Abstract
It has been proposed that γ-protocadherins (Pcdh-γs) are involved in the establishment of specific patterns of neuronal connectivity. Contrary to the other Pcdh-γs, which are expressed in the embryo, Pcdh-γC5 is expressed postnatally in the brain, coinciding with the peak of synaptogenesis. We have developed an antibody specific for Pcdh-γC5 to study the expression and localization of Pcdh-γC5 in brain. Pcdh-γC5 is highly expressed in the olfactory bulb, corpus striatum, dentate gyrus, CA1 region of the hippocampus, layers I and II of the cerebral cortex, the molecular layer of the cerebellum. Pcdh-γC5 is expressed in both neurons and astrocytes. In hippocampal neuronal cultures, and in the absence of astrocytes, a significant percentage of synapses, more GABAergic than glutamatergic, have associated Pcdh-γC5 clusters. Some GABAergic axons show Pcdh-γC5 in the majority of their synapses. Nevertheless, many Pcdh-γC5 clusters are not associated with synapses. In the brain, a significant number of Pcdh-γC5 clusters are located at contact points between neurons and astrocytes. Electron microscope immunocytochemistry of the rat brain shows that i) Pcdh-γC5 is present in some GABAergic and glutamatergic synapses both pre- and postsynaptically; ii) Pcdh-γC5 is also extrasynaptically localized in membranes and in cytoplasmic organelles of neurons and astrocytes; and iii) that Pcdh-γC5 is also localized in perisynaptic astrocyte processes. The results support the notion that i) Pcdh-γC5 plays a role in synaptic specificity and/or synaptic maturation, and ii) that Pcdh-γC5 is involved in neuron-neuron synaptic interactions and in neuron-astrocyte interactions, including perisynaptic neuron-astrocyte interactions.
Keywords: astrocytes, synapse formation, glia, GABAergic synapse, glutamatergic synapse, protocadherin
INTRODUCTION
Clustered protocadherins (Pcdhs) belong to the cadherin superfamily of cell adhesion proteins (Redies et al., 2000; Frank and Kemler, 2002; Junghans et al., 2005; Morishita and Yagi, 2007; Shapiro et al., 2007; Yagi, 2008). Clustered Pcdhs are predominantly expressed in the CNS and it has been proposed that they are involved in the recognition between pre-and post-synaptic contacts and in the establishment of specific patterns of neuronal connectivity (Kohmura et al., 1998; Shapiro and Colman, 1999; Wang et al., 2002; Kallenbach et al., 2003; Phillips et al., 2003; Esumi et al., 2005; Frank et al., 2005; Kaneko et al., 2006). In mammals, the genes for the clustered Pcdhs are organized in three families (Pcdh-α, β and γ), which are arranged in tandem, clustered in a small genome locus on a single chromosome (Wu and Maniatis, 1999; Wu et al., 2001; Wu, 2005). In human, rat and mouse, the Pcdh-γ gene cluster contains 22 variable exons, being the Pcdh-γC5 exon located at the downstream end of the Pcdh-γ cluster. Each variable exon combines, by cis-splicing of the mRNA, with three downstream constant exons that encode the C-terminus, which is common to the 22 members of the Pcdh-γ family. Each variable exon encodes six extracellular cadherin repeats, the transmembrane domain and the proximal moiety of the cytoplasmic domain. The three constant exons encode the distal (C-terminus) moiety of the cytoplasmic domain. Supplementary Fig. S1 shows a diagram of the exon organization of the Pcdh-γ cluster and the topology of the Pcdh-γC5 protein in relationship to the mRNA splicing.
An antibody that recognizes all Pcdh-γs (Phillips et al., 2003) and several mouse knock out (KO) mutants, in which the whole Pcdh-γ family has been deleted (Wang et al., 2002; Hambsch et al., 2005; Weiner et al., 2005; Lefebvre et al., 2008; Garret and Weiner, 2009), have been used for studying the distribution and function of the combined Pcdh-γ family. Specific hybridization probes have also been used to study the brain distribution and developmental expression of the mRNAs of the individual members of the Pcdh-γ family by in situ hybridization and Northern blot analysis (Frank et al., 2005; Zou et al., 2007; Allen Brain Atlas http://www.brain-map.org). Only recently some specific antibodies for some members of the Pcdh-γ family and some tagged constructs for expression studies in host cells have been developed (Frank et al., 2005; Haas et al., 2005; Reiss et al., 2006; Fernández-Monreal et al., 2009). Nevertheless, the understanding of the expression and functional roles of the majority of the individual members of the Pcdh-γ family is unknown or very limited. In this study we have concentrated on Pcdh-γC5 not only because the expression and subcellular localization of this protein is unknown, but because unlike the other members of the Pcdh-γ family, which are expressed in the embryo, Pcdh-γC5 expression in the brain occurs after the second postnatal week, coinciding with the peak of synaptogenesis. This developmental time-course makes Pcdh-γC5 our selected candidate for studying the possible role Pcdh-γs play in the establishment of specific patterns of neuronal connectivity.
MATERIALS AND METHODS
Animals
All the animal protocols have been approved by the Institutional Animal Care and Use Committee of the University of Connecticut and followed the National Institutes of Health guidelines.
Antibodies and antibody characterization
Table I summarizes the primary antibodies used in this communication.
Table I.
Primary Antibodies
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| β2/3 | affinity-purified bovine GABAAR | Mouse monoclonal hybridoma supernatant, clone 62-3G1, made in our laboratory | 1:5 for EM postembedding |
| GAD | Purified rat GAD | Sheep polyclonal, gift from Dr. Irwin J. Kopin, Lot# 1440-4 | 1:500 for immunofluorescence |
| γ2 | AA 1–15 of rat GABAAR γ2 subunit | Guinea pig polyclonal, made in our laboratory | 1:1000 for immunofluorescence |
| gephyrin | Affinity-purified rat glycine receptor protein complex | Synaptic Systems, Cat# 147011, clone mAb7a, Lot# 147011/14, mouse monoclonal | 1:500 for immunofluorescence |
| GFAP | Bovine spinal cord homogenate | PharMingen, Cat# 60311D, clone 4A11, Lot# MO50728, mouse monoclonal | 1:600 for immunofluorescence |
| GLAST | Synthetic peptide from the carboxy-terminus of rat GLAST (QLIAQD NEPEKPVADSETKM) | Millipore, Cat# AB1782, Lot# 22061280, Guinea pig polyclonal | 1:2000 for immunofluorescence |
| GluR2 | Recombinant fusion protein TrpE-GluR2 (N-terminal portion, AA 175–430) | Millipore, Cat# MAB397, mouse monoclonal, clone 6C4, Lot# 19090047 | 1:100 for EM postembedding |
| MAP2 | Recombinant MAP2 protein | Novus, Cat# NB300-213, Lot# D1, Chicken polyclonal | 1:20000 for immunofluorescence |
| Pan-Pcdh -γ | AA 808–931 (C-terminal constant domain) of mouse Pcdh-γA1 | N e u r o M a b, C a t # 73–185, mouse monoclonal, clone N159/5, Lot# 437-3VA-24 | 1:15 for immunofluorescence |
| Pan-Pcdh -γA | AA 720–804 (variable cytoplasmic domain) of mouse Pcdh-γA3 | NeuroMab, Cat#73-178, mouse monoclonal, clone N144/32, Lot# 437-3VA-12 | 1:15 for immunofluorescence |
| Pcdh-γC5 | AA 1–14 (N-terminus variable domain) of rat Pcdh- γC5 | Rabbit polyclonal, made in our laboratory | 1:50 to 1:30 for immunofluorescence; 1:100 for light microscopy immunocytochemistry; 1:3 to 1:5 for EM postembedding; 1:75 to 1:150 for EM preembedding and 1:5 for immunoblot |
| PSD-95 | GST fusion protein encoding the full-length rat PSD-95 | Chicken polyclonal antiserum UCT-C1, gift from Dr. Randall S. Walikonis | 1:1000 for immunofluorescence |
| PSD-95 | Recombinant rat PSD-95 | Millipore, Cat# MAB 1596, Lot# LV1453199, mouse monoclonal, clone 6G6-1C9 | 1:100 for immunofluorescence |
| vGAT | Synthetic peptide (SLEGLIEAYRTNAED) of rat vGAT | Chemicon, Cat# AB5855, Lot# 24030142, Guinea pig polyclonal | 1:2000 for immunofluorescence |
| vGlut1 | Synthetic peptide (GATHSTVQPPRPPPPVRDY) of rat vGLUT1 | Chemicon, Cat# AB5905; Lot# 24080852, Guinea pig polyclonal | 1:10000 for immunofluorescence |
| S-100β | Purified bovine brain S-100β preparation | Sigma, Cat# S2532, Lot# 085K4880, clone SH-B1, mouse monoclonal | 1:2000 for immunofluorescence |
A novel rabbit (Rb) antibody was raised to a synthetic peptide of the deduced amino acid sequence of the rat Pcdh-γC5 (GenBank accession number GQ131870). The antibody Pcdh-γC5 to amino acids 1–14 (QLRYSVVEESEPGT-C) of the extracellular variable region recognizes a peptide unique to Pcdh-γC5. The synthetic peptide was covalently coupled via cysteine, to keyhole limpet hemocyanin and injected into a New Zealand rabbit in complete Freund’s adjuvant for the first immunization and incomplete Freund’s adjuvant for all subsequent immunizations. Sera were collected after four months of immunizations and the Pcdh-γC5 antibody was affinity-purified on the immobilized antigenic peptide. For all light and electron microscopy (EM) immunocytochemistry, immunoblot and immunofluorescence experiments we used the antibody affinity-purified on immobilized antigenic peptide. Antibody specificity was determined by i) sequence specificity as determined from Entrez protein database; ii) ELISA, showing binding to the antigenic peptide; iii) immunobloting of brain tissue, showing specific immunoreactivity with a 120kD peptide band; iv) displacement of immunoreactivity by antigenic peptide in both immunoblots and light microscopy immunocytochemistry; v) the presence of very strong Pcdh-γC5 immunofluorescence in HEK293 cells transfected with non-tagged Pcdh-γC5 or with Pcdh-γC5 tagged at the C-terminus with EGFP in comparison with the background immunofluorescence of the non-transfected cells; vi) the absence of significant Pcdh-γC5 immunofluorescence of HEK293 cells transfected with Pcdh-γC3-EGFP or Pcdh-α4–EGFP over the background fluorescence of non-transfected cells; vii) in cultured hippocampal neurons, the anti-Pcdh-γC5 immunofluorescent clusters are also immunopositive with a mouse (Ms) mAb anti-Pan-Pcdh-γ antibody in double-label experiments (Supplementary Fig. S3); viii) the distribution of the immunoreactivity in various brain regions is consistent with the distribution of Pcdh-γC5 mRNA revealed by in situ hybridization (see below); and ix) the reported postnatal developmental expression of Pcdh-γC5 mRNA, which is unique among Pcdhs, corresponds to the postnatal development of the immunoreactivity in the brain (see below).
The guinea pig (GP) anti-rat γ2 (to amino acids 1–15 QKSDDDYEDYASNKT) GABAAR subunit antibody was raised in our laboratory and affinity-purified on the corresponding immobilized peptide antigen. Triple-label immunofluorescence in cultured hippocampal neurons with this antibody shows clusters that are highly colocalized with the clusters immunolabeled with antibodies to other GABAAR subunits such as Rb anti-γ2, Rb anti-α1, Rb anti–α2 and Ms monoclonal antibody (mAb) anti-β2/3. Immunofluorescence is blocked by the antigenic peptide. In addition, the clusters show apposition to GABAergic presynaptic glutamate decarboxylase (GAD)-containing terminals (Christie et al., 2002a, 2002b; Christie and De Blas, 2003; Charych et al., 2004a, 2004b; Li et al., 2005a; Serwanski et al., 2006; Li et al., 2007; Yu and De Blas, 2008; Yu et al. 2008; Li et al., 2009).
The Ms mAb anti-β2/3 GABAAR subunit (clone 62-3G1) was also raised in our laboratory to the affinity-purified bovine GABAAR (De Blas et al., 1988; Vitorica et al., 1988). This mAb recognizes an N-terminal epitope that is common to the rat β2 and β3 subunits but not present in the β1 subunit (Ewert et al., 1992). In immunoblots of rat brain membranes or affinity-purified GABAARs, this antibody specifically recognizes a 55–57 kD protein band corresponding to the β2/β3 GABAAR subunits (Vitorica et al., 1988; Moreno et al., 1994; Miralles et al., 1999). Immunoreactivity in immunoblots and immunocytochemistry was blocked by affinity-purified GABAARs (De Blas et al., 1988; Vitorica et al., 1988) Triple-label immunofluorescence of cultured hippocampal neurons shows high colocalization of immunolabeled β2/3 clusters with that of other GABAAR subunits in apposition to GAD-containing GABAergic presynaptic terminals (Christie et al., 2002a, 2002b; Riquelme et al., 2002; Christie and De Blas, 2003; Serwanski et al., 2006; Yu et al., 2007).
The monoclonal mouse anti-gephyrin (mAb 7a) to affinity-purified rat glycine receptor protein complex (Pfeiffer et al., 1984) was purchased from Synaptic Systems (Gottingen, Germany, Catalog number 147011, clone mAb7a, Lot 147011/14). In immunoblots of purified glycine receptor, this mAb binds to a 93 kD protein band and, to a lesser extent, to a 48 kD protein band (Pfeiffer et al., 1984). Immunoreactivity is absent in a gephyrin knockout mouse but not in the wild-type mouse (Feng et al., 1998). This antibody has been thoroughly characterized and widely used in the literature to localize gephyrin by light microscopy and EM immunocytochemistry in the rat brain and in neuronal cultures (Kirsch and Betz, 1993, 1995; Giustetto et al., 1998; Kneussel et al., 1999; Christie et al., 2002a; Christie and De Blas, 2003; Christie et al., 2006).
The GP anti-vGlut1 (to synthetic peptide GATHSTVQPPRPPPPVRDY of rat vGLUT1) was from Chemicon (Temecula, CA; catalog number AB5905; Lot number 24080852). In immunoblots of a synaptic membrane fraction from rat cerebral cortex, this antiserum recognizes a ~60 kD protein band corresponding to vGlut1, which is a marker for glutamatergic nerve terminals (Melone et al., 2005; Panzanelli et al., 2007). Double-label immunofluorescence tests done in our laboratory have shown identical labeling in both rat brain sections and cultured hippocampal neurons of this antibody and a Rb anti-vGlut1 (Synaptic system, catalogue 135002).
The Ms mAb to the GluR2 subunit of the AMPA receptor (to amino acids 175–430, catalog number MAB397, clone 6C4, lot number 19090047) was from Chemicon. This antibody specifically recognizes a 102 kD protein in immunoblots of rat brain and HEK293 cells transfected with GluR2 but not other AMPA/kainate GluR receptor subunits (Vissavajjhala et al., 1996). In the wild-type mouse, this antibody specifically reacts with the GluR2 protein band in immunoblots and shows immunoreactivity in brain tissue by immunocytochemistry. The GluR2 knockout mouse shows no reactivity in the same assays (Sans et al., 2003, Medvedev et al., 2008).
The sheep anti-GAD (lot number 1440-4) was a gift of Dr. Irwin J. Kopin (NINDS, Bethesda). This antibody to purified rat GAD recognizes a 65 kDa protein in rat brain immunoblots. The antibody precipitated GAD from rat brain and detected purified GAD in crossed immunoelectrophoresis (Oertel et al., 1981a, 1981b). The GP anti-vGAT (vesicular GABA transporter) antiserum to the synthetic peptide SLEGLIEAYRTNAED of rat vGAT was from Chemicon (catalog number AB5855, lot number 24030142). The specificity has been tested by immunofluorescence in brain sections and in culture showing a staining pattern similar to that of other vGAT antisera and by adsorption of the antiserum with the immunogen peptide. (Li et al., 2005b; Panzanelli et al., 2007). We have previously shown that the aforementioned anti-GAD and anti-vGAT immunoreactivities specifically colocalize at GABAergic presynaptic terminals (Li et al., 2005b) and that the immunoreactivity is apposed to that of postsynaptic GABAARs and gephyrin (Christie et al., 2002a, 2002b; Christie and De Blas, 2003).
The Ms mAb to recombinant rat PSD-95 protein was from Millipore (Billerica, MA; catalog number MAB1596, clone 6G6-1C9, Lot LV1453199). This antibody reacts with a 95 kDa protein in immunoblots of rat brain (Su et al., 2007). In cultured hippocampal neurons this antibody reacts with a protein that colocalizes with NR1 and NR2A (Fong et al., 2002). The chicken anti-PSD-95 antibody (antiserum UCT-C1, to the GST fusion protein encoding the full-length rat PSD-95) was a gift from Dr. Randall Walikonis from our department. The chicken anti-PSD antibody specifically reacts with a 95kD protein in rat brain immunoblots. In cultured hippocampal neurons, the immunoreactivity localizes at glutamatergic synapses (Tyndall and Walikonis, 2006; Moon et al., 2009). We did specificity tests of the chicken anti-PSD in hippocampal cultures by triple-label immunofluorescence with the aforementioned Ms mAb anti-PSD-95 antibody and the GP anti-vGlut1 (see below). The Ms and chicken anti-PSD-95 antibodies gave identical labeling of PSD-95 clusters apposed to vGlut1 puncta.
The Ms mAb to Pan-Pcdh-γ (Clone N159/5, to amino acids 808–931 of the constant cytoplasmic domain of mouse Pcdh-γA1 that is shared by all 22 Pcdh-γs) and the Ms mAb to Pan-Pcdh-γA (clone N144/32, to amino acids 720–804 of the variable cytoplasmic domain of mouse Pcdh-γA3), were from NeuroMab (Davis, CA). In immunoblots, both antibodies recognize a 100kD protein band in rat and mouse brain but not in the Pcdh-γ knockout mouse. The specificity of the antibodies was also tested by showing specific immunoreactivity with the corresponding protein bands in immunoblots of COS cells transfected with i) GFP-tagged Pcdh-γA3, Pcdh-γB2 or Pcdh-γC4 constructs, for mAb Pan-Pcdh-γ antibody, and ii) GFP-tagged Pcdh-γA3 or Pcdh-γA4, for mAb Pan-Pcdh-γA antibody (manufacturer’s technical information).
The Ms mAb anti-glial fibrillary acidic protein (GFAP) was from Pharmingen (to a bovine spinal cord homogenate, San Diego, CA; catalog number 60311D, lot number MO50728; clone 4A11). This antibody specifically recognizes a 50kD protein in immunoblots of extract from mixed glial-neuronal cultures but not from pure neuronal cultures. This antibody has been used for the identification of astrocytes (Fried et al., 2004).
The Ms mAb anti-S-100 (β-subunit) to a purified bovine brain S-100β preparation, was from Sigma (St. Louis, MO; catalog number S2532, clone SH-B1). It recognizes specifically a 10kD protein band in immunoblots of lumbar spinal cord from rat or wild-type mouse but not from a S-100β knockout mouse (Tanga et al., 2006). This antibody shows immunolabeling of spinal cord sections of the wild-type but not the knockout mouse.
The GP anti-glial glutamate transporter (GLAST) to the synthetic peptide QLIAQDNEPEKPVADSETKM of the carboxy-terminus of rat GLAST was from Millipore (catalog number AB1782; lot number 22061280). In immunoblots of glial cultures from rat cortex (Figiel and Engele, 2000) or from rat brain striatum (Chung et al., 2008), this antibody specifically recognizes a 65 kD protein band
The chicken anti-MAP2 (to recombinant MAP2 protein) was from Novus Biologicals (Littleton, CO; catalog number NB300-213). The specificity of this antibody has been tested using immunofluorescence with mixed glia-neuron cultures. The perikarya and dendrites of neurons are strongly and specifically labeled with the MAP2 antibody, while the axons of the neurons and the processes of all other cell types in these cultures (astrocytes, oligodendrocytes, microglia, endothelia and fibroblasts) are not labeled (manufacturer’s technical information). In addition, we have tested the specificity of this antibody by showing in double-label experiments, identical immunofluorescence to that of Ms mAb to MAP2 (Sigma. St. Louis, MO; catalog number M9942, clone HM-2, lot number 037K4805).
Fluorophore-labeled fluorescein isothiocyanate (FITC), Texas Red or aminomethylcoumarin (AMCA) species-specific anti-IgG antibodies were made in donkey (Jackson ImmunoResearch Laboratories, West Grove, PA) and used for immunofluorescence in cell cultures. For confocal microscopy the secondary antibodies were made in donkey (labeled with Cy3 or Cy5 from Jackson ImmunoResearch Laboratories) or in goat (labeled with Alexa 488 from MP Biomedicals, Solon, OH). The colloidal gold-labeled (10 nm diameter) goat anti-mouse secondary antibody was from ICN (Irvine, CA) and the colloidal gold-labeled, goat anti-Rb IgG (18 nm diameter) secondary antibody was from Jackson ImmunoResearch Laboratories.
Preparation of rat brain subcellular fractions
All steps for preparation of various rat brain fractions were performed at 4°C. For homogenate preparation, forebrains (including the telencephalon but not the diencephalon) from Sprague Dawley (SD) female rats of various ages were homogenized with a glass/Teflon homogenizer (1g of tissue/10ml of 10% sucrose, 50mM Tris-HCl, 1mM phenylmethylsulfonyl fluoride, pH 7.4) containing a protease inhibitor cocktail (Roche, Indianapolis, IN). The homogenate was centrifuged for 10 min at 1,000×g to eliminate nuclei and cell debris and the supernatant was used for immunobloting to investigate Pcdh-γC5 expression during rat brain development.
For brain membrane preparation, homogenates from the forebrain (telencephalon) of adult female rats were centrifuged for 10 min at 1,000×g and the supernatant was centrifuged at 100,000×g for 1hr. The pellet was suspended in 5mM Tris-HCl pH 7.4 and subjected to homogenization in a glass Dounce homogenizer, followed by centrifugation at 12,000×g for 30 min and suspension of the pellet in 50mM Tris-HCl, pH 7.4.
The preparation of crude synaptosomal (P2), purified synaptosomal (P2B) and “one triton” postsynaptic density (PSD), type-I PSD glutamatergic and type-II PSD GABAergic fractions was described elsewhere (Li et al., 2007). The “one triton” PSD fraction was prepared from purified synaptosomes as described elsewhere (Li et al., 2007). For the preparation of type-I and type-II PSD fractions, the “One Triton PSD” pellet was suspended in 0.32 M sucrose, centrifuged at 201,800× g for 16 hours through a continuous sucrose gradient (0.32–2.0 M) and fractionated as described elsewhere (Li et al., 2007). Sucrose fraction #6 (ρ = 1.10 g/ml) is enriched in GABAergic type-II PSDs whereas sucrose #10 (ρ = 1.20 g/ml) is enriched in glutamatergic type-I PSDs (Li et al., 2007). The protein concentrations of different brain fractions were measured by using Micro BCA Protein Assay Reagent Kit (Pierce, Rockford, IL).
Immunoblots for Figure 1K were done according to the procedure of De Blas and Cherwinski (1983). For Figs 1L and 2A the immunoreactive protein bands were visualized with a peroxidase-conjugated secondary antibody followed by a chemiluminiscence reaction (SuperSignal West Pico Trial Kit, Thermo Scientific, Rockford, IL) and exposure to CL-Xposure Film (Pierce, Rockford, IL). Quantification of the intensity of the bands was done with Quantity One software (Bio-Rad, Hercules, CA).
Fig. 1. Immunocytochemical localization of Pcdh-γC5 in the rat brain and immunoblots of brain fractions.
(A) Immunocytochemistry of a parasagittal section of a P30 rat brain with anti-Pcdh-γC5. The Pcdh-γC5 is highly expressed in the olfactory bulb, olfactory tubercle, cerebral cortex, corpus striatum, dentate gyrus, CA1 region of the hippocampus, cerebellum, medial cerebellar nucleus, substantia nigra reticulata and pontine nuclei. (B) Olfactory bulb. (C) Layers I – III of the cerebral cortex. (D) Corpus Striatum. (E) Cerebellum. (F) Hippocampus. (G) The CA2-CA3 region of the hippocampus. (H) The CA1 region of the hippocampus. Note the accumulation of Pcdh-γC5 immunoreactivity in both the neuropil and perikaryon of pyramidal cells. (I) High magnification of the stratum radiatum (SR) of CA1. Note the granular aspect of the immunostaining in the neuropil (arrows). (J) Dentate gyrus and hilus. Scale bar = 3mm in A, 67μm in B, 50μm in C, E and J, 100μm in D, G and H, 250μm in F and 10μm in I. (K) Immunoblot of a rat brain membrane fraction shows that Pcdh-γC5 antibody recognizes a ~120kD protein band that is displaceable by the antigenic peptide (50μg/ml). (L) Immunoblot of various rat brain fractions with Pcdh-γC5 antibody. The 120kD Pcdh-γC5 protein band is present in various brain fractions including the crude synaptosomal (P2), microsomal (P3), synaptosomal (P2B), “One triton” postsynaptic density (PSD), type-II (GABAergic) PSD and type-I (glutamatergic) PSD fractions. The amount of protein loaded in each lane was 4.3μg. The abbreviations used in the panels are: Cerebellum (CB), cerebral cortex (CC), corpus striatum (St), dentate gyrus (DG), external plexiform layer (EP), granule cell layer (GR), glomerular layer (GL), hilus (H), hippocampus (HP), medial cerebellar nucleus (Med), molecular layer (ML), olfactory bulb (OB), olfactory tubercle (Tu), pontine nuclei (Pn), Purkinje cell layer (PK), stratum oriens (SO), stratum pyramidale (SP), stratum radiatum (SR) and substantia nigra reticulata (SNR).
Fig. 2. Pcdh-γC5 expression during rat brain development.
(A) Immunoblots of rat forebrain (telencephalon) homogenates of various ages (42μg of protein per lane). The Pcdh-γC5 antibody recognizes a 120kD protein band (arrow), whose immunoreactivity is displaced by the antigenic peptide (50μg/ml). Between P0 and P7 two proteins of higher Mr (arrowhead) are also recognized by the antibody. However this immunoreactivity is non-specific because the antigenic peptide does not block it and the protein band disappears after P7. An anti-actin antibody showed no significant difference in the protein load in the various lanes. (B) Quantification by densitometry of the Pcdh-γC5 protein band. (C) Light microscopy immunocytochemistry shows the expression of Pcdh-γC5 during the postnatal development of the rat brain. Little or no Pcdh-γC5 is expressed at P0 or P7. At P14 and onwards the brains showed Pcdh-γC5 immunoreactivity, which was blocked by the antigenic peptide (50μg/ml, only shown in this figure for P30). (D–K) Light microscopy immunocytochemistry. Postnatal development of Pcdh-γC5 immunoreactivity in the cerebellum. The single asterisk labels the molecular layer, the circle labels the granule layer, the arrow points to the Purkinje cell layer and the double asterisk labels the deep cerebellar nuclei. (L–S) Light microscopy immunocytochemistry. Postnatal development of Pcdh-γC5 immunoreactivity in the olfactory bulb. For abbreviations in L–S, see the legend to Fig. 1. The displacement of the immunoreaction by the antigenic peptide in cerebellum and olfactory bulb is shown in panels H–K and P–S respectively. Scale bar = 5mm in C; 550μm in D–K and 230 μm in L–S.
Immunocytochemistry of rat brain sections
This procedure has be described elsewhere (De Blas, 1984; Charych et al., 2004a, 2004b). Briefly SD rats of the specified ages were anesthetized (with 60 mg/kg ketamine-HCl, 8mg/kg xylazine, 2mg/kg acepromazine maleate) followed by perfusion through the ascending aorta with PB (27mM NaH2PO4, 92mM Na2HPO4, pH 7.4) and 4% PLP fixative (4% paraformaldehyde, 1.37% lysine, 0.21% sodium periodate in 0.1M phosphate buffer, pH 7.4). Brains were cryoprotected, frozen and sectioned with a freezing microtome. Parasagittal brain sections (thickness: 70μm thick for P0 and P7, 40μm thick for P14 and P21 and 25μm for other ages) were incubated with the anti-Pcdh-γC5 antibody in 0.3% Triton X-100 in PB at 4°C overnight. The washed sections were incubated with biotinylated anti-rabbit IgG and avidin-biotin-horseradish peroxidase complex (ABC procedure, Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA) in 1% normal goat serum. To visualize the reaction, sections were incubated with 3-3′ diaminobenzidine tetrahydrochloride (DAB) in the presence of 0.03% cobalt chloride, 0.03% nickel ammonium sulfate and 0.01% H2O2. Sections were then washed and mounted on gelatin-coated glass slides. No tissue immunolabelling was detected when the primary antibody was incubated with 25 μg/ml of antigenic peptide or when the primary antibody was omitted.
Cell cultures
Hippocampal neuronal cultures were prepared according to Goslin et al. 1998 as described elsewhere (Christie et al., 2002a, 2002b; Christie and De Blas, 2003). Briefly, dissociated neurons from embryonic day 18 (E18) Sprague-Dawley (SD) rat hippocampi were plated at low density (3000–8000 cells per 18mm diameter coverslip) and maintained in glial cell conditioned medium up to 21 days. Astrocytes are seldom found in these neuronal cultures (Supplementary Fig. S2).
Astrocyte cultures were prepared from the cerebral cortex of postnatal day 0 (P0) SD rats as described by Goslin et al. (1998). Briefly, glial cells were plated in glial culture medium (DMEM with 0.6% D-glucose, 26 mM NaHCO3, and 10% horse serum) in a 5% CO2 atmosphere, at 37°C for about 9 days until confluent. Cells were harvested after treatment with trypsin/EDTA followed by incubation with horse serum. Cells were collected after centrifugation and stored in liquid nitrogen in 10% DMSO/glial culture medium. After thawing, cells were plated on poly-L-lysine coated glass coverslips and maintained on glial culture medium for four days followed by immunofluorescence.
In utero electroporation
Embryonic rat brain gene transfer using in utero electroporation was done as described by Li et al. (2005b). Briefly, pregnant Wistar rats at 14 daygestationwere anesthetized as described above and a laparotomy was performed. The uterine horns were gently pulled out and 1–3 μl of a sterile mixture of 0.5 μg /μl of pLZRS-CA-gapEGFP plasmid (gift from Drs. A. Okada and S. K. McConnell, Stanford University, Stanford) and Fast Green (2 mg/ml; Sigma, St. Louis) were microinjected by pressure with a picospritzer through the uterine wall into the lateral ventricles of the embryos with a sterile glass capillary pipette. Electroporation was carried out by a brief (1–2 msec) discharge of a 500-μF capacitor charged to 50–100 V with a power supply. The voltage pulse was discharged with a pair of sterile gold/copper alloy oval plates (1×0.5 cm) after gently pinching the head of each embryo through the uterus. After electroporation, the uterus was returned to the body cavity and the incision was closed sewing it up with sterile surgical suture. The pups were sacrificed at 35 days after birth.
Immunofluorescence
Immunofluorescence of fixed hippocampal and glial cultures was done as described elsewhere (Christie et al., 2002a, 2002b; Christie et al., 2006). Briefly, cells on glass coverslips were fixed in 4% paraformaldehyde, 4% sucrose in PBS (all the reagents were prepared in PBS) for 15 minutes. The free aldehyde groups were quenched with 50mM NH4Cl in PBS for 10min. Permeabilization was done with 0.25% Triton X-100 for 5 minutes followed by incubation with 5% normal donkey serum (NDS) in 0.25% Triton X-100 for 30 min. The coverslips were incubated overnight at 4°C with a mixture of primary antibodies raised in different species, in the presence of 0.25% Triton X-100, followed by incubation with a mixture of species-specific secondary anti-IgG antibodies raised in donkey and conjugated to Texas Red, FITC or AMCA fluorophores in 0.25% Triton X-100 at room temperature for 1 hr. The coverslips were mounted on glass slides with Prolong Gold anti-fade mounting solution (Invitrogen, Eugene, OR)
For immunofluorescence of brain slices, free-floating sections from P83 rat brains (or P35 for brains subjected to in utero electroporation), were prepared as described above for immunocytochemistry. Brain sections were incubated with 2% normal goat serum (NGS), 0.3% Triton X-100 in PB for 1hr at RT followed by incubation with a mixture of primary antibodies raised in different species in 1% NGS, 0.3% Triton X-100 in PB at 4°C overnight. After washes with PB, the sections were incubated with a mixture of Cy3-labeled donkey anti-Rb, Alexa Fluor 488-labeled goat anti-Ms (or anti-chicken) and Cy5-labeled donkey anti-GP (or anti-Ms) IgG secondary antibodies for 1.5hrs at RT. After washes with PB the sections were mounted on gelatin-coated glass slides with Prolong Gold anti-fade mounting solution and analyzed by laser confocal microscopy. Pepsin treatment of the fixed free floating sections (0.15 mg/ml in 0.2N HCl at 37 °C for 10 min) prior to the aforementioned treatment with NGS and Triton X-100, aiming to facilitate the access of the antibody to the antigen, did not affect the localization of Pcdh-γC5 immunoreactivity in the brain when compared with sections not treated with pepsin.
Image acquisition, analysis and quantification
Fluorescence images of neuronal cultures were collected using a Nikon Plan Apo 60×/1.40 objective (a Nikon Plan Fluor 20×/0.45 objective was used for Fig.S2 A and B) on a Nikon Eclipse T300 microscope with a Photometrics CoolSNAP HQ2 CCD camera, driven by IPLab 4.0 (Scanalytics, Rockville, MD) acquisition software. Brain section immunofluorescence images were acquired on a Leica TCS SP2 laser confocal microscope using a HCX PL Apo 100×/1.40-0.7 oil CS objective lens and a pinhole set at 1Airy unit. Figures 8 and 9 show single optical sections (0.1 μm thick) and Fig. 7 shows maximal projections of stacks of 10 consecutive images (each 0.1 μm thick). For qualitative analysis, images were processed and merged for color colocalization using Photoshop 7.0 (Adobe, San Jose, CA) adjusting brightness and contrast as described elsewhere (Christie et al., 2002).
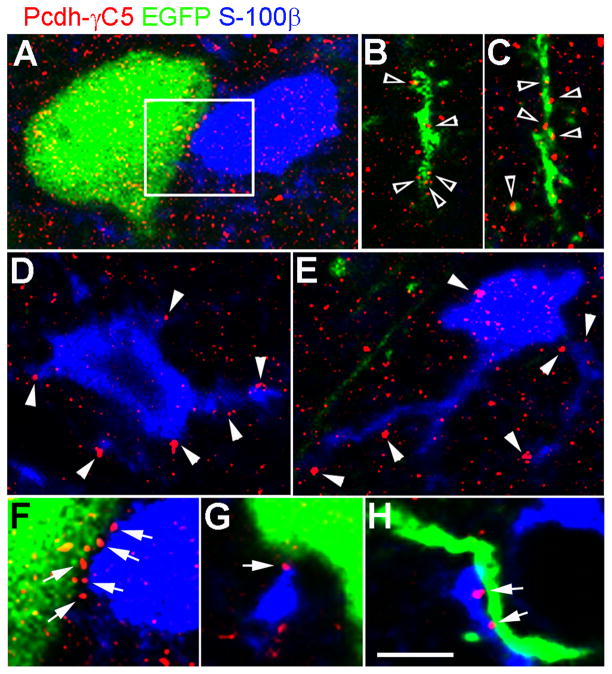
Fig. 8. In the intact brain Pcdh-γC5 forms clusters at contact point between neurons and astrocytes.
(A–H) Laser confocal microscopy of the cerebral cortex of a P35 rat brain in which a limited population of neurons are labeled by EGFP (green) by in utero electroporation. Single optical section 0.1μm thick. The tissue was triple-label with EGFP fluorescence (green) and antibodies to Pcdh-γC5 (red) and S-100β (blue). Pcdh-γC5 forms clusters on the neuronal soma (A) and dendrites (empty arrowheads in B and C), astrocyte cell body and processes (D and E), at contact points between neuron and astrocyte (arrows in F, G and H). Note the presence Pcdh-γC5 clusters at the edge of astrocytes (arrowheads in D and E). The scale bar = 5 μm in panels A to E and 2.8 μm in panels F to H.
Fig. 9. Synaptic and perisynaptic Pcdh-γC5 clusters in the intact brain.
Laser confocal microscopy of the CA1 region of the hippocampus. (A–B) Some Pcdh-γC5 clusters colocalize (arrows) with the GABAergic synaptic markers gephyrin and vGAT. (C–D) Some Pcdh-γC5 clusters (arrows) are adjacent to the GABAergic synaptic markers vGAT and gephyrin (arrowheads). (E–F) Some Pcdh-γC5 clusters colocalize (arrows) with the glutamatergic synaptic markers PSD-95 and vGlut1. (G–H) Some Pcdh-γC5 clusters (arrows) are adjacent to the glutamatergic synaptic markers PSD-95 and vGlut1 (arrowheads). (I–J) Some Pcdh-γC5 clusters (arrows) are on S-100β+ astrocytic processes adjacent to vGAT puncta (arrowheads). (K–L) Some Pcdh-γC5 clusters (arrows) that colocalize (K) or are adjacent to vGAT puncta (arrowhead, L) are not associated with the astrocytic marker S-100β. (M–N) Some Pcdh-γC5 clusters (arrows) adjacent to the glutamatergic synaptic marker PSD-95 (arrowheads) are on S-100β+ astrocytic processes. (O–P) Some Pcdh-γC5 clusters (arrows) that colocalize (O) or are adjacent to PSD-95 clusters (arrowhead, P) are not associated with the astrocyte marker S-100β. Scale bar = 1μm.
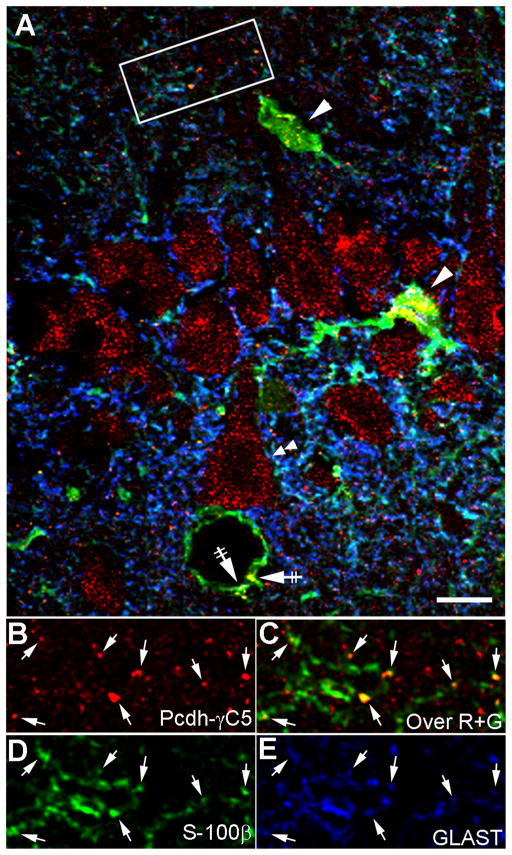
Fig. 7. In the intact brain, Pcdh-γC5 clusters are present in neurons and astrocytes.
Triple-label laser confocal microscopy of the CA1 region of the hippocampus in the P83 rat brain with Rb anti-Pcdh-γC5 (red), mouse anti-S-100β (green) and GP anti-GLAST (blue). (A) Pcdh-γC5 clusters are present in the soma of neurons (double arrowheads), cell bodies of astrocytes (arrowheads) and the astrocytic end-feet contacting blood vessels (crossed arrows). (B–E) The high magnification image of the inset box in panel A shows that Pcdh-γC5 clusters are present in astrocytic processes (arrows) revealed with antibodies to S-100β and GLAST. The scale bar = 10 μm in A and 6μm in B–E.
For quantification in hippocampal cultures of cluster density (Pcdh-γC5, nonsynapticγ2, PSD95 and vGlut1) and colocalization, three independent immunofluorescence experiments were performed for each combination of antibodies. A total of 60 dendritic fields (86μm2 average) were analyzed from 30 neurons (10 randomly selected pyramidal neurons in each experiment). The maximum intensities of the fluorophore channel were normalized and the low intensity and diffuse non-clustered background fluorescence signal seen in the dendrites was subtracted. The total number of clusters counted was 2275 for Pcdh-γC5, 1092 for nonsynaptic γ2, 1267 for PSD-95 and 1263 for vGlut1. Cluster density was calculated as number of clusters per 100 μm2 of dendritic surface. In these low density cultures, neurons have relatively low GABAergic innervation. For the quantification of the percentage of GABAergic synapses that have associated Pcdh-γC5 clusters, 30 randomly selected neurons (3 independent experiments, 10 neurons per experiment) were analyzed. In each neuron, 15 GABAergic synapses (GAD puncta+ andγ2 cluster+) were randomly selected and analyzed for the association of Pcdh-γC5 clusters. Thus, a total of 450 GABAergic synapses (GAD+ andγ2+) were analyzed. A cluster in a fluorescence channel was considered to co-localize with a cluster in the other channel when >66% of surface of one of the clusters overlapped with the other cluster. For the quantification of Pcdh-γC5 cluster size, 30 dendrites from 15 randomly selected neurons (2 dendrites/neuron, 5 neurons per experiment, 3 independent experiments) were analyzed. The size of 914 Pcdh-γC5 clusters was measured with IPLab 4.0 software. All the values were reported as mean ± standard error of the mean (SEM).
Preembedding EM immunocytochemistry
This procedure has been described elsewhere (Charych et al., 2004a, 2004b; Li et al., 2009). Briefly, 45-day-old SD female rats were anesthetized as described above and perfused through the aorta with PB followed by either 4% paraformaldehyde, 0.1% glutaraldehyde in PB, pH 7.4, fixative or PLP fixative (periodate/lysine/4% paraformaldehyde fixative, McLean and Nakane, 1974) as indicated above. 50μm thick parasagittal vibratome (Leica VT1000S, Vienna, Austria) sections were incubated with 3% normal goat serum in PBS at RT for 1 hr and then incubated with the Pcdh-γC5 antibody in PBS overnight at 4 °C followed by the ABC procedure described above. After development of the DAB reaction and washes, the tissue was incubated with 1% osmium tetroxide in PB at room temperature for 45 minutes and dehydrated by sequential incubations in 50%, 70%, 85%, and 95% ethanol 3 times each for 5min and in 100% ethanol 3 times for 15min at 4°C. The dehydrated sections were embedded in Embed 812 resin (Electron Microscopy Sciences, Hatfield, PA) between two aclar strips and polymerized at 60°C for 48hrs. Ultrathin sections (70–80 nm thick) were cut on a Leica Ultracut UCT (Leica Mikrosysteme GmbH, Wien, Austria). Sections were counterstained at RT with 2% uranyl acetate for 1 min and with lead citrate for 1 min.
Postembedding EM immunogold labeling and quantification
For all panels of Fig. 10, except G, the tissue preparation, freeze substitution and postembedding immunogold labeling were done as reported previously (Rubio and Wenthold, 1997; Riquelme et al., 2002; Charych et al., 2004a, 2004b; Li et al., 2005a; Serwanski et al., 2006; Rubio et al., 2008). Briefly, 70-day old SD female rats were anesthetized as described above and perfused with Ringer’s solution, pH 6.9 at room temperature quickly followed by 4% paraformaldehyde, 0.5% glutaraldehyde in 0.1M PB, pH 7.4. Vibratome sections (300–500μm thick) were cryoprotected with 2M sucrose in PB and plunge-frozen in liquid propane cooled by liquid nitrogen (−186 °C). Sections were incubated with 1.5% uranyl acetate in anhydrous methanol at −90°C for 30hrs and infiltrated with Lowicryl HM20 resin (Polysciences, Warrington, PA, USA) followed by polymerization with UV light for 72hrs in a freeze substitution instrument (Leica AFS, Vienna, Austria) in a temperature gradient (−45°C to 0°C). Sections (70–80 nm thick) were cut from the embedded tissue block and collected on 400-mesh gold-gilded nickel grids coated with a Coat-Quick ‘G’ pen (Daido Sangyo, Japan). Immunogold labeling was performed according to a double-sided immunoreaction procedure as described elsewhere (Riquelme et al., 2002). The tissue sections were incubated with either Rb Pcdh-γC5 antibody alone or a mixture of Rb Pcdh-γC5 and mouse mAb to β2/3 GABAAR subunit or to GluR2 AMPA receptor subunit, followed by incubation with a mixture of goat anti-rabbit and goat anti-mouse species-specific anti-IgG secondary antibodies labeled with colloidal gold particles of 18 and 10 nm diameter respectively. The tissue sections were counterstained with 2% uranyl acetate and then with 2% lead citrate. No immunolabeling was observed when the primary antibody was omitted. For panel G of figure 10 the Lowicryl-embedded tissue blocks were a generous gift of Drs. Peter Somogyi (MRC Anatomical Neuropharmacology Unit, Oxford, UK) and Zoltan Nusser (Institute of Experimental Medicine, Budapest, Hungary), who have described the preparation of the blocks elsewhere (Nusser et al., 1998). These tissue blocks were sectioned in our laboratory and the ultrathin sections were subjected to the same immunogold labeling procedure described above. All sections were visualized with a Tecnai Biotwin 12KV transmission electron microscope (FEI Corporations, Eindhoven, Holland). EM images were stored in Adobe Photoshop 7.0 and contrast/brightness was adjusted.
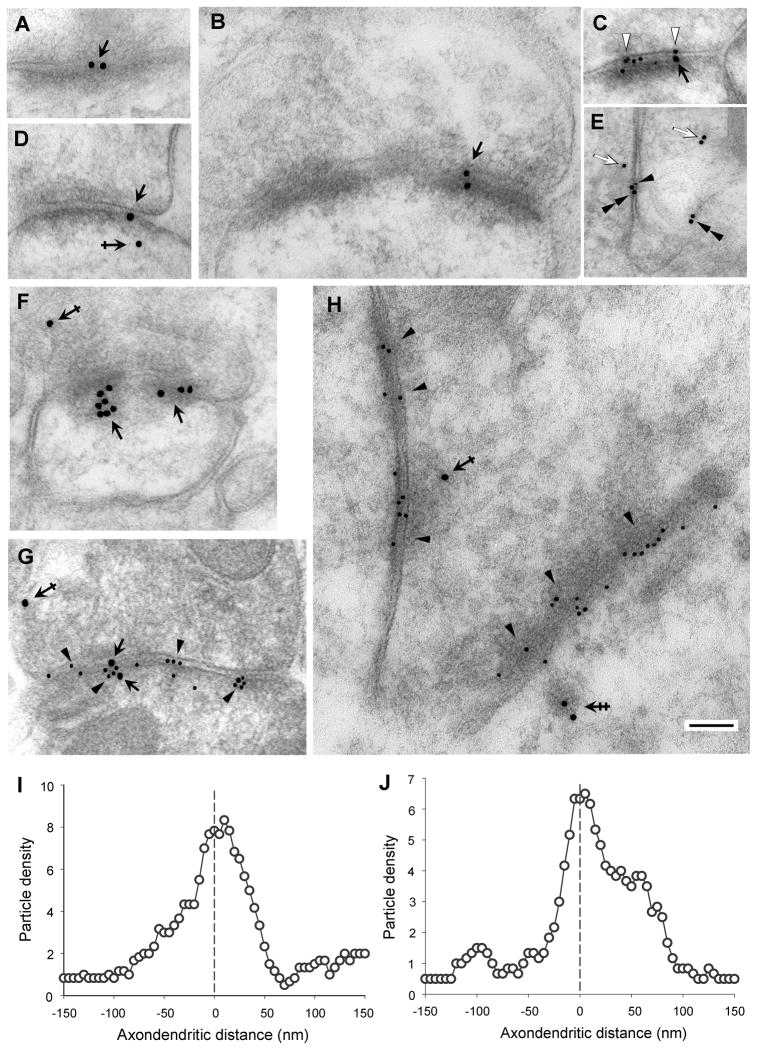
Fig. 10. Post-embedding EM immunogold of rat brain with anti-Pcdh-γC5 antibody.
(A–H) Post-embedding EM immunogold labeling with Rb Pcdh-γC5 in single-label experiment (A, B, D and F) or in double-label experiment with mouse mAb anti-GluR2 (C) or mouse mAb anti-β2/3 GABAAR (E, G and H). In all panels except E, where there is no synapse, the presynaptic terminal is located above the synaptic cleft. The larger gold particles (18 nm diameter) correspond to Pcdh-γC5 immunoreactivity, while the small particles (10 nm) correspond to GluR2 immunoreactivity in C or to β2/3 GABAAR immunoreactivity in E, G and H. The Pcdh-γC5 immunoreactivity is associated with the synaptic complex (A,B,C,D,F, and G, black arrows), presynaptic terminals and postsynaptic cytoplasm (D, F, G and H, single and double crossed arrows) and non-synaptic membranes (E, double arrowheads) and cytoplasm (E, white arrows). The double-crossed arrow in H points to Pcdh-γC5 immunoreactivity associated with a postsynaptic vesicular structure. Single black arrowheads point to GABAAR immunoreactivity (small particles) in GABAergic synapses, while in E the single black arrowhead points to a GABAAR gold particle associated with a non-synaptic membrane. White arrowheads in C point to GluR2 immunoreactivity. Thin sections are from the cerebral cortex (A,B,D, E and F) or from the molecular layer of the cerebellum (C, G and H). The scale bar represents 100 nm in all panels except in panel E (160nm). (I and J) Axodendritic distribution of Pcdh-γC5 gold particles (n=167) in GABAergic synapses (I) or in glutamatergic/type I synapses (J, n=133). Gold particles are distributed in 5 nm bins according to the distance from the center of each gold particle to the midline of the synaptic cleft defined as the zero point. Negative and positive values represent presynaptic and postsynaptic localization respectively. Particle density is the number of particles in a 5-nm bin. The graph was smoothed using a six-point weighted running average.
We used the method of Valtschanoff and Weinberg (2001) for the quantification of the distribution of the Pcdh-γC5 gold particles at the synapse as we have described elsewhere (Serwanski et al., 2006). The axodendritic distance of each gold particle to the middle of the synaptic cleft was measured as a perpendicular line between the center of the gold particle and the midline of the synaptic cleft. The distances of the particles were sorted into 5 nm bins for plotting particle density. A negative or positive number indicates that the particle was located on the presynaptic or postsynaptic side of the midline, respectively. Binned data were smoothed with a six-point weighted running average using SigmaPlot 10.01 (Systat Software Inc., Chicago, IL).
RESULTS
Generation of a specific anti-Pcdh-γC5 antibody and immunocytochemical localization of Pcdh-γC5 in the rat brain
We have developed a novel rabbit antibody to the N-terminus of the rat Pcdh-γC5 that specifically recognizes Pcdh-γC5 but not other members of the Pcdh-γ family. The epitope recognized by this antibody is identical in rat, mouse and human. In immunoblots of a rat brain membrane fraction, the affinity-purified antibody specifically recognized a single 120,000 Mr polypeptide (Fig. 1K). The immunoreactivity was blocked by the antigenic peptide (Fig. 1K). This Mr is in agreement with the reported ~120,000 Mr of the Pcdh-γ family (Frank et al., 2005). Other specificity tests were described in Materials and Methods.
Light microscopy immunocytochemistry of P30 rat brain parasagittal sections showed that Pcdh-γC5 is highly expressed in the olfactory bulb (OB), cerebral cortex (CC), corpus striatum (St), olfactory tubercle (Tu), dentate gyrus (DG), hippocampus (HP), cerebellum (CB) in this order of intensity (Fig. 1A). Lower levels of expression were found in the substantia nigra, reticulata (SNR), pontine nuclei (Pn) and the medial cerebellar nucleus (Med). The immunoreactivity was particularly high in the external plexiform layer (EP) and granule cell layer (GR) of the olfactory bulb (Fig. 1B), and in layers I to III of the cerebral cortex (Fig.1C). There was also high immunoreactivity in the corpus striatum (Fig. 1D). In the hippocampus, the CA1 region had the highest while the CA3 region had the lowest immunoreactivity (Fig. 1F–H). In the hippocampus there was immunoreactivity in the soma of the pyramidal cells of the stratum pyramidale (SP, Fig. 1G and 1H) as well as in the synaptic layers. Thus in the CA1 region (Fig. 1H) there was strong punctate staining in the stratum radiatum (SR), as shown at high magnification (Fig. 1I, arrows). The presence of these Pcdh-γC5 granules in the neuropil and their size (0.2–1.5 μm in diameter) is consistent with the notion that they are synaptic. However, confocal studies shown below, indicate that many of these granules (clusters) are not synaptically localized. Note that the Fig. 1I panel is showing structures at considerably higher magnification than in the other panels, as indicated in the figure legend. Immunoreactivity was also strong in the molecular layer (ML) of the dentate gyrus (Fig. 1J). In the cerebellum (Fig.1E), the immunoreactivity was high in the soma and dendrites of the Purkinje cells (PK) and in the molecular layer (ML). Our immunocytochemistry data on the distribution of the Pcdh-γC5 protein in the rat brain are consistent with the in situ hybridization data on the distribution of Pcdh-γC5 mRNA in the rat brain (Zou et al., 2007) and mouse brain (Allen Brain Atlas http://www.brain-map.org, Entrez Gene ID 93708, http://mouse.brain-map.org/brain/Pcdhgc5.html?ispopup=true).
Subcellular fractionation of the rat brain (Fig. 1L) shows that there is an enrichment of Pcdh-γC5 in the postsynaptic density fractions such as i) the “one triton” PSD fraction (PSD), which is enriched in both GABAergic and glutamatergic postsynaptic densities; ii) the type-II PSD fraction, which is enriched in GABAergic postsynaptic densities and iii) the type-I PSD fraction, which is enriched in glutamatergic postsynaptic densities, when compared with the crude synaptosomal P2, microsomal P3 and synaptosomal P2B fractions. The characterization of the type-I PSD and type-II PSD postsynaptic density fractions has been reported elsewhere (Li et al., 2007). These results support the notion that Pcdh-γC5 is present in glutamatergic and GABAergic synapses.
Pcdh-γC5 is not expressed until the second postnatal week of the rat brain development
Immunoblots with rat forebrain (telencephalon) homogenates of various ages, from embryonic day 18 (E18) to postnatal day 90 (P90) showed that the 120kD Pcdh-γC5 protein band (Fig. 2A, arrow), whose immunoreaction was blocked by the antigenic peptide, is not significantly expressed until the second postnatal week, reaching a peak by P21 followed by a slight decrease in expression (Fig. 2A and B). There were also two immunoreactive protein bands of higher Mr that were present between P0 and P7, but the corresponding immunoreactivity was not displaceable by the antigenic peptide (Fig. 2A, arrowhead), indicating that the immunoreactivity was non-specific. These non-specific immunoreactive protein bands disappeared after P7.
Light microscopy immunocytochemistry experiments (Fig. 2C) show that the developmental expression of Pcdh-γC5 immunoreactivity in the rat brain agrees with that of the immunoblots. There was little or no specific immunoreactivity at P0 and P7. At P14 there was immunoreactivity in the regions that showed immunoreactivity in the adult. The typical pattern of expression in the adult was fully developed by P21. At all ages in which Pcdh-γC5 was clearly expressed (P14–P90), the immunoreactivity was displaced by the antigenic peptide, (shown in Fig. 2C for P30).
In the cerebellum, the time-course of the expression of the Pcdh-γC5 immunoreactivity largely coincided with that of the development of GABAergic synapses. Thus, specific Pcdh-γC5 immunoreactivity in Purkinje cells, molecular layer and deep cerebellar nuclei is absent at P0 and P7, but is well developed in these structures at P14 and fully developed at P21 (Fig. 2 D–G). We consider non-specific the immunoreactivity not displaced by the peptide antigen. Thus, the weak immunoreactivity observed at P0 and P7 in the cerebellum is non-specific, since is not displaced by the peptide antigen (shown for P7 in Fig 2D and H), while the immunoreactivity at P14 and onwards is displaceable by the peptide. At P14 there is some immunoreactivity in the Purkinje cells that is not displaced by the antigenic peptide (Fig. 2I, arrow). The presence of non-specific immunoreactivity at the earlier postnatal ages coincided with the presence of non-specific protein bands in the immunoblots from P0 to P7, whose immunoreactivity is not displaceable by the antigenic peptide (Fig. 2A, arrowheads).
It has been shown that GABAergic synapses on Purkinje cells start appearing at P7–P10 in the soma followed by a large increase in the number of GABAergic synapses in the Purkinje cell dendrites at P14 and P21 (Altman, 1972; Takayama, 2005; Viltono et al., 2008). This time-course coincides with the increase in specific Pcdh-γC5 immunoreactivity at P14 and P21 in the molecular layer and Purkinje cells (Fig 2D–G, single asterisk and arrow respectively). Also the formation of the glutamatergic synapses from parallel fibers on Purkinje cell dendrites and the ensheathment of the Purkinje cells by the Bergmann glia occurs during the same time-frame (Altman, 1972). In the deep cerebellar nuclei, some GABAergic synaptic activity from the Purkinje cells is recorded at P9 but it highly increases at P14 (Gambino et al., 2009), also coinciding with the time-course of specific Pcdh-γC5 immunoreactivity (Fig. 2 D and E, double asterisks). These results are consistent with the hypotheses that Pcdh-γC5 plays a role in synaptogenesis and that glial cells are involved in this process, since Pcdh-γC5 is expressed by both neurons and astrocytes as shown below.
In the olfactory bulb (OB), significant Pcdh-γC5 immunoreactivity develops between P7 and P14, being fully developed by P21 (Fig. 2L–O). At P0, there is no specific immunoreactivity displaceable by the antigenic peptide while at P7, very low levels of immunoreactivity displaceable by the antigenic peptide might be present in the external plexiform layer (EP) (Fig. 2L and P, arrowhead). If any, the specific signal is so weak that it is not detected by the immunoblots at P7 (Fig. 2A, arrows). At P14 and later, the highest levels of expression of Pcdh-γC5 in the OB (and in the brain) occurs in the EP (Fig. 2C, M–O). At these ages the immunoreactivity is displaced by the peptide antigen (Fig. 2Q–S). Specific Pcdh-γC5 immunoreactivity in the glomeruli and in the granule cell layer also shows at P14 but not at P7 (Fig. 2 L–O). It has been shown that high density levels of granule to mitral (GR/M) GABAergic synapses in the EP is reached by P15-P20 followed by a slight increase up to P44 (Hinds and Hinds, 1976). Nevertheless, a significant number of GABAergic GR/M synapses in the EP are present at P5 and P7, age at which very little, if any, specific Pcdh-γC5 immunoreactivity in the EP is detected (Fig. 2L, arrowhead). Glutamatergic mitral to granule (M/GR) synapses in the EP develop earlier, showing a very high density by P5 and P7. The glutamatergic synapses in the glomeruli from the olfactory receptor axons develop even earlier showing a significant number of these synapses at birth (Hins and Hinds, 1976). Therefore, in the OB there is a closer correlation between the time-course of Pcdh-γC5 expression and the development of GABAergic synapses in the EP than with that of glutamatergic synapses. Nevertheless, the appearance of clear Pcdh-γC5 immunoreactivity at P14 shows a delay with respect to the formation of significant number of the GABAergic synapses at P7.
The time-course of the Pcdh-γC5 protein expression in the rat brain shown by immunobloting and immunocytochemistry is identical to that of the Pcdh-γC5 mRNA expression in the mouse brain, as revealed by Northern blot analysis (Frank et al., 2005).
In cultured hippocampal neurons Pcdh-γC5 forms clusters, which are localized in a subset of GABAergic and glutamatergic synapses and also extrasynaptically
We have studied the expression and localization of Pcdh-γC5 in cultured hippocampal neurons (21DIV). At this age, the majority of pyramidal cells and interneurons show Pcdh-γC5 clusters both in dendrites and soma as shown by immunofluorescence with anti-Pcdh-γC5 (Fig. 3). In dendrites, Pcdh-γC5 clusters were found at a density of 38.4±1.4 (mean± SEM) clusters/100 μm2, n=2275, with an average size of 0.06±0.01μm2, n=914 (Fig. 3A and B, arrowheads). Some larger (and brighter) Pcdh-γC5 clusters (0.14±0.1 μm2, n=714, p<0.001) were also present in the soma and in the proximal segment of 5 % of the neurons (Fig. 3A and B, arrows), likely representing neurons with high levels of expression of Pcdh-γC5. This might be a reflection of the higher levels of Pcdh-γC5 expression in CA1 over CA3 as shown above (Fig. 1A and F). The larger and brighter Pcdh-γC5 clusters were found in some neurons regardless of whether they were pyramidal cells or interneurons. In 14 DIV or younger cultures, the majority of neurons showed few or no Pcdh-γC5 clusters, in agreement with the development of Pcdh-γC5 expression in the rat brain during the second and third postnatal weeks.
Fig. 3. Pcdh-γC5 expression in cultured hippocampal neurons.
(A) Immunofluorescence of cultured hippocampal neurons (21 DIV) with the Rb anti-Pcdh-γC5 antibody. (B) Enlargement of the inset box in A. Arrows point to larger Pcdh-γC5 normally found in the soma and proximal dendrites. Arrowheads point to smaller clusters that are present in dendrites. (C–F) Triple-label immunofluorescence with GP anti-γ2 (C), Rb anti-Pcdh-γC5 (D), sheep anti-GAD (E) and overlay (F). (G–J) Triple label immunofluorescence with mouse anti-PSD95 (G), Rb anti-Pcdh-γC5 (H), GP anti-vGluT1 (I) and overlay (J). Arrows show GABAergic (C–F) and glutamatergic (G–J) synapses that have colocalizing Pcdh-γC5 clusters. (K–R) High magnification images show the association of Pcdh-γC5 with GABAergic synapses. Pcdh-γC5 clusters colocalize with synaptic γ2 clusters (K–N, arrows) or partially colocalize with synaptic γ2 clusters (O and P, crossed arrows) or partially or fully colocalize with GAD terminals but not with γ2 (O–R, arrowheads). (S–Z) Association of Pcdh-γC5 with glutamatergic synapses. Pcdh-γC5 clusters colocalize with synaptic PSD-95 (S–V, arrows) or partially colocalize with synaptic PSD-95 clusters (X and Y, crossed arrows) or partially or fully colocalize with vGlut1 terminals but not PSD-95 (W, X, Z, arrowheads). (ZZ) Quantification of the % of GABAergic and glutamatergic synapses that show associated Pcdh-γC5 clusters. Scale bar = 13μm for A, 5μm for B–J and 1.5μm for K–Z. * p<0.05 in Student’s t test.
A significant number of GABAergic synapses had associated Pcdh-γC5 clusters as shown by triple-label immunofluorescence with anti-GAD and anti-γ2 GABAAR subunit antibodies (Fig. 3C–F arrows, and Fig. 3ZZ). We have previously shown that these GAD+ and γ2+ contacts correspond to synapses with actively recycling synaptic vesicles (Christie et al. 2002b). We found that 45±5% of the GABAergic synapses (GAD+ and γ2+) had associated Pcdh-γC5 clusters. In 29±3% of the GABAergic synapses, the synaptic γ2 clusters and Pcdh-γC5 clusters colocalized (>66% of the surface of the Pcdh-γC5 cluster had overlap with γ2cluster, as shown in Fig. 3K–N, arrows). In 12±2% of the GABAergic synapses, the Pcdh-γC5 clusters were in contact or partially overlapping (between 1% to 66%) with the synaptic γ2 clusters (Fig. 3O and P, crossed arrows). In 4±2% of the GABAergic synapses, the Pcdh-γC5 clusters were associated, with partial or complete overlap, with the GAD terminal but had no overlap with the postsynaptic γ2clusters of these synapses (Fig. 3O–R, arrowheads).
Some non-synaptic (GAD−) γ2 clusters (22±1%) also showed colocalizing Pcdh-γC5 clusters, indicating that the colocalization of γ2 and Pcdh-γC5 clusters does not require the presence of presynaptic GAD-containing terminal. Nevertheless, the presence of a GAD-containing terminal in a GABAergic synapse significantly increased the colocalization of synaptic γ2 clusters with Pcdh-γC5 clusters, as shown above (29±3% vs. 22±1%, p=0.034).
A significantly smaller percentage (33±4%, p=0.023) of glutamatergic synapses (Glut1+ and PSD-95+), compared with GABAergic synapses (45±5%), had associated Pcdh-γC5 clusters (Fig. 3G–J, arrows and Fig. 3ZZ). In 17±3% of the glutamatergic synapses, the synaptic PSD-95and Pcdh-γC5 clusters colocalized (Fig. 3S–V, arrows). In 13±2% of the glutamatergic synapses, the Pcdh-γC5 clusters were in contact or partially overlapping with the synaptic PSD-95clusters (Fig. 3X and Y, crossed arrows). In 2±1% of the glutamatergic synapses, the Pcdh-γC5 clusters were associated with vGlut1+ terminal but had no overlap with the postsynaptic PSD-95 clusters of the same synapses (Fig. 3W, X and Z, arrowheads).
Nevertheless, the majority of the Pcdh-γC5 clusters (63±4%) were not associated with GABAergic or glutamatergic synaptic markers. Thus, only a subset of Pcdh-γC5 clusters are synaptic and only a subset of synapses (GABAergic and glutamatergic) have associated Pcdh-γC5 clusters. The aforementioned large and very bright Pcdh-γC5 clusters that are present in the soma and proximal dendrites of some neurons were not associated with synapses. The majority of Pcdh-γC5 clusters associated with synapses were localized in dendrites.
It is worth mentioning that astrocytes are largely absent from these cultures. In these hippocampal neuronal cultures, the Pcdh-γC5 clusters are expressed in neurons as determined by the colocalization of Pcdh-γC5 immunofluorescence with MAP+ neurons and the absence of GFAP immunofluorescence in these cells (supplementary Fig. S2 A, C and D). A few isolated GFAP+ astrocytes (6±2% of the cells) are present in these cultures (supplementary Fig. S2 B). The Pcdh-γC5 clusters associated with synapses in these hippocampal cultures are of neuronal origin.
We have also investigated the clustering of Pcdh-γC5 in relationship with that of other Pcdh-γs in double-labeling experiments by using the Rb Ab to Pcdh-γC5 in combination with i) a Pan-Pcdh-γ Ms mAb, which recognizes all the members in Pcdh-γ family, or ii) a Pan-Pcdh-γA, which recognize all Pcdh-γA members of the Pcdh-γ family (Supplementary Fig. S3). In 21 DIV hippocampal neurons, the majority (91±2%) of Pcdh-γC5 clusters were also immunoreactive with the Pan-Pcdh-γ antibody and 74±3% of Pan-Pcdh-γ clusters were immunoreactive with the Pcdh-γC5 antibody. Fewer Pcdh-γC5 clusters (54±3%) were Pan-Pcdh-γA positive. Also 53±4% of Pan-Pcdh-γA clusters were Pcdh-γC5 positive. These results indicate that: i) some Pcdh-γC5 clusters contain other Pcdh-γs, such as Pcdh-γAs; ii) that there are neuronal clusters that contain Pcdh-γC5 but do not contain Pcdh-γAs; and iii) that there are neuronal Pcdh-γ clusters that contain Pcdh-γAs but do not contain Pcdh-γC5. This difference in Pcdh-γs cluster composition is consistent with the existence of a neuronal Pcdh-γ recognition code.
Some GABAergic axons have Pcdh-γC5 in the majority of their synapses
Also consistent with a role of Pcdh-γC5 in synaptic specificity, is that although 45±5% of the GABAergic synapses (GAD+ and γ2+) had associated Pcdh-γC5 clusters, the majority of the GABAergic synapses made by some axons had associated Pcdh-γC5. Fig. 4A shows a GABAergic axon that innervates two different neurons. The majority of the GABAergic synapses made by this axon on neurons 1 and 2 (GAD+ and γ2+) have associated Pcdh-γC5 clusters (Fig. 4B–I, arrows). Alternatively, two GABAergic axons, presumably from two different GABAergic cells that innervated the same pyramidal cell, showed clear differences in the percentage of GABAergic synapses having associated Pcdh-γC5 clusters (Fig. 5). In this example, most GABAergic synapses from axon 1 (Fig. 5B–E, arrows) and some GABAergic synapses from axon 2 (Fig. 5F–I, arrows) had associated Pcdh-γC5 clusters. Nevertheless, several GABAergic synapses from axon 2 did not have associated Pcdh-γC5 clusters (Fig. 5F–I, arrowheads). Moreover, some Pcdh-γC5 clusters associated with GABAergic synapses from axon 2 are very small. There are also axons whose GABAergic synapses seldom show associated Pcdh-γC5. These results indicate that GABAergic synapses from different axons show differential association with Pcdh-γC5 clusters, supporting the notion that Pcdh-γC5 plays a role in neuronal recognition and connectivity.
Fig. 4. Some GABAergic axons have Pcdh-γC5 in the majority of their synapses.
(A) Overlay of triple-label immunofluorescence of 21 DIV cultured hippocampal neurons showing the same axon (arrowheads) contacting two different pyramidal cells (neuron 1 and neuron 2). (B–I) Enlargements of the inset boxes in A shows GABAergic synapses from the same axon on neuron 1 (B–E) and neuron 2 (F–I). Triple-label immunofluorescence with Rb anti-Pcdh-γC5 (green), GP anti-γ2 (red) and sheep anti-GAD (blue) shows that the majority of the GABAergic synapses (GAD+ and γ2+, arrows) from this axon on the two neurons have colocalizing Pcdh-γC5 clusters. Scale bar = 24 μm for A and 10μm for B–I.
Fig. 5. Two GABAergic axons contacting the same neuron show differential extent of Pcdh-γC5 localization to their synapses.
(A) Overlay of triple-label immunofluorescence of 21 DIV cultured hippocampal neurons showing two axons contacting a pyramidal cell (axon 1 and axon 2). (B–I) Enlargements of the insets in A shows GABAergic synapses (arrows) from axon 1 (B–E) and axon 2 (F–I). Triple-label immunofluorescence with Rb anti-Pcdh-γC5 (green), GP anti-γ2 (red) and sheep anti-GAD (blue). The majority of the GABAergic synapses (GAD+ and γ2+, arrows) from axon 1 (B–E) have colocalizing Pcdh-γC5 clusters. In contrast, axon 2 has some GABAergic synapses with Pcdh-γC5 (F–I, arrows) and some without Pcdh-γC5 (F–I, arrowheads). Scale bar = 20μm for A and 13μm for B–I.
Pcdh-γC5 is also expressed by cultured astrocytes
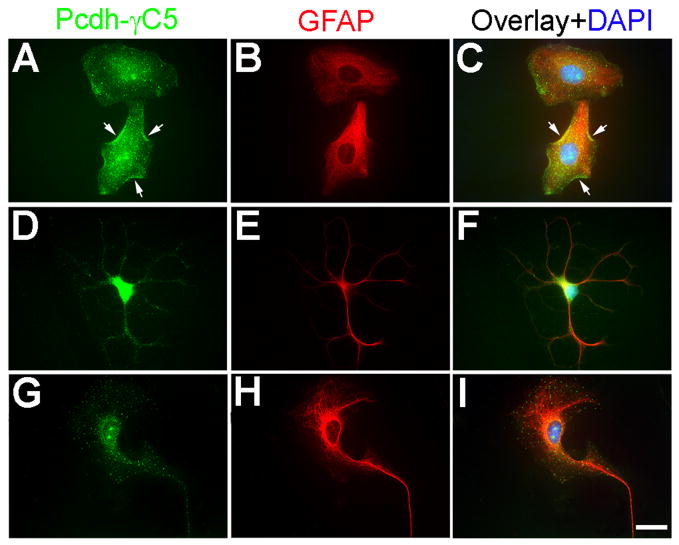
We have investigated whether Pcdh-γC5 is also expressed by cultured astrocytes identified by glial fibrillary acidic protein (GFAP) immunoreactivity. All the GFAP+ cells, regardless of their morphology, had Pcdh-γC5 clusters, as shown in (Fig. 6). The astrocytes GFAP+ cells (Fig. 6B, E and H, red) showed Pcdh-γC5 clusters in the cell body and processes (Fig. 6A, D and G, green). Some of the clusters were associated with the plasma membrane (Fig. 6A and C, arrows). Thus, Pcdh-γC5 is expressed by both neurons and astrocytes, forming clusters in both cell types.
Fig. 6. Pcdh-γC5 is expressed by cultured astrocytes.
Triple-label fluorescence of cultured astrocytes with Rb anti-Pcdh-γC5 (green, A, D, G), mouse anti-GFAP (red, B, E, H) and the nuclear stain DAPI (blue, C, F, I). The Pcdh-γC5 is expressed in astrocytes with various morphologies. The Pcdh-γC5 immunofluorescence is frequently shown in the form of clusters both in the cell body and processes. Some of the clusters are clearly associated with the plasma membrane (A, C, arrows). Scale bar=20μm.
In the intact brain Pcdh-γC5 forms clusters both in neurons and astrocytes, often in contact points between neurons and astrocytes
Laser confocal microscopy of adult brain sections (Fig. 7 and 8) showed that, like in cultures, neurons and astrocytes have Pcdh-γC5 clusters. These clusters are similar to the granular staining observed by immunocytochemistry in brain sections (Fig. 1I). Some Pcdh-γC5 clusters were bright and abundant in the perikaryon of some neurons (Fig. 7A, the double arrowhead points to Pcdh-γC5 clusters in the soma of a pyramidal cell in hippocampal CA1). Triple label immunofluorescence with the astrocytic markers S-100β and GLAST shows that in the brain, there are Pcdh-γC5 clusters localized in the cell body of astrocytes (Fig. 7A arrowheads) and in the astrocytic end-feet contacting blood vessels (Fig. 7A, crossed arrows). The Pcdh-γC5 clusters frequently colocalized with astrocytic processes, which were labeled with S-100β and GLAST (Fig. 7B–E, arrows). These results show that Pcdh-γC5 clusters are present in the cell body and processes of astrocytes.
To investigate whether some Pcdh-γC5 clusters were associated with contact points between astrocytes and neurons, we studied the brain of rats that have been subjected to in utero electroporation with an EGFP plasmid. The EGFP labeling, limited to a relatively small population of transfected neurons, allowed us to identify individual neurons and processes, which is an advantage over using an antibody to a general neuronal marker. Figure 8 combines the transgenic expression of EGFP (green) in the soma and dendrites of some neurons of the cerebral cortex with the immunolabeling of astrocytes (blue) and Pcdh-γC5 clusters (red). These single optical sections showed the presence of Pcdh-γC5 clusters in the cell body of neurons (green) and astrocytes (blue) as shown in Fig. 8A, D and E. Pcdh-γC5 clusters were also present in neuronal dendrites (green, Fig. 8B and C, empty arrowheads) and astrocytic processes (blue, Fig. 8D, E). There were Pcdh-γC5 clusters associated with the edge of the astrocyte at both the cell body and processes (Fig. 8D and E, arrowheads). Some of these clusters were clearly located at points of contacts between neurons and astrocytes. Sometimes the contact points were between the cell body of the neuron and the cell body of the astrocyte (Fig. 8A and F, arrows; panel F shows at higher magnification the boxed area in A). Sometimes the contact points were between astrocyte processes and neuronal soma (Fig. 8G, arrow) and sometimes between neuronal dendrites and glial processes (Fig. 8H, arrows).
In single optical sections, some GABAergic and glutamatergic synapses had associated Pcdh-γC5 clusters. These clusters sometimes colocalized with both vGAT and gephyrin (Fig 9A and B, arrows) or with both vGlut1 and PSD-95 (Fig. 9E and F, arrows). Sometimes the Pcdh-γC5 clusters were perisynaptic (arrows Fig. 9C, D, G and H), being adjacent to the GABAergic (Fig. 9C and D, arrowheads) or glutamatergic (Fig. 9G and H, arrowheads) synapses. Triple-label immunofluorescence combining a synaptic marker and an astrocytic marker, showed that some perisynaptic Pcdh-γC5 clusters were on astrocytic (S-100β+, green) processes (arrows, Fig. 9I, J, M and N) that were adjacent to GABAergic (Fig. 9I and J, arrowheads) or glutamatergic (Fig. 9M and N, arrowheads) synaptic markers. However, other Pcdh-γC5 clusters (arrows, Fig. 9K, L) coinciding (Fig. 9K) or being adjacent to the GABAergic synaptic marker vGAT (Fig. 9L, arrowhead) or coinciding (Fig. 9O) or being adjacent to the glutamatergic synaptic marker PSD-95 (Fig. 9P, arrowhead) were not in astrocytic processes, as shown by the absence of S-100β immunoreactivity. These results show that some perisynaptic Pcdh-γC5 clusters are on astrocyte processes but that other synaptic and perisynaptic Pcdh-γC5 clusters are not on astrocyte processes, in agreement with the aforementioned results on cultured hippocampal neurons, which also show synaptic and perisynaptic Pcdh-γC5 clusters in the absence of astrocytes.
Postembedding EM immunogold
Single-label (Fig. 10A, B, D and F) or double-label (Fig. 10C, E, G and H) postembedding EM immunogold showed that a significant number of gold particles corresponding to Rb anti-Pcdh-γC5 immunoreactivity were associated with the synaptic complex (Fig. 10A, B, C, D, F and G, black arrows) but not exclusively, since Pcdh-γC5 gold particles were also associated with the plasma membrane of the presynaptic terminals and the presynaptic cytoplasm (Fig. 10F, G and H, single crossed arrow) as well as with the postsynaptic cytoplasm (Fig. 10D, single crossed arrow) in dendrites and dendritic spines, vesicular structures in the postsynaptic cytoplasm (Fig. 10H, double crossed arrow), non-synaptic membranes (Fig. 10E, double arrowheads) and the cytoplasm not associated with synapses (Fig. 10E, white arrows). Quantification showed that 27% of the Pcdh-γC5 gold particles (n=918) were associated with synapses (within 200 nm from the synaptic cleft), 20% were associated with non-synaptic plasma membranes including neuron/astrocyte membrane contacts and 53% were intracellular, not associated with synapses and present in both neurons and astrocytes.
Some of the labeled synapses had morphology corresponding to asymmetrical Gray’s type-I glutamatergic synapses (Fig. 10A, B, C, D and F). Double-label immunogold with mouse anti-GluR2 subunit of the AMPA receptor showed that some type-I synapses had both GluR2 (Fig. 10C, white arrowheads) and Pcdh-γC5 immunoreactivity (Fig. 10C black arrow). Other synapses labeled with Pcdh-γC5 gold particles had symmetrical type-II morphology corresponding to GABAergic synapses. This was confirmed by double-label postembedding EM immunogold with anti-β2/3 GABAAR antibody. Figure 10G shows a GABAergic synapse labeled with both anti-Pcdh-γC5 (arrows, large gold particles) and anti-β2/3 GABAAR (arrowheads, small particles). A crossed arrow in Fig. 10G shows a Pcdh-γC5 particle in the presynaptic terminal. Fig. 10H shows two GABAergic synapses from the same terminal labeled with anti-β2/3 GABAAR (small particles, arrowheads). A Pcdh-γC5 large gold particle is localized in the presynaptic terminal (Fig. 10H, single crossed arrow) while two Pcdh-γC5 large gold particles are in the postsynaptic cytoplasm associated with a vesicular structure (Fig. 10H, double crossed arrow). Although synaptic labeling was clearly shown in some synapses, the Pcdh-γC5 immunolabeling showed considerably fewer gold particles than that of β2/3 GABAARs, the latter showing many gold particles decorating GABAergic synapses (Fig. 10G and H). This could be in part due to the Pcdh-γC5 epitope being sensitive to the fixation and postembedding procedures, which might partially denature the epitope. We have found that the presence of glutaraldehyde in the fixative, required in the postembedding procedure to reasonably preserve the structure of the tissue, lowers the intensity of the Pcdh-γC5 immunoreactivity in the brain sections.
The localization of an individual gold particle might not correspond to the exact localization of the antigen. For an 18 nm gold particle, the antigen could be located up to 32 nm respectively, from the center of the gold particle (Kellenberger and Hayat, 1991). This is due to the combined size of the primary and secondary antibodies plus the size of the gold particle. This consideration is particularly important when determining the localization of an antigen in the pre- vs. post-synaptic membrane, since the distance between the pre- and post-synaptic membrane (~20 nm) is smaller than the theoretical maximal distance (32 nm) between the actual location of the Pcdh-γC5 epitope and the center of the 18 nm gold particle. Therefore, quantitative analysis of the distribution of gold particles is necessary to ascertain the pre- or post-synaptic localization of Pcdh-γC5. The axodendritic distribution of Pcdh-γC5 immunogold particles (n=167) in GABAergic synapses, identified by double labeling with the anti-β2/3 mAb (Fig. 10G), showed that the Pcdh-γC5 particle density was highest at 10–15 nm from the middle line of the synaptic cleft coinciding with the postsynaptic membrane (Fig. 10I). Moreover, 74% of all gold particles located within 50nm on each side of the synaptic cleft midline (n=117) were within the range expected for an antigen localized at the postsynaptic membrane and 64% of the particles were within the range expected for an antigen localized in the presynaptic membrane (±32 nm from the postsynaptic or presynaptic membrane respectively).
Similarly, the axodendritic distribution of Pcdh-γC5 gold particles in Gray’s type-I glutamatergic synapses identified by either double labeling with anti-GluR2 or by their Gray’s type-I morphology (n= 133, Fig. 10J) showed that the Pcdh-γC5 particle density was highest at 5–10 nm from the middle line of the synaptic cleft coinciding with the postsynaptic membrane. Moreover, of all the gold particles located within 50nm on each side of the synaptic cleft midline (n=71) of glutamatergic synapses, 97% were within the range expected for an antigen localized at the postsynaptic membrane and 83% within the range expected for an antigen localized in the presynaptic membrane. In GABAergic and glutamatergic synapses there were a significant number of gold particles in both the presynaptic and the postsynaptic cytoplasm, beyond the 32 nm from the pre- or the postsynaptic membrane respectively, which are likely due to the presence of Pcdh-γC5 in pre and postsynaptic organelles. Some could represent Pcdh-γC5 located on exo- or endocytic vesicles (i.e. Fig. 10H, double crossed arrow).
Therefore, although the particle distribution in both GABAergic and glutamatergic synapses is biased towards the postsynaptic membrane, the data are consistent with the presence of Pcdh-γC5 in both presynaptic and postsynaptic membranes.
Preembedding EM immunocytochemistry
We have also done preembedding EM immunocytochemistry (Fig. 11). This is not the technique of choice for localizing protein epitopes facing the synaptic cleft, like the N-terminus of Pcdh-γC5, since in the absence of detergents, the antibody penetration into the tightly packed cleft structure is very poor. Detergents that facilitate the antibody penetration, like Triton X-100, are incompatible with the preservation of the tissue morphology at the EM level. However, EM preembedding immunocytochemistry in the absence of detergents can efficiently be used to label Pcdh-γC5 localized in cytoplasmic organelles. We have done the pre-embedding technique by fixing the brain with two different fixatives, one containing glutaraldehyde (and paraformaldehyde) as in Fig. 11A, C, F and G, and another fixative (PLP) that contains no glutaraldehyde (but contains paraformaldehyde) as in Fig. 11B, D and E. Preembedding EM immunocytochemistry shows the presence of Pcdh-γC5 immunoreactivity in intracellular organelles such as the endoplasmic reticulum of some neurons (Fig. 11A, arrows) and the cisternae of the Purkinje cells in the cerebellum (Fig. 11B, arrows). Immunoreactivity was also found in some synapses with type-I morphology presumably glutamatergic (Fig. 11C and D, crossed arrows), some synapses with type-II morphology, presumably GABAergic (Fig. 11E, arrow) and some glial processes adjacent to some synapses (Fig. 11F and G double crossed arrows). Note that not all synapses show immunoreactivity. Thus in Fig. 11D one synapse (crossed arrow) had immunoreactivity while two others (arrows) did not. As indicated above, this technique does not label well antigens present in the synaptic cleft. Nevertheless, some labeling in the cleft and synaptic membranes (pre and postsynaptic) is present (Fig. 11C and E). These experiments also show the presence of a significant amount of Pcdh-γC5 immunoreactivity in the postsynaptic cytoplasm not associated with the postsynaptic density (Fig. 11C and D, arrowheads). This immunoreactivity might represent a subsynaptic intracellular pool of Pcdh-γC5. Fig. 11F and G also show that strong Pcdh-γC5 immunoreactivity (double crossed arrows) is present in some glial processes adjacent to synapses that don’t show obvious Pcdh-γC5 immunoreactivity (Fig. 11F and G single arrowheads). Thus these EM results show the presence of Pcdh-γC5 in a subset of GABAergic and glutamatergic synapses and also in some perisynaptic astrocytic processes, which is consistent with the Pcdh-γC5 immunofluorescence results in culture and in brain sections, which also show labeling in these structures.
Fig. 11. Pre-embedding EM immunocytochemistry of the rat brain with anti-Pcdh-γC5 antibody.
(A) Immunoreactivity with the Pcdh-γC5 antibody concentrates in the endoplasmic reticulum of some neurons (arrows). (B) Immunoreactivity in Purkinje cell cisternae (arrows). (C and D) Some synapses with type-I morphology show immunoreactivity in synaptic membranes (C, arrow) and particularly in the postsynaptic density (C and D, crossed arrows). Immunoreactivity was also found in the postsynaptic cytoplasm separated from the postsynaptic density (C and D, arrowheads). Note in D that one synapse shows immunoreactivity (crossed arrows) while two others show no immunoreactivity (arrows). (E) Some synapses with type-II morphology show immunoreactivity (arrow) in the pre- and post-synaptic membranes and in the synaptic cleft. (F and G) Some glial processes (double crossed arrows) adjacent to unlabeled synapses (single arrowheads) show strong immunoreactivity. Little or no accumulation of immunoreactivity is found in the same glial processes distant from the synapses (double arrowheads). Samples are from hippocampus (A), cerebellum (B, D and E) and cerebral cortex (C, F and G). The brain was fixed wit either 4% paraformaldehyde, 0.1% glutaraldehyde (A, C, F and G) or with PLP (B, D and E). The scale bar represents 100 nm for C, E, F and G; 162 nm for D; 270 nm for B; and 325 nm for A.
DISCUSSION
An antibody to the constant C-terminus region that is common to all Pcdh-γs, has been used by others to determine the combined cellular and subcellular localization in the brain at the EM level and in hippocampal neuronal cultures by immunofluorescence of the 22 members of the Pcdh-γ family (Phillips et al., 2003). This study concluded that Pcdh-γs are localized in synapses, but not exclusively. The same conclusion was derived from studying a mouse where all Pcdh-γs were expressed in the form of Pcdh-γ–GFP fusion proteins (Wang et al., 2002). These studies on the combined expression of the 22 members of the Pcdh-γ family did not reveal whether there is differential expression and/or localization among individual Pcdh-γs and whether some are synaptic and others are non-synaptic. Nevertheless, little is known about the cellular and subcellular localization of individual Pcdh-γ in the brain due to the absence of antibodies specific for most of the individual members of the Pcdh-γ family. An antibody specific for Pcdh-γA3 has recently been developed and has been used to study the trafficking of overexpressed Pcdh-γA3 in cultured neurons (Fernández-Monreal et al., 2009). Limited immunocytochemistry data at the light microscopy level have been presented for Pcdh-γC3, Pcdh-γB4 and Pcdh-γA1 (Frank et al., 2005). We have now developed a specific antibody to Pcdh-γC5 aiming to study the expression, specific localization and function of Pcdh-γC5 in the brain. By using the anti-Pcdh-γC5 antibody we have shown that the Pcdh-γC5 protein expression in the rat brain occurs postnatally after the second postnatal week, peaking during the third week, closely following the time course of the mRNA expression in the mouse (Frank et al., 2005). The postnatal development of Pcdh-γC5 is unique among Pcdh-γs since the mRNAs of the other members of the Pcdh-γ family (and of all the other clustered Pcdhs) are already expressed in the embryo, some as early as E10 (Frank et al., 2005). The postnatal expression of Pcdh-γC5 coincides with the developmental peak of synaptogenesis and neural maturation (ie. in the cerebellum, as shown above) being consistent with the hypothesis that Pcdh-γ and other clustered Pcdhs are involved in the establishment of specific patterns of neuronal connectivity (Kohmura et al., 1998; Shapiro and Colman, 1999; Wang et al., 2002; Kallenbach et al., 2003; Phillips et al., 2003; Esumi et al., 2005; Frank et al., 2005; Kaneko et al., 2006). Nevertheless, the experimental evidence in support of this hypothesis is rather limited.
A KO mouse lacking the whole Pcdh-γ cluster (the 22 members of the family), showed reduced density of GABAergic and glutamatergic synapses in the spinal cord (Wang et al., 2002). These mutants also showed apoptosis of interneurons in the spinal cord (see also Prasad et al., 2008), which has been interpreted as Pcdh-γs having a role in neuronal survival, thus complicating the interpretation of the results in terms of a possible synaptic role of the Pcdh-γs. Weiner et al. (2005) generated a Pcdh-γ KO mutant that also lacked the proapoptotic protein Bax. These double KO animals had normal neuronal survival but reduced number of glutamatergic and GABAergic synapses and smaller excitatory and inhibitory synaptic currents in the spinal cord. These results support the notion that Pcdh-γs are involved in synaptic connectivity. None of the aforementioned Pcdh-γ KO mice survived after birth (see also Hambsch et al., 2006). Nevertheless, hippocampal cultures from the Pcdh-γ KO mice survived past three weeks and formed glutamatergic and GABAergic synapses (Wang et al., 2002). Thus, the synaptic connectivity dependence on Pcdh-γs might vary depending on the CNS region and the neuronal type. A double conditional KO showing retina-specific deletions of Pcdh-γs did not show reduced synaptic density in the retina (Lefebvre et al., 2008) although had thinner synaptic layers and a reduced number of retina cells.
Our immunofluorescence and EM immunocytochemistry experiments show that Pcdh-γC5 is associated with a subset of GABAergic and glutamatergic synapses even in the absence of astrocytes. We have also shown by quantitative EM immunogold analysis of brain sections that Pcdh-γC5 is present in both pre and postsynaptic structures. In addition, there is enrichment of Pcdh-γC5 in various brain fractions enriched in PSDs (“one triton” PSD fraction, type-I glutamatergic PSD fraction and type-II GABAergic PSD fractions). These results are consistent with a synaptic function of neuronal Pcdh-γC5. Moreover, some data are consistent with the notion that Pcdh-γC5 is involved in the specificity of neuronal connectivity. Thus, the majority of the synapses made by some GABAergic axons on some neurons had associated Pcdh-γC5. Other GABAergic synapses made by other axons showed little association with Pcdh-γC5.
Using an antibody that recognizes all members of the Pcdh-γ family, Phillips et al. (2003) reported that in hippocampal cultures the Pcdh-γs, when found at synapses, were mostly associated with excitatory synapses. In the case of Pcdh-γC5 we find that a significantly higher percentage of GABAergic synapses over glutamatergic synapses have associated Pcdh-γC5 clusters. Therefore some members of the Pcdh-γ family might preferentially associate with some GABAergic synapses (like Pcdh-γC5) while other Pcdh-γs might preferentially associate with some glutamatergic synapses. This hypothesis will be tested as more antibodies specific for individual Pcdh-γs are developed. Alternatively, the preferential localization in GABAergic or glutamatergic synapses of specific members of the Pcdh-γ family might be related more to the “matching” of the Pcdh-γs expressed by the pre- and post-synaptic neurons. This notion is supported by i) the differential extent of localization of Pcdh-γC5 in the GABAergic synapses made by different axons; and ii) the heterogeneity of the Pcdh-γ cluster composition. Thus, some Pcdh-γC5 clusters contain Pcdh-γAs while some Pcdh-γC5 clusters don’t contain Pcdh-γAs. In turn, some Pcdh-γA clusters contain, and some others do not contain, Pcdh-γC5.
The quantification of the gold particles in the EM immunogold experiments is consistent with the localization of Pcdh-γC5 in both the presynaptic and the postsynaptic membranes. Our results also agree with the notion that clustered Pcdhs, as a class of molecules, can be localized pre- and post-synaptically (Wang et al., 2002; Phillips et al., 2003; Blank et al., 2004; Frank et al., 2005). Nevertheless, our results go beyond showing that an individual member of the Pcdh-γ family can be localized in the pre- and postsynaptic membrane of an individual synapse. This has relevant implications since our results are consistent with the existence of trans-homophilic interactions between the presynaptic and postsynaptic Pcdh-γC5. Others have shown that in host cells, Pcdh-γs preferentially have trans-homophilic interactions (Sano et al., 1993; Obata et al., 1995; Fernández-Monreal et al., 2009) and that Pcdh-γs can regulate cell adhesion (Reiss et al., 2006). We have also shown that other Pcdh-γs can colocalize with Pcdh-γC5 in the same cluster. The heterogeneity of the Pcdh-γ cluster composition and the trans-homophilic interactions of Pcdh-γs, could regulate the strength of the adhesion between the pre and postsynaptic membranes and the establishment of specific patterns of neuronal connectivity. The presence of various Pcdh-γ in the same cluster is also consistent with the notion that these molecules can form cis-heteromers (Murata et al., 2004). The adhesion between pre and postsynaptic membranes and between neurons and astrocytes could be regulated by controlled proteolysis of Pcdh-γs. It has been shown that Pcdh-γA3 and Pcdh-γC3 present at the plasma membrane undergo proteolytic cleavage (Haas et al., 2005; Hambsch et al., 2005; Reiss et al., 2006; Bonn et al., 2007). The proteolysis of Pcdh-γs could control the cell adhesion properties of these molecules (Reiss et al., 2006). Moreover, the cytoplasmic fragment generated by proteolytic cleavage might signal to the nucleus activating the transcription of the Pcdh-γ locus (Haas et al., 2005; Hambsch et al., 2005; Junghans et al., 2005).
Nevertheless, the majority of Pcdh-γC5 clusters in the brain are not associated with synapses. We have found that i) many non-synaptic Pcdh-γC5 clusters are localized in the soma and dendrites of neurons; ii) many Pcdh-γC5 clusters are present in astrocytes in both the cell body and processes; iii) Pcdh-γC5 clusters are also found at contact points between neurons and astrocytes; and iv) some Pcdh-γC5 clusters in astrocytic processes are perisynaptic. Garrett and Weiner (2009), based on a confocal microscopy study on conditional mouse KO for all the Pcdh-γ family, have proposed that Pcdh-γs are present in astrocytic perisynaptic processes. Confocal analysis does not have the resolution to directly demonstrate the existence of Pcdh-γ in astrocytic perisynaptic processes. Our EM immunocytochemistry data have shown that Pcdh-γC5 can be localized in perisynaptic astrocytic processes, in agreement with Garrett and Weiner’s conclusions. Moreover, Garret and Weiner (2009) have also shown that deletion of the Pcdh-γ family in astrocytes delays both excitatory and inhibitory synapse formation in spinal cord neurons in vivo and in culture. The authors proposed that the Pcdh-γs that are present in perisynaptic astrocytic processes promote synaptogenesis, which is in agreement with our observation with the localization of Pcdh-γC5. Nevertheless, synaptogenesis occurs in the absence of astrocytes (as we have shown above) and in the total absence of Pcdh-γs expression in the mouse Pcdh-γ KOs (Wang et al., 2002; Weiner et al., 2005). Moreover, we have found that Pcdh-γC5 clusters that are present at contact points between neurons and astrocytes, are not necessarily perisynaptic, suggesting a role for the Pcdh-γC5-mediated neuronal-astrocyte interactions wider than promoting neuronal connectivity.
Supplementary Material
Acknowledgments
This research was supported by the National Institute of Neurological Disorders and Stroke grants NS 38752 and NS 39287.
We would like to thank Drs. Peter Somogyi (MRC Anatomical Neuropharmacology Unit, Oxford, UK) and Zoltan Nusser (Institute of Experimental Medicine, Budapest, Hungary), for the block of brain tissue embedded in lowicryl HM20 for Fig. 10G. We thank Mr. Bevan Bonhomme and Ms. Haiyan Xiao for testing the specificity of the anti-Pcdh-γC5 antibody in HEK293 cells transfected with the Pcdh-γC3 and Pcdh-α4 constructs. We thank Dr. Tzu-Ting Chiou for testing the immunofluorescence of mouse mAbs to PanPcdh-γ, Pan Pcdh-γA, Pcdh-γA3 and Pcdh-γB2 in hippocampal cultures. We thank Dr. Marcus Frank, University of Freiburg, Germany, for the Pcdh-γC3 constructs and Drs. Martin Schwarz and Peter Seeburg, Max-Planck Institute-Heidelberg, Germany, for the Pcdh-α4 constructs that we have used for testing the specificity of our antibody to Pcdh-γC5 in transfected HEK293 cells. We thank Dr. Akiko Nishiyama from our department for providing Embed 812, and the antibodies to GLAST and S-100β and Dr. Randall Walikonis from our department for providing the chicken anti-MAP2 and the chicken anti-PSD95 antibodies. We thank Mr. Jabari Bailey and Mr. Rajvir Jutla for their help in the light microscopy immunocytochemistry experiments and brain sectioning. We also thank Dr. Marco Sassoè-Pognetto, University of Turin, Italy, for indicating some useful references on the formation of synaptic contacts in cerebellum and olfactory bulb during development.
Abbreviations
- ABC
avidin-biotin-horseradish peroxidase complex
- AMCA
aminomethylcoumarin
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- DAB
3-3′ diaminobenzidine tetrahydrochloride
- DAPI
4′,6-diamidino-2-phenylindole
- DIV
days in culture
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DMSO
dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- EGFP
enhanced green fluorescent protein
- ELISA
Enzyme-linked immunosorbent assay
- EM
electron microscope(y)
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GABA
γ-aminobutyric acid
- GABAAR
γ-aminobutyric acid type A receptor
- GAD
glutamic acid decarboxylase
- GFAP
glial fibrillary acidic protein
- GLAST
glial glutamate transporter
- GP
guinea pig
- HEK293
human embryonic kidney 293 cells
- KO
knock out mouse
- mAb
monoclonal antibody
- NDS
normal donkey serum
- NGS
normal goat serum
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- Pcdh
protocadherin
- PSD
postsynaptic density
- Rb
rabbit
- RT
room temperature
- SD
Sprague Dawley
- SDS-PAGE
sodium dodecyl sulphate- polyacrylamide gel electrophoresis
- vGAT
vesicular GABA transporter
- vGlut1
vesicular glutamate transporter 1
LITERATURE CITED
- Altman J. Postnatal development of the cerebellar cortex in the rat. II. Phases in the maturation of Purkinje cells and of the molecular layer. J Comp Neurol. 1972;145:399–463. doi: 10.1002/cne.901450402. [DOI] [PubMed] [Google Scholar]
- Blank M, Triana-Baltzer GB, Richards CS, Berg DK. Alpha-protocadherins are presynaptic and axonal in nicotinic pathways. Mol Cell Neurosci. 2004;26:530–543. doi: 10.1016/j.mcn.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Bonn S, Seeburg PH, Schwarz MK. Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing. Mol Cell Biol. 2007;27:4121–4132. doi: 10.1128/MCB.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charych EI, Yu W, Li R, Serwanski DR, Miralles CP, Li X, Yang BY, Pinal N, Walikonis R, De Blas AL. A four PDZ domain-containing splice variant form of GRIP1 is localized in GABAergic and glutamatergic synapses in the brain. J Biol Chem. 2004a;279:38978–38990. doi: 10.1074/jbc.M405786200. [DOI] [PubMed] [Google Scholar]
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem. 2004b;90:173–189. doi: 10.1111/j.1471-4159.2004.02481.x. [DOI] [PubMed] [Google Scholar]
- Christie SB, De Blas AL. GABAergic and glutamatergic axons innervate the axon initial segment and organize GABAA receptor clusters of cultured hippocampal pyramidal cells. J Comp Neurol. 2003;456:361–374. doi: 10.1002/cne.10535. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Yu W, Daniels SB, Cantino ME, De Blas AL. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res. 2002a;136:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- Christie SB, Miralles CP, De Blas AL. GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons. J Neurosci. 2002b;22:684–697. doi: 10.1523/JNEUROSCI.22-03-00684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Yang B-Y, De Blas AL. Clustered and non-Clustered GABAA Receptors in cultured Hippocampal Neurons. Mol Cell Neurosci. 2006;31:1–14. doi: 10.1016/j.mcn.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Chung EK, Chen LW, Chan YS, Yung KK. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J Comp Neurol. 2008;511:421–437. doi: 10.1002/cne.21852. [DOI] [PubMed] [Google Scholar]
- De Blas AL. Monoclonal antibodies to specific astroglial and neuronal antigens reveal the cytoarchitecture of the Bergmann alia fibers in the cerebellum. J Neurosci. 1984;4:265–273. doi: 10.1523/JNEUROSCI.04-01-00265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Blas AL, Cherwinski HM. Detection of antigens on nitrocellulose paper immunoblots with monoclonal antibodies. Anal Biochem. 1983;133:214–219. doi: 10.1016/0003-2697(83)90245-2. [DOI] [PubMed] [Google Scholar]
- De Blas AL, Vitorica J, Friedrich P. Localization of the GABAA receptor in the rat brain with a monoclonal antibody to the 57,000 Mr peptide of the GABAA receptor/benzodiazepine receptor/Cl-channel complex. J Neurosci. 1988;8:602–614. doi: 10.1523/JNEUROSCI.08-02-00602.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert M, de Blas AL, Mohler H, Seeburg PH. A prominent epitope on GABAA receptors is recognized by two different monoclonal antibodies. Brain Res. 1992;569:57–62. doi: 10.1016/0006-8993(92)90368-j. [DOI] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-α gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Feng G, Tintrup H, Kirsch J, Nichol MC, Kuhse J, Betz H, Sanes JR. Dual requirement for gephyrin in glycine receptor clustering and molybdoenzyme activity. Science. 1998;282:1321–1324. doi: 10.1126/science.282.5392.1321. [DOI] [PubMed] [Google Scholar]
- Figiel M, Engele J. Pituitary adenylate cyclase-activating polypeptide (PACAP), a neuron-derived peptide regulating glial glutamate transport and metabolism. J Neurosci. 2000;20:3596–3605. doi: 10.1523/JNEUROSCI.20-10-03596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DK, Rao A, Crump FT, Craig AM. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium/ calmodulin-dependent kinase II. J Neurosci. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Monreal M, Kang S, Phillips GR. Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons. Mol Cell Neurosci. 2009;40:344–353. doi: 10.1016/j.mcn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Ebert M, Shan W, Phillips GR, Arndt K, Colman DR, Kemler R. Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci. 2005;29:603–616. doi: 10.1016/j.mcn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Frank M, Kemler R. Protocadherins. Curr Opin Cell Biol. 2002;14:557–562. doi: 10.1016/s0955-0674(02)00365-4. [DOI] [PubMed] [Google Scholar]
- Fried G, Andersson E, Csöregh L, Enmark E, Gustafsson JA, Aanesen A, Osterlund C. Estrogen receptor beta is expressed in human embryonic brain cells and is regulated by 17beta-estradiol. Eur J Neurosci. 2004;20:2345–2354. doi: 10.1111/j.1460-9568.2004.03693.x. [DOI] [PubMed] [Google Scholar]
- Gambino F, Kneib M, Pavlowsky A, Skala H, Heitz S, Vitale N, Poulain B, Khelfaoui M, Chelly J, Billuart P, Humeau Y. IL1RAPL1 controls inhibitory networks during cerebellar development in mice. Eur J Neurosci. 2009;30:1476–1486. doi: 10.1111/j.1460-9568.2009.06975.x. [DOI] [PubMed] [Google Scholar]
- Garrett AM, Weiner JA. Control of CNS synapse development byγ-protocadherin-mediated astrocyte-neuron contact. J Neurosci. 2009;29:11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustetto M, Kirsch J, Fritschy JM, Cantino D, Sassoe-Pognetto M. Localization of the clustering protein gephyrin at GABAergic synapses in the main olfactory bulb of the rat. J Comp Neurol. 1998;395:231–244. doi: 10.1002/(sici)1096-9861(19980601)395:2<231::aid-cne7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Goslin K, Asmussen H, Banker G. Rat hippocampal neurons in low density culture. In: Banker G, Goslin K, editors. Culturing Nerve Cells. 2. MIT Press; Cambridge, MA: 1998. pp. 339–370. [Google Scholar]
- Haas IG, Frank M, Véron N, Kemler R. Presenilin-dependent processing and nuclear function of gamma-protocadherins. J Biol Chem. 2005;280:9313–9319. doi: 10.1074/jbc.M412909200. [DOI] [PubMed] [Google Scholar]
- Hambsch B, Grinevich V, Seeburg PH, Schwarz MK. γ-Protocadherins, presenilin-mediated release of C-terminal fragment promotes locus expression. J Biol Chem. 2005;280:15888–15897. doi: 10.1074/jbc.M414359200. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Hinds PL. Synapse formation in the mouse olfactory bulb. I. Quantitative studies. J Comp Neurol. 1976;169:15–40. doi: 10.1002/cne.901690103. [DOI] [PubMed] [Google Scholar]
- Junghans D, Haas IG, Kemler R. Mammalian cadherins and protocadherins: about cell death, synapses and processing. Curr Opin Cell Biol. 2005;17:446–452. doi: 10.1016/j.ceb.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kallenbach S, Khantane S, Carroll P, Gayet O, Alonso S, Henderson CE, Dudley K. Changes in subcellular distribution of protocadherin gamma proteins accompany maturation of spinal neurons. J Neurosci Res. 2003;72:549–556. doi: 10.1002/jnr.10618. [DOI] [PubMed] [Google Scholar]
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T. Allelic gene regulation of Pcdh-α and Pcdh-γ clusters involving both monoallelic and biallelic expression in single purkinje cells. J Biol Chem. 2006;281:30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- Kellenberger E, Hayat MA. In: Colloidal Gold: Principles, Methods, and Applications. Hayat MA, editor. Vol. 3. San Diego, CA: Academic Press, Inc; 1991. pp. 21–24. Chapter 1. [Google Scholar]
- Kirsch J, Betz H. Widespread expression of gephyrin, a putative glycine receptor-tubulin linker protein, in rat brain. Brain Res. 1993;621:301–310. doi: 10.1016/0006-8993(93)90120-c. [DOI] [PubMed] [Google Scholar]
- Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneussel M, Brandstatter JH, Laube B, Stahl S, Muller U, Betz H. Loss of postsynaptic GABA(A) receptor clustering in gephyrin-deficient mice. J Neurosci. 1999;19:9289–9297. doi: 10.1523/JNEUROSCI.19-21-09289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–4151. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, Huganir RL, De Blas AL. GRIP1 in GABAergic synapses. J Comp Neurol. 2005a;488:11–27. doi: 10.1002/cne.20566. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie SB, Miralles CP, Bai J, LoTurco JJ, De Blas AL. Disruption of GABAA Receptor Clusters Leads to Decreased GABAergic Innervation of Pyramidal Neurons. J Neurochem. 2005b;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Li X, Serwanski DR, Miralles CP, Bahr BA, De Blas AL. Two pools of Triton X-100-insoluble GABAA receptors are present in the brain, one associated to lipid rafts and another one to the post-synaptic GABAergic complex. J Neurochem. 2007;102:1329–1345. doi: 10.1111/j.1471-4159.2007.04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Serwanski DR, Miralles CP, Nagata K, De Blas AL. Septin 11 is present in GABAergic synapses and plays a functional role in the cytoarchitecture of neurons and GABAergic synaptic connectivity. J Biol Chem. 2009;284:17253–17265. doi: 10.1074/jbc.M109.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Melone M, Burette A, Weinberg RJ. Light microscopic identification and immunocytochemical characterization of glutamatergic synapses in brain sections. J Comp Neurol. 2005;492:495–509. doi: 10.1002/cne.20743. [DOI] [PubMed] [Google Scholar]
- Medvedev NI, Rodríguez-Arellano JJ, Popov VI, Davies HA, Tigaret CM, Schoepfer R, Stewart MG. The glutamate receptor 2 subunit controls post-synaptic density complexity and spine shape in the dentate gyrus. Eur J Neurosci. 2008;27:315–325. doi: 10.1111/j.1460-9568.2007.06005.x. [DOI] [PubMed] [Google Scholar]
- Miralles CP, Li M, Mehta AK, Khan ZU, De Blas AL. Immunocytochemical localization of the β3 subunit of the gamma-aminobutyric acid (A) receptor in the rat brain. J Comp Neurol. 1999;413:535–548. [PubMed] [Google Scholar]
- Moon IS, Cho SJ, Seog DH, Walikonis R. Neuronal activation increases the density of eukaryotic translation initiation factor 4E mRNA clusters in dendrites of cultured hippocampal neurons. Exp Mol Med. 2009;41:601–610. doi: 10.3858/emm.2009.41.8.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JI, Piva MA, Miralles CP, De Blas AL. Immunocytochemical localization of the beta 2 subunit of the gamma-aminobutyric acid A receptor in the rat brain. J Comp Neurol. 1994;350:260–271. doi: 10.1002/cne.903500209. [DOI] [PubMed] [Google Scholar]
- Morishita H, Yagi T. Protocadherin family: diversity, structure, and function. Curr Opin Cell Biol. 2007;19:584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Murata Y, Hamada S, Morishita H, Mutoh T, Yagi T. Interaction with protocadherin-gamma regulates the cell surface expression of protocadherin-alpha. J Biol Chem. 2004;279:49508–49516. doi: 10.1074/jbc.M408771200. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to ssynaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel WH, Schmechel DE, Tappaz ML, Kopin IJ. Production of a specific antiserum to rat brain glutamic acid decarboxylase by injection of an antigen-antibody complex. Neuroscience. 1981a;6:2689–2700. doi: 10.1016/0306-4522(81)90113-5. [DOI] [PubMed] [Google Scholar]
- Oertel WH, Schmechel DE, Mugnaini E, Tappaz ML, Kopin IJ. Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neuroscience. 1981b;6:2715–2735. doi: 10.1016/0306-4522(81)90115-9. [DOI] [PubMed] [Google Scholar]
- Obata S, Sago H, Mori N, Rochelle JM, Seldin MF, Davidson M, St John T, Taketani S, Suzuki ST. Protocadherin Pcdh2 shows properties similar to, but distinct from, those of classical cadherins. J Cell Sci. 1995;108:3765–3773. doi: 10.1242/jcs.108.12.3765. [DOI] [PubMed] [Google Scholar]
- Panzanelli P, Fritschy JM, Yanagawa Y, Obata K, Sassoè-Pognetto M. GABAergic phenotype of periglomerular cells in the rodent olfactory bulb. J Comp Neurol. 2007;502:990–1002. doi: 10.1002/cne.21356. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F, Simler R, Grenningloh G, Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci USA. 1984;81:7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Tanaka H, Frank M, Elste A, Fidler L, Benson DL, Colman DR. γ-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci. 2003;23:5096–5104. doi: 10.1523/JNEUROSCI.23-12-05096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad T, Wang X, Gray PA, Weiner JA. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: insights from genetic analyses of the protocadherin-gamma gene cluster. Development. 2008;135:4153–4164. doi: 10.1242/dev.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redies C, Ast M, Nakagawa S, Takeichi M, Martinez-de-la-Torre M, Puelles L. Morphologic fate of diencephalic prosomeres and their subdivisions revealed by mapping cadherin expression. J Comp Neurol. 2000;421:481–514. doi: 10.1002/(sici)1096-9861(20000612)421:4<481::aid-cne3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Haas IG, Schulte M, Ludwig A, Frank M, Saftig P. Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion. J Biol Chem. 2006;281:21735–21744. doi: 10.1074/jbc.M602663200. [DOI] [PubMed] [Google Scholar]
- Riquelme R, Miralles CP, De Blas AL. Bergmann glia GABAA receptors concentrate on the glial processes that wrap inhibitory synapses. J Neurosci. 2002;22:10720–10730. doi: 10.1523/JNEUROSCI.22-24-10720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Gudsnuk KA, Smith Y, Ryugo DK. Revealing the molecular layer of the primate dorsal cochlear nucleus. Neuroscience. 2008;154:99–113. doi: 10.1016/j.neuroscience.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Tanihara H, Heimark RL, Obata S, Davidson M, St John T, Taketani S, Suzuki S. Protocadherins: a large family of cadherin-related molecules in central nervous system. EMBO J. 1993;12:2249–2256. doi: 10.1002/j.1460-2075.1993.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sans N, Vissel B, Petralia RS, Wang YX, Chang K, Royle GA, Wang CY, O’Gorman S, Heinemann SF, Wenthold RJ. Aberrant formation of glutamate receptor complexes in hippocampal neurons of mice lacking the GluR2 AMPA receptor subunit. J Neurosci. 2003;23:9367–9373. doi: 10.1523/JNEUROSCI.23-28-09367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Colman DR. The diversity of cadherins and implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- Su KY, Chien WL, Fu WM, Yu IS, Huang HP, Huang PH, Lin SR, Shih JY, Lin YL, Hsueh YP, Yang PC, Lin SW. Mice deficient in collapsin response mediator protein-1 exhibit impaired long-term potentiation and impaired spatial learning and memory. J Neurosci. 2007;27:2513–2524. doi: 10.1523/JNEUROSCI.4497-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama C. Formation of GABAergic synapses in the cerebellum. Cerebellum. 2005;4:171–177. doi: 10.1080/14734220510008012. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Raghavendra V, Nutile-McMenemy N, Marks A, Deleo JA. Role of astrocytic S100beta in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience. 2006;140:1003–1010. doi: 10.1016/j.neuroscience.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Tyndall SJ, Walikonis RS. The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell Cycle. 2006;5:1560–1568. doi: 10.4161/cc.5.14.2918. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viltono L, Patrizi A, Fritschy JM, Sassoè-Pognetto M. Synaptogenesis in the cerebellar cortex: differential regulation of gephyrin and GABAA receptors at somatic and dendritic synapses of Purkinje cells. J Comp Neurol. 2008;508:579–591. doi: 10.1002/cne.21713. [DOI] [PubMed] [Google Scholar]
- Vissavajjhala P, Janssen WG, Hu Y, Gazzaley AH, Moran T, Hof PR, Morrison JH. Synaptic distribution of the AMPA-GluR2 subunit and its colocalization with calcium-binding proteins in rat cerebral cortex: an immunohistochemical study using a GluR2-specific monoclonal antibody. Exp Neurol. 1996;142:296–312. doi: 10.1006/exnr.1996.0199. [DOI] [PubMed] [Google Scholar]
- Vitorica J, Park D, Chin G, De Blas AL. Monoclonal antibodies and conventional antisera to the GABAA receptor/benzodiazepine receptor/Cl− channel complex. J Neurosci. 1988;8:615–622. doi: 10.1523/JNEUROSCI.08-02-00615.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Weiner JA, Levi S, Craig AM, Bradley A, Sanes JR. γ-protocadherins are required for survival of spinal interneurons. Neuron. 2002;36:843–854. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Wang X, Tapia JC, Sanes JR. γ-protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:5–7. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. Comparative genomics and diversifying selection of the clustered vertebrate protocadherin genes. Genetics. 2005;169:2179–2188. doi: 10.1534/genetics.104.037606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhension genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang T, Cheng JF, Kim Y, Grimwood J, Schmutz J, Dickson M, Noonan JP, Zhang MQ, Myers RM, Maniatis T. Comparative DNA sequence analysis of mouse and human protocadherin gene clusters. Genome Res. 2001;11:389–404. doi: 10.1101/gr.167301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T. Clustered protocadherin family. Dev Growth Differ. 2008;50(Suppl 1):S131–140. doi: 10.1111/j.1440-169X.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Yu W, Jiang M, Miralles CP, Li RW, Chen G, De Blas AL. Gephyrin clustering is required for the stability of GABAergic synapses. Mol Cell Neurosci. 2007;36:484–500. doi: 10.1016/j.mcn.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Charych EI, Serwanski DR, Li RW, Ali R, Bahr BA, De Blas AL. Gephyrin interacts with the glutamate receptor interacting protein 1 isoforms at GABAergic synapses. J Neurochem. 2008;105:2300–2314. doi: 10.1111/j.1471-4159.2008.05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, De Blas AL. Gephyrin expression and clustering affects the size of glutamatergic synaptic contacts. J Neurochem. 2008;104:830–845. doi: 10.1111/j.1471-4159.2007.05014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C, Huang W, Ying G, Wu Q. Sequence analysis and expression mapping of the rat clustered protocadherin gene repertoires. Neuroscience. 2007;144:579–603. doi: 10.1016/j.neuroscience.2006.10.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.