Abstract
Cerebrovascular disease may contribute to the development and progression of Alzheimer’s disease (AD). This study investigated whether impairments in cerebral hemodynamics can be detected in early-stage AD. Nine patients with mild AD and eight cognitively normal controls matched for age underwent brain magnetic resonance imaging and neuropsychological evaluation, followed by assessment of steady-state cerebral blood flow velocity (CBFV, transcranial Doppler), blood pressure (BP, Finapres), and cerebrovascular resistance index (BP/CBFV). Cerebral hemodynamics were quantified using spectral and transfer function analysis of BP and CBFV in rest, during standing up after squat, and during repeated squat-stand maneuvers. Compared to controls, AD patients had lower CBFV and higher cerebrovascular resistance index, unexplained by brain atrophy. Low-frequency variability of BP was enhanced, suggesting impaired arterial baroreflex function. However, CBFV variability was reduced despite enhanced BP variability, and dynamic cerebral autoregulation was not impaired. In conclusion, despite a distinct pattern of altered cerebral hemodynamics, AD patients may have normal autoregulation. However, the challenges for autoregulation in AD are higher, as our data show enhanced BP fluctuations. Increased cerebral vasoconstriction or reduced vasomotion also may attenuate CBFV variability.
Keywords: Alzheimer’s disease, cardiovascular physiology, cerebral autoregulation, transcranial Doppler ultrasonography
INTRODUCTION
Vascular pathology plays an important role in the development and progression of Alzheimer’s disease (AD) [1–6], and vascular risk factors increase the risk of developing the disease [7]. Brain autopsy studies have observed that both large and small-vessel disease are extensively present in AD [1,8]. In animal models of AD, these changes led to impaired cerebral autoregulation – the vascular mechanisms to maintain cerebral perfusion – thereby increasing the risk of brain ischemia [9]. However, at present it remains unknown whether cerebral autoregulation is also impaired in AD. This is of particular relevance as there is preliminary evidence which suggests that blood pressure (BP) regulation may be affected in AD [10,11], e.g., manifested as a reduced baroreflex function [12].
Transcranial Doppler ultrasonography(TCD) allows measurements of cerebral blood flow velocity (CBFV) in the major cerebral arteries. Registered together with BP, synchronized data of beat-to-beat variability in BP and CBFV are obtained, and transfer function analysis, a method based on spectral analysis of beat-to-beat data, can then be used to quantify dynamic cerebral autoregulation [13,14]. In this pilot study, we used this method to assess BP regulation and dynamic cerebral autoregulation in patients with early AD.
MATERIALS AND METHODS
Subjects
After having screened 11 patients and 12 controls, we included 9 patients with a diagnosis of probable AD according to NINCDS-ADRDA criteria, with a clinical dementia rating scale of 0.5 (n = 3) or 1.0 (n = 6), and 8 controls, matched for age and level of education, recruited from the Alzheimer Disease Center at UT Southwestern Medical Center, Dallas. Subjects with acute or chronic knee or hip conditions, which would prevent them from performing squat-stand maneuvers (2 controls), and subjects without an adequate acoustic window, preventing high quality TCD signal detection (1 control, 1 AD), were excluded during the initial screening process. Subsequently, 2 subjects (1 control, 1 AD) were excluded because of poor finger BP and TCD signal quality during measurements. The study was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas. All participants signed an informed consent form. In AD patients, additional informed consent was obtained by proxy. AD patients and their controls underwent a comprehensive clinical-neuropsychological evaluation and brain imaging using magnetic resonance imaging (MRI). Characteristics of the study participants are listed in Table 1.
Table 1.
Characteristics of the study participants
| Characteristic | Alzheimer’s disease (N = 9) |
Aging controls (N = 8) |
P value |
|---|---|---|---|
| Age-yrs | 67.9 (5.5) | 64.5 (4.0) | 0.17 |
| Gender -male:female | 3:6 | 4:4 | |
| MMSE | 25 (3.2) | 29 (0.5) | 0.03 |
| Word list recall (number of words) | 2 (1.9) | 8 (1.3) | 0.0001 |
| Paragraph recall (sentences) | 1 (2.1) | 18 (3.9) | 0.0001 |
| TMT-A-T score | 40 (18) | 58 (15) | 0.068 |
| TMT-B-T score | 40 (11) | 59 (10) | 0.014 |
| CDR-sum of boxes | 4 (1.2) | 0.1 (0.2) | 0.0001 |
| Total brain volume (ml) | 1081 (65) | 1108 (79) | 0.53 |
| White matter lesions (ml) | 14 (10.4) | 8 (5.8) | 0.25 |
| Vascular risk factors (no. of subjects) | |||
| Hypertension | 4 | 3 | |
| Diabetes | 0 | 0 | |
| Smoking | 0 | 0 | |
| Medication | |||
| Antihypertensive drugs | 4 | 3 | |
| Lipid-lowering drugs | 4 | 2 | |
| Cholinesterase-inhibitor | 8 | – | |
| NMDA-receptor antagonist | 8 | – |
MMSE: Mini-Mental State Examination; TMT: trail making test (parts A and B); CDR: clinical dementia rating scale.
Cerebral autoregulation
The brain can adapt cerebrovascular resistance in response to changes in BP to maintain a relatively stable CBF [14,15]. Static autoregulation refers to the stability of CBF within a certain range of BP values that fall within the upper and lower limits of autoregulation [15]. This concept, however, is based on BP and CBF measurements averaged over at least several minutes. Recent methods allow the measurement of changes in CBF to brief (seconds) changes in BP to reflect rapid adaptation of the cerebral vasculature. This concept is known as dynamic cerebral autoregulation [14].
Hemodynamic measurements
All measurements were performed at the same time of day (morning), in the same quiet laboratory with constant ambient temperature.
BP was measured in the finger by photoplethysmography (Finapres, Ohmeda, Englewood, CO), a validated method for hemodynamic research [16].
The hand with the Finapres cuff was held steadily in place at heart level, with the arm supported by straps attached to a custom-designed vest, and the hand fixed against the chest with a Velcro glove. CBFV was obtained in the middle cerebral artery (MCA) on one side by transcranial Doppler ultrasonography (DWL Elektronische Systeme, Germany) [13]. End-tidal CO2 was monitored with a nasal cannula using capnography (Criticare Systems, Inc., Waukesha, WI). In addition, peripheral arterial saturation (pulse-oximetry) and 3-lead electrocardiography were recorded.
After at least 10 min rest in sitting position, 5-min segments of BP and CBFV data were recorded during spontaneous respiration. To quantify dynamic cerebral autoregulation, these data were used for spectral analysis of spontaneous oscillations in BP and CBFV. This method is known as transfer function analysis of spontaneous oscillations in BP and CBFV [13].
To enhance the magnitude of alterations in BP and CBF, and to mimic such changes in daily life associated with changes in body posture, oscillations in BP and CBFV were induced by repeated squat-stand maneuvers [17]. After careful instruction and practice, participants were coached into performing these maneuvers at a frequency of 0.025 Hz (20 s squat followed by 20 s standing up) for 5 min, 0.05 Hz (10 s squat, 10 s stand) for 4 min and at 0.1 Hz (5 s squat, 5 s stand) for 3 min, separated by 10 min of recovery. Physical assistance during standing up from squat was provided to aid the performance of these maneuvers, and patients and controls were provided auditory support by a metronome as well as verbal instruction to maintain precise rhythm of the maneuvers.
The AD patients had their caregiver present in the laboratory during all tests and were constantly monitored by a research nurse and a geriatrician. Neither patients nor controls showed signs of stress or anxiety before, during, or after testing.
Transfer function analysis of dynamic cerebral autoregulation
The method of transfer function analysis, which provides the parameters of gain, phase, and coherence, has been described in detail previously [13].
Transfer gain quantifies how the amplitudes of the changes (oscillations) in BP at different frequencies are transmitted to CBFV; a lower gain implies that these oscillations are reduced (damped) either by dynamic cerebral autoregulation or increases in the cerebrovascular impedance [18].
Transfer function phase reflects the time relationship between oscillations in BP and CBFV. Coherence can be compared to a linear correlation coefficient and quantifies to what extent changes in CBFV are linearly associated with changes in BP.
MRI measurements
T2-weighted and Fluid-Attenuated Inversion Recovery (FLAIR) images were obtained using a Philips Intera 1.5T scanner. Images were converted from DICOM to ANALYZE format to facilitate analysis. Segmentation of skull and connective tissue was conducted using the skull strip function in the DICOM image editor MRIcro. White matter lesion volume and whole brain volume were measured using an image processing tool developed with MATLAB (The MathWorks, Inc., Natick, MA) [19].
Standing up following a single squat
To investigate the effect of a single hypotensive challenge to cerebral perfusion, we measured the hemodynamic effects of a single squat (60 s) followed by standing up in 5 aging controls and 6 AD patients.
Statistical analysis
Comparisons were made between the AD patients and the healthy age-matched controls. For normally distributed data, two-tailed Student t-test was used. Not all data resulting from transfer function analysis were normally distributed. Here, Student t-test was repeated after log-transformation of the data, and in addition non-parametric testing was used (Wilcoxon rank test). Linear regression analysis was used to explore the relationship between BP and CBFV spectral power. Statistical significance was defined as p < 0.05. Data are presented as mean and standard deviation (SD).
RESULTS
Patient characteristics
AD patients had relatively mild cognitive deficits consistent with early stage of the disease (Table 1) but were clearly impaired in episodic memory. As expected due to the diagnostic criteria for probable AD, patients had no overt cerebrovascular disease on MRI. However, volumetric analysis revealed a trend towards more white matter lesions in AD. Whole brain volume was not significantly different.
Baseline hemodynamics are presented in Table 2. Despite the early stage of disease, AD patients had a substantially lower CBFV and higher cerebrovascular resistance compared with the controls.
Table 2.
Baseline hemodynamic parameters
| Alzheimer’s Disease (N = 9) |
Aging Controls (N = 8) |
P Value | |
|---|---|---|---|
| Systolic blood pressure-mmHg | 140 (19.8) | 126 (26.5) | 0.23 |
| Diastolic blood pressure-mmHg | 74 (9.0) | 73 (9.8) | 0.91 |
| Mean arterial pressure-mmHg | 101 (12.4) | 95 (15.8) | 0.39 |
| Mean arterial pressure-mmHg (from Finapres) | 98 (12.4) | 94 (10.9) | 0.39 |
| Cerebral blood flow-velocity-cm/s | 38 (7.1) | 55 (19.0) | 0.05 |
| Cerebrovascular resistance-mmHg/cm/s | 2.7 (0.7) | 1.9 (0.6) | 0.03 |
Cerebral hemodynamics
Figure 1 shows an example of spontaneous oscillations in BP and CBFV, as well as the oscillations that were induced by repeated squat-stand maneuvers, in one patient with AD and one control subject. Note that oscillations are larger in AD for BP, but smaller for CBFV, compared to the healthy control.
Fig. 1.
Spontaneous and induced oscillations in blood pressure and cerebral blood flow velocity. Example from two representative subjects, one with AD and one normal control, demonstrating spontaneous oscillations in blood pressure and cerebral blood flow-velocity over a period of 5 min (panel A). Panel B through D depict the effect of repeated squat-stand maneuvers at different intervals on these oscillations. Recording periods are 5, 4 and 3 min respectively. Panel B shows how repeated squat-stand intervals at 20 s lead to oscillations at 0.025 Hz. Panel C: 10 s intervals with oscillations at 0.05 Hz. Panel D: 5 s intervals with oscillations at 0.1 Hz.
Figure 2 displays the results of spectral analysis of oscillations in BP and CBFV. Note the larger spectral power in AD for BP oscillations, but not for CBFV.
Fig. 2.
Spectral density plots of oscillations in blood pressure and cerebral blood flow velocity. Individual power spectral density plots for aged controls and AD patients, for blood pressure (upper pane), and cerebral blood flow-velocity (lower pane). Spectral power is a measure indicating the strength of oscillations at the specified frequency. The Y-axis shows the magnitude of the observed oscillations, and the X-axis displays the frequency of these oscillations. Note the much stronger oscillations in blood pressure in the very low frequency range (0.02–0.07 Hz) in AD patients, with reduced oscillations in cerebral blood flow-velocity.
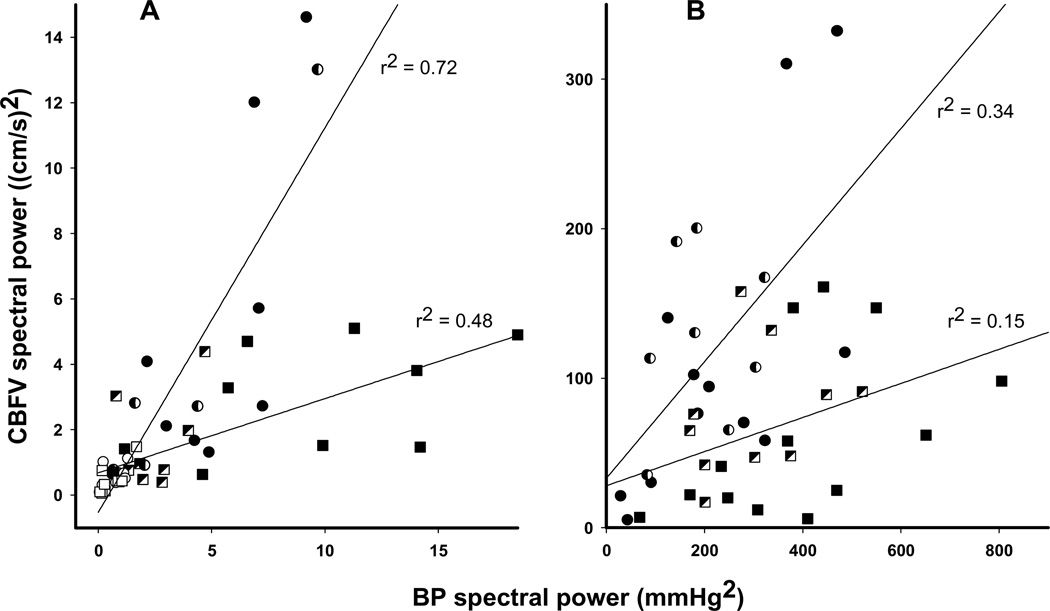
The relationship between BP and CBFV spectral power, for spontaneous as well as for induced oscillations, is plotted in Fig. 3. Compared with healthy controls, patients with AD display a low CBFV spectral power relative to higher BP spectral power, both for spontaneous oscillations and for oscillations induced by the repeated squat-stand maneuvers. In other words, in AD, larger variability in BP was associated with reduced, rather than increased, variability in CBFV.
Fig. 3.
Correlation between spectral power of blood pressure (PSBP) and cerebral blood flow velocity (PSCBFV). A: spontaneous oscillations during rest. B: oscillations induced by repeated squat-stand maneuvers at 0.025, 0.05 and 0.1 Hz. For each individual, PSBP is plotted against PSCBFV. ■: AD patients. ●: normal controls. Filled symbols: very low frequency (0.02–0.07 Hz, A) and 0.025 and 0.05 Hz maneuvers (B), half-filled symbols: low frequency (0.07–0.2 Hz, A) and 0.1 Hz maneuvers (B), open symbols: high frequency (0.2–0.35 Hz, A). The correlation between PSBP and PSCBFV (p < 0.05) is less strong in AD patients than in controls. In addition, the slope of the regression lines differs between patients and controls (A: 1.18 vs. 0.23, B: 0.39 vs. 0.11, p = 0.01), indicating lower PSCBFV relative to PSBP in AD patients.
Group mean values for BP and CBFV spectral power are presented in Table 3.
Table 3.
Spectral power of spontaneous and induced oscillations in blood pressure and cerebral blood flow velocity
| Alzheimer’s Disease | Aging Controls | P Value | |
|---|---|---|---|
| Spontaneous oscillations | N = 9 | N = 8 | |
| PS BP – mmHg2 | |||
| VLF | 9.6 (5.5) | 5.6 (2.4) | 0.08 |
| LF | 2.4 (1.4) | 2.6 (3.1) | 0.84 |
| HF | 0.5 (0.6) | 0.6 (0.5) | 0.69 |
| PS CBFV – (cm/s)2 | |||
| VLF | 3.0 (1.7) | 5.5 (5.1) | 0.18 |
| LF | 1.5 (1.4) | 2.8 (4.2) | 0.43 |
| HF | 0.4 (0.5) | 0.5 (0.4) | 0.68 |
| Induced oscillations at 0.025 Hz | N = 4 | N = 4 | |
| PS BP – mmHg2 | 239 (150.6) | 86.3 (67.3) | 0.11 |
| PS CBFV – (cm/s)2 | 11.8 (7.3) | 39.5 (42.9) | 0.27 |
| Induced oscillations at 0.05 Hz | N = 9 | N = 8 | |
| PS BP – mmHg2 | 460.9 (185.4) | 306.8 (131.2) | 0.06 |
| PS CBFV – (cm/s)2 | 84.3 (55.5) | 149.6 (109.1) | 0.16 |
| Induced oscillations at 0.1 Hz | N = 9 | N = 8 | |
| PS BP – mmHg2 | 315 (117.8) | 195.3 (90.8) | 0.03 |
| PS CBFV – (cm/s)2 | 77.8 (45.4) | 126 (58.5) | 0.08 |
BP: blood pressure; CBFV: cerebral blood flow-velocity; PS: power spectrum, indicating the magnitude of the observed oscillations within the specified frequency range; VLF: very low frequency oscillations, 0.02–0.07 Hz; LF: low frequency, 0.07–0.2 Hz; HF: high frequency, 0.2–0.35 Hz. Spontaneous oscillations were recorded during five minutes of rest in sitting position. Induced oscillations were provoked by repeated squat-stand maneuvers at 20/20, 10/10 and 5/5 s intervals (0.025, 0.05 and 0.1 Hz respectively).
Individual differences in the magnitude of BP oscillations could not be explained by a higher or lower baseline BP, or by a history of hypertension.
Estimates of transfer function gain, phase, and coherence are depicted in Fig. 4. Transfer function gain for induced oscillations at the frequencies of 0.05 and 0.1 Hz was reduced by ~30% in AD relative to normal controls (p = 0.01). However, for spontaneous oscillations this difference was not significant (p = 0.18).
Fig. 4.
Transfer function analysis of spontaneous and induced oscillations in blood pressure and cerebral blood flow velocity. Group-averaged data showing results of transfer function analysis of spontaneous oscillations (left) in blood pressure (BP) and cerebral blood flow-velocity (CBFV) as well as of oscillations induced by repeated squat-stand maneuvers (right). ■: AD patients.●: normal controls. *: p < 0.05; ** p < 0.01. N = 9 (AD patients) resp. 8 (controls) for spontaneous oscillations and maneuvers at 0.05 and 0.1 Hz; n = 4 for maneuvers at 0.025 Hz.
The reduction in mean BP upon standing up after a single squat was slightly larger [39 mm Hg (SD 16)] in AD than in controls [26 mm Hg (SD 11)], or in relative change, a reduction of 33 versus 26% (p = 0.2). The orthostatic BP reduction following squat led to a reduction in mean CBFV of 12 (SD 4) versus 21 (SD 26) cm/s in AD versus controls, or 26 and 29% (p = 0.77). However, AD patients reached lower diastolic flow-velocity values during standing [12 (SD 7) versus 24 (SD 11) cm/s, p = 0.02].
DISCUSSION
With non-invasive monitoring of BP and CBFV using TCD, we observed a distinct pattern of changes in cerebral hemodynamics in patients with mild AD. However, dynamic cerebral autoregulation was not impaired. In summary, the observed alterations consisted of a reduced CBFV with elevated cerebrovascular resistance, not explained by brain atrophy; and reduced CBFV oscillations, despite increases in oscillations in BP, suggesting cerebral vasoconstriction. Although enhanced damping of flow oscillations occurs in AD, CBFV, especially diastolic CBFV, was profoundly reduced during transient orthostatic hypotension. This suggests that autoregulation in AD may fail to compensate for the globally reduced CBF and instability of BP leading to brain hypoperfusion under orthostatic stress.
Reduced CBFV in AD
Baseline CBFV was reduced by 40% in AD compared to age-matched controls, coupled with a 40% increase in cerebrovascular resistance. Although we only measured CBFV in the MCA (providing blood flow to ~70% of the brain), our study is consistent with findings of a reduction in total CBF of ~20% early in the disease process of AD [20,21]. It has been argued that reduction in CBF in AD could be explained by brain atrophy or by a reduction in brain metabolic demand [22]. However, differences in individual whole brain volume between our AD patients and their controls could not explain their differences in CBFV (Tables 1 and 2), suggesting that changes in CBF were not related exclusively to brain atrophy [21,23]. In addition, a reduction in brain metabolic demand in the mild stage of AD (CDR 0.5–1) is not likely to explain a 40% reduction in CBF under resting conditions [20].
Enhanced BP oscillations with reduced CBFV oscillations in AD
The finding of increased oscillations in BP, combined with reduced oscillations in CBFV, in patients with AD was unexpected and has not been reported previously. BP oscillations in the very low frequency range (between 0.02 and 0.07 Hz) are characterized by periods of 14–50 s, which translate into intervals of spontaneously elevated BP that last ~7–25 s, alternated with intervals of reduced BP of that same approximate length. The high BP spectral power in AD patients signifies the magnitude of these changes in BP (Table 3). In this study, we cannot determine the cause of these enhanced oscillations. Most likely, these data indicate that in early AD alterations occur in BP regulation, which may be related to the central impairment of baroreflex function [12]. The right insular region has an important role in the regulation of autonomic function [10,11]. As this region is affected by AD neuropathology early in the course of disease, it has been suggested that AD may be associated with autonomic dysfunction in baroreflex control of arterial pressure [11]. In a similar vein, pathology of the insula and amygdala has been implicated in central autonomic disturbances including sudden cardiac death [10].
Oscillations in CBFV were reduced in AD. This finding is striking considering that, because of the stronger oscillations in BP, oscillations in CBFV were expected to be stronger, not weaker, in AD. This may be explained by enhanced cerebral vasoconstriction or vascular deformation that have been linked to AD, such as arteriolar luminal obstruction, microvascular deformation, or endothelial dysfunction associated with amyloid-β [3–6,24]. This interpretation may bear implications for studies using functional magnetic resonance imaging (fMRI) in AD. fMRI measures neuronal activity indirectly through the blood oxygen level dependent hemodynamic response function. It is interesting to note that the amplitude of this hemodynamic response is reduced in early AD during brain activation [25]. Although this is usually taken to represent altered neural activity and/or neurovascular coupling, in the light of the present study it may well be driven primarily by the increases in vascular resistance or impedance in AD.
Dynamic cerebral autoregulation in AD
This is the first study to assess dynamic cerebral autoregulation in patients with AD; previous human studies have investigated vasomotor reactivity to CO2 but not pressure autoregulation. Dynamic autoregulation reduces the impact on CBF of slow (low frequency) changes in BP, but becomes progressively ineffective for more rapid changes in BP (high frequency), consistent with a “high-pass filter”. Consequently, reduction in transfer function gain (and increase in phase) at lower frequencies of BP oscillations is interpreted as normal autoregulation [14]. According to this model, dynamic autoregulation was not impaired in AD, as this would have resulted in an increased rather than decreased transfer function gain, as well as reductions in phase. This is in contrast with the findings in an AD animal model where cerebral autoregulation was impaired even before vascular deposition of amyloid-β had occurred [9]. However, direct comparisons between studies in humans and animals may be difficult. In addition, only static, not dynamic, autoregulation was assessed in animal studies [9].
The lower gain (reduced CBFV oscillations) in AD may suggest that cerebral vasoconstrictive and/or vasodilatory responses to changes in BP were enhanced, thus leading to reductions in CBFV variability. This interpretation, however, is not supported by the observations during orthostatic hypotension (induced by the single squat-stand maneuver), where it was clear that cerebral autoregulation could not prevent a substantial and prolonged reduction in CBFV in AD. We therefore conclude that the reduced transfer function gain in AD most likely reflects increased cerebrovascular resistance and/or impedance associated with cerebral vasoconstriction. This is consistent with recent findings of increased cerebrovascular resistance and reduced cerebrovascular compliance in AD using perfusion MRI [21].
The reduction in gain in AD was significant only for the squat-stand maneuvers. Most likely, this is because gain calculations are statistically more reliable as the signal to noise ratio is improved due to the much larger and more coherent oscillations in BP and CBFV [17].
Study limitations
Certain limitations of this study need to be addressed. First, this is an explorative study in a small number of subjects. Second, AD patients were treated with the cholinesterase-inhibitor donepezil and the NMDA-receptor antagonist memantine, and there is evidence to suggest that cholinesterase-inhibitors may augment CBF and improve neurovascular function [26]. This could mean that the deleterious neurovascular effects of AD have been underestimated in this study. Third, some patients and controls were hypertensive or used antihypertensive drugs. Previous research has shown that neither mild to moderate hypertension per se, nor the use of antihypertensive medication, affects dynamic cerebral autoregulation [18]. Furthermore, exclusion of these patients would seriously hamper the generalizability of this study. Finally, the methodological caveats of using TCD to estimate changes in CBF have been discussed in detail before [14].
Conclusion
Patients with early AD had a distinct pattern of altered cerebral hemodynamics including reduced CBFV and increased cerebral vascular resistance. Induced BP fluctuations that might be encountered during daily life were markedly exaggerated, consistent with impaired baroreflex function. Although these BP fluctuations induced smaller changes in CBFV in AD patients (consistent with either improved dynamic autoregulation or increased cerebrovascular impedance), CBF was severely compromised during orthostatic stress, demonstrating reduced vasomotor reserve in patients with AD.
ACKNOWLEDGMENTS
This study was supported in part by Internationale Stichting Alzheimer Onderzoek (ISAO), Kelly King Foundation and NIH P30 AG12300.
Carlos Marquez de la Plata carried out the MRI volumetric analysis and Arenda van Beek analyzed the single squat-stand data. Dr. Claassen has received lecture fees from Janssen-Cilag and is a member of a Data Safety Monitoring Board for GSK.
REFERENCES
- 1.Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, Miklossy J. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 2.Aliev G, Smith MA, Obrenovich ME, de la Torre JC, Perry G. Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Alzheimer disease. Neurotox Res. 2003;5:491–504. doi: 10.1007/BF03033159. [DOI] [PubMed] [Google Scholar]
- 3.Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, Deane R, Brooks AI, Kanagala S, Rubio A, Sagare A, Liu D, Li F, Armstrong D, Gasiewicz T, Zidovetzki R, Song X, Hofman F, Zlokovic BV. Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med. 2005;11:959–965. doi: 10.1038/nm1287. [DOI] [PubMed] [Google Scholar]
- 5.Chow N, Bell RD, Deane R, Streb JW, Chen J, Brooks A, Van NW, Miano JM, Zlokovic BV. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci U S A. 2007;104:823–828. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer EP, Ulmann-Schuler A, Staufenbiel M, Krucker T. Altered morphology and 3D architecture of brain vasculature in a mouse model for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2008;105:3587–3592. doi: 10.1073/pnas.0709788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Oijen M, de Jong FJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403–410. doi: 10.1002/ana.21073. [DOI] [PubMed] [Google Scholar]
- 8.Jellinger KA, Attems J. Prevalence and impact of cerebrovascular pathology in Alzheimer’s disease and parkinsonism. Acta Neurol Scand. 2006;114:38–46. doi: 10.1111/j.1600-0404.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 9.Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002;283:H315–H323. doi: 10.1152/ajpheart.00022.2002. [DOI] [PubMed] [Google Scholar]
- 10.Cheung RT, Hachinski V. The insula and cerebrogenic sudden death. Arch Neurol. 2000;57:1685–1688. doi: 10.1001/archneur.57.12.1685. [DOI] [PubMed] [Google Scholar]
- 11.Royall DR, Gao JH, Kellogg DL., Jr Insular Alzheimer’s disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses. 2006;67:747–758. doi: 10.1016/j.mehy.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Szili-Torok T, Kalman J, Paprika D, Dibo G, Rozsa Z, Rudas L. Depressed baroreflex sensitivity in patients with Alzheimer’s and Parkinson’s disease. Neurobiol Aging. 2001;22:435–438. doi: 10.1016/s0197-4580(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 14.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 15.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 16.Sammons EL, Samani NJ, Smith SM, Rathbone WE, Bentley S, Potter JF, Panerai RB. Influence of noninvasive peripheral arterial blood pressure measurements on assessment of dynamic cerebral autoregulation. J Appl Physiol. 2007;103:369–375. doi: 10.1152/japplphysiol.00271.2007. [DOI] [PubMed] [Google Scholar]
- 17.Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol. 2008;103:153–160. doi: 10.1152/japplphysiol.90822.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Witkowski S, Fu Q, Claassen JA, Levine BD. Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension. 2007;49:1149–1155. doi: 10.1161/HYPERTENSIONAHA.106.084939. [DOI] [PubMed] [Google Scholar]
- 19.Marquez de la Plata C, Ardelean A, Koovakkattu D, Srinivasan P, Miller A, Phuong V, Harper C, Moore C, Whittemore A, Madden C, Diaz-Arrastia R, Devous M., Sr Magnetic resonance imaging of diffuse axonal injury: quantitative assessment of white matter lesion volume. J Neurotrauma. 2007;24:591–598. doi: 10.1089/neu.2006.0214. [DOI] [PubMed] [Google Scholar]
- 20.Nagata K, Buchan RJ, Yokoyama E, Kondoh Y, Sato M, Terashi H, Satoh Y, Watahiki Y, Senova M, Hirata Y, Hatazawa J. Misery perfusion with preserved vascular reactivity in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:272–281. doi: 10.1111/j.1749-6632.1997.tb48479.x. [DOI] [PubMed] [Google Scholar]
- 21.Bateman GA, Levi CR, Schofield P, Wang Y, Lovett EC. Quantitative measurement of cerebral haemodynamics in early vascular dementia and Alzheimer’s disease. J Clin Neurosci. 2006;13:563–568. doi: 10.1016/j.jocn.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Shih WJ, Ashford JW, Coupal JJ, Ryo YU, Stipp VV, Magoun SL, Gross K. Consecutive brain SPECT surface three-dimensional displays show progression of cerebral cortical abnormalities in Alzheimer’s disease. Clin Nucl Med. 1999;24:773–777. doi: 10.1097/00003072-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Spilt A, Weverling-Rijnsburger AW, Middelkoop HA, van Der Flier WM, Gussekloo J, de Craen AJ, Bollen EL, Blauw GJ, van Buchem MA, Westendorp RG. Late-onset dementia: structural brain damage and total cerebral blood flow. Radiology. 2005;236:990–995. doi: 10.1148/radiol.2363041454. [DOI] [PubMed] [Google Scholar]
- 24.de la Torre JC. Cerebromicrovascular pathology in Alzheimer’s disease compared to normal aging. Gerontology. 1997;43:26–43. doi: 10.1159/000213834. [DOI] [PubMed] [Google Scholar]
- 25.Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage. 2005;26:1078–1085. doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Claassen JA, Jansen RW. Cholinergically mediated augmentation of cerebral perfusion in Alzheimer’s disease and related cognitive disorders: the cholinergic-vascular hypothesis. J Gerontol A Biol Sci Med Sci. 2006;61:267–271. doi: 10.1093/gerona/61.3.267. [DOI] [PubMed] [Google Scholar]