Abstract
Background & Aims
The human di/tripeptide transporter hPepT1 is abnormally expressed in colons of patients with inflammatory bowel disease, although its exact role in pathogenesis is unclear. We investigated the contribution of PepT1 to intestinal inflammation in mouse models of colitis and the involvement of the nucleotide-binding oligomerization domain 2 (NOD2) signaling pathway in the pathogenic activity of colonic epithelial hPepT1.
Methods
Transgenic mice were generated in which hPepT1 expression was regulated by the β-actin or villin promoters; colitis was induced using 2,4,6-trinitrobenzene sulfonic acid (TNBS) or dextran sodium sulfate (DSS) and the inflammatory responses were assessed. The effects of NOD2 deletion in the hPepT1 transgenic mice was also studied, to determine the involvement of the PepT1–NOD2 signaling pathway.
Results
TNBS and DSS induced more severe levels of inflammation in β-actin–hPepT1 transgenic mice than wild-type littermates. Intestinal epithelial cell (IEC)-specific hPepT1 overexpression in villin-hPepT1 transgenic mice increased the severity of inflammation induced by DSS, but not TNBS. Bone marrow transplantation studies demonstrated that hPepT1 expression in IECs and immune cells has an important role in the proinflammatory response. Antibiotics abolished the effect of hPepT1 overexpression on the inflammatory response in DSS-induced colitis in β-actinh–PepT1 and villin-hPepT1 transgenic mice, indicating that commensal bacteria are required to aggravate intestinal inflammation. Nod2−/−, β-actin–hPepT1 transgenic/Nod2−/−, and villinhPepT1 transgenic/Nod2−/− littermates had similar levels of susceptibility to DSS-induced colitis, indicating that hPepT1 overexpression increased intestinal inflammation in a NOD2-dependent manner.
Conclusions
The PepT1–NOD2 signaling pathway is involved in aggravation of DSS-induced colitis in mice.
Keywords: IBD, mouse model, immune response, bacteria-derived peptides
Introduction
PepT1 is a di/tripeptide transporter highly expressed in epithelial cells of the small intestine, but expressed only poorly or not at all in the colon 1. Our group previously observed that colonic PepT1 expression is enhanced under conditions of chronic inflammation, such as IBD 1, a finding that has been confirmed by other investigators 2. One normal transport function of gut epithelial cells is the absorption of small peptides from the diet, mediated by peptide transport activity 3, 4. This is achieved by the action of an apical membrane protein termed human intestinal H-coupled oligonucleotide transporter (hPepT1), that co-transports peptides with H+ 5, 6. The specificity of hPepT1 is broad and includes many di- and tri-peptides in addition to various peptide-derived drugs 7–13.
Commensal bacteria that colonize the human colon produce significant amounts of di/tripeptides. We were the first to report that PepT1 transports the small formylated bacterial peptide fMLP 14, 15. We have since shown that other bacteria-derived peptides such as muramyl dipeptide (MDP) and Tri-DAP may also be transported by hPepT1 16, 17. Small bacterial peptides occur at substantially lower levels in the small intestine compared to the colon. Interestingly, hPepT1 expression is normally restricted to the small intestine, a site in which small bacterial peptide concentrations are low, reflecting the sparse bacterial load of this tissue relative to that of the colon. Thus, the profile of hPepT1 expression along the normal human digestive tract is such that access of small bacterial peptides to hPepT1 is minimized, to reduce intracellular uptake of such peptides. We found that this normal expression pattern becomes altered in patients with chronic ulcerative colitis (UC) or Crohn’s disease 1, in whom expression of hPepT1 occurs in the colon. The transporter consequently mediates intracellular accumulation of small prokaryotic materials.
We have shown that intracellular accumulation of bacterial products such as tri-DAP, fMLP, and MDP may trigger signals that lead to initiation of intestinal inflammatory responses 14, 16, 17. Transport of fMLP into Caco2-BBE cells stimulates NF-κB and AP-1 activity, which may in turn initiate inflammatory responses in IECs 18, 19. MDP and Tri-DAP also induce NF-κB activation in Caco2-BBE cells, confirmed by secretion of the chemokine IL-8 and monocyte chemoattractant protein-1 (MCP-1) 16, 17. Interestingly, mucosal IL-8 and MCP-1 are highly expressed in intestinal regions affected by IBD. IL-8 and MCP-1 are chemoattractants for neutrophils and monocytes, respectively. The finding that hPepT1-mediated transport of bacterial oligopeptides into intestinal epithelial cells stimulates both IL-8 secretion 14, 18 and increases neutrophil transepithelial migration 14, 18 suggests a cascade of signaling events are activated upon bacterial peptide transport.
In addition to maintaining efficient physical and biological barriers, the intestinal epithelium plays a role in inducing innate and adaptive immunity. Sensing pathogens is the first step in mounting an effective immune response required for elimination of the invading organism and establishing protective immunity. NOD-like receptors (NLRs), consisting of more than 20 related family members, are present in the cytosol and recognize intracellular ligands 20–23. NOD1 is activated by peptides that contain a diaminophilic acid, such as the PepT1 substrate Tri-DAP, and NOD2 recognizes muramyl dipeptides including the PepT1 substrate MDP. Thus, it has been suggested that PepT1 transport activity plays an important role in determining the intracellular levels of ligands for NOD1 and NOD2, which in turn control the extent of activation of downstream inflammatory pathways 20–23.
Based on our reports on the role played by hPepT1 in intestinal inflammation 1, 14–18,24–26, Zucchelli et al. 27 tested hPepT1 polymorphisms for association with IBD. The authors reported that a functional hPepT1 SNP (rs2297322) was associated with IBD in two cohorts of Swedish and Finnish patients 27. In addition, they observed that the transport activity of hPepT1 SNP (rs2297322) was higher compared to WT hPepT1 27. Importantly, this new finding, together with previous data showing aberrant colonic PepT1 expression in IBD patients 1, 2, provide solid evidence of the pathological relevance of intestinal PepT1.
To investigate the in vivo pathogenic role of PepT1 in intestinal inflammation, we generated two transgenic mouse lines in which hPepT1 expression was driven either by a β-actin promoter, which results in hPepT1 expression in all tissues, or a villin promoter, which drives hPepT1 expression specifically in IECs, and assessed inflammatory responses using murine models of colitis. The effect of NOD2 deletion on such responses in the transgenic mice was further studied to explore the potential involvement of the PepT1/NOD2 signaling pathway in colitis.
Results
Characterization of β-actin-hPepT1 transgenic mice
hPepT1 expression is upregulated in IBD 1, 2, therefore we generated a transgenic mouse line in which hPepT1 expression was driven by the β-actin promoter (β-actin-hPepT1 mice), to investigate the potential pathogenic role of hPepT1. β-actin-hPepT1 mice exhibited ubiquitous expression of hPepT1 in all tested tissues (Figure 1A-i). The animals appeared healthy; body weight, breeding biology, and general appearance were normal. The morphology of various tissues, including the gastrointestinal tract, appeared unchanged (Supplementary Figure 1S1). Immunohistochemical analysis verified hPepT1 membrane staining of colonocytes in β-actin-hPepT1 mice, but not in WT mice (Figure 1Aii). Also, hPepT1 was highly expressed in apical membrane vesicles prepared from colonocytes of β-actin-hPepT1 mice and mediated the transport of Lysine-Proline-Valine, a known substrate of hPepT1 27 (Figure 1Ai–iv).
Figure 1. hPepT1 over-expression increases the susceptibility of mice to DSS-induced colitis.
(A) Characterization of β-actin-hPepT1 mice. Western blotting analysis showing hPepT1 expression in tissues of WT and β-actin-hPepT1 animals (A-i). hPepT1 expression in colonic brush border membrane vesicles of WT and β-actin-hPepT1 animals (A-ii). Immunohistochemical analysis of hPepT1 using colonic sections from WT and β-actin-hPepT1 animals (A-iii). hPepT1-mediated uptake of KPV by colonic brush border membrane vesicles isolated from β-actin-hPepT1mice (A-iv). (B–F) Gender- and age-matched WT and β-actin-hPepT1 littermates were given water (H2O) or 3% (w/v) DSS (DSS) for 8 days. Percentage change in body weight, clinical score, and colon length were assessed at day 8 post-treatment (B). Representative photographs obtained by endoscopy on the day of sacrifice (C). Histological score assessed at the end of DSS treatment. Representative H&E-stained distal colonic sections are shown. Bars =100 µm (D). Neutrophil infiltration into the colon was quantified by the measurement of MPO activity (E). Levels of colonic cytokine/chemokine mRNA measured by qRT-PCR (F). Data are means± SEMs (n=9/group/condition) from two experiments that yielded similar results (*P < .05; **P < .005).
hPepT1 expression aggravates the susceptibility of mice to DSS-induced colitis
Next, we investigated the effect of hPepT1 overexpression on intestinal inflammation induced by DSS treatment, an experimental model of human UC 28. DSS treatment caused more severe body weight loss, rectal bleeding, and diarrhea, yielding a higher overall clinical score, in β-actin-hPepT1 mice compared to WT littermates (Figure 1B). Shortening of the colon, a macroscopic indication of colitis, was greater in DSS-treated β-actin-hPepT1 mice compared to DSS-treated WT animals (β-actin-hPepT1 mice: 48% vs WT: 58%) (Figure 1B). To more accurately assess colonic inflammation, colonoscopy was performed, revealing severe inflammation with markedly bloody diarrhea in the colon of β-actin-hPepT1 mice, whereas WT animals exhibited only mild inflammation (Figure 1C). In addition, histological examination of colonic sections revealed complete disruption of the colonic architecture in β-actin-hPepT1 mice, with a large increase in immune cell infiltration into the mucosa and submucosa upon DSS treatment (Figure 1D). Consistent with these results, DSS treatment induced an increase in the colonic activity of myeloperoxidase (MPO), an indicator of neutrophil infiltration, in β-actin-hPepT1 mice (Figure 1E). Most importantly, the colonic mRNA expression levels of pro-inflammatory cytokines (IFN-γ, IL-1β, IL-6, and TNF-α) were dramatically elevated in β-actin-hPepT1 animals treated with DSS (Figure 1F).
We next examined the effect of hPepT1 expression on the ability of mice to recover from DSS-induced colitis. During the recovery phase (when DSS-containing solution was replaced by regular water), more than 20% of the β-actin-hPepT1 mice died, whereas none of the WT animals expired (Supplementary Figure S2). Together, these results demonstrate that overexpression of hPepT1 aggravates DSS-induced colitis and compromises intestinal recovery in mice.
hPepT1 expression aggravates the susceptibility of mice to TNBS-induced colitis
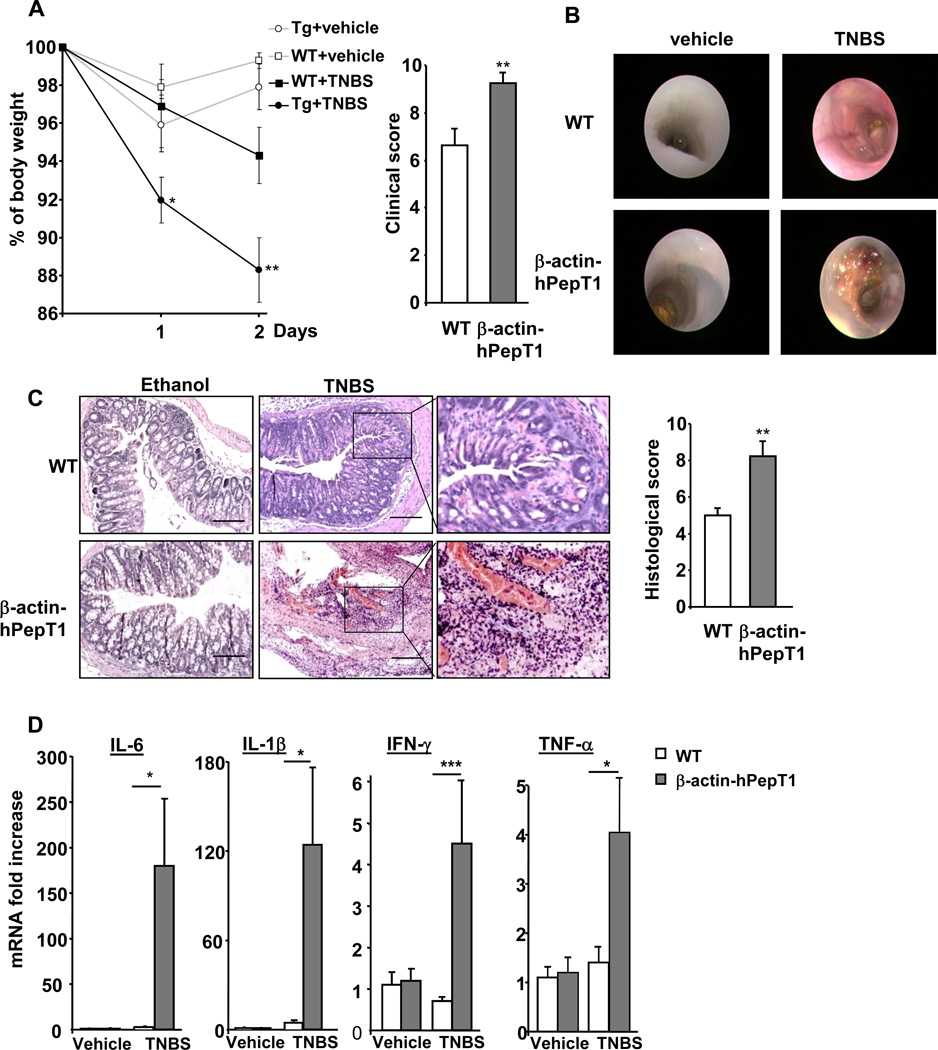
The effect of hPepT1 overexpression on TNBS-induced colitis, a well-known model of human Crohn’s disease 29, was also investigated. β-actin-hPepT1 mice lost more weight than WT littermates 1 and 2 days after TNBS treatment, and had higher clinical scores compared to WT animals (Figure 2A and 2B). Consistent with these observations, colonoscopy revealed severe inflammation with markedly bloody diarrhea in the colons of β-actin-hPepT1 mice, whereas WT animals had only mild inflammation (Figure 2B). Consistently, the colons of β-actin-hPepT1 mice exhibited more pronounced histological damage, with massive infiltration of immune cells, compared to WT animals (Figure 2C). Finally, the greater severity of colonic inflammation in β-actin-hPepT1 mice was confirmed by an increase in expression of mRNAs encoding pro-inflammatory cytokines (Figure 2D). These results indicate that β-actin-hPepT1 mice are more susceptible to TNBS-induced colitis.
Figure 2. hPepT1 over-expression increases the susceptibility of mice to TNBS-induced colitis.
WT and β-actin-hPepT1 mice were given intrarectal water (vehicle) or TNBS dissolved in 50% (v/v) ethanol. Mice were sacrificed 2 days post-treatment. Percentage changes in body weight of β-actin-hPepT1 mice animals during treatment (*P < .05; **P < .005) vs. WT-TNBS animals. Clinical scores were assessed on the day of sacrifice (A). Representative photographs obtained by endoscopy on the day of sacrifice. Bars =100 µm (B). Representative H&E-stained distal colonic sections and histological scores assessed on the day of sacrifice (C). Levels of cytokine/chemokine mRNA as determined by qRT-PCR in the colons of control and TNBS-treated mice (D). Data are means ± SEMs (n=9/group/condition) from two experiments that yielded similar results (*P < .05; **P < .005; ***P < .001).
hPepT1 expression in IECs and in immune cells may play an important role in the pro-inflammatory response
To understand the role played by hPepT1 in the pathogenesis of colitis, we determined the contribution of mucosal versus immune cell-derived hPepT1 to the development of colitis. We generated bone marrow chimeras of WT or β-actin-hPepT1 mice. Bone marrow transplantation was verified by RT-PCR and Western blotting. WT mice that received myeloid cells from β-actin-hPepT1 animals expressed hPepT1 at both the mRNA and protein levels (Figure 3A, B). Interestingly, β-actin-hPepT1 mice that received WT bone marrow exhibited the same sensitivity to TNBS-induced colitis as WT animals injected with β-actin-hPepT1 bone marrow, as assessed by measuring the levels of colonic mRNAs encoding pro-inflammatory cytokines (Figure 3C). In addition, WT animals injected with WT bone marrow were less sensitive to TNBS-induced colitis when compared to β-actin-hPepT1 mice that received WT bone marrow or to WT animals injected with β-actin-hPepT1 bone marrow (Figure 3C). Finally, β-actin-hPepT1 mice that received β-actin-hPepT1 bone marrow were more sensitive to TNBS-induced colitis compared to β-actin-hPepT1 mice that received WT bone marrow or WT animals injected with β-actin-hPepT1 bone marrow (Figure 3C). These results suggest that hPepT1 expression in IECs and immune cells play an important role in the pro-inflammatory response.
Figure 3. hPepT1 expression in IECs and in immune cells may play an important role in the pro-inflammatory response.
Bone marrow transplants were performed (D: donor; R: recipient). RT-PCR (A) and Western blotting (B) analyses confirmed hPepT1 expression in bone marrow in mice receiving hPepT1 mouse-derived marrow. Mice were given intrarectal TNBS dissolved in 50% (v/v) ethanol, and were sacrificed 2 days post-treatment. Levels of colonic mRNAs encoding cytokines/chemokines were quantified by qRT-PCR in the colons of control and TNBS-treated mice (C). Data are means ± SEMs (n=9/group/condition) from two experiments that yielded similar results (*P < .05).
Characterization of villin-hPepT1 transgenic mice
To examine the role played by hPepT1 in IECs (including colonocytes) during intestinal inflammation, we generated a transgenic mouse model in which hPepT1 was specifically expressed in IECs under the control of a villin promoter (to yield villin-hPepT1 mice). Western blotting confirmed expression of hPepT1 in the colonocytes of such animals, but not those of WT mice (Figure 4A-i). The mice exhibited normal body weight, breeding behavior, and general appearance. The morphology of the gastrointestinal tract appeared normal (Supplementary Figure S3). Immunohistochemical analysis verified hPepT1 membrane staining of colonocytes in villin-hPepT1 mice, but not in WT mice (Figure 4A-ii). In addition, apical membrane vesicles prepared from colonocytes of villin-hPepT1 mice expressed high levels of functional hPepT1 exhibiting transport activity (Figure 4Aiii and 4Aiv). Together, the results clearly show that villin-hPepT1 mice overexpress hPepT1 specifically in the colonic mucosa, which has also been observed in IBD patients 1. Therefore, villin-hPepT1 animals serve as an appropriate model to investigate the pathogenic role of hPepT1 in IBD.
Figure 4. IEC-specific hPepT1 overexpression increases the susceptibility of mice to DSS-induced colitis.
(A) Characterization of villin-hPepT1 mice. IEC-specific hPepT1 expression in villin-hPepT1 mice, but not in WT animals shown by western blotting (A-i). hPepT1 expression in colonic brush border membrane vesicles prepared from WT or villin-hPepT1 mice (A-ii). hPepT1 expression assessed by immunohistochemical analysis of colonic sections of WT and villin-hPepT1 animals (A-iii). hPepT1 mediated KPV transport in colonic brush border membrane vesicles prepared from villin-hPepT1 but not in WT mice (A-iv). (B–F) Gender- and age-matched WT and villin-hPepT1 littermates were given water (H2O) or 3% (w/v) DSS for 8 days. Percent change in body weight, clinical score, and colon length were assessed at day 8 post-treatment (B). Representative photographs obtained by endoscopy on the day of sacrifice (C). Histological score assessed at the end of DSS treatment. Representative H&E-stained distal colonic sections are shown. Bars =100 µm (D). Neutrophil infiltration into the colon quantified by measurement of MPO activity (E). Levels of colonic mRNAs encoding cytokines/chemokines, as determined by qRT-PCR (F). Data are means ± SEMs (n=9/group/condition) from two experiments that yielded similar results (*P < .05; **P < .005; ***P < .001).
Colonic hPepT1 expression aggravates DSS-induced colitis in mice
To assess the effect of hPepT1 expression in colonocytes during inflammation, colitis was induced using DSS. DSS treatment induced severe weight loss in villin-hPepT1 mice, associated with a higher total clinical score, compared to WT animals (Figure 4B). The severity of DSS-induced colitis in villin-hPepT1 transgenic mice was accompanied by a rise in inflammation, with massive mucosal erythema and bleeding (Figure 4C). In villin-hPepT1 mice, DSS treatment increased histological damage as shown by almost complete crypt disruption and inflammatory infiltration throughout the mucosa and submucosa (Figure 4D), consistent with a marked increase in colonic MPO activity (Figure 4E) compared to WT mice. Importantly, villin-hPepT1 animals exhibited an increase in DSS-induced colonic levels of the pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and IFN-γ, compared to WT animals (Figure 4F). The effect of colonic hPepT1 expression on the ability of mice to recover from DSS-induced colitis was also examined. After 8 days of DSS treatment, mice were switched to regular water for an additional 8 days. The mortality rate of villin-hPepT1 mice (25%) during the recovery period was markedly greater than that of WT animals (0%) (Supplementary Figure S2). Collectively, these results demonstrate that IEC-specific hPepT1 expression exacerbates DSS-induced colitis and reduces intestinal recovery and healing after induction of inflammation.
Colonic hPepT1 expression does not affect intestinal inflammation induced by TNBS
The effect of colonic hPepT1 expression on TNBS-induced colitis was also investigated. In contrast to β-actin-hPepT1 mice, villin-hPepT1 animals did not show increased weight loss compared to WT mice at 1 and 2 days post-TNBS treatment (Figure 5A). There were no significant differences in the total clinical score (Figure 5B), inflammation detected by colonoscopy (Figure 5C), histology (Figure 5D) or mRNA levels of the pro-inflammatory cytokines IL-1β, IL-6, IFN-γ, and TNF-α (Figure 5E). These results suggest that hPepT1 expression specifically in the colon does not contribute to TNBS-induced colitis.
Figure 5. IEC-specific hPepT1 overexpression does not increase the susceptibility of mice to TNBS-induced colitis.
WT and villin-hPepT1 mice were given intrarectal water (control) or TNBS dissolved in 50% (v/v) ethanol. Mice were sacrificed 2 days post-treatment. (A) Percent change in body weight during treatment. (B) Clinical score on the day of sacrifice. (C) Representative photographs obtained by endoscopy on the day of sacrifice. (D) Representative H&E-stained distal colonic sections and histological scores assessed on the day of sacrifice. (E) Levels of mRNAs encoding cytokines/chemokines (as determined by qRT-PCR) in the colons of control and TNBS-treated mice. Data are means ± SEMs (n=9/group/condition) from two experiments that yielded similar results. NS, not statistically significant.
Antibiotic treatment decreases the severity of colitis in hPepT1 transgenic mice
hPepT1 transports proinflammatory bacterial peptides, therefore we hypothesized that overexpression of functional hPepT1 in transgenic mice would lead to an increase in the intracellular concentration of bacterial products, thus aggravating proinflammatory responses in experimental colitis models. To test this idea, WT and β-actin-hPepT1 littermates were treated for 2 weeks with broad-spectrum antibiotics including vancomycin, metronidazole, neomycin, and ampicillin, a combination previously shown to deplete enteric microbial communities 30, prior to induction of colitis with DSS. Feces were collected immediately prior to and after antibiotic treatment and the numbers of cultivable fecal bacteria were quantified to confirm bacterial depletion. Antibiotic treatment markedly reduced the total number of bacteria in both WT and β-actin-hPepT1 mice (Figure 6A). DSS induced mild colonic inflammation, with no significant difference evident between WT and β-actin-hPepT1 mice, as assessed by measuring induction of pro-inflammatory cytokine mRNA levels (Figure 6B). IL-1β, IL-6 and IFNγ expressions were lower in antibiotic+DSS-treated β-actin-hPepT1 mice (Figure 6B) compared to β-actin-hPepT1 animals given DSS alone (Figure 1F). However, TNF-α expression was two-fold higher in antibiotic+DSS-treated β-actin-hPepT1 (Figure 6B) mice compared to β-actin-hPepT1 animals given DSS alone (Figure 1F). The apparent discrepancy may be due to the low levels of TNF-α mRNA expression in DSS-treated mice compared to that of the other cytokines tested. Overall, antibiotic treatment eliminated the difference in cytokine mRNA levels apparent when levels were evaluated in β-actin-PepT1, villin-PepT1 and WT mice after receiving DSS treatment alone. Similarly, no significant differences in DSS-induced colonic inflammatory features were observed in antibiotic-treated villin-hPepT1 mice and control littermates (data not shown). These results suggest that under inflammatory conditions expression of hPepT1 aggravates intestinal inflammation by transporting proinflammatory bacterial peptides into cells.
Figure 6. hPepT1 overexpression that increases the susceptibility of mice to DSS-induced colitis requires the presence of bacterial flora.
WT and β-actin hPepT1 mice were treated with broad-spectrum antibiotics A) Cultivable bacteria present in the feces before (pre-treatment) and after treatment (with antibiotics), and in water (vehicle)-treated mice, were counted. B) After antibiotic treatment, β-actin hPepT1 and WT mice were given water or a 3% (w/v) DSS solution for 8 days and the levels of colonic mRNAs encoding cytokines/chemokines were measured by qRT-PCR. Data are means ± SEMs (n=9/group/condition) from two experiments that yielded similar results (*P < .05; **P < .005; ***P < .001 vs. water). NS, not statically significant.
NOD2 increases the severity of colitis in hPepT1 transgenic mice
Many NLRs have been shown to detect microbial ligands in the cytoplasm. The prototypic NLR family member NOD2/caspase recruitment domain (CARD) 15 senses MDP formed from bacterial peptidoglycans, which induces intestinal epithelial inflammation in a hPepT1-dependent manner 16, 17, 27. To determine the role played by NOD2 and hPepT1 during colitis β-actin-hPepT1 mice were crossed with Nod2−/− mice, and the resulting pups were backcrossed with Nod2−/− animals to generate β-actin-hPepT1 Tg/Nod2−/− mice or control littermates (Nod2−/− mice). When colitis was induced with DSS, no significant difference in body weight loss, histological damage, or pro-inflammatory cytokine mRNA expression levels, was evident when β-actin-hPepT1 Tg/Nod2−/− and Nod2−/− mice were compared (Figure 7 A–C). Similar results were obtained when colitis was induced by TNBS (data not shown). Villin-hPepT1 Tg/Nod2−/− and Nod2−/− mice showed similar extents of DSS-induced colitis as measured by body weight loss, histological damage and pro-inflammatory cytokine mRNA expression levels (Figure 7 D–F), suggesting the inflammatory activity of hPepT1 is NOD2-dependent. Data in Figure 7 indicate that loss of NOD2, irrespective of hPepT1 expression, can induce DSS colitis to similar levels. In contrast, when NOD2 is expressed in either villin-PepT1-hPepT1 or wild-type mice, DSS induced colitis is aggravated in such animals upon PepT1 overexpression (from villin-PepT1), as shown in Figure 4. Together, the data show that an active PepT1/NOD2 signaling pathway activity is required to aggravate induced colitis in hPepT1-transgenic mice.
Figure 7. NOD2 expression increases the severity of colitis in hPepT1 transgenic mice.
(A–C) Nod2−/− and β-actin-hPepT1 Tg/Nod2−/− mice were given water (control) or a 3% (w/v) DSS solution for 7 days. Percent change in body weight, assessed at day 7 post-treatment (A). Representative H&E-stained distal colonic sections are shown. Bars =100 µm (B). Levels of colonic mRNAs encoding cytokines/chemokines were analyzed by qRT-PCR (C). (D–F) Nod2−/− and villin-hPepT1 Tg/Nod2−/− mice were given water (control) or 3% (w/v) DSS solution for 7 days. Percentage change in body weight, assessed at day 7 post-treatment (D). Representative H&E-stained distal colonic sections are shown. Bars =100 mm (E). Levels of colonic mRNA encoding cytokines/chemokines were analyzed by qRT-PCR (F). Data are means ± SEMs (n=9/group/condition); the experiment was repeated twice with similar results (*P < .05; **P < .005; **).
Discussion
Although PepT1 expression is upregulated in the colon of IBD patients, the role played by hPepT1 remains unknown. Neither β-actin-hPepT1 nor villin-hPepT1 transgenic mice differed from control WT animals in the absence of colitis induction. The colonic lumen contains high bacterial mass and, consequently, bacteria-derived pro-inflammatory di/tripeptides such as MDP, fMLP, and Tri-DAP can be transported by colonic hPepT1 in hPepT1 transgenic animals. However, we found that simple coexistence of colonic hPepT1 and hPepT1-transportable bacterial pro-inflammatory di/tripeptides was not sufficient to induce intestinal inflammation in transgenic animals. This appears to contradict the finding that colonic PepT1 induced by bowel resection may transport oligopeptides and potentially cause colonic mucosal damage by delivering bacteria-derived fMLP into rat colonic cells 31. However, the perfusion model used resulted in the accumulation of amounts of fMLP that may be greater than the physiological fMLP concentration derived from prokaryotic products in the lumen of the colon. We cannot exclude the possibility that ectopic expression of hPepT1 in colonic epithelial cells may affect the overall electrophysiological properties of the colonic epithelium. Under normal conditions, PepT1 is not expressed in colonic epithelial cells. Induction of such expression, in apical plasma membrane domains, may initiate changes in colonic epithelial function(s). Colonic PepT1 expression introduces an absorptive function (of di/tripeptides) not found in normal colonocytes. The potential effects of ectopic expression of hPepT1 in colonic epithelial cells require further investigation.
We have previously shown that PepT1 is expressed in immune cells such as macrophages and transports small bacteria-derived di/tripeptides such as fMLP 24, 25. Such peptides may access immune cells because of the decrease in intestinal barrier permeability that occurs during intestinal inflammation 32, 33. We have also suggested that expression of PepT1 in immune cells may influence the immune response via transportation of small bacteria-derived peptides. Here, we show that ectopic hPepT1 expression in immune cells does not cause intestinal inflammation when colitis is not induced. This may be explained by the fact that intestinal barrier function is not affected in transgenic hPepT1 mice and small bacteria-derived peptides cannot access the immune system. However, we show that TNBS, which is known to disrupt intestinal barrier functions, induces worse intestinal inflammation in β-actin-hPepT1, but not villin-hPepT1 mice, compared to WT littermates. In addition, our bone marrow transplant experiments demonstrated that hPepT1 expression in IECs and immune cells may play an important role in TNBS-induced colitis in β-actin-hPepT1 mice. These results may indicate that bacteria-derived peptides have access to immune cells expressing hPepT1 in β-actin-hPepT1 mice, but not in villin-hPepT1 transgenic animals.
Next, we used DSS to induce acute colitis with ulceration, a condition typical of human UC. After DSS treatment all measured inflammatory indicators, such as MPO levels in the colonic mucosa, weight loss and pro-inflammatory cytokine concentrations, were higher in β-actin hPepT1 transgenic mice than in WT animals, suggesting DSS induces an inflammatory context that creates a proinflammatory role for hPepT1. We speculate that the initial inflammation removes the mucus layer, leading to direct access of bacterial proinflammatory peptides to hPepT1 present at the surface of IECs. It is possible that the inflammation of colonic epithelial cells activates hPepT1 transport activity because of a decreased intraluminal pH in the inflamed colon. An increase in hPepT1-mediated transport may enhance the accumulation of proinflammatory bacterial di/tripeptides inside cells. This proinflammatory role for hPepT1 could result from transporter expression in colonic epithelial and/or immune cells, as hPepT1 is expressed in both cell types in hPepT1 mice. To explore the possibility that epithelial hPepT1 expression was sufficient to aggravate colitis, we used a villin-PepT1 transgenic mouse line that expresses hPepT1 specifically in the intestine, including the colonic mucosa. We found that colitis induced by DSS was aggravated in villin-PepT1 mice compared to WT controls, suggesting that hPepT1 expressed by colonic epithelial cells plays a role in exacerbating intestinal colitis.
NOD1 is activated by peptides that contain a diaminophilic acid, such as the PepT1 substrate tri-DAP and NOD2 recognizes the PepT1 substrate MDP. Thus, PepT1 transport activity plays an important role in intracellular loading of ligands for NOD1 and NOD2 that determine the activation level of downstream inflammatory pathways, including NF-κB 20–23. Here, we demonstrate that antibiotic treatment decreases the severity of colitis in hPepT1 transgenic mice, suggesting that bacteria and/or bacterial products such as fMLP, MDP, and tri-DAP are crucial for aggravation of induced colitis in hPepT1 transgenic animals. We also demonstrated that hPepT1 transgenic mice lacking NOD2 expression do not show aggravation of induced colitis, compared to WT animals. Together, these data suggest that the PepT1/NOD2 signaling pathway is required to aggravate induced colitis in hPepT1 transgenic mice.
We have demonstrated a causal link between the sensing of PepT1-transported small bacterial products by NOD2, and the development of inflammatory disorders. Our present study elucidated the role of PepT1 in modulation of antimicrobial function, and the participation of this protein in the regulatory mechanisms of the adaptive immune system. In conclusion, our present study; together with previous data showing aberrant colonic PepT1 expression in IBD patients; and the ability of PepT1 to transport bacterial peptides such as fMLP, MDP, and DAP that activate inflammatory signaling pathways in an NOD-dependent manner, provide solid evidence for the physiopathological relevance of intestinal PepT1 in intestinal inflammation.
Materials and Methods
Generation of hPepT1 transgenic mice
The hPepT1 full-length protein was cloned using the following primers: Forward: 5’-CGC CAT GGG AAT GTC CAA ATC-3’; Reverse: 5’-CCC CGG TTA AGT GTC TTT GTC TAC-3’. hPepT1 was then sub-cloned into the pβAct-3×HA vector (which contain the β-actin promoter see map Supplementary Figure S2) and the pBS KS Villin MES vector 34 (which contain the villin promoter see map Figure S4-1). Transgenic founder mice (FVB/N background) generated by pronuclear injection (Emory transgenic mouse and gene targeting core facility) were identified by PCR using the following primers: β-Actin Forward: 5’-CTC CAC CAA ACG CAG ACA CA-3’; Villin Forward: 5’-TCC TGT GTG CTA TCA CAG CC; Reverse: 5’-AGT TCG CCA GCC TAG TGG AT-3’. Homozygous mouse lines were then generated and their genotype was confirmed by crossing the selected homozygous transgenic hPepT1 mice with WT mice to generate offspring that were 100% heterozygous for hPepT1. Experiments were performed using homozygous mice. For transgenic experiments, 2 founder lines showing similar results were used. hPepT1 transgenic mice were backcrossed 10 times with WT C57/BL6 mice and then crossed with NOD2 KO mice (C57/BL6 background) obtained from Charles River. Histological examinations were performed independently by pathologists from Charles River. All animal procedures were approved by the Animal Care Committee of Emory University and were conducted in accordance to the Guide for the Care of Use of Laboratory Animals from the US Public Health Service.
Induction of colitis
Colonoscopy
Bone marrow transplantation
Bone marrow transplantation was performed, as described previously 35. Briefly, the femur and tibia were removed and stripped off all muscle and sinew, and bone marrow cells were harvested by flushing the bone cavity with basal marrow medium (Iscove’s medium; Cambrex). After washing with PBS, bone marrow cells were resuspended in basal marrow medium. Approximately 5 × 106 cells in 50 µl were transplanted retro-orbitally. Four treatment groups with 6 animals per group were used (WT→WT, WT→hPepT1+/+, hPepT1+/+→WT, and hPepT1+/+→hPepT1+/+). Mice were given neomycin at 2 mg/ml for the first week of post-transplantation. Five weeks after transplantation, we induced colitis by 3% DSS and mice were assessed daily for rectal bleeding, weight loss, and diarrhea. At the end of the experimental period, mice were sacrificed and engraftment was verified by genotyping bone marrow cells using the following specific primers: hPepT1 forward: 5’-ACC ATA CGT TTG TGG CTC TG-3’; hPepT1 reverse: 5’-GAG GTG ACT GCT TGT CCA ATT-3’.
Broad spectrum antibiotic treatment
Mice were treated for 4 weeks with ampicillin (1 g/l; Cellgro), vancomycin (500 mg/l; plantMedia), neomycin sulfate (1 g/l; bioworld), and metronidazole (1 g/l; mpBio) as described 36.
Myeloperoxidase (MPO) activity in the colon
Protein extraction and Western blot analysis
RNA extraction and RT-PCR
Real-time RT-PCR
Preparation of mouse colonic apical membrane vesicles and in vivo uptake experiment
Histology
Immunohistochemistry
Statistical analysis
Supplementary Material
Acknowledgements
The authors thank Dr Mauricio Rojas, Emory University, Division of Pulmonary, for his help with the bone marrow chimera mice.
Grant support: This work was supported by grants from the Department of Veterans Affairs and National Institute of Diabetes and Digestive and Kidney Diseases Grants R24-DK-064399 (center grant), R56-DK-061941 (to D. Merlin), and RO1-DK55850 (to S. V. Sitaraman). D. Merlin is a recipient of a Senior Research Award from the Crohn's and Colitis Foundation of America.
Abbreviations
- IBD
inflammatory bowel disease
- NOD2
nucleotide-binding oligomerization domain 2
- TNBS
2,4,6-Trinitrobenzene sulfonic acid
- DSS
dextran sodium sulfate
- WT
wild-type
- IEC
intestinal epithelial cell
- MDP
muramyl dipeptide
- UC
ulcerative colitis
- NLR
NOD-like receptor
- MPO
myeloperoxidase
Footnotes
No conflicts of interest exists.
Author contributions – G. Dalmasso and H.T. Nguyen developed experimental concepts and designs; acquired data; analyzed and interpreted data; drafted the manuscript; critically revised the manuscript for important intellectual content and performed statistical analyses on experiments. D. Merlin developed experimental concepts and designs; interpreted data; and critically revised the manuscript for important intellectual content. S. A. Ingersoll and S. Ayyadurai acquired data and reviewed the manuscript. H. Laroui, M.A. Charania, Y.Yan and S. V. Sitaraman reviewed and offered insight on writing the manuscript.
References
- 1.Merlin D, Si-Tahar M, Sitaraman SV, et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120:1666–1679. doi: 10.1053/gast.2001.24845. [DOI] [PubMed] [Google Scholar]
- 2.Wojtal KA, Eloranta JJ, Hruz P, et al. Changes in mRNA expression levels of solute carrier transporters in inflammatory bowel disease patients. Drug Metab Dispos. 2009;37:1871–1877. doi: 10.1124/dmd.109.027367. [DOI] [PubMed] [Google Scholar]
- 3.Mathews DM, Adibi SA. Peptide absorption. Gastroenterology. 1976;71:151–161. [PubMed] [Google Scholar]
- 4.Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol. 2003;285:G779–G788. doi: 10.1152/ajpgi.00056.2003. [DOI] [PubMed] [Google Scholar]
- 5.Fei YJ, Kanai Y, Nussberger S, et al. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- 6.Liang R, Fei YJ, Prasad PD, et al. Human intestinal H+/peptide cotransporter. Cloning, functional expression, and chromosomal localization. J Biol Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- 7.Bretschneider B, Brandsch M, Neubert R. Intestinal transport of beta-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into Caco-2 cell monolayers and the transepithelial flux. Pharm Res. 1999;16:55–61. doi: 10.1023/a:1018814627484. [DOI] [PubMed] [Google Scholar]
- 8.Brodin B, Nielsen CU, Steffansen B, et al. Transport of peptidomimetic drugs by the intestinal Di/tri-peptide transporter, PepT1. Pharmacol Toxicol. 2002;90:285–296. doi: 10.1034/j.1600-0773.2002.900601.x. [DOI] [PubMed] [Google Scholar]
- 9.de Vrueh RL, Smith PL, Lee CP. Transport of L-valine-acyclovir via the oligopeptide transporter in the human intestinal cell line, Caco-2. J Pharmacol Exp Ther. 1998;286:1166–1170. [PubMed] [Google Scholar]
- 10.Friedman DI, Amidon GL. Intestinal absorption mechanism of dipeptide angiotensin converting enzyme inhibitors of the lysyl-proline type: lisinopril and SQ 29,852. J Pharm Sci. 1989;78:995–998. doi: 10.1002/jps.2600781205. [DOI] [PubMed] [Google Scholar]
- 11.Kramer W, Girbig F, Gutjahr U, et al. Interaction of renin inhibitors with the intestinal uptake system for oligopeptides and beta-lactam antibiotics. Biochim Biophys Acta. 1990;1027:25–30. doi: 10.1016/0005-2736(90)90043-n. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen CU, Supuran CT, Scozzafava A, et al. Transport characteristics of L-carnosine and the anticancer derivative 4-toluenesulfonylureido-carnosine in a human epithelial cell line. Pharm Res. 2002;19:1337–1344. doi: 10.1023/a:1020306926419. [DOI] [PubMed] [Google Scholar]
- 13.Daniel H. Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- 14.Merlin D, Steel A, Gewirtz AT, et al. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest. 1998;102:2011–2018. doi: 10.1172/JCI4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buyse M, Charrier L, Sitaraman S, et al. Interferon-gamma increases hPepT1-mediated uptake of di-tripeptides including the bacterial tripeptide fMLP in polarized intestinal epithelia. Am J Pathol. 2003;163:1969–1977. doi: 10.1016/s0002-9440(10)63555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vavricka SR, Musch MW, Chang JE, et al. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409. doi: 10.1053/j.gastro.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 17.Dalmasso G, Nguyen HT, Charrier-Hisamuddin L, et al. PepT1 mediates transport of the proinflammatory bacterial tripeptide L-Ala-{gamma}-D-Glu-meso-DAP in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G687–G696. doi: 10.1152/ajpgi.00527.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buyse M, Tsocas A, Walker F, et al. PepT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol. 2002;283:C1795–C1800. doi: 10.1152/ajpcell.00186.2002. [DOI] [PubMed] [Google Scholar]
- 19.Chiu NM, Chun T, Fay M, et al. The majority of H2-M3 is retained intracellularly in a peptide-receptive state and traffics to the cell surface in the presence of N-formylated peptides. J Exp Med. 1999;190:423–434. doi: 10.1084/jem.190.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 21.Tanabe T, Chamaillard M, Ogura Y, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597. doi: 10.1038/sj.emboj.7600175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franchi L, Warner N, Viani K, et al. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Bourhis L, Benko S, Girardin SE. Nod1 and Nod2 in innate immunity and human inflammatory disorders. Biochem Soc Trans. 2007;35:1479–1484. doi: 10.1042/BST0351479. [DOI] [PubMed] [Google Scholar]
- 24.Charrier L, Merlin D. The oligopeptide transporter hPepT1: gateway to the innate immune response. Lab Invest. 2006;86:538–546. doi: 10.1038/labinvest.3700423. [DOI] [PubMed] [Google Scholar]
- 25.Charrier L, Driss A, Yan Y, et al. hPepT1 mediates bacterial tripeptide fMLP uptake in human monocytes. Lab Invest. 2006;86:490–503. doi: 10.1038/labinvest.3700413. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen HT, Dalmasso G, Powell KR, et al. Pathogenic bacteria induce colonic PepT1 expression: an implication in host defense response. Gastroenterology. 2009;137:1435–1447. doi: 10.1053/j.gastro.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zucchelli M, Torkvist L, Bresso F, et al. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1562–1569. doi: 10.1002/ibd.20963. [DOI] [PubMed] [Google Scholar]
- 28.Dalmasso G, Charrier-Hisamuddin L, Nguyen HT, et al. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–178. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Shi B, Song D, Xue H, et al. PepT1 mediates colon damage by transporting fMLP in rats with bowel resection. J Surg Res. 2006;136:38–44. doi: 10.1016/j.jss.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 32.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 34.Pinto D, Robine S, Jaisser F, et al. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 35.Iyer SS, Ramirez AM, Ritzenthaler JD, et al. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296:L37–L45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.