Abstract
HIV-associated nephropathy is characterized by renal podocyte proliferation and dedifferentiation. This study found that all-trans retinoic acid (atRA) reverses the effects of HIV-1 infection in podocytes. Treatment with atRA reduced cell proliferation rate by causing G1 arrest and restored the expression of the differentiation markers (synaptopodin, nephrin, podocin, and WT-1) in HIV-1–infected podocytes. It is interesting that both atRA and 9-cis RA increased intracellular cAMP levels in podocytes. Podocytes expressed most isoforms of retinoic acid receptors (RAR) and retinoid X receptors (RXR) with the exception of RXRγ. RARα antagonists blocked atRA-induced cAMP production and its antiproliferative and prodifferentiation effects on podocytes, suggesting that RARα is required. For determination of the effect of increased intracellular cAMP on HIV-infected podocytes, cells were stimulated with either forskolin or 8-bromo-cAMP. Both compounds inhibited cell proliferation significantly and restored synaptopodin expression in HIV-infected podocytes. The effects of atRA were abolished by Rp-cAMP, an inhibitor of the cAMP/protein kinase A pathway and were enhanced by rolipram, an inhibitor of phosphodiesterase 4, suggesting that the antiproliferative and prodifferentiation effects of atRA on HIV-infected podocytes are cAMP dependent. Furthermore, both atRA and forskolin suppressed HIV-induced mitogen-activated protein kinase 1 and 2 and Stat3 phosphorylation. In vivo, atRA reduced proteinuria, cell proliferation, and glomerulosclerosis in HIV-1–transgenic mice. These findings suggest that atRA reverses the abnormal phenotype in HIV-1–infected podocytes by stimulating RARα-mediated intracellular cAMP production. These results demonstrate the mechanism by which atRA reverses the proliferation of podocytes that is induced by HIV-1.
HIV-associated nephropathy (HIVAN) is the most common cause of chronic renal failure in HIV-1–seropositive patients (1). The key histologic features of HIVAN are collapsing FSGS and microcystic tubular dilation (2). A unique characteristic of HIVAN is that podocytes, which normally are quiescent and highly differentiated cells, undergo marked proliferation and dedifferentiation (3,4). Expression of HIV-1 viral proteins is critical for pathogenesis as shown in HIV-1–transgenic mice (Tg26) as well as humans (5,6). Podocytes from HIV-1–transgenic mice exhibit increased growth rate and loss of contact inhibition when compared with those of control littermates (7). In vitro, infection of podocytes with HIV-1 also causes proliferation and dedifferentiation (8). We have shown that the HIV-1 nef gene is the major determinant of podocyte proliferation and dedifferentiation (8,9) by inducing Src-dependent mitogen-activated protein kinase (MAPK) 1 and 2/Stat3 activation (10).
Retinoids are derivatives of vitamin A and have multiple cellular functions, including inhibition of proliferation, induction of cell differentiation, regulation of apoptosis, and inhibition of inflammation (11). During kidney development, retinoic acid (RA) affects tubulogenesis and nephron number (12). In addition to their established benefits in treating some malignancies, retinoids have been found to provide protection in several experimental models of kidney disease (13–16). In rat models of acute and chronic mesangioproliferative glomerulonephritis, retinoids preserve renal function, decrease albuminuria, and reduce glomerular and tubular damage (13,14). In the rat model of puromycin aminonucleoside–induced nephrosis, retinoids prevent proteinuria from developing by protecting podocytes from injury (15,16).
Retinoids exert their effects by binding two families of nuclear receptors, retinoic acid receptors (RAR) and retinoid X receptors (RXR). All-trans-RA (atRA) binds and activates the RAR (RAR α, β, and γ), whereas 9-cis RA binds and activates both the RAR and the RXR (RXR α, β, and γ) (17). RAR are expressed in many tissues, including the kidney (18). They affect gene transcription either directly by binding to the RA-response elements of a promoter region (18,19) or indirectly by modulating transcription factors such as activator-protein 1 (20,21) or NF-κB (22). Activation of cytosolic signaling molecules by retinoids also has been reported as an important pathway to induce leukemia cell differentiation (23). Retinoids directly alter the activity of various protein kinases, including MAPK and protein kinase C isoforms (24,25). A recent study reported that atRA induced rapid cAMP production and increases protein kinase A (PKA) activity in acute myeloblastic leukemia cells, leading to cell differentiation (26). A synergistic effect between atRA and inhibitors of phosphodiesterase also was observed for myeloid differentiation (27).
On the basis of the antiproliferative nature of atRA and the pathogenesis of HIVAN (podocyte proliferation and dedifferentiation), we hypothesized that retinoids may exert a beneficial effect on podocytes that are infected with HIV-1. In this study, we show that atRA can inhibit HIV-1–induced podocyte proliferation and dedifferentiation through activation of an RARα-mediated cAMP/PKA pathway.
Materials and Methods
Infection of Conditionally Immortalized Murine Podocytes with HIV-1 Expressing or Control Vector
Conditionally immortalized murine podocytes were isolated as described previously (28). These cells proliferate under permissive conditions (IFN-γ at 33°C) but differentiate under nonpermissive conditions (37°C). The HIV-1 constructs were described previously (8). Briefly, the HIV-1 gag/pol deleted construct pNL4–3:d1443 was derived from the provirus pNL4–3. A fragment that contained the EGFP gene (from pEGFP-C1; Clontech, Palo Alto, CA) was inserted at the SphI/MscI gag/pol deletion site. The expression of HIV-1 genes was confirmed by Western blot analysis. The HIV-1 gag/pol genes and VSV.G envelope glycoprotein were provided in trans using pCMV R8.91 and pMD.G plasmids, respectively (gifts of Dr. Didier Trono, Salk Institute, La Jolla, CA). As a negative control, virus also was produced from pHR-CMV-IRES2-GFP-ΔB, which contains the HIV-1 long-term repeat and EGFP. As the pilot study, we performed experiments in both freshly infected podocytes and a subset of established podocytes. We obtained similar results; therefore, we used here the established HIV-infected podocytes. In all experiments, cells were grown at 37°C on type 1 collagen–coated dishes for 10 d to inactivate the temperature-sensitive T antigen and to allow for differentiation. By Western blot, we confirmed that T antigen was absent in these cells.
Cell Growth Assay
Control and HIV-1–infected podocytes were plated on collagen-coated 24-well plates at a density of 20,000 cells/well. Podocytes were cultured further for 3 to 5 d with atRA or 9-cis RA (0.1 to 10 μM; Sigma, St. Louis, MO), RARα agonists (Am580 and Ro-23-4217), RARα antagonist, and RXR agonist Ro-25-7386, and 4-hydroxyphenylretinamide, a poor activator of RAR, which served as a negative control (Roche, Basel, Switzerland) (29). For testing of PKA pathway involvement, podocytes were cultured with H89 (10 μM; Calbiochem, San Diego, CA), rolipram (10 μM; Calbiochem), Rp-cAMP (100μM; Sigma), Forskolin (Sigma), or various combinations as indicated in the figures. Vehicle alone served as a control. After intervention, cells were trypsinized and counted. For studying contact inhibition, podocytes were cultured for 10 d under nonpermissive conditions and were plated at 80% confluence on collagen-coated dishes. Podocytes were cultured further with atRA (10 μM) or vehicle (DMSO), and photographs were taken on day 7. 3-(4,5-dimethylthiazol)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay for cell proliferation also was performed after 3 d using a protocol that was provided by manufacturer (Promega, Madison, WI).
Flow Cytometry
After virus infection, podocytes were cultured under nonpermissive conditions for 10 d and then incubated with atRA (10 μM) or vehicle for 4 additional days. Cells were trypsinized, washed with 1× PBS, and fixed with ice-cold 80% ethanol. Cells were pelleted, resuspended in 1× PBS, and passed through a 25-G needle to create a single suspension. RNase (1 mg/ml) and propidium iodine (400 μg/ml) were added, and cells were incubated for 30 min at 37°C. Cell-cycle analysis was performed on a FACSCalibur flow cytometer, and data were analyzed with CellQuest software (Becton Dickinson, Mountain View, CA).
Apoptosis Study
Apoptosis was determined by Annexin V FITC apoptosis detection kit (BD Bioscience, San Jose, CA). This combined staining using Annexin V and propidium iodide allowed us to distinguish early apoptotic cells from necrotic or later apoptotic cells. After staining, apoptotic cells were measured by flow cytometry.
Northern Blot Analysis
Total RNA was extracted using Trizol (Life Technologies BRL, Grand Island, NY). Probes were generated by reverse transcriptase–PCR of RNA that were isolated from glomeruli of a normal mouse. The cDNA probes were radiolabeled with [32P-α] dCTP by random oligonucleotide priming and hybridized either with Hybrisol (Intergen, Purchase, NY) or with ULTRAhyb (Ambion, Austin, TX).
Real-Time PCR
Real-time PCR was performed with a Roche LightCycler with a Qiagen QuantiTect One Step RTPCR SYBR green kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Validated primer sets for nephrin and tubulin were obtained from Qiagen. Melting-curve analysis was performed to check for a single amplicon that was verified by size using gel electrophoresis. LightCycler analysis software was used for determining crossing points using the second derivative method. Data were analyzed by the 2–△△ CT method as described by Livak and Schmittgen (30) and are presented as fold increase normalized to housekeeping genes.
Assay for cAMP
Intracellular cAMP was measured using a cAMP Biotrak enzyme immunoassay system (Amersham Biosciences, Piscataway, NJ). After being serum-starved overnight, cells were stimulated with different analogs of retinoids (0.1 to 10 μM) with or without rolipram (10 μM) for 2, 5, 10, and 30 min as indicated. Cells were lysed, and 100 μl of cell lysates was used for the assay. A nonacetylation enzyme immunoassay procedure was used to measured intracellular cAMP production with a standard curve in a range from 12.5 to 3200 fmol/well.
Western Blot
Podocytes were lysed with a buffer that contained 1% NP40, a protease inhibitor cocktail and tyrosine and serine-threonine phosphorylation inhibitors. Cell lysates were subjected to Western blot analysis using the following specific antibodies: Anti–phospho-MAPK1,2, anti-MAPK1,2, anti–phospho-Stat3, and anti-Stat3 from Cell Signaling Laboratory (Beverly, MA). Anti-Nef antibody was obtained from FIT Biotech Oyj Plc (Tampere, Finland). Anti-podocin antibody was a gift from Dr. Peter Mundel (Mount Sinai School of Medicine, NY).
In Vivo Studies
Experimental Design
Tg26 and their littermates were given intraperitoneal injection of atRA at 16 mg/kg or vehicle alone (corn oil) three times per week from 10 d of age to 4 mo of age (five mice per group).
Urine Analysis
Urine was collected at 20-d intervals. Urine was assayed for protein excretion using sulfosalicylic acid and creatinine excretion using a colorimetric microplate assay based on the Jaffe reaction (Oxford Biomedical Research, Oxford, MI).
Histology and Sclerosis Scoring
Kidney tissue was collected at 120 d and fixed in formalin or methyl Carnoy fixed tissue. Periodic acid-Schiff staining was performed. A glomerulosclerosis index and interstitial disease index were measured on tissue from all animals. A total of 50 glomeruli were observed per tissue sample, and the percentage of segmental sclerosis was recorded as 0, 0 to 25, 25 to 50, 50 to 75, or 75 to 100%. Collapsed glomerular tufts also were recorded. The degree of interstitial disease, mostly tubular dilation, was recorded as an overall impression of the biopsy with the same percentage range.
Immunohistochemical analysis for nuclear cell proliferation–associated antigen (Ki67) to assess proliferation was performed with mouse anti-Ki67 1:100 (BD Pharmingen, San Diego, CA) and followed by biotinylated horse anti-mouse IgG antibodies (1:100; both from Vector Laboratories, Burlingame, CA). The ABC elite kit was used to enhance the signal, and staining was visualized by reaction with 3,3′-diaminobenzidine. The number of Ki67-positive nuclei per glomerulus was scored in a total of 50 glomeruli per mouse.
Statistical Analyses
For the cell proliferation and cAMP assays, the data are means ± SD of the mean. For Northern blot and Western blot analysis, all experiments were repeated at least three times. Representative experiments are shown below. Statistically significant differences between the means were determined by unpaired t test or the Mann-Whitney U test where appropriate. Significance was defined as a P ≤ 0.05.
Results
RA Inhibits Proliferation and Restores Differentiation Markers in HIV-1–Infected Podocytes
We found that atRA (10 μM) markedly decreased cell proliferation rate in both control and HIV-1–infected podocytes (Figure 1A). There was no nuclear fragmentation in the cells that were treated with up to 10 μM atRA, suggesting that cell toxicity or increased apoptosis was not responsible for these findings. This was confirmed further by analysis of apoptosis cells using Annexin V and propidium iodide staining. The antiproliferative effects of atRA were reversible because HIV-infected podocytes recovered a high growth rate when atRA was removed from culture medium (data not shown). atRA (10 μM) also inhibited the contact-independent growth of podocytes that was induced by HIV-1 infection (Figure 1B). Podocytes that were treated with atRA were enlarged and grew in a monolayer, suggesting that these cells had converted back to a more differentiated state. For further exploration of the effective dosage of atRA on podocyte proliferation, both control and HIV-infected podocytes were incubated with atRA at different concentrations. We found that atRA significantly inhibited HIV-induced proliferation at 0.5 μM (Figure 1C).
Figure 1.
(A) All-trans retinoic acid (atRA) inhibits HIV-1–induced podocyte proliferation. After differentiation at 37°C for 10 d, Mock or HIV-1–infected podocytes were plated on collagen-coated six-well plates at 20,000 cells/well with or without atRA (10 μM) for 7 d. Bars represent mean cell number ± SD of six samples. (B) atRA inhibits HIV-induced loss of contact inhibition. Mock- or HIV-1–infected podocytes were plated on collagen-coated six-well plates at 80% confluence. Cells were cultured further with or without atRA (10 μM). Photographs were taken on day 7. C. Dosage response of retinoids on podocyte proliferation. After differentiation, control podocytes (◆) and HIV-infected podocytes (■) were incubated in collagen-coated dishes for 3 d with or without atRA at concentrations of 0.1, 0.5, 1, or 10 μM. Cell number was counted. Means ± SD of three independent experiments are shown. *P < 0.001 verus cells without atRA treatment. (D) Effects of atRA on mRNA expression levels of WT-1, synaptopodin, cyclin A, cyclin E, and glyceraldehyde-3-phosphate dehydrogenase (G3PDH). Normal podocytes (Mock) or HIV-1–infected podocytes (HIV) were cultured for 3 d with or without atRA (10 μM). Total RNA (10 μg/lane) was analyzed by Northern blotting. This is a representative blot of three independent experiments. (E) Dosage response of retinoids on synaptopodin expression. HIV-infected podocytes were treated with various concentrations (0.1 to 10 μM) of atRA or 9-cis-RA for 3 d. Total RNA (10 μg/lane) was analyzed by Northern blotting for synaptopodin and GAPDH. This is a representative blot of three independent experiments. (F) Effects of atRA on podocin expression. HIV-infected podocytes were treated with atRA (1 μM) for 3 d. Podocin expression was determined by Western blot using anti-podocin antibody. This is a representative blot of three independent experiments. (G) Effects of atRA on nephrin expression. HIV-infected podocytes were treated with atRA (1 μM) for 3 d. Nephrin/tubulin ratios were determined by real-time PCR. The fold of increased as compared with control podocytes are expressed. *P < 0.05 versus control (CL); **P < 0.05 versus HIV-infected podocytes; n = 3. Magnification, ×200.
FACS analysis was performed on HIV-1– and mock-infected podocytes that were treated with atRA for 7 d. As shown in Table 1, the percentage of HIV-1–infected podocytes in the G0/G1 phase was one third that of mock-infected podocytes (22.1 versus 61%). Treatment of HIV-1–infected podocytes with atRA resulted in an increase in the percentage of cells in G1 to 53.6% with a decrease in the fraction of cells in the S phase. These results demonstrate that atRA inhibits the proliferation of HIV-1–infected podocytes by inducing G1 arrest.
Table 1.
Effects of atRA on podocyte cell cyclea
| Parameter | % AP | %G0/G1 | %S | %G2/M |
|---|---|---|---|---|
| Mock | 0.2 | 61 | 14.7 | 24.1 |
| HIV | 0.7 | 22.1 | 71 | 6.2 |
| Mock + atRA | 0 | 71.1 | 6.9 | 22 |
| HIV + atRA | 5.3 | 53.6 | 15.9 | 25.2 |
HIV-1– or mock-infected podocytes were plated on collagen-coated plates and cultured for 14 d with or without all-trans retinoic acid (atRA). Cell-cycle distribution was analyzed as described in Materials and Methods. The G0/G1, S, and G2/M phases of frequency distribution were determined. AP, hypodiploid DNA content (apoptotic population of cells).
RA has been shown to inhibit G1-to-S transition by regulating the expression of cell-cycle proteins (31,32). We found that atRA inhibited the transcription of cyclin A and cyclin E in HIV-1–infected podocytes (Figure 1D). These findings suggest that atRA blocks HIV-1–induced G1→S progression through down-regulation of cyclin A and cyclin E expression.
Because HIV-1 infection also is known to suppress expression of podocyte differentiation markers, we examined whether atRA could reverse these effects as well. HIV-1–infected podocytes were treated with atRA (10 μM) or vehicle for 3 d. As shown by Northern blot, atRA significantly increased the mRNA levels of WT-1 and synaptopodin in HIV-infected podocytes (Figure 1D). The effect of atRA and 9-cis RA on synaptopodin expression was dosage dependent, as shown in Figure 1E. By real-time PCR, we found that nephrin mRNA levels also increased after atRA treatment in both control and HIV-infected podocytes (Figure 1E). Similarly, Western blot analysis showed that atRA stimulated podocin expression in both control and HIV-infected podocytes (Figure 1F). These data suggest that treatment with atRA induces podocyte differentiation.
RA Induces a Rapid Increase in Intracellular cAMP in Podocytes through RARα
Recently, it was shown that atRA stimulates intracellular cAMP generation in leukemia cells, suggesting a critical cytosolic pathway for myeloid differentiation (26). Because activation of the cAMP pathway generally is considered protective of injury in podocytes (33), we examined whether cAMP mediates the effects of atRA on podocytes. We found that atRA induced a three- to four-fold increase in intracellular cAMP production in control podocytes. This stimulation was amplified when cells were pretreated with rolipram, a phosphodiesterase 4 (PDE4) inhibitor (Figure 2A). A similar pattern of stimulation was observed in HIV-1–infected podocytes (Figure 2B). Once again, stimulation of cAMP production by atRA and 9-cis RA were dosage dependent (Figure 2, C and D).
Figure 2.
(A and B) atRA stimulates intracellular cAMP production. After differentiation at 37°C, control podocytes (A) and HIV-infected podocytes (B) were cultured on collagen-coated six-well dishes and were stimulated with atRA (10 μM) for 2, 5, 10, 20, and 30 min with or without preincubation with rolipram (100 μM). Intracellular cAMP levels were determined using ELISA. The representative experiment of three independent assays is shown. (C and D) Dosage response of atRA and 9-cis-RA on cAMP production. Control podocytes were cultured on collagen-coated six-well dishes and were stimulated with various concentration of atRA or 9-cis-RA for 2, 5, and 10 min after preincubation with rolipram (100 μM). The representative experiment of three independent assays is shown.
Next, we investigated whether podocytes have retinoid receptors and whether these receptors mediate the actions of atRA on HIV-infected podocytes. By Northern blot, we found that podocytes express RARα, RARβ, RARγ, RXRα, and RXRβ (Figure 3A). We also found that HIV infection significantly suppressed expression of two major receptors in podocytes (RARα and RXRα; Figure 3A). Using receptor-specific agonists and antagonists, we evaluated the roles of these receptors in mediating the effects of RA on podocytes. The RARα antagonist (Ro-41-5253) blocked atRA-induced cAMP production (Figure 3B). Conversely, the RXR agonist (Ro-25-7386) had only a minor effect on atRA-induced cAMP production (Figure 3C). These data suggest that RARα but not RXR mediates atRA-induced cAMP production. The mechanism by which this occurs remains to be determined. We examined whether RARα also mediates the phenotypic effects of atRA on podocytes. As shown in Figure 3D, RARα agonists (Am580 and Ro-23-4217) but not RXR agonist (Ro-25-7386) significantly inhibited HIV-induced proliferation, similar to atRA and 9-cis-RA. Furthermore, the RARα antagonist Ro-41-5253 blocked the inhibitory effects of atRA. The RARα agonists also restored expression of synaptopodin in HIV-infected podocytes similar to what was observed with atRA, and the RARα antagonist blocked the effect of atRA on synaptopodin expression (Figure 3E). Therefore, although the expression is suppressed partially in HIV-infected podocytes, RARα still plays a critical role in mediating the effects of retinoids on podocytes.
Figure 3.
(A) HIV reduces expression of retinoic acid receptor α (RARα) and retinoid X receptor α (RXRα). Northern blotting of RARα, RARβ, RARγ, RXRα, and RXRβ in control podocytes (Mock) and HIV-1–infected podocytes (HIV) that were treated with or without atRA (10 μM). HIV reduces the expression of both RARα and RXRα. Twenty micrograms per lane of total RNA was loaded. The representative blots of three independent experiments are shown. (B) Effects of RARα antagonist on atRA-induced cAMP production. After differentiation at 37°C, podocytes were cultured on collagen-coated six-well dishes and stimulated with atRA (1 μM) for 2, 5, and 10 min with or without preincubation with 10 μM of RARα antagonist (Ro-41-5253). DMSO served as a control. All cells were pretreated with rolipram. Intracellular cAMP level was determined using ELISA. A representative experiment from three independent assays is shown. (C) Effect of RXR agonist on intracellular cAMP production. Podocytes were cultured on collagen-coated six-well dishes and stimulated with RXR agonist (Ro-25-7186; 0.1 and 1 μM) for 2, 5, and 10 min after preincubation with rolipram. Intracellular cAMP level was determined using ELISA. A representative experiment from three independent assays is shown. (D) Effects of RARα and RXR agonists and RARα antagonist on HIV-induced podocyte proliferation. After differentiation, control podocytes and HIV-infected podocytes were incubated in collagen-coated dishes for 3 d with DMSO (control), atRA (1 μM), RARα agonists (Am580; 1 μM), RXR agonist (Ro-25-7386; 1 μM), 9-cis-RA (1 μM), or both atRA and RARα antagonist (Ro-41-5253). Cell number was counted. Mean ± SD of three independent experiments is shown. *P < 0.001. (E) Effects of RARα and RXR agonists and RARα antagonist on synaptopodin expression in HIV-infected podocytes. HIV-infected podocytes were treated with atRA (1 μM), 4-hydroxyphenylretinamide (poor activator of RAR; 1 μM), Am580 (RARα agonist; 1 μM), Ro-23-4272 (RARα agonist; 1 μM), or Ro-25-7386 (RXR agonist; 1 μM) for 3 d. Additional cells were preincubated with Ro-41-5253 (RARα antagonist; 10 μM) and then treated with atRA (1 μM). Total RNA (10 μg/lane) was analyzed by Northern blotting for synaptopodin and GAPDH. This is a representative blot of three independent experiments.
Activation of the cAMP/PKA Pathway by atRA Reverses HIV-1–Induced Proliferation and Dedifferentiation
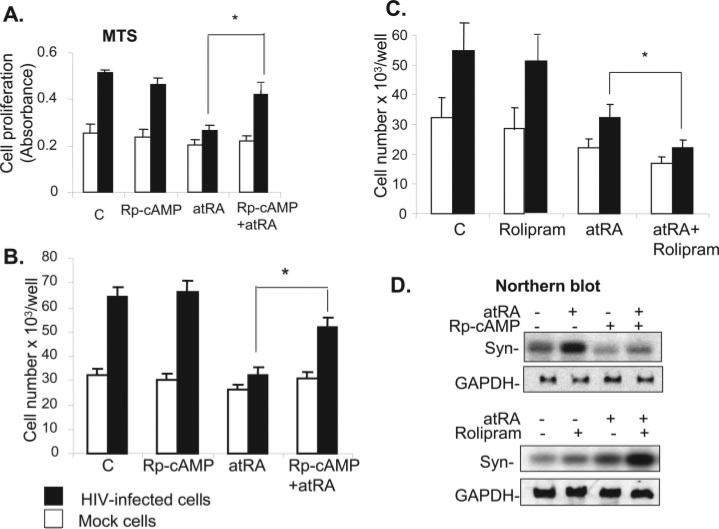
Because atRA induces cAMP production in podocytes, we investigated whether activation of the cAMP/PKA pathway was sufficient to reverse the effects of HIV-1 on podocytes. HIV-1 podocytes were treated with forskolin (to stimulate adenylyl cyclase) or exogenous 8-bromo-cAMP (a cAMP analogue). Both compounds inhibited HIV-induced podocyte proliferation and restored the expression of synaptopodin (Figure 4). These data suggest that activation of the cAMP/PKA pathway could be the mechanism by which atRA reverses the effects of HIV-1 on podocytes Therefore, we examined whether the actions of atRA were mediated by activation of the cAMP/PKA pathway. Rp-cAMP (a competitor of endogenous cAMP) significantly diminished the inhibitory effect of atRA on HIV-1–induced podocyte proliferation as assessed by the MTS cell proliferation assay (Figure 5A) and cell counting (Figure 5B). Treatment of cells with both rolipram and atRA to increase cAMP production were additive in inhibiting podocyte proliferation (Figure 5C). We also found that Rp-cAMP blocked the ability of atRA to stimulate the expression of synaptopodin in HIV-infected podocytes, whereas the effects of atRA were enhanced by rolipram (Figure 5D). These data indicate that the activation of the cAMP pathway mediates the effects of atRA on podocytes.
Figure 4.
(A) Forskolin (FK) and cAMP inhibit HIV-induced podocyte proliferation. After differentiation, control podocytes and HIV-infected podocytes were incubated in collagen-coated dishes with or without FK (30 μM) and cAMP (100 μM) for 3 d. Cell number was counted. Mean ± SD of five independent experiments is shown. *P < 0.001 versus HIV-infected cells. (B) FK and cAMP restore synaptopodin expression. The HIV-infected cells that were treated with FK and cAMP were subjected to Northern blot analysis for synaptopodin and GAPDH. A representative blot of three independent experiments is shown.
Figure 5.
(A and B) Rp-cAMP (Rp) blocks antiproliferative effect of atRA on podocytes. Differentiated podocytes were pretreated with Rp-cAMP (100 μM) for 30 min and then were stimulated with or without atRA (10 μM) for another 3 d. Cells were lysed for MTS assay using the Promega kit (A) or for counting cell number (B). Mean ± SD of four independent experiments is shown. *P < 0.001. (C) A synergic effect is observed between atRA and rolipram. Podocytes were pretreated with rolipram (10 μM) for 30 min and then stimulated with atRA (10 μM) for another 3 d. The cell number was counted. Mean ± SD of three independent experiments is shown. *P < 0.01. (D) atRA induces synaptopodin expression in a protein kinase A (PKA)-dependent pathway. Under the same culture conditions as above, cells were used for Northern blot analysis for synaptopodin and GAPDH. A representative blot of three independent experiments is shown.
atRA Inhibits HIV-Induced MAPK1,2 and Stat3 Phosphorylation
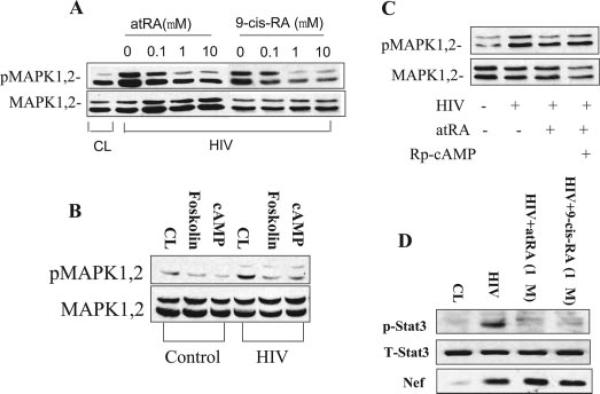
We reported previously that HIV stimulates the Ras/Raf/MAPK1,2 pathway, leading to podocyte proliferation through Nef (10). We examined whether atRA used a cross-talk mechanism with cAMP to affect MAPK1,2 phosphorylation in podocytes. It is interesting that both atRA and 9-cis RA decreased HIV-induced MAPK1,2 phosphorylation in a dosage-depen dent manner (Figure 6A). Forskolin and 8-bromo-cAMP also inhibited HIV-induced MAPK1,2 phosphorylation (Figure 6B), whereas Rp-cAMP abolished the inhibitory effect of atRA on MAPK1,2 activation (Figure 6C). Together, these data indicate that atRA inhibits MAPK1,2 phosphorylation through activation of the cAMP pathway. We also found that both atRA and 9-cis-RA suppressed phosphorylation of Stat3 (Tyr705; Figure 6D). As a control, we did not see changes in Nef expression in HIV-infected podocytes after atRA treatment (Figure 6D).
Figure 6.
atRA inhibits HIV-induced mitogen-activated protein kinase (MAPK) 1 and 2 and Stat3 phosphorylation. (A) HIV-infected podocytes were incubated with atRA or 9-cis-RA at 0.1 to 10 μM overnight in serum-free medium. Control podocytes served as a control (CL). Cells were lysed for Western blot of phospho and total MAPK1,2. (B) Control and HIV-infected podocytes were incubated with FK (30 μM), cAMP (100 μM), or vehicle overnight in serum-free medium. Cells then were lysed for Western blot of phospho and total MAPK1,2. (C) Cells were preincubated with Rp-cAMP (100 μM) for 30 min and then stimulated with or without atRA (10 μM) overnight in serum-free medium, and Western blots were performed for phospho and total MAPK1,2. A representative blot from a minimum of three independent experiments is shown for all experiments. (D) HIV-infected podocytes were treated with atRA (1 μM) overnight in serum-free medium. Control podocytes served as a control (CL). Cells were lysed for Western blot of phospho and total Stat3 and Nef. A representative blot from a minimum of three independent experiments is shown for all experiments.
In Vivo Study
To confirm our in vitro findings, we examined whether treating HIV-1–transgenic mice (Tg26) with atRA ameliorates HIV-associated kidney disease. As shown in Figure 7, Tg26 mice developed more proteinuria and glomerulosclerosis than their littermates. When Tg26 mice were treated with atRA, they exhibited significantly reduced proteinuria, cell proliferation, and glomerulosclerosis, compared with nontreated Tg26 mice. These data indicate that atRA exerts protective effects in vivo.
Figure 7.
atRA improves kidney disease in HIV-1–transgenic mice. (A) Mean urine protein/urine creatinine (Upro/Ucr) ratio measured at 20-d intervals between 40 and 120 d of age. HIV-Tg26 mice had a significant increase in Upro/Ucr ratio at day 60 when compared with wild type (WT) mice. atRA treatment decreased Upro/Ucr ratio in HIV-Tg26 to WT levels. (B) The number of glomeruli with segmental sclerosis or collapsed tufts was increased in HIV-Tg26 mice at day 120 when compared with WT mice. atRA treatment significantly decreased the number of glomeruli with segmental sclerosis and completely abrogated tuft collapse. (C) Mean average of Ki67-positive nuclei was increased in HIV-Tg26 mice at day 120 when compared with WT mice. atRA treatment decreased the average number of Ki67-positive nuclei to WT levels.
Discussion
atRA Changes HIV-Infected Podocytes from a Pathologic Phenotype to a More Differentiated Phenotype
This study demonstrates that atRA can reverse the pathologic changes that are observed in HIV-infected podocytes (cell dedifferentiation and proliferation) and in HIV-1–transgenic mice (proteinuria and glomerulosclerosis). Recently, retinoids were shown to improve kidney diseases in several experimental animal models, including anti–Thy-1 nephritis (13,14), puromycin aminonucleoside–induced nephrosis (15,16), lupus nephritis (34), and anti–glomerular basement membrane nephritis (35,36). atRA prevents the decrease in synaptopodin, nephrin, and podocin staining in an anti–glomerular basement membrane glomerular injury model (36).
HIV-associated nephropathy is characterized by podocyte proliferation, loss of contact inhibition, and dedifferentiation, similar to malignant cells (7–10). In this study, we found that RA blocked HIV-1–induced proliferation and contact-independent growth primarily through G1 arrest and decreased expression of cyclin A and cyclin E in HIV-1–infected podocytes. atRA also induced podocyte differentiation manifested by increased expression of WT-1, nephrin, podocin, and synaptopodin. These findings provide a rationale for using RA to treat HIVAN and other diseases of podocyte proliferation, such as idiopathic collapsing FSGS.
atRA Reverses Abnormal Phenotype of HIV-Infected Podocytes through Stimulation of Intracellular cAMP Production
Several studies demonstrated that atRA induces rapid cAMP production and increases PKA activity in acute myeloblastic leukemia cells (26). Our results demonstrate significant stimulation of cAMP production within minutes when podocytes are treated with either atRA or 9-cis-RA. This stimulation was amplified when cells were pretreated with rolipram, an inhibitor of PDE4. Podocytes express several G protein–coupled receptors (GPCR), including the β2-adrenergic receptor, the D1-dopamine receptor, and the prostaglandin IP and EP4 receptors (33,37). Activation of these receptors results in generation of intracellular cAMP. Induction of the cAMP–PKA pathway in podocytes regulates cell morphology, actin assembly, and matrix production (33). In addition, cAMP seems to attenuate the effect of hormones that activate the Ca2+/PKC pathway (38). Overall, the cAMP pathway seems to exert a protective effect on podocytes (33). In this study, we found that the cAMP/PKA pathway mediates the beneficial effects of atRA on podocytes because Rp-cAMP blocked atRA-induced differentiation of podocytes. We also found that rolipram enhanced the atRA effect. These data indicate that atRA exerts its effect on HIV-1–infected podocytes through activation of the cAMP pathway. Treatment with atRA alone or in combination with PDE inhibitors could be an effective treatment strategy (in combination with antivirals) for HIVAN.
RARα Mediates the Effects of atRA on HIV-Infected Podocytes
Although receptor-independent effects of retinoids have been described, most studies have shown that RAR and/or RXR play a role in retinoid-induced cellular effects through either genomic or nongenomic pathways (18,24,26). It was surprising that RARα, which is suppressed in HIV-infected cells, still played a critical role in atRA's effects on podocyte pheno-type as supported by our data using the RARα agonist and antagonist. How RARα mediates atRA-induced cAMP production, however, remains unclear. Estrogen can induce cAMP production through activation of adenylyl cyclase (39), and a recent study suggested that estrogen binds to intracellular GPCR (40). Further studies are required to determine whether there are GPCR that bind atRA.
atRA Inhibits HIV-Induced MAPK1,2 and Stat3 Phosphorylation
In previous studies, we found that HIV Nef induced MAPK1,2 and Stat3 activation in podocytes (10,41). Inhibition of MAPK1,2 or Stat3 activation suppressed Nef-induced cell proliferation and dedifferentiation (10). Here, we found that atRA inhibited both MAPK1,2 and Stat3 phosphorylation in podocytes. atRA probably reduces MAPK phosphorylation through activation of MAPK phosphatase 1, as this was reported previously (42). It has been reported that atRA can inhibit Stat3 activation in leukemia cells (43).
Conclusion
We found that atRA inhibits proliferation and induces differentiation in HIV-infected podocytes through RARα-mediated cAMP/PKA activation. These data provide new insights into the underlying molecular mechanisms of the podocyte disease of HIVAN as well as a novel therapeutic strategy for its treatment.
Acknowledgments
This work was supported by National Institutes of Health grant P01DK056492 (to P.K.) and grant GM-54508 (to R.I.). J.H. is supported by a K08 award (DK-65495). S.N. is supported by an individual National Research Service Award grant (GM-65065).
We thank Rafael Mira Lopez for providing RAR and RXR agonists and antagonist and for thoughtful discussion and suggestions.
Footnotes
J.C.H. and T.-C.L. contributed equally to this work.
Disclosures
None.
References
- 1.Monahan M, Tanji N, Klotman PE. HIV-associated nephropathy: An urban epidemic. Semin Nephrol. 2001;21:394–402. doi: 10.1053/snep.2001.23771. [DOI] [PubMed] [Google Scholar]
- 2.D'Agati V, Appel GB. HIV infection and the kidney. J Am Soc Nephrol. 1997;8:138–152. doi: 10.1681/ASN.V81138. [DOI] [PubMed] [Google Scholar]
- 3.Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 4.Barisoni L, Mokrzycki M, Sablay L, Nagata M, Yamase H, Mundel P. Podocyte cell cycle regulation and proliferation in collapsing glomerulopathies. Kidney Int. 2000;58:137–143. doi: 10.1046/j.1523-1755.2000.00149.x. [DOI] [PubMed] [Google Scholar]
- 5.Bruggeman LA, Dikman S, Meng C, Quaggin SE, Coffman TM, Klotman PE. Nephropathy in human immunodeficiency virus-1 transgenic mice is due to renal transgene expression. J Clin Invest. 1997;100:84–92. doi: 10.1172/JCI119525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruggeman LA, Ross MD, Tanji N, Cara A, Dikman S, Gordon RE, Burns GC, D'Agati VD, Winston JA, Klotman ME, Klotman PE. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz EJ, Cara A, Snoeck H, Ross MD, Sunamoto M, Reiser J, Mundel P, Klotman PE. Human immunodeficiency virus-1 induces loss of contact inhibition in podocytes. J Am Soc Nephrol. 2001;12:1677–1684. doi: 10.1681/ASN.V1281677. [DOI] [PubMed] [Google Scholar]
- 8.Husain M, Gusella GL, Klotman ME, Gelman IH, Ross MD, Schwartz EJ, Cara A, Klotman PE. HIV-1 Nef induces proliferation and anchorage-independent growth in podocytes. J Am Soc Nephrol. 2002;13:1806–1815. doi: 10.1097/01.asn.0000019642.55998.69. [DOI] [PubMed] [Google Scholar]
- 9.Sunamoto M, Husain M, He JC, Schwartz E, Klotman PE. Critical role for Nef in HIV-1-induced podocyte dedifferentiation. Kidney Int. 2003;64:1695–1701. doi: 10.1046/j.1523-1755.2003.00283.x. [DOI] [PubMed] [Google Scholar]
- 10.He JC, Husain M, Sunamoto M, D'Agati VD, Klotman ME, Iyengar R, Klotman PE. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114:643–651. doi: 10.1172/JCI21004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans TR, Kaye SB. Retinoids: Present role and future potential. Br J Cancer. 1999;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilar J, Gilbert T, Moreau E, Merlet-Benichu C. Metanephros organogenesis is highly stimulated by vitamin A derivatives in organ culture. Kidney Int. 1996;49:1478–1487. doi: 10.1038/ki.1996.208. [DOI] [PubMed] [Google Scholar]
- 13.Lehrke I, Schaier M, Schade K, Morath C, Waldherr R, Ritz E, Wagner J. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am J Physiol Renal Physiol. 2002;282:F741–F751. doi: 10.1152/ajprenal.00026.2001. [DOI] [PubMed] [Google Scholar]
- 14.Wagner J, Dechow C, Morath C, Lehrke I, Amann K, Waldherr R, Floege J, Ritz E. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol. 2000;11:1479–1487. doi: 10.1681/ASN.V1181479. [DOI] [PubMed] [Google Scholar]
- 15.Moreno-Manzano V, Mampaso F, Sepulveda-Munoz JC, Alique M, Chen S, Ziyadeh FN, Iglesias-de la Cruz MC, Rodriguez EN, Orellana JM, Reyes P, Arribas I, Xu Q, Kitmura M, Lucio Cazana FJ. Retinoids as a potential treatment for experimental puromycin-induced nephrosis. Br J Pharmacol. 2003;139:823–831. doi: 10.1038/sj.bjp.0705311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki A, Ito T, Imai E, Yamato M, Iwatani H, Kawachi H, Hori M. Retinoids regulate the repairing process of the podocytes in puromycin aminonucleoside-induced nephrotic rats. J Am Soc Nephrol. 2003;14:981–991. doi: 10.1097/01.asn.0000057857.66268.8f. [DOI] [PubMed] [Google Scholar]
- 17.Allenby G, Bocquel MT, Saunders M, Kazmer S, Speck J, Rosenberger M, Lovey A, Kastner P, Grippo JF, Chambon P, Arthur AL. Retinoic acid receptors and retinoid X receptors: Interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993;90:30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Q, Lucio-Cazana J, Kitamura M, Ruan X, Fine LG, Norman JT. Retinoids in nephrology: Promises and pitfalls. Kidney Int. 2004;66:2119–2131. doi: 10.1111/j.1523-1755.2004.66002.x. [DOI] [PubMed] [Google Scholar]
- 19.Gronemeyer H, Miturski R. Molecular mechanisms of retinoid action. Cell Mol Biol Lett. 2001;6:3–52. [PubMed] [Google Scholar]
- 20.Benkoussa M, Brand C, Delmotte MH, Formstecher P, Lefebvre P. Retinoic acid receptors inhibit AP1 activation by regulating extracellular signal-regulated kinase and CBP recruitment to an AP1-responsive promoter. Mol Cell Biol. 2002;22:4522–4534. doi: 10.1128/MCB.22.13.4522-4534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonson MS. Anti-AP-1 activity of all-trans retinoic acid in glomerular mesangial cells. Am J Physiol. 1994;267:F805–F815. doi: 10.1152/ajprenal.1994.267.5.F805. [DOI] [PubMed] [Google Scholar]
- 22.Na SY, Kang BY, Chung SW, Han SJ, Ma X, Trinchieri G, Im SY, Lee JW, Kim TS. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFkappaB. J Biol Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 23.Licht J, Zelent A. Retinoid and growth factor receptor signaling in APL. Blood. 2005;105:1381–1382. [Google Scholar]
- 24.Radominska-Pandya A, Chen G, Czernik PJ, Little JM, Samokyszyn VM, Carter CA, Nowak G. Direct interaction of all-trans-retinoic acid with protein kinase C (PKC). Implications for PKC signaling and cancer therapy. J Biol Chem. 2000;275:22324–22330. doi: 10.1074/jbc.M907722199. [DOI] [PubMed] [Google Scholar]
- 25.Hong HY, Varvayanis S, Yen A. Retinoic acid causes MEK-dependent RAF phosphorylation through RARalpha plus RXR activation in HL-60 cells. Differentiation. 2001;68:55–66. doi: 10.1046/j.1432-0436.2001.068001055.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q, Tao J, Zhu Q, Jia PM, Dou AX, Li X, Cheng F, Waxman S, Chen GQ, Chen SJ, Lanotte M, Chen Z, Tong JH. Rapid induction of cAMP/PKA pathway during retinoic acid-induced acute promyelocytic leukemia cell differentiation. Leukemia. 2004;18:285–292. doi: 10.1038/sj.leu.2403226. [DOI] [PubMed] [Google Scholar]
- 27.Parrella E, Gianni M, Cecconi V, Nigro E, Barzago MM, Rambaldi A, Rochette-Egly C, Terao M, Garattini E. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the cAMP and the retinoic acid signaling pathways. J Biol Chem. 2004;279:42026–42040. doi: 10.1074/jbc.M406530200. [DOI] [PubMed] [Google Scholar]
- 28.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 29.Apfel C, Bauer F, Crettaz M, Forni L, Kamber M, Kaufmann P, LeMotte P, Pirson W, Klaus M. A retinoic acid receptor alpha antagonist selectively counteracts retinoic acid effects. Proc Natl Acad Sci U S A. 1992;89:7129–7133. doi: 10.1073/pnas.89.15.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real time quantitative PCR and the 2–deltadelta CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Seewaldt V, Kim JH, Caldwell LE, Johnson BS, Swisshelm K, Collins SJ. All-trans-retinoic acid mediates G1 arrest but not apoptosis of normal human mammary epithelial cells. Cell Growth Differ. 1997;8:631–641. [PubMed] [Google Scholar]
- 32.Teixeira C, Pratt MA. CDK2 is a target for retinoic acid-mediated growth inhibition in MCF-7 human breast cancer cells. Mol Endocrinol. 1997;11:1191–1202. doi: 10.1210/mend.11.9.9977. [DOI] [PubMed] [Google Scholar]
- 33.Endlich N, Endlich K. cAMP pathway in podocytes. Microsc Res Tech. 2002;57:228–231. doi: 10.1002/jemt.10079. [DOI] [PubMed] [Google Scholar]
- 34.Perez de Lema G, Lucio-Cazana FJ, Molina A, Luckow B, Schmid H, de Wit C, Moreno-Manzano V, Banas B, Mampaso F, Schlondorff D. Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney Int. 2004;66:1018–1028. doi: 10.1111/j.1523-1755.2004.00850.x. [DOI] [PubMed] [Google Scholar]
- 35.Oseto S, Moriyama T, Kawada N, Nagatoya K, Takeji M, Ando A, Yamamoto T, Imai E, Hori M. Therapeutic effect of all-trans retinoic acid on rats with anti-GBM antibody glomerulonephritis. Kidney Int. 2003;64:1241–1252. doi: 10.1046/j.1523-1755.2003.00219.x. [DOI] [PubMed] [Google Scholar]
- 36.Vaughan MR, Pippin JW, Griffin SV, Krofft R, Fleet M, Haseley L, Shankland SJ. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 2005;68:133–144. doi: 10.1111/j.1523-1755.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 37.Bek M, Nusing R, Kowark P, Henger A, Mundel P, Pavenstadt H. Characterization of prostanoid receptors in podocytes. J Am Soc Nephrol. 1999;10:2084–2093. doi: 10.1681/ASN.V10102084. [DOI] [PubMed] [Google Scholar]
- 38.Endlich N, Nobiling R, Kriz W, Endlich K. Expression and signaling of parathyroid hormone-related protein in cultured podocytes. Exp Nephrol. 2001;9:436–443. doi: 10.1159/000052643. [DOI] [PubMed] [Google Scholar]
- 39.Aronica SM, Lee Kraus W, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: Stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1572–1573. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 41.Husain M, D'Agati V, He JC, Klotman ME, Klotman PE. HIV-1 Nef induces dedifferentiation of podocytes in vivo: A characteristic feature of HIVAN. AIDS. 2005;19:1975–1980. doi: 10.1097/01.aids.0000191918.42110.27. [DOI] [PubMed] [Google Scholar]
- 42.Xu Q, Konta T, Furusu A, Nakayama K, Lucio-Cazana J, Fine LG, Kitamura M. Transcriptional induction of mitogen-activated protein kinase phosphatase 1 by retinoids: Selective roles of nuclear receptors and contribution to the antiapoptotic effect. J Biol Chem. 2002;277:41693–41700. doi: 10.1074/jbc.M207095200. [DOI] [PubMed] [Google Scholar]
- 43.Dong S, Chen SJ, Tweardy DJ. Cross-talk between retinoic acid and STAT3 signaling pathways in acute promyelocytic leukemia. Leuk Lymphoma. 2003;44:2023–2029. doi: 10.1080/1042819031000116670. [DOI] [PubMed] [Google Scholar]