Abstract
Prions consist mainly, if not entirely, of PrPSc, an aggregated conformer of the host protein PrPC. Prions come in different strains, all based on the same PrPC sequence, but differing in their conformations. The efficiency of prion transmission between species is usually low, but increases after serial transmission in the new host, suggesting a process involving mutation and selection. Even within the same species, the transfer of prions between cell types entails a selection of favoured 'substrains', and propagation of prions in the presence of an inhibitory drug can result in the appearance of drug-resistant prion populations. We propose that prion populations are comprised of a variety of conformers, constituting 'quasi-species', from which the one replicating most efficiently in a particular environment is selected.
Keywords: mutation, quasi-species, seeding hypothesis, selection, strain
See Glossary for abbreviations used in this article.
Glossary.
Brain [22L] brain infected with 22L prions
DAPH-12 4,5-bis-4-methoxyanilinophthalimide
EGCG epigallocatechin-3-gallate
ELISA enzyme-linked immunosorbent assay
GPI glycosylphosphatidylinositol
NaPTA sodium phosphotungstate
PK1 [22L] PK1 cells infected with 22L prions
PMCA protein misfolding cyclic amplification
Prnp murine prion protein gene
PrP prion protein
PrPC PrP, cellular (normal) form
PrPSc PrP, scrapie (pathogenic) form
RML Rocky Mountain Laboratory (prion strain)
rPrPSc proteinase-K-resistant PrPSc
sPrPSc proteinase-K-sensitive PrPSc
Introduction
Prions are infectious agents that cause transmissible spongiform encephalopathies (TSEs). They are comprised mainly, if not entirely, of PrPSc, an aggregated conformer of the normal host glycoprotein, PrPC (Prusiner, 1991). Propagation of PrPSc occurs through seeded polymerization, a process by which PrPC is recruited into the PrPSc aggregate, and adopts its 'abnormal' conformation (Gajdusek, 1988; Jarrett & Lansbury, 1993). PrPSc has physicochemical properties that distinguish it from PrPC, such as aggregation, precipitability by NaPTA and exposure of distinctive epitopes. We distinguish between rPrPSc, which is largely resistant to proteinase K, and sPrPSc, which is susceptible to proteinase K (Cronier et al, 2008; Nazor et al, 2005; Safar et al, 1998; Tzaban et al, 2002) but resistant to pronase E digestion (D'Castro et al, 2010). Both forms of PrPSc, but not PrPC, are precipitable by NaPTA (Safar et al, 1998).
The mature form of the murine PrP protein consists of 208 amino acids, comprises two potential N-glycosylation sites—which might or might not carry complex glycans—and is attached to the outer surface of the plasma membrane by a GPI anchor. PrP is encoded by a single-copy gene, known as Prnp in mice, which encompasses a coding sequence devoid of introns. Interestingly, inbred mice homozygous for Prnp can indefinitely propagate more than 20 strains of prions (Bruce, 2003). These strains were originally characterized by different incubation times and neuropathology after injection into various mouse lines. Subsequently, it was found that individual strains are associated with physicochemical differences of PrPSc (Baron et al, 2011; Bessen & Marsh, 1992; Collinge et al, 1996; Kuczius & Groschup, 1999; Peretz et al, 2001a; Telling et al, 1996), as well as with distinct tropism for cultured cell lines (Mahal et al, 2007). It is currently believed that 'strain-ness' is encoded by the conformation of PrP (Bessen & Marsh, 1992; Collinge et al, 1996; Telling et al, 1996); the role of glycosylation, if any, is still unclear (Sidebar A; Lawson et al, 2005; Piro et al, 2009; Tuzi et al, 2008; Cancellotti et al, 2010).
Sidebar A | In need of answers.
What role does glycosylation have in specifying 'strain-ness'?
What is the atomic structure of sPrPSc and rPrPSc?
Do features other than conformation underlie strain specificity?
Which cellular components enable prion replication?
What determines cell tropism of prion strains?
How do strain mutations come about?
Many, but apparently not all, mammalian species can develop prion disease, spontaneously or as a consequence of infection. In humans, sporadic Creutzfeldt–Jakob disease—which has a worldwide incidence of about 10−6—is believed to be initiated by spontaneous conversion of PrPC to PrPSc (Gajdusek, 1991), as are atypical bovine spongiform encephalopathy (BSE) and atypical scrapie in sheep (Baron et al, 2007). Given appropriate conditions, prion disease can spread in a population, as exemplified by scrapie in sheep almost worldwide, BSE in the UK and chronic wasting disease in cervids in the USA.
Transmission barriers
In general, there is a considerable barrier to the transmission of prions between animal species, so that even massive intracerebral inoculation causes disease in only a few animals (low 'attack rate') and/or only after long incubation times, if at all ('transmission barrier'; Collinge, 1999). Importantly, when prions are serially transmitted from initial trans-species recipient to other animals of the same species, attack rates increase and incubation times decrease, reflecting adaptation to the new host (Bruce & Fraser, 1991; Kimberlin & Walker, 1977).
To what is the transmission barrier due? Differences in the PrP sequence between donor and recipient make an important contribution, as shown by several instances in which a barrier was overcome or diminished by replacing the PrP gene of the recipient by its counterpart from the donor (Browning et al, 2004; Prusiner et al, 1990); this might be referred to as a 'sequence barrier'. Sequence barriers are common between animal species, but can occur within a single species, for example between C57BL/6 and VM mice (Bruce, 1993), the PrPs of which—encoded by Prnpa and Prnpb (formerly sincS7 and SincP7)—differ by just two residues (Westaway et al, 1987). The barrier might, to a large extent, be due to conformational restrictions imposed by sequence differences. However, even if the PrP sequences of a donor and recipient are congruent, transmission barriers can still occur, as in the case of variant Creutzfeldt–Jakob disease prions from humans inoculated into mice that express human PrPC (Hill et al, 1997). This indicates that some components in the recipient that are essential for prion replication—such as 'protein X' proposed by Prusiner and colleagues (Kaneko et al, 1997; Telling et al, 1995)—might not interact appropriately with the donor's PrP, constituting what might be called a 'cell barrier'. Finally, two prion strains originating from the same donor species, and therefore having the same PrP sequence, might show differing infectivities towards a recipient expressing the same PrP. For example, sporadic but not variant Creutzfeldt–Jakob disease prions are highly infectious to mice transgenic for human PrP (Hill et al, 1997), thus exemplifying a 'strain barrier'.
Prion strain mutation and selection in vivo
It was observed early on that prions derived from a pool of scrapie-infected sheep brain gave rise to different strains in mice, depending on the genotype of the mice and the passage history of the prions (Bruce, 2003). It was not immediately clear whether this was the consequence of heterogeneity in the original source, mutation during transmission, or both. Prion cloning by endpoint dilution cast light on this question. In this procedure, mice are inoculated with prions at dilutions such that only one in ten or more develop the disease; the transmission is repeated 2–3 times. When uncloned 22C prions raised in Prnpa mice were passaged through Prnpb mice, a new prion strain, 22H, was recovered. However, when cloned 22C prions were subjected to the same procedure, the prion strain remained unchanged, indicating that the uncloned 22C sample had been a mixture of at least two strains (Bruce, 2003). Conversely, transfer of cloned 139A prions from mouse to hamster and back to mouse resulted in a new strain, 139H/M, and this was attributed to mutation of the agent—at the time firmly believed to be a nucleic-acid-containing entity (Kimberlin et al, 1987). If a mutation is defined as a heritable change of any replicating entity, as we propose, then the phenomenon observed by Kimberlin was indeed a mutation, even if we now attribute it to a change in the conformation of PrPSc rather than in the sequence of a nucleic acid.
The adaptation of prions from one host species to another—as reflected by attaining minimal incubation times—usually takes two or more serial transmissions (Bruce & Fraser, 1991). According to the seeding model (Gajdusek, 1988; Jarrett & Lansbury, 1993), the first step in PrPSc replication requires the addition of host PrPC to the incoming PrPSc aggregate. Because the amino acid sequences differ, the host PrPC might be unable to adopt the conformation required for stable addition to the seed, and a conformational change or mutation of the seed might first be required to allow addition. Such mutations might be favoured in very short seeds, the conformation of which might be less stable than that of longer entities. Alternatively, the host PrPC might assume a conformation that allows it to add to the seed, but is different from that within the seed; the mutation would then be a consequence of unfaithful replication. Even if endowed with the capacity to propagate, the mutated seed might do so only very slowly in the novel environment, and several rounds of mutation and selection might be required to achieve a replication rate that leads to pathogenesis. An informative experiment, reported by Hill and colleagues, showed that inoculation of Sc237 prions from hamsters into mice could, after two years, lead to accumulation of PrPSc without causing disease. They also showed that the resulting prion population was able to elicit disease in both mice and hamsters, albeit after long incubation times, but with 100% attack rate (Hill et al, 2000). This suggests the slow evolution of a heterogeneous population, comprised of prions able to adapt efficiently to both hamsters and mice. The spectrum of conformations that PrPSc can adopt in a particular host is constrained by the PrP sequence (Angers et al, 2010).
More recently, Prusiner and colleagues generated prions by inoculating mice with polymerized recombinant PrP and found that, upon further transmission to mice, a variety of distinct strains emerged, as assessed by incubation time and conformational stability. Serial propagation of such synthetic prions led to selection of more rapidly propagating strains (Colby et al, 2009; Legname et al, 2004, 2005, 2006).
In conclusion, there is clear evidence from animal experiments that naturally occurring prions are subject to mutation and selection.
Prion strains in cultured cells
It was recognized early on that most cell lines are not susceptible to chronic infection by prions, and those that are, are often susceptible to some strains and not others (Bosque & Prusiner, 2000; Lehmann, 2005; Nishida et al, 2000; Rubenstein et al, 1992; Schatzl et al, 1997; Vorberg et al, 2004). The capacity of a strain to infect a cell line can be determined by the standard scrapie cell assay (SSCA): cells are exposed to a serial dilution of prions, propagated for three splits, and the proportion of PrPSc-accumulating cells is determined by an ELISA (Fig 1). The infectivity of a sample is expressed as the 'response index', namely the reciprocal of the dilution giving rise to a specified proportion of PrPSc-positive cells (Klohn et al, 2003). Making use of the differential cell tropism of prion strains, Mahal and colleagues developed the cell panel assay (CPA), which allows the characterization of several prion strains, in particular 22L, RML, ME7 and 301C, on a panel of four cell lines, PK1, CAD, R33 and LD9; strains are characterized by their differential ability to infect the various cell lines (Table 1; Mahal et al, 2007). Another characteristic parameter is the extent to which chronic infection of PK1 cells is inhibited by swainsonine (swa), an inhibitor of α-mannosidase II that causes misglycosylation of N-glycosylated proteins. A typical response to swa is two logs of inhibition for RML and 79A, one log for 139A and none for 22L (Browning et al, 2011).
Figure 1.
The SSCA. Typically, a sample is serially diluted 1:10 and added to monolayers of susceptible cells in 96-well plates for 3 days. The cells are subjected to three 1:10 splits, 20,000 cells are filtered onto plates and rPrPSc-containing cells are identified by subjecting them to proteinase K treatment followed by an enzyme-linked immunosorbent assay. A plot of infected cells ('spots') versus the logarithm of the dilution allows quantification (Klohn et al, 2003; Mahal et al, 2007). SSCA, standard scrapie cell assay.
Table 1. Susceptibility of various cell lines to murine prion strains.
| CAD | LD9 | PK1 | PK1 + swa | R33 | |
|---|---|---|---|---|---|
| 22L | + | + | + | + | + |
| RML | + | + | + | − | − |
| ME7 | + | + | − | − | − |
| 301C | + | − | − | − | − |
Susceptibility was assessed by the SSCA (standard scrapie cell assay). The data are simplified from Mahal et al (2007) and Browning et al (2011).
Selection of prion substrains
Brain-derived 22L prions can chronically infect PK1 cells in the presence of swa ('swa-resistant') and R33 cells ('R33-competent'); however, when transferred to cultured cells these properties change. To monitor the adaptation of prions to cell culture, PK1 cells were exposed to 22L-infected brain homogenate, and the prions produced were analysed by using the CPA at various times after infection (Fig 2A). After 9 days—the earliest time point examined (P0)—the prion population was still R33-competent and largely swa-resistant, but after 13, 27 and 40 doublings (P4, P8 and P12, respectively), it was fully R33-incompetent and swa-sensitive—that is, 'PK1-adapted'. Because these changes in properties occur gradually, it seems likely that they reflect the adaptation of the 22L prion population from the brain to the PK1 cell environment, rather than being due to recruitment of some cell-specific components, which would have resulted in an immediate change. Indeed, when the PK1-adapted prions were again propagated in the brain, they reacquired their initial properties as seen in the CPA and elicited histopathology indistinguishable from that of 'authentic' brain-derived 22L prions (Li et al, 2010). A likely explanation for these observations is that a prion strain population is a quasi-species (Eigen, 1971), consisting of a major component and many variants, which are constantly being generated and selected against in a particular environment, as described earlier for RNA viruses and retroviruses (Domingo et al, 1978, 2006) and also proposed for prions ('cloud model' in Collinge & Clarke (2007); Li et al, 2010). When changing hosts, a different variant, or 'substrain', might be selected, and the composition of the population could change accordingly (Fig 2A).
Figure 2.
22L prions adapt to their environment. (A) Top: PK1 cells were infected with brain-derived 22L prions containing a majority of 'brain-competent' prions (blue) and multiple 'variant' prions (red, green, yellow and grey)—of which one species is 'PK1-competent, swa-sensitive' (orange)—and passaged for 12 1:10 splits. With successive passages (P0 to P12), the PK1-competent prions are selected and become the main component of the quasi-species population. Bottom: The serial dilutions of the prion samples from the splits indicated were assayed on PK1 cells, PK1 cells + swa and R33 cells by the SSCA (standard scrapie cell assay). (B) Chronically 22L-infected PK1 cells were propagated for ten 1:20 splits in the absence of swa (bottom row, C0 to C10); the 'PK1-competent, swa-sensitive' prions (orange) remained the dominant population. When propagated in the presence of swa (top row, S1 to S10), the prion population (orange) decreased markedly in S1, followed by a gradual increase of swa-resistant (red) prions. When, after five splits in the presence of swa, the population was further propagated in the absence of swa (middle row, SC6 to SC10), the 'PK1-competent, swa-sensitive' prions (orange) eventually became dominant, presumably because they have a slight growth advantage over the other prions (red) in the absence of swa. swa, swainsonine.
To analyse whether there is a similar change in properties observed in the CPA when transferring 22L prions to a different cell line, R33 cells were inoculated. Both PK1 and R33 cells were derived from murine N2a cells; PK1 was selected for high and R33 for low susceptibility to RML prions (Klohn et al, 2003). In this case, no significant change in the properties of the 22L was detected, even after 80 doublings (Mahal et al, 2010); retention of R33 competence was not surprising, as it was a condition for survival in the new environment, but swa resistance was also retained. However, when the prions were transferred from the R33 cells to PK1 cells, they gradually became swa-sensitive and R33-incompetent, as they had when they were transferred directly from the brain to PK1 cells. By contrast, when transfer and propagation were carried out in the presence of swa, the prions not only remained swa-resistant, as might be expected, but also continued to be R33-competent, again suggesting that the two properties were linked (Mahal et al, 2010).
We assume that prion properties such as cell tropism and response to swa are encoded in the conformation of PrPSc. Whether this conformation is modulated by other cellular components—such as small RNAs—remains unknown. Notably, some groups describe a requirement of RNA or poly(rA) for the PMCA-mediated generation of infectious prions from purified PrPC or recombinant PrP (Deleault et al, 2003, 2007; Wang et al, 2010), whereas others do not see this requirement (Kim et al, 2010). How can PrPSc conformation encode substrain characteristics such as cell tropism? The level of prions in a cell is determined by the rate of synthesis on the one hand and the rate of depletion by partitioning, secretion and degradation on the other. Only if the rate of synthesis is equal to or exceeds that of depletion can chronic infection occur. The structure of PrPSc is probably an important determinant for the relevant rate constants, in particular of synthesis and degradation. As mentioned above, different strains of PrPSc show distinct physicochemical properties. However, we have not detected differences in the conformational stability or susceptibility to degradation of PrPSc associated with substrains, probably because these differences are too discrete to be detectable by relatively crude methods.
Selection of drug-resistant mutants
Can the swa-sensitive PK1-adapted 22L prion population convert to swa resistance when propagated in the presence of swa? After one passage (S1) in the presence of swa, the infectivity level of the population dropped precipitously, by more than 90%, but after the second passage, infectivity increased and the prions were fully swa-resistant when assayed on PK1 cells (Fig 2B). After the eighth passage (S8), the level of infectivity was as high as before the propagation in swa, and the prions were swa-resistant. To determine whether the transition to swa resistance was reversible, cells that had been passaged five times in the presence of swa (S5) were propagated in the absence of swa: the prions continued to be swa-resistant for four splits (SC9), but after the fifth split they had become swa-sensitive. The conversion to swa resistance required only a few passages because swa-sensitive prions were unable to replicate in the presence of swa, while the reverse process took longer, presumably because the swa-sensitive prions have only a slight replicative advantage over their swa-resistant counterparts.
Is the misglycosylation of PrPSc caused by swa the process that renders prions resistant to the drug? The answer is clearly no, as 22L prions propagated in the presence of swa (S5) were fully drug-resistant after five splits; when swa was withdrawn, PrPSc was again normally glycosylated after propagation for two further splits (SC8), but continued to be fully swa-resistant (Li et al, 2010).
The 22L prions propagated for ten splits in the presence or absence of swa (S10 and C10, respectively) showed distinctive behaviour not only in their drug resistance, but also in that S10 prions were R33-competent whereas C10 prions were R33-incompetent (Li et al, 2010), and are thus strain variants, or substrains. Cell lysate or concentrated conditioned medium from the two variants, containing equal amounts of PrPSc, were injected into mouse brain and caused disease. Interestingly, the C10 prions, assayed as lysates, had a significantly longer incubation time than their S10 counterparts, which had almost the same incubation time as the 22L brain homogenate. Nonetheless, after passaging through mouse brain, the CPA properties of S10- and C10-derived prions were indistinguishable from those of authentic 22L-infected brain homogenate. In summary, 22L prions propagated in PK1 cells adapt to the cellular environment and in doing so become swa-sensitive and R33-incompetent, unless constrained by the presence of swa, under which conditions they remain more similar to brain prions. When returned to brain, the incubation time for the swa-sensitive prions is longer than that for the swa-resistant prions, perhaps reflecting the time required to select a brain-adapted population. The various transmissions to which 22L prions were subjected, all of which entailed adaptation to a changed environment with the possible exception of brain 22L transferred to R33 cells, are shown in Fig 3.
Figure 3.
22L prions transferred into various environments acquire distinct properties. (A) 22L prions from brain (blue) gradually become swa-sensitive and R33-incompetent (orange) when passaged in PK1 cells, and (B) regain their original properties when returned to brain (Li et al, 2010). (C) 22L prions from brain (blue) were transferred to R33 cells (green) and remain swa-resistant and R33-competent; it is not clear whether other properties change (Mahal et al, 2010). (D) R33-adapted prions (green) when transferred to PK1 cells in the absence of swa, become swa-sensitive and R33-incompetent (orange), but when (E) transferred to PK1 cells in the presence of swa, they remain swa-resistant and R33-competent (red; Mahal et al, 2010). (F) swa-resistant, R33-competent prions from PK1 cells propagated in the presence of swa (red) become swa-sensitive and R33-incompetent (orange) when propagated in PK1 cells in the absence of swa. (H) swa-resistant, R33-competent prions from PK1 cells propagated in the presence of swa (red) have a higher specific infectivity when inoculated into brain than swa-sensitive and R33-incompetent (orange) prions from PK1 cells propagated in the absence of swa. All populations are depicted as quasi-species, comprising one major substrain and a few variants. swa, swainsonine.
Similarly, Ghaemmaghami and colleagues reported that treatment with quinacrine of RML-infected mice resulted at first in a reduction of PrPSc in the brain, followed by an increase of PrPSc with moderately diminished conformational stability and distinct conformation-dependent immunoassay properties (Ghaemmaghami et al, 2009). Moreover, in RML-infected, non-dividing N2a cells exposed to quinacrine, quinacrine-resistant PrPSc accumulated; this was not the case in dividing cells, showing that the propagation rate of PrPSc was lower than the rate of cell division.
Interestingly, the prionogenic domain of the yeast prion Sup35—a protein that is completely different from PrP—also gave rise to novel, drug-resistant strains when propagated in the presence of the inhibitor EGCG. A second inhibitor, DAPH-12—which acts by a different mechanism—synergized with EGCG to enhance inhibition (Roberts et al, 2009; Wang et al, 2008).
Heterogeneity of prion strain populations
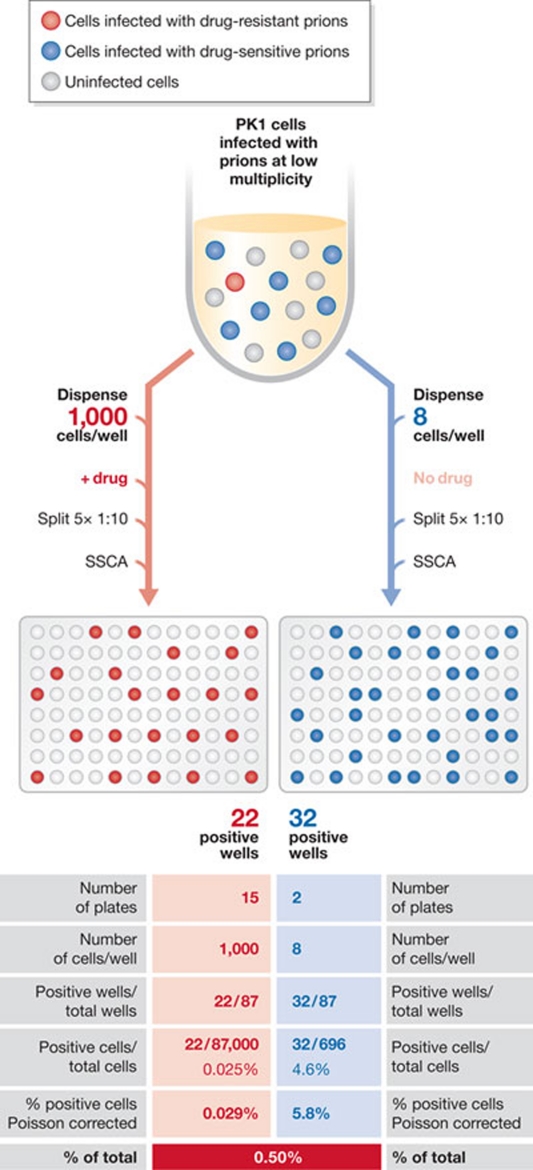
Does a PK1-cell-adapted 22L prion population, which as a whole is swa-sensitive, contain a low level of swa-resistant variants even before exposure to the inhibitor? To answer this question, we performed a frequency assay (Li et al, 2010): PK1 cells were exposed to prions secreted by 22L-infected PK1 cells in the absence or presence of swa and an appropriate number of cells was distributed into individual wells of a 96-well plate. After five splits, any well that had received one or more prion-infected cells became massively infected and could be scored by using the SSCA. From the proportion of infected wells in the swa-exposed and control plates, the abundance of pre-existing swa-resistant prions was calculated to be about 0.5% (Fig 4).
Figure 4.
Determining the proportion of pre-existing drug-resistant prions using a frequency assay. PK1 cells are exposed to prions secreted by PK1 cells chronically infected with 22L in the presence or absence of the drug of interest, and pools of 1,000 infected cells (with drug) or eight infected cells (without drug, +992 uninfected cells) are plated in 96-well plates. Cells are grown to confluence and split 1:10 five times in the continued presence or absence of the drug. In these conditions, any well originally containing one or more infected cells becomes positive in the standard scrapie cell assay (SSCA). In this example, 22 of 87,000 cells became PrPSc-positive in the presence of the drug, and 32 of 696 cells in its absence. Correcting for the Poisson distribution, about 0.5% of the prions were resistant before exposure to the drug. Modified from (Li et al, 2010).
To determine how heterogeneity is generated, eight clones of 22L prions were created by infection at endpoint dilution of swa-sensitive prions secreted by PK1 cells chronically infected with 22L. The clones were serially propagated, and the appearance of swa-resistant mutants was monitored by challenging the prion populations with swa and determining whether the population became swa-resistant; swa-sensitive populations that acquire swa resistance after exposure to swa are designated 'swa-competent'. In two clones, competence was achieved after 31 doublings, in one after 53 and in two others after 86 doublings, whereas the remaining clones had not become swa-competent at that stage. One of the clones that remained swa-sensitive continued to be swa-incompetent for 116 doublings (Li et al, 2010, 2011). These findings imply that some clones have a higher mutation rate than others, and that mutability itself is a variable property of prion substrains (Fig 5A). Interestingly, all swa-incompetent clones became indistinguishable from wild-type 22L—that is, swa-resistant and R33-competent—when propagated in mouse brain. This is probably because even prions with a low mutation rate, when replicating for approximately 200 days under strong selection pressure, gave rise to a wild-type 22L population (Li et al, 2011).
Figure 5.
Cloned prions give rise to quasi-species populations. (A) During propagation, variant prions are generated by mutation. Mutation rates might differ; for example, swa-resistant prions (red) arise stochastically in clone 1 at stage c, in clone 2 at stage d, and in clone 3 not at all during the observation period (scheme based on the experiment of figure 3A in Li et al, (2010)). (B) Conjectural free energy landscape for prion strains and substrains. Substrains are distinguishable collectives of prions that can interconvert reproducibly and readily because they are separated by activation energy barriers that can be overcome in a particular environment under physiological conditions. Different strains are separated by high-energy barriers that are rarely, if ever, overcome in a particular environment. The extent to which the individual wells are populated (red blocks) is governed by the accumulation rate of the prion substrain in that environment. When the environment changes, such as when prions are transferred between tissues, different substrains might be favoured.
Closing thoughts
Classical work clearly established that the TSE agent, as it was then called, can undergo mutation and selection when transferred between animal hosts. In fact, these were the properties that served as the main argument in favour of the agent being virus-like (Bruce & Dickinson, 1987), an idea that is still supported by some (Miyazawa et al, 2011), despite definitive evidence to the contrary (Castilla et al, 2008; Kim et al, 2010; Wang et al, 2010).
The newer results discussed here show that transfer not only between species, but also between cellular environments within the same species, elicits prion adaptation. We have shown that multiply passaged prion populations are heterogeneous, heterogeneity, importantly, arises in cloned prion populations as they are propagated, and the mutation rate can differ for different clones (Fig 5A; Li et al, 2011). Different substrains become major components as the population adapts to the environment (Fig 5B; Li et al, 2010). This conforms to the predicted behaviour of a quasi-species, which results when populations have a high mutation rate and constantly eliminate less fit mutants, thereby giving rise to a dynamic steady state of variants.
Heterogeneity and the underlying mutations are probably due to changes in PrPSc conformation; because they arise readily and are reversible in the cases discussed above, we assume that the activation energy required for the transition is low, allowing the formation of a substantial number of distinct conformers. The conjectural free energy landscape shown in Fig 5B depicts two series of wells separated by low activation energy barriers labelled A1–A10 and B1–B8, corresponding to substrains of strains A and B. The two sets of substrains are separated by a high-energy barrier, which prevents interconversion of strains. The extent to which a well is populated, provided it is thermodynamically accessible, depends on the replicative potential of the substrain. When a population is transferred to a different environment, the free energy profile might change, allowing the population of wells that were not accessible in the first environment and leading to a different dominant substrain (Mahal et al, 2010).
How many murine prion strains and substrains can be encoded by a particular PrP sequence? Even if PrPSc conformations are not modulated by covalently linked glycans or by non-PrP components, their number is probably exceedingly large (Sawaya et al, 2007). In the case of a protein monomer, the activation energy barriers between different conformational states are so low that only the lowest free energy state is substantially populated at room temperature or above. However, in a multimer consisting of a large number of subunits, a vast number of conformations that would be unstable in a monomer are stabilized because the activation energy required to simultaneously change the conformation of an assembly of monomers is additive or close to additive and exceeds thermal energy at physiological conditions. Thus, prion and virus populations exhibit an unexpected commonality: both are heterogeneous and achieve heterogeneity by mutations, and as a consequence are subject to selection in distinct environments, but virus mutations are due to nucleotide changes and prion mutations to conformational states of a protein.
The conclusion that prions constitute quasi-species populations subject to mutation and selection has an impact on our thinking about therapeutic approaches. Because of a prion's potential to acquire drug resistance, a more effective approach would be to starve it of its precursor, PrPC, either by abrogating its synthesis at the transcriptional or translational level, or by using a drug or antibody (Enari et al, 2001; Peretz et al, 2001b) to prevent its transport to the site of PrPC-to-PrPSc conversion, hinder its conversion and/or promote its accelerated turnover. PrP depletion has no deleterious effects, at least in mice (Büeler et al, 1992; Mallucci et al, 2002) and cattle (Richt et al, 2007). Drugs directly targeting PrPSc are more likely to engender resistance, because small conformational differences could abrogate binding; this problem might be countered by the use of two or more drugs with different target sites (Duennwald & Shorter, 2010; Roberts et al, 2009). A further therapeutic option would be the induction of autophagy, which has been shown to clear PrPSc from infected cell cultures (Aguib et al, 2009; Heiseke et al, 2010). However, it should be noted that prion-infected cell cultures are poor models for therapeutic drugs, because cell division potently contributes to PrPSc depletion and thus a modest effect of a drug could lead to clearance of infection, which is not the case with non-dividing neurons in the brain. So far, despite considerable efforts by numerous groups, there is no effective therapy on the horizon.
Acknowledgments
The work performed in Scripps Florida was supported by grants from the National Institutes of Health (1RO1NSO59543, 1RO1NSO67214) and by a generous donation from the Alafi Family Foundation to C.W. We thank D. Eisenberg for helpful advice and C. Lasmézas for critical reading of the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aguib Y, Heiseke A, Gilch S, Riemer C, Baier M, Schatzl HM, Ertmer A (2009) Autophagy induction by trehalose counteracts cellular prion infection. Autophagy 5: 361–369 [DOI] [PubMed] [Google Scholar]
- Angers RC et al. (2010) Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science 328: 1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron GS, Hughson AG, Raymond GJ, Offerdahl DK, Barton KA, Raymond LD, Dorward DW, Caughey B (2011) Effect of glycans and the glycophosphatidylinositol anchor on strain dependent conformations of scrapie prion protein: improved purifications and infrared spectra. Biochemistry 50: 4479–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron T, Biacabe AG, Arsac JN, Benestad S, Groschup MH (2007) Atypical transmissible spongiform encephalopathies (TSEs) in ruminants. Vaccine 25: 5625–5630 [DOI] [PubMed] [Google Scholar]
- Bessen RA, Marsh RF (1992) Biochemical and physical properties of the prion protein from two strains of the transmissible mink encephalopathy agent. J Virol 66: 2096–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosque PJ, Prusiner SB (2000) Cultured cell sublines highly susceptible to prion infection. J Virol 74: 4377–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning S, Baker CA, Smith E, Mahal SP, Herva ME, Demczyk CA, Li J, Weissmann C (2011) Abrogation of complex glycosylation by swainsonine results in strain- and cell-specific inhibition of prion replication. J Biol Chem 19 September [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, Miller MW, Williams ES, Hoover E, Telling GC (2004) Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol 78: 13345–13350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce E (1993) Scrapie strain variation and mutation. In Spongiform Encephalopathies, vol. 49 (ed Allen IV), pp 822–838. New York, USA: Churchill Livingstone [DOI] [PubMed] [Google Scholar]
- Bruce ME (2003) TSE strain variation. Br Med Bull 66: 99–108 [DOI] [PubMed] [Google Scholar]
- Bruce ME, Dickinson AG (1987) Biological evidence that scrapie agent has an independent genome. J Gen Virol 68: 79–89 [DOI] [PubMed] [Google Scholar]
- Bruce ME, Fraser H (1991) Scrapie strain variation and its implications. Curr Top Microbiol Immunol 172: 125–138 [DOI] [PubMed] [Google Scholar]
- Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C (1992) Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356: 577–582 [DOI] [PubMed] [Google Scholar]
- Cancellotti E, Bradford BM, Tuzi NL, Hickey RD, Brown D, Brown KL, Barron RM, Kisielewski D, Piccardo P, Manson JC (2010) Glycosylation of PrPC determines timing of neuroinvasion and targeting in the brain following transmissible spongiform encephalopathy infection by a peripheral route. J Virol 84: 3464–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla J, Morales R, Saa P, Barria M, Gambetti P, Soto C (2008) Cell-free propagation of prion strains. EMBO J 27: 2557–2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby DW, Giles K, Legname G, Wille H, Baskakov IV, DeArmond SJ, Prusiner SB (2009) Design and construction of diverse mammalian prion strains. Proc Natl Acad Sci USA 106: 20417–20422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge J (1999) Variant Creutzfeldt–Jakob disease. Lancet 354: 317–323 [DOI] [PubMed] [Google Scholar]
- Collinge J, Clarke AR (2007) A general model of prion strains and their pathogenicity. Science 318: 930–936 [DOI] [PubMed] [Google Scholar]
- Collinge J, Sidle KC, Meads J, Ironside J, Hill AF (1996) Molecular analysis of prion strain variation and the aetiology of 'new variant' CJD. Nature 383: 685–690 [DOI] [PubMed] [Google Scholar]
- Cronier S, Gros N, Tattum MH, Jackson GS, Clarke AR, Collinge J, Wadsworth JD (2008) Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem J 416: 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Castro L, Wenborn A, Gros N, Joiner S, Cronier S, Collinge J, Wadsworth JD (2010) Isolation of proteinase K-sensitive prions using pronase E and phosphotungstic acid. PLoS ONE 5: e15679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleault NR, Lucassen RW, Supattapone S (2003) RNA molecules stimulate prion protein conversion. Nature 425: 717–720 [DOI] [PubMed] [Google Scholar]
- Deleault NR, Harris BT, Rees JR, Supattapone S (2007) Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA 104: 9741–9746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo E, Sabo D, Taniguchi T, Weissmann C (1978) Nucleotide sequence heterogeneity of an RNA phage population. Cell 13: 735–744 [DOI] [PubMed] [Google Scholar]
- Domingo E, Martin V, Perales C, Grande-Perez A, Garcia-Arriaza J, Arias A (2006) Viruses as quasispecies: biological implications. Curr Top Microbiol Immunol 299: 51–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duennwald ML, Shorter J (2010) Countering amyloid polymorphism and drug resistance with minimal drug cocktails. Prion 4: 244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigen M (1971) Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58: 465–523 [DOI] [PubMed] [Google Scholar]
- Enari M, Flechsig E, Weissmann C (2001) Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci USA 98: 9295–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajdusek DC (1988) Transmissible and non-transmissible amyloidoses: autocatalytic post-translational conversion of host precursor proteins to β-pleated configurations. J Neuroimmunol 20: 95–110 [DOI] [PubMed] [Google Scholar]
- Gajdusek DC (1991) The transmissible amyloidoses—genetical control of spontaneous generation of infectious amyloid proteins by nucleation of configurational change in host precursors—Kuru–CJD–GSS–Scrapie–BSE. Eur J Epidemiol 7: 567–577 [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami S, Ahn M, Lessard P, Giles K, Legname G, DeArmond SJ, Prusiner SB (2009) Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog 5: e1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiseke A, Aguib Y, Schatzl HM (2010) Autophagy, prion infection and their mutual interactions. Curr Issues Mol Biol 12: 87–97 [PubMed] [Google Scholar]
- Hill AF, Joiner S, Linehan J, Desbruslais M, Lantos PL, Collinge J (2000) Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci USA 97: 10248–10253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P (1997) The same prion strain causes vCJD and BSE. Nature 389: 448–450; 526 [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury PJ (1993) Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73: 1055–1058 [DOI] [PubMed] [Google Scholar]
- Kaneko K, Zulianello L, Scott M, Cooper CM, Wallace AC, James TL, Cohen FE, Prusiner SB (1997) Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc Natl Acad Sci USA 94: 10069–10074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI et al. (2010) Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285: 14083–14087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin RH, Walker C (1977) Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol 34: 295–304 [DOI] [PubMed] [Google Scholar]
- Kimberlin RH, Cole S, Walker CA (1987) Temporary and permanent modifications to a single strain of mouse scrapie on transmission to rats and hamsters. J Gen Virol 68: 1875–1881 [DOI] [PubMed] [Google Scholar]
- Klohn PC, Stoltze L, Flechsig E, Enari M, Weissmann C (2003) A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci USA 100: 11666–11671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczius T, Groschup MH (1999) Differences in proteinase K resistance and neuronal deposition of abnormal prion proteins characterize bovine spongiform encephalopathy (BSE) and scrapie strains. Mol Med 5: 406–418 [PMC free article] [PubMed] [Google Scholar]
- Lawson VA, Collins SJ, Masters CL, Hill AF (2005) Prion protein glycosylation. J Neurochem 93: 793–801 [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB (2004) Synthetic mammalian prions. Science 305: 673–676 [DOI] [PubMed] [Google Scholar]
- Legname G, Nguyen HO, Baskakov IV, Cohen FE, Dearmond SJ, Prusiner SB (2005) Strain-specified characteristics of mouse synthetic prions. Proc Natl Acad Sci USA 102: 2168–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legname G, Nguyen HO, Peretz D, Cohen FE, DeArmond SJ, Prusiner SB (2006) Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA 103: 19105–19110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann S (2005) Prion propagation in cell culture. Methods Mol Biol 299: 227–234 [DOI] [PubMed] [Google Scholar]
- Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C (2010) Darwinian evolution of prions in cell culture. Science 327: 869–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mahal SP, Demczyk CA, Weissmann C (2011) Mutability of prions. EMBO Rep (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahal SP, Baker CA, Demczyk CA, Smith EW, Julius C, Weissmann C (2007) Prion strain discrimination in cell culture: the cell panel assay. Proc Natl Acad Sci USA 104: 20908–20913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahal SP, Browning S, Li J, Suponitsky-Kroyter I, Weissmann C (2010) Transfer of a prion strain to different hosts leads to emergence of strain variants. Proc Natl Acad Sci USA 107: 22653–22658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallucci GR, Ratte S, Asante EA, Linehan J, Gowland I, Jefferys JG, Collinge J (2002) Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J 21: 202–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K, Emmerling K, Manuelidis L (2011) Replication and spread of CJD, kuru and scrapie agents in vivo and in cell culture. Virulence 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazor KE et al. (2005) Immunodetection of disease-associated mutant PrP, which accelerates disease in GSS transgenic mice. EMBO J 24: 2472–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Harris DA, Vilette D, Laude H, Frobert Y, Grassi J, Casanova D, Milhavet O, Lehmann S (2000) Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J Virol 74: 320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D, Scott MR, Groth D, Williamson RA, Burton DR, Cohen FE, Prusiner SB (2001a) Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci 10: 854–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz D et al. (2001b) Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412: 739–743 [DOI] [PubMed] [Google Scholar]
- Piro JR, Harris BT, Nishina K, Soto C, Morales R, Rees JR, Supattapone S (2009) Prion protein glycosylation is not required for strain-specific neurotropism. J Virol 18: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB (1991) Molecular biology of prion diseases. Science 252: 1515–1522 [DOI] [PubMed] [Google Scholar]
- Prusiner SB et al. (1990) Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63: 673–686 [DOI] [PubMed] [Google Scholar]
- Richt JA et al. (2007) Production of cattle lacking prion protein. Nat Biotechnol 25: 132–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BE, Duennwald ML, Wang H, Chung C, Lopreiato NP, Sweeny EA, Knight MN, Shorter J (2009) A synergistic small-molecule combination directly eradicates diverse prion strain structures. Nat Chem Biol 5: 936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein R, Deng H, Race RE, Ju W, Scalici CL, Papini MC, Kascsak RJ, Carp RI (1992) Demonstration of scrapie strain diversity in infected PC12 cells. J Gen Virol 73: 3027–3031 [DOI] [PubMed] [Google Scholar]
- Safar JG, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB (1998) Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4: 1157–1165 [DOI] [PubMed] [Google Scholar]
- Sawaya MR et al. (2007) Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447: 453–457 [DOI] [PubMed] [Google Scholar]
- Schatzl HM, Laszlo L, Holtzman DM, Tatzelt J, DeArmond SJ, Weiner RI, Mobley WC, Prusiner SB (1997) A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol 71: 8821–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telling GC, Scott M, Mastrianni J, Gabizon R, Torchia M, Cohen FE, DeArmond SJ, Prusiner SB (1995) Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83: 79–90 [DOI] [PubMed] [Google Scholar]
- Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, Mastrianni J, Lugaresi E, Gambetti P, Prusiner SB (1996) Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274: 2079–2082 [DOI] [PubMed] [Google Scholar]
- Tuzi NL et al. (2008) Host PrP glycosylation: a major factor determining the outcome of prion infection. PLoS Biol 6: e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzaban S, Friedlander G, Schonberger O, Horonchik L, Yedidia Y, Shaked G, Gabizon R, Taraboulos A (2002) Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41: 12868–12875 [DOI] [PubMed] [Google Scholar]
- Vorberg I, Raines A, Story B, Priola SA (2004) Susceptibility of common fibroblast cell lines to transmissible spongiform encephalopathy agents. J Infect Dis 189: 431–439 [DOI] [PubMed] [Google Scholar]
- Wang F, Wang X, Yuan CG, Ma J (2010) Generating a prion with bacterially expressed recombinant prion protein. Science 327: 1132–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H et al. (2008) Direct and selective elimination of specific prions and amyloids by 4,5-dianilinophthalimide and analogs. Proc Natl Acad Sci USA 105: 7159–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, Prusiner SB (1987) Distinct prion proteins in short and long scrapie incubation period mice. Cell 51: 651–662 [DOI] [PubMed] [Google Scholar]