treated HeLa cells were classified into cisternae, vesicles and tubules and counted. Mean values±s.d. (n=7–8). (D) The depletion of WAC alters the subcellular localization of VCIP135. NRK cells were transfected with siRNA1 specific to WAC. The cells were incubated for 26 h and then fixed. Golgi (green) and VCIP135 (red) were stained by monoclonal anti-GM130 antibodies and polyclonal anti-VCIP135 antibodies, respectively. Bar=10 μm.
Since WAC forms a complex with VCIP135 in Golgi membranes (Figure 2A), another question is whether the subcellular localization of VCIP135 is altered when WAC expression is suppressed in living cells. The localization of VCIP135 in WAC siRNA-treated cells is shown in Figure 5D. The depletion of WAC (Supplementary Figure S1C) caused the partial dissociation of VCIP135 from the Golgi (Figure 5D, bottom left panel), although some VCIP135 still remained in the Golgi. Considering our biochemical result that VCIP135 directly binds to WAC (Figure 2B and C), it is likely that part of VCIP135 in the Golgi requires its binding to WAC for its Golgi localization.
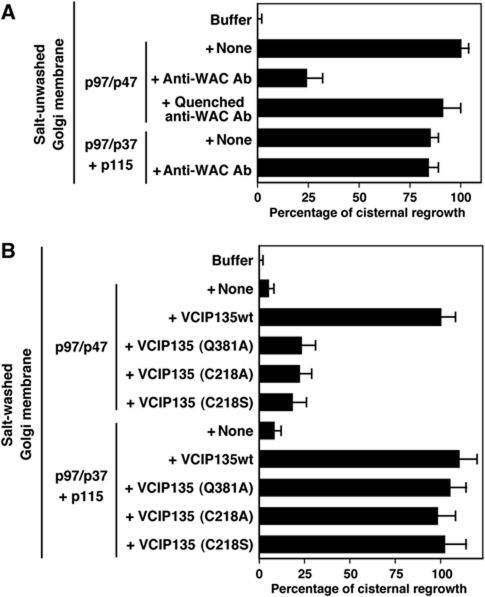
To clarify the molecular function of WAC in Golgi biogenesis, we performed the in vitro Golgi reformation assay (Uchiyama et al, 2006). As shown in Figure 6A, cisternal growth was observed by incubating Golgi fragments with the p97/p47 complex (the control). Polyclonal antibodies to WAC inhibited the p97/p47-mediated cisternal growth by ∼75% compared with the control. This inhibition was rescued by quenching the antibodies with recombinant WAC protein. The same antibodies had no effect on p97/p37-mediated cisternal growth. Together, these results strongly suggest that WAC is specifically involved in the p97/p47 membrane fusion pathway.
Figure 6.
WAC is required for p97/p47-mediated Golgi membrane fusion. (A) Anti-WAC antibodies inhibited p97/p47-mediated Golgi reformation. For in vitro Golgi reformation, Golgi fragments were mixed with the indicated components (p97, 60 μg/ml; p47, 30 μg/ml; p37, 20 μg/ml; p115, 40 μg/ml) together with anti-WAC antibodies. Antibodies to WAC were polyclonal and affinity-purified. After incubation at 37 °C for 60 min, the membranes were fixed, processed and the percentage of membrane in cisterna was determined. Mean±s.d. (n=5); 0% represents the buffer (25.2% in cisternal membranes), 100% represents +p97/p47 (52.1% in cisternal membranes). (B) VCIP135(Q381A), which lacks binding affinity to WAC, did not function in p97/p47-mediated Golgi reformation. In all, 1 M KCl-washed Golgi fragments were incubated with several components or their combinations; p97 (60 μg/ml), p47 (30 μg/ml), p37 (20 μg/ml), p115 (40 μg/ml), VCIP135wt (18 μg/ml), VCIP135(Q381A) (18 μg/ml), VCIP135(C218A) (18 μg/ml), VCIP135(C218S) (18 μg/ml). The percentage of membrane in cisterna was determined. Mean±s.d. (n=5–7); 0% represents the buffer (25.3% in cisternal membranes), 100% represents +p97/p47+VCIP135wt (54.5% in cisternal membranes).
We also tested VCIP135(Q381A), which lacks binding affinity to WAC but maintains its binding affinity to p97 as well as its deubiquitinating activity at the same level as the wild type (Figure 3B–D), in the in vitro Golgi reformation assay (Figure 6B). Golgi membranes were washed with 1 M KCl to remove membrane-bound VCIP135 before being used for the assays. WAC still remained in Golgi membranes after the salt-wash treatment. When the salt-washed membranes were incubated with p97/p47 and VCIP135, cisternal growth was observed. However, when VCIP135(Q381A) was added together with p97/p47, there was no cisternal growth. Other VCIP135 mutants, C218A and C218S, which lack deubiquitinating activities (Figure 3D, lanes 4 and 5) (Wang et al, 2004), also caused no cisternal growth even in the presence of p97/p47. In contrast to the p97/p47 pathway, all mutants, including VCIP135(Q381A), still functioned at the same level as the wild type in the p97/p37 pathway. All of these results indicate that WAC is required specifically for p97/p47-mediated Golgi membrane fusion.
Since the p97/p47 pathway is required only for organelle reassembly at mitosis (Uchiyama et al, 2003), the function of WAC is thought to be necessary for Golgi reassembly at mitosis. To confirm this, we investigated Golgi reassembly at the end of mitosis in WAC-depleted cells (Supplementary Figure S3). In WAC-depleted cells, Golgi reassembly was partially inhibited at cytokinesis (Supplementary Figure S3, panels m–o). This in vivo result supports the requirement of WAC in p97/p47-mediated Golgi reassembly at mitosis.
VCIP135 is required for ER membrane fusion mediated by p97/p47 and p97/p37, although its deubiquitinating activity is not necessary
Although immunofluorescence staining revealed that WAC mainly localizes to the Golgi and the nucleus, some WAC was present in the cytosol (Figure 1B). Biochemical experiments using subcellular fractionation also showed that WAC existed in the cytosol (Supplementary Figure S1A). As VCIP135, a binding partner of WAC, localizes to the ER as well as the Golgi (Uchiyama et al, 2002), there are some possibilities that WAC may also have a role in ER membrane fusion together with VCIP135. On the other hand, the role of VCIP135 in ER biogenesis still remains unclear. We previously reported that VCIP135 also functions in ER biogenesis by showing that the microinjection of anti-VCIP135 antibodies into living cells disrupted ER network structures (Uchiyama et al, 2002). There has been no detailed study since then on its role in ER biogenesis. Therefore, before investigating the requirement of WAC for ER membrane fusion, we first tried to clarify the function of VCIP135 using an in vitro ER reformation assay.
In our in vitro ER reformation assay, the membranes labelled with a fluorescent dye were incubated with cytosol in a narrow space between two coated coverslips (at a distance of 6.0 μm). The coated coverslip allowed the adhesion of the branched portion of the ER network on its surface and stabilized the reformed network structures, which enabled us to measure the degree of network formation. After incubation, the ER networks reformed on the surface of a coverslip were observed and quantified by confocal microscopy without fixation. When membranes were incubated together with cytosol, ER networks were reformed (Figure 7A, panel b). The addition of antibodies to p47, p37 and VCIP135 partially inhibited this ER reformation, and these inhibitions were rescued by quenching the antibodies with their antigens (Figure 7Ac–e and B). These in vitro results show that p47, p37 and VCIP135 function in ER membrane fusion. In contrast, anti-WAC antibodies showed no inhibition (Figure 7Af and B). This is the first report on the in vitro functions of VCIP135 and p37 in ER network formation, although we previously showed their in vivo functions (Uchiyama et al, 2002, 2006).
Figure 7.
p47, p37 and VCIP135 function in ER membrane fusion. For in vitro ER reformation, salt-unwashed membranes (20 μg) labelled with a fluorescent dye were mixed with HeLa cytosol (60 μg) and indicated antibodies (0.5 μg). All antibodies were polyclonal and affinity-purified. The reaction mixture was incubated for 2 h at 25°C in a narrow space between two coverslips. The ER network reformed on the surface of a coverslip was observed by confocal microscopy without fixation. (A) Representative images. Bar=10 μm. (B) The number of three-way junctions was counted as a parameter of network formation. Mean±s.d. (n=6–8); 0% represents +buffer (no cytosol) (0.0 per (10 μm)2), 100% represents +cytosol (no antibodies) (107.0 per (10 μm)2).
In the case of Golgi membrane fusion, the p97 pathways require VCIP135, and the p97/p47 pathway especially requires its deubiquitinating function as well as its activator, WAC (Figure 6). In ER membrane fusion, do the p97 pathways also require VCIP135 and its deubiquitinating activity? To answer this question, we performed in vitro ER reformation assays and the results are presented in Figure 8. Endogenous VCIP135, p47 and p37 were removed from the membranes by salt-wash and from the cytosol by the depletion with p97-beads (Hetzer et al, 2001), and recombinant VCIP135, p47 and p37 were added instead. When recombinant VCIP135 was not added, ER reformation was not observed even in the presence of either recombinant p47 or p37 (Figure 8Ab and Cb). When VCIP135wt was added together with either p47 or p37, ER network structures were reformed (Figure 8Ac and Cc). These data indicate that the p97/p47 and p97/p37 pathways require VCIP135 for their ER membrane fusion.
Figure 8.
The deubiquitinating activity of VCIP135 is not necessary for p97-mediated ER membrane fusion. In the in vitro ER reformation assay, salt-washed membranes labelled with a fluorescent dye and cytosol depleted by p97-beads were used to remove endogenous VCIP135. As the cytosol contained p97, exogenous p97 was not added. (A) VCIP135 mutants, which lack deubiquitinating activities, functioned in p97/p47-mediated ER reformation. Representative images of salt-washed membranes (10 μg) incubated with cytosol depleted by p97-beads (60 μg) and indicated components; p47 (60 μg/ml), VCIP135wt (40 μg/ml), VCIP135(C218A) (40 μg/ml), VCIP135(C218S) (40 μg/ml). Bar=10 μm. (B) The number of three-way junctions was counted in the images obtained as in (A) by confocal microscopy as a parameter of network formation. Mean±s.d. (n=8); 0% represents +depleted cytosol alone (0.0 per (10 μm)2), 100% represents +p47+VCIP135wt (56.8 per (10 μm)2). (C) VCIP135 mutants, which lack deubiquitinating activities, functioned in p97/p37-mediated ER reformation. Representative images of salt-washed membranes (10 μg) incubated with cytosol depleted by p97-beads (60 μg) and indicated components; p37 (60 μg/ml), VCIP135wt (40 μg/ml), VCIP135(C218A) (40 μg/ml), VCIP135(C218S) (40 μg/ml). Bar=10 μm. (D) The number of three-way junctions was counted in the images obtained as in (C). Mean±s.d. (n=8); 0% represents +depleted cytosol alone (0.0 per (10 μm)2), 100% represents +p37+VCIP135wt (65.3 per (10 μm)2).
We next tested using VCIP135 mutants (C218S, C218A), which lack deubiquitinating activities (Figure 3D, lanes 4 and 5), whether the deubiquitinating activity of VCIP135 is necessary in these two p97 pathways. Interestingly, even when VCIP135(C218S) was added instead of VCIP135wt together with either p47 or p37, ER network reformation was observed (Figure 8Ad and Cd) and there was no significant difference in the degree of ER network formation between VCIP135wt and its mutants (C218S and C218A) (Figure 8B and D). These results demonstrate that ER membrane fusion mediated by p97/p47 and p97/p37 requires VCIP135 but not its deubiquitinating activity.
We finally investigated the requirement of WAC, an activator of VCIP135 deubiquitinating enzyme, in ER membrane fusion. In an in vitro ER reformation assay, we also tested VCIP135(Q381A), which lacks WAC-binding affinity. As presented in Figure 9A and B, VCIP135(Q381A) was as effective as VCIP135wt in both the p97/p47 and p97/p37 pathways. These results indicate that the binding of VCIP135 to WAC may be unnecessary in ER membrane fusion. Figure 9C shows the results of WAC siRNA experiments. Since ER structures are easily damaged and lost after fixation, we used a stable cell line expressing GFP-tagged HSP47, an ER protein, for in vivo experiments and observed ER structures in living cells without fixation, by confocal microscopy. The depletion of WAC by WAC siRNA caused no obvious morphological changes in the ER structures of living cells (middle and right panels). Additionally, anti-WAC antibodies did not inhibit ER reformation in the in vitro assay (Figure 7Af and B). Considering both in vivo and in vitro results, it can be concluded that WAC is not required for ER membrane fusion, although VCIP135 is involved in p97-mediated ER membrane fusion. This strongly supports the finding that VCIP135 deubiquitinating activity is unnecessary for p97-mediated ER membrane fusion.
Figure 9.
WAC is not required for ER membrane fusion. (A) VCIP135(Q381A), which lacks binding affinity to WAC, functioned in p97/p47-mediated ER reformation. In the in vitro ER reformation assay, salt-washed membranes and cytosol depleted by p97-beads were used as in Figure 8. Salt-washed membranes (10 μg) incubated with cytosol depleted by p97-beads (60 μg) and the indicated components; p47 (60 μg/ml), VCIP135wt (40 μg/ml), VCIP135(Q381A) (40 μg/ml). The number of three-way junctions was counted by confocal microscopy as a parameter of network formation. Mean±s.d. (n=8); 0% represents +depleted cytosol alone (0.0 per (10 μm)2), 100% represents +p47+VCIP135wt (68.5 per (10 μm)2). (B) VCIP135(Q381A) functioned in p97/p37-mediated ER reformation. Salt-washed membranes (10 μg) incubated with cytosol depleted by p97-beads (60 μg) and the indicated components; p37 (60 μg/ml), VCIP135wt (40 μg/ml), VCIP135(Q381A) (40 μg/ml). The number of three-way junctions was counted. Mean±s.d. (n=9); 0% represents +depleted cytosol alone ([0.0 per (10 μm)2), 100% represents +p37+VCIP135wt (43.5 per (10 μm)2). (C) The depletion of WAC had no effect on ER network structures in living cells. HeLa cells expressing GFP-tagged HSP47 were transfected with either mock or two distinct siRNA duplexes specific to WAC. After incubation for 30 h, ER structures were observed in living cells without fixation by confocal microscopy. Representative images of the ER after the transfection are shown. N, nucleus. Bar=5 μm.
Discussion
We previously identified VCIP135 as an essential factor involved in the p97/p47 membrane fusion pathway and showed that it is required for Golgi biogenesis in vivo (Uchiyama et al, 2002). Wang et al (2004) subsequently showed that VCIP135 possesses deubiquitinating activity, which is essential for p97/p47-mediated Golgi membrane fusion. We later identified another p97 membrane fusion pathway, the p97/p37 pathway, and reported that this pathway also requires VCIP135, but not its deubiquitinating activity (Uchiyama et al, 2006). VCIP135 therefore seems to work in two distinct manners, one via its deubiquitinating activity as observed in the p97/p47 pathway, and the other in a ubiquitin-independent manner as observed in the p97/p37 pathway. This causes a big question: how does VCIP135 choose either of its two distinct functions? Does VCIP135 have specific adaptor proteins which guide it to one of its functions? In this study, we identify WAC as a candidate for such a specific adaptor protein. WAC directly binds to VCIP135 and activates its deubiquitinating activity. Suppression of WAC expression in living cells caused the dissociation of VCIP135 from the Golgi and Golgi fragmentation. An in vitro Golgi reformation assay revealed that WAC was required for p97/p47-mediated Golgi membrane fusion, but not for p97/p37-mediated Golgi membrane fusion. In contrast to Golgi membrane fusion, the role of VCIP135 in ER membrane fusion still remains unclear. We previously suggested from in vivo observations that VCIP135 might also be necessary for ER biogenesis, but since then there have been no studies on its function in ER membrane fusion. In this study, we clarified using an in vitro assay that both the p97/p47 and p97/p37 pathways required VCIP135 in their ER membrane fusion as well as in their Golgi membrane fusion. Interestingly, in contrast to Golgi membrane fusion, neither of the two pathways required its deubiquitinating activity in their ER membrane fusion. This is consistent with the finding that WAC, an activator of VCIP135 deubiquitinase, does not function in ER membrane fusion.
In Golgi membrane fusion, although both the p97/p47 and p97/p37 pathways require VCIP135, only the p97/p47 pathway requires its deubiquitinating activity (Figure 6B). WAC binds to VCIP135 in Golgi membranes (Figure 2A) and functions only in Golgi membrane fusion mediated by the p97/p47 pathway but not by the p97/p37 pathway (Figure 6A). The deubiquitinating activity of VCIP135 per se is very low, and WAC binds to VCIP135 and dramatically enhances this activity (Figure 4). Taking all of these facts into consideration, WAC appears to function in the p97/p47 pathway by activating VCIP135 deubiquitinating activity. In other words, WAC may have a role as a specific adaptor protein, which directs VCIP135 towards its deubiquitinating function in the p97/p47 pathway. Several lines of evidence also suggest another role of WAC, as a ‘receptor’ of VCIP135 in Golgi membranes. WAC can directly bind to VCIP135 in vitro (Figure 2B), and actually forms a complex with VCIP135 in Golgi membranes (Figure 2A). VCIP135 was partially dissociated from the Golgi in cells in which WAC expression was suppressed (Figure 5D, left bottom panel). Moreover, after VCIP135 was stripped from Golgi membranes with salt-wash treatment, WAC still remained in Golgi membranes (Figure 4C). These results indicate that WAC also works as a ‘receptor’ of VCIP135 in Golgi membranes. In summary, WAC first functions as a ‘receptor’ of VCIP135 to capture it in Golgi membranes and subsequently activates its deubiquitinating activity. Notably, A20 deubiquitinating enzyme, which belongs to the same deubiquitinase family (OTU family) as VCIP135, has a binding protein, TAX1BP1 (Shembade et al, 2010). TAX1BP1 interacts with ubiquitinated substrates and recognizes their polyubiquitin chains (Shembade et al, 2007; Iha et al, 2008). TAX1BP1 therefore functions as a ‘ubiquitin receptor’ protein that connects A20 deubiquitinating enzyme with ubiquitinated substrates. Similarly, WAC might have a third role to guide VCIP135 deubiquitinating enzyme to its substrate. In addition, taking into account that TAX1BP1 and A20 also form a complex with the ubiquitin ligases Itch and RNF11 (Shembade et al, 2008, 2009), WAC might also form a complex with a ubiquitin ligase. Based on these ideas, we are now purifying WAC-containing protein complexes from Golgi extracts in order to identify ubiquitinated substrates and the ubiquitin ligases involved.
In p97-mediated membrane fusion, VCIP135 has ubiquitin-independent and ubiquitin-dependent functions. In this study, we showed that VCIP135 usually functions in a ubiquitin-independent manner and that its ubiquitin-dependent function is necessary only for p97/p47-mediated Golgi membrane fusion. Concerning its ubiquitin-independent function, VCIP135 is thought to assist p97/p47 and p97/p37 in priming SNARE complexes. We have shown that VCIP135 binds to the p97/p47(p37)/SNARE complex in Golgi membranes and dissociates it via p97-catalysed ATP hydrolysis (Uchiyama et al, 2002, 2006). This reaction mediated by VCIP135 is ubiquitin-independent. Similarly, there are some possibilities that VCIP135 might work for SNARE priming in ER membranes, although SNAREs in ER membranes still remain unclear. On the other hand, we have not got any clue to its ubiquitin-dependent function of VCIP135. Nevertheless, the comparison between the p97/p47 and p97/p37 pathways in Golgi membrane fusion brings out one speculation on the requirement of ubiquitin in p97/p47-mediated Golgi membrane fusion. The p97/p37 pathway utilizes the p115–GM130 tethering in the same way as the NSF pathway (Uchiyama et al, 2006). In contrast, since the p97/p47 pathway requires neither p115 nor GM130, it is entirely unclear what tethering system is required in p97/p47-mediated Golgi membrane fusion. One interesting idea is that the ubiquitination may be necessary for the formation of a tethering protein complex, which may be dissociated through VCIP135-mediated deubiquitination after membrane fusion.
In an in vitro assay, we could not observe ER reformation in the absence of the cytosol, which is consistent with the report by the Mattaj group (Hetzer et al, 2001). In contrast, the group of Rapoport observed ER reformation even in the absence of the cytosol, when the reaction was performed in a buffer containing 0.2 M KCl (Voeltz et al, 2006). They also reported that the same membranes could not form network structures in a reaction buffer containing low salt, when incubated in the absence of the cytosol (Dreier and Rapoport, 2000; Voeltz et al, 2006). Both the Mattaj group and we performed assays in a buffer containing low salt. One possible explanation for these observations is that the membranes per se have the ability to form network structures but such abilities are weakened under low-salt conditions by the binding of some negative regulatory factor to the membranes. We also succeeded in reforming network structures using 0.5 M KCl-washed membranes in the presence of the cytosol as well as components of the p97 pathway, but the resulting structures were morphologically different from those obtained by the group of Rapoport from the membranes alone in 0.2 M KCl-containing buffer: for example, the ER tubes reformed by the p97 pathway were much thicker. It is therefore likely that the ER membrane fusion mediated by p97 may be different from that occurring in the absence of the cytosol.
There is still controversy on the role of p97 and its yeast orthologue, Cdc48p, in ER membrane fusion. There have been many reports on the p97 pathway in ER membrane fusion. Originally, Latterich et al (1995) reported on the ER membrane fusion mediated by Cdc48p using an in vitro system. The group of Mattaj subsequently showed the function of p97 and p47 in ER membrane fusion using an in vitro assay (Hetzer et al, 2001). We previously clarified the functions of p47, VCIP135 and p37 in an in vivo system (Uchiyama et al, 2002, 2006), and in this report have shown their functions using an in vitro system. On the other hand, the group of Rapoport reported that yeast cells with a temperature-sensitive mutant in Cdc48p showed normal ER structures even at nonpermissive temperature, indicating that Cdc48p, the yeast orthologue of p97, is not required for ER biogenesis (Prinz et al, 2000). In this study, we tried to clarify the orthologue families of the components involved in the p97 membrane fusion pathway. The results are presented in Supplementary Figures S4–S6. The orthologues of p37 and p47 exit widely from plants to mammals (Supplementary Figure S4), while the orthologues of VCIP135 and WAC can be found only in vertebrates (Supplementary Figures S5 and S6). These facts suggest that p97-mediated membrane fusion may function only in vertebrates, which causes the different requirement of the p97 pathway between yeasts and mammals.
We finally want to discuss on the function of WAC in the nucleus (Figure 1B). Quite recently, Zhang and Yu (2011) have reported that WAC interacts with RNF20/40 and promotes its E3 ligase activity. WAC also interacts RNA polymerase II and targets RNF20/40 to associate with RNA polymerase II complex for H2B ubiquitination at active transcription sites. It is unclear whether there is any relationship between the two distinct functions of WAC in the Golgi and the nucleus, or whether WAC has just multiple functions. Nevertheless, considering that WAC can interact with both a ubiquitin ligase and a deubiquitinase, several speculations are brought about. Although VCIP135 mainly localizes to the ER and Golgi, some VCIP135 are also present in the nucleus (Uchiyama et al, 2002). Since WAC can form a complex with VCIP135 as we observed in the Golgi, it is plausible that WAC may interact with VCIP135 deubiquitinase in the nucleus. This speculation leads to the interesting hypothesis that WAC might target VCIP135 deubiquitinase to associate with RNA polymerase II complex for H2B deubiquitination. On the other hand, there is some possibility that WAC might also interact with a ubiquitin ligase in the Golgi. Taking into account that A20 deubiquitinase, another member of OTU family, also forms a complex with the ubiquitin ligases Itch and RNF11 (Shembade et al, 2008, 2009), this idea seems to be very promising.
Materials and methods
Proteins and antibodies
p97, p37, p47, VCIP135 and p115 were prepared as reported previously (Rabouille et al, 1995; Kondo et al, 1997; Uchiyama et al, 2002, 2006). Polyclonal antibodies to p47, p37, p97 and VCIP135 were prepared as described previously (Uchiyama et al, 2002, 2003, 2006). Monoclonal antibodies to p97, His-tag, GM130 and ubiquitin were purchased from Progen, Qiagen, BD Transduction and Santa Cruz, respectively.
Identification and cDNA cloning of WAC
The ORF of VCIP135 was subcloned into the yeast expression vector pGBKT7. The plasmid was transformed into yeast strain AH109 and a yeast two-hybrid screen was performed essentially according to the manufacturer's instructions using a GAL4 DNA activation domain fusion library in pACT2 (mouse 17d embryo MATCHMAKER cDNA Library, Clontech). In all, 5 × 106 colonies were screened and 98 colonies were positive. Two of them encoded WAC. Its full cDNA sequence (BC080851) was identified by an EST database search. The clone containing the full cDNA sequence of WAC was isolated from a rat liver cDNA library (Clontech) by PCR (primers: 5′-CTGCCGCCCCGTAGTTGGCAC-3′, 5′-GTTACACATGGCTTCCATGGCTTC-3′). This clone was sequenced, and a start codon was confirmed: it had a stop codon immediately upstream of the start codon. Its nucleotide sequence is available in the DNA data bank of Japan database under the accession code AB574169.
The ORF of WAC was subcloned into pET22b and pGEX6P-1 for the production of His-tagged and GST-tagged recombinant protein, respectively. His–WAC and GST–WAC were expressed in E. coli and purified with Ni-beads and GSH-beads, respectively, followed by further purification using 5–30% sucrose gradient centrifugation. His-tagged WAC was used to raise rabbit anti-WAC polyclonal antibodies.
Isolation of a VCIP135 mutant, which lacks binding affinity to WAC
VCIP135 mutants were generated as previously described (Reddy and Seaman, 2001; Kaneko et al, 2010). In order to generate PCR products with mutations randomly distributed throughout VCIP135(1–611), the cDNAs of VCIP135(1–312) and (312–611) were amplified by a PCR reaction in which dATP was limited to 10% of the other dNTPs. Full-length cDNAs of VCIP135 in which mutations were randomly introduced at its amino-terminal half were generated using each PCR product by a PCR reaction and then subcloned into pGBKT7 vector. The ORF of WAC was also subcloned into pACT2 vector. Both plasmids were co-transformed into yeast strain AH109 and a yeast two-hybrid screen was performed.
Biochemical experiments
Binding experiments were carried out in buffer A (20 mM Hepes, 1 mM MgCl2, 1 mM ATP, 1 mM DTT, 10% glycerol, pH 7.4) containing 0.15 M KCl and 1% Triton X-100. For immunoprecipitation experiments using Golgi membranes, highly purified Golgi membranes (Hui et al, 1998) (0.45 mg) were solubilized in 100 μl of buffer A containing 0.1 M KCl, 0.5% Triton X-100 and protease inhibitor cocktail (EDTA-free, Roche), and mixed with polyclonal antibodies to WAC (3 μg). WAC and its binding proteins were precipitated by protein G-beads.
The tissue distribution of WAC was studied with denaturing immunoprecipitation as previously described (Uchiyama et al, 2003). Each rat tissue (10 mg wet) was homogenized and extracted, followed by immunoprecipitation using an excess amount of anti-WAC antibodies (1 μg). The amounts of WAC in the precipitates were estimated by western blotting.
The deubiquitinating activity of VCIP135 was assayed basically according to the method of Wang et al (2004). Briefly, VCIP135 and the indicated proteins were incubated with a mixture of oligo-ubiquitin chains (UC-230, BostonBiochem) in 20 μl of buffer A containing 0.1 M KCl and 1 mg/ml BSA for 4 h at 30°C. Aliquots of the reaction mixture were analysed by western blotting using a monoclonal antibody to ubiquitin.
The deubiquitinating functions of VCIP135 and its mutants were also tested using the extract from mitotic Golgi fragments, as follows. Mitotic Golgi fragments (15 μg) were prepared as previously reported (Uchiyama et al, 2003) and washed with 1 M KCl, followed by solubilization with 0.5% CHAPS. The extracts were incubated together with either VCIP135 or its mutants (1.8 μg) in 25 μl of buffer A containing 0.1 M KCl and 0.5% CHAPS for 2 h at 30°C. The amounts of generated monoubiquitin were estimated by western blotting using a monoclonal antibody to ubiquitin.
Knockdown of WAC by siRNA
WAC was targeted with independent siRNAs (B-Bridge International): 5′-CCAGUUACUCUCCACAAGA -3′ (WAC siRNA1), 5′-CCAGUGGAAUGGAAGACAA-3′ (WAC siRNA2). Each 15 nM siRNA duplex was transfected into either HeLa or NRK cells using lipofectamine RNAimax (Invitrogen).
Microscopic studies on cultured cells
For observation of Golgi structures, cells grown on coverslips were fixed with 3% PFA/PBS for 5 min, permeabilized with 0.1%. saponin for 2 min and stained with polyclonal antibodies to either WAC or VCIP135 and monoclonal antibodies to GM130. For observation of ER structures, we used a stable Hela cell line expressing GFP-tagged HSP47, an ER protein (Uchiyama et al, 2002). ER structures were observed in living cells, without fixation, using confocal microscopy.
HeLa cells grown on coverslips were fixed 32 h after WAC siRNA treatment, embedded into Epon, ultrathin sectioned and observed by an electron microscope (JEM-1230, JEOL). The Golgi area was defined by the boundary enclosing the Golgi stacks, tubulo-reticular networks and tubules and all the vesicles that were within 100 nm of these membranes. Membrane profiles in the Golgi area were divided into three categories: cisternae, vesicles and tubules. The relative proportion of each category of membranes was counted using an intersection method, as previously described (Shorter et al, 1999).
In vitro Golgi reformation assay
The in vitro Golgi reformation assay was performed as reported previously (Shorter et al, 1999). All proteins added in this assay were prepared as recombinant proteins from E. coli.
In vitro ER reformation assay
Xenopus egg cytosol and membrane were prepared according to the method of Hetzer et al (2001) with the following modifications. The light membrane fraction was stained with 0.05 mg/ml CM-DiI (Molecular Probes), diluted with 20 vol. of buffer B (50 mM KCl, 20 mM HEPES, 2.5 mM MgCl2, pH 7.4) containing 0.25 M sucrose, and centrifuged onto a cushion of buffer B containing 1 M sucrose at 20 000 g for 30 min at 4°C. The membrane was suspended in buffer B with 0.5 M sucrose, aliquoted and stored at −80°C. For the preparation of salt-washed membrane, membranes were washed with 0.5 M KCl immediately before the assay. HeLa cytosol was prepared as reported previously (Rabouille et al, 1995). The cytosol was dialysed against buffer B containing 0.25 M sucrose and 1 mM DTT, aliquoted and stored at −80°C. To remove p97-binding proteins, the desalted Xenopus egg cytosol (40 mg) was depleted with p97-beads (100 μg p97) three times. Neither p47, p37 nor VCIP135 could be detected in the depleted cytosol by western blotting.
Membrane (not salt-washed, 20 μg: salt-washed, 10 μg), cytosol (60 μg) and indicated proteins were mixed in 10 μl of reaction buffer B containing 0.25 M sucrose, 1 mM DTT, 0.2 mM GTP and ATP regenerating system (1 mM ATP, 20 mM creatine phosphate, 1 mg/ml creatine kinase). The reaction mixture was incubated for 2 h at 25°C in a narrow space between two coverslips. The coverslips coated with polyethyleneimine were used to stick the branched portion of the reformed ER network on their surfaces. Some silica-based spacer beads (6.0 μm in diameter, Ube-Nitto Kasei, Japan), which are usually used in liquid crystal displays and semiconductor chips, were also added into the reaction mixture to control the precise distance between the two coverslips. After incubation, the ER network reformed on the surface of a coverslip was observed by confocal microscopy (LSM510, Zeiss) without fixation. Obtained images were analysed using Image-Pro Plus software (Media Cybernetics).
Supplementary Material
Acknowledgments
We thank Y Shimoji and H Yokozawa for their kind assistance with the analysis of the images obtained in an in vitro ER reformation assay; Y Ogawa and M Komae for their technical assistance with the purification of recombinant proteins; HA Popiel for her kind assistance in preparation of the manuscript. This work is supported by grants to HK from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author contributions: GT, YK, KU, TK and HK performed the biochemical and molecular cell biological studies; HT performed the bioinformatic studies.
Footnotes
The authors declare that they have no conflict of interest.
References
- Dreier L, Rapoport TA (2000) In vitro formation of the endoplasmic reticulum occurs independently of microtubules by a controlled fusion reaction. J Cell Biol 148: 883–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW (2001) Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol 3: 1086–1091 [DOI] [PubMed] [Google Scholar]
- Hui N, Nakamura N, Slusarewicz P, Warren G (1998) Purification of rat liver Golgi stacks. In Cell Biology: A Laboratory Handbook, J Ceils (ed), Vol. 2, pp 46–55. Orlando: Academic Press [Google Scholar]
- Iha H, Peloponese JM, Verstrepen L, Zapart G, Ikeda F, Smith CD, Starost MF, Yedavalli V, Heyninck K, Dikic I, Beyaert R, Jeang KT (2008) Inflammatory cardiac valvulitis in TAX1BP1-deficient mice through selective NF-kappaB activation. EMBO J 27: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Tamura K, Totsukawa G, Kondo H (2010) Isolation of a point-mutated p47 lacking binding affinity to p97ATPase. FEBS Lett 584: 3873–3877 [DOI] [PubMed] [Google Scholar]
- Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature 388: 75–78 [DOI] [PubMed] [Google Scholar]
- Latterich M, Frohlich KU, Schekman R (1995) Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell 82: 885–893 [DOI] [PubMed] [Google Scholar]
- Mellman I, Simons K (1992) The Golgi complex: in vitro veritas? Cell 68: 829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA (2000) Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol 150: 461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C, Kondo H, Newman R, Hui N, Freemont P, Warren G (1998) Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell 92: 603–610 [DOI] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters JM, Warren G (1995) An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell 82: 905–914 [DOI] [PubMed] [Google Scholar]
- Reddy JV, Seaman MN (2001) Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol Biol Cell 12: 3242–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Liebl DJ, Harhaj EW (2007) Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J 26: 3910–3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE, Harhaj EW (2008) The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol 9: 254–262 [DOI] [PubMed] [Google Scholar]
- Shembade N, Ma A, Harhaj EW (2010) Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327: 1135–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW (2009) The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. EMBO J 28: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter J, Watson R, Giannakou ME, Clarke M, Warren G, Barr FA (1999) GRASP55, a second mammalian GRASP protein involved in the stacking of Golgi cisternae in a cell-free system. EMBO J 18: 4949–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama K, Jokitalo E, Kano F, Murata M, Zhang X, Canas B, Newman R, Rabouille C, Pappin D, Freemont P, Kondo H (2002) VCIP135, a novel essential factor for p97/p47-mediated membrane fusion, is required for Golgi and ER assembly in vivo. J Cell Biol 159: 855–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama K, Jokitalo E, Lindman M, Jackman M, Kano F, Murata M, Zhang X, Kondo H (2003) The localization and phosphorylation of p47 are important for Golgi disassembly-assembly during the cell cycle. J Cell Biol 161: 1067–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama K, Totsukawa G, Puhka M, Kaneko Y, Jokitalo E, Dreveny I, Beuron F, Zhang X, Freemont P, Kondo H (2006) p37 is a p97 adaptor required for Golgi and ER biogenesis in interphase and at the end of mitosis. Dev Cell 11: 803–816 [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124: 573–586 [DOI] [PubMed] [Google Scholar]
- Wang Y, Satoh A, Warren G, Meyer HH (2004) VCIP135 acts as a deubiquitinating enzyme during p97-p47-mediated reassembly of mitotic Golgi fragments. J Cell Biol 164: 973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G (1995) Intracellular membrane morphology. Philos Trans R Soc Lond B Biol Sci 349: 291–295 [DOI] [PubMed] [Google Scholar]
- Zhang F, Yu X (2011) WAC, a functional partner of RNF20/40, regulates histone H2B ubiquitination and gene transcription. Mol Cell 41: 384–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.