Abstract
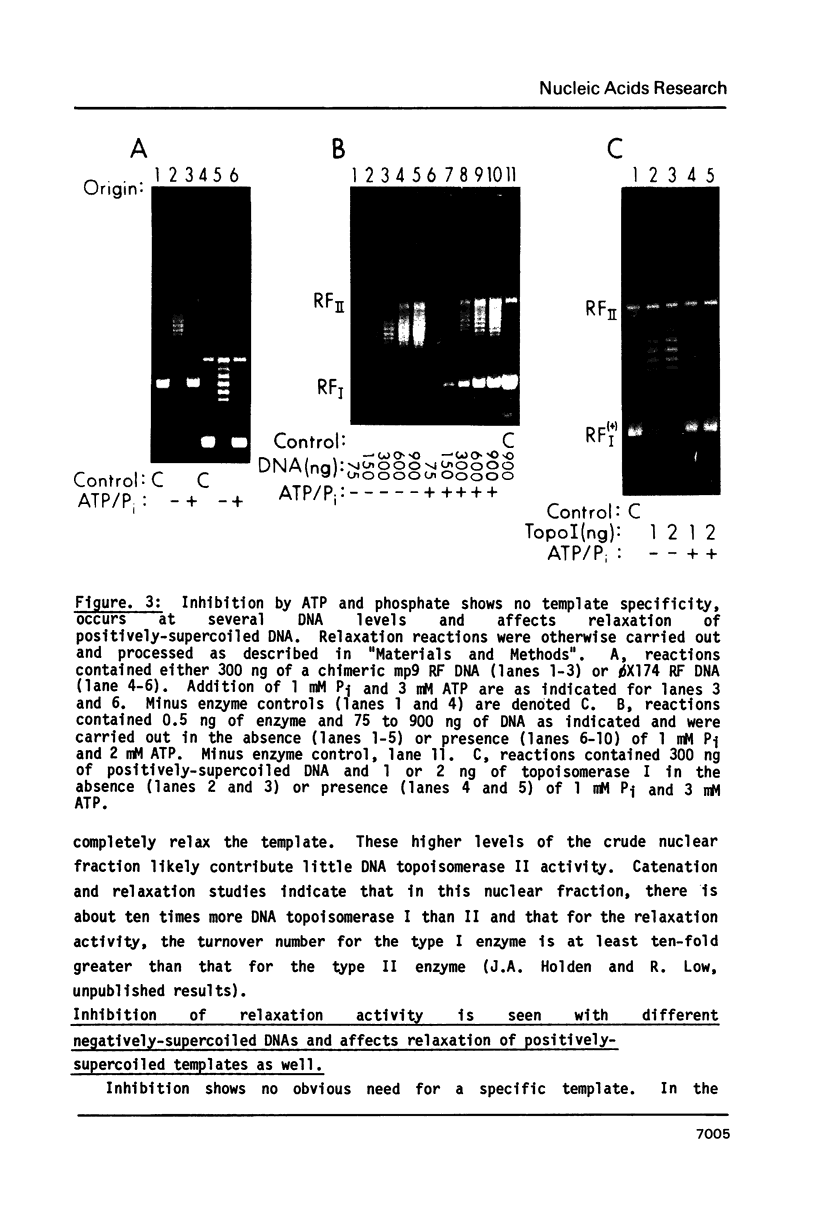
The relaxation activity of DNA topoisomerase I from HeLa cell nuclei is strongly inhibited by a variety of purine nucleotides in the presence but not absence of 1 mM potassium phosphate. For ATP, 3-4 mM causes nearly complete inhibition. The 2'-and 3'-AMP isomer are active as well in the presence of 1 mM phosphate, but the 5'-AMP isomer and adenosine are inert. At 3 mM ATP, the titration curve for phosphate is sigmoidal with inhibition beginning abruptly at about 0.5 mM. The negatively-supercoiled DNA isolated from an "inhibited" reaction is relaxed as well as the standard DNA template in the absence of ATP and phosphate suggesting that inhibition does not result from an alteration of the template which protects against its relaxation. Relaxation of positively-supercoiled DNA is also inhibited. Catalysis by E. coli DNA topoisomerase I and HeLa DNA topoisomerase II is not inhibited at concentrations of ATP and phosphate sufficient to cause 80-90% inhibition of HeLa type 1 enzyme.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerboom T. P., Bookelman H., Zuurendonk P. F., van der Meer R., Tager J. M. Intramitochondrial and extramitochondrial concentrations of adenine nucleotides and inorganic phosphate in isolated hepatocytes from fasted rats. Eur J Biochem. 1978 Mar 15;84(2):413–420. doi: 10.1111/j.1432-1033.1978.tb12182.x. [DOI] [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Been M. D., Burgess R. R., Champoux J. J. Nucleotide sequence preference at rat liver and wheat germ type 1 DNA topoisomerase breakage sites in duplex SV40 DNA. Nucleic Acids Res. 1984 Apr 11;12(7):3097–3114. doi: 10.1093/nar/12.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been M. D., Champoux J. J. Breakage of single-stranded DNA by eukaryotic type 1 topoisomerase occurs only at regions with the potential for base-pairing. J Mol Biol. 1984 Dec 15;180(3):515–531. doi: 10.1016/0022-2836(84)90025-1. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Cozzarelli N. R. Catenation and knotting of duplex DNA by type 1 topoisomerases: a mechanistic parallel with type 2 topoisomerases. Proc Natl Acad Sci U S A. 1981 Feb;78(2):843–847. doi: 10.1073/pnas.78.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc Natl Acad Sci U S A. 1972 Jan;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. Proteins that affect DNA conformation. Annu Rev Biochem. 1978;47:449–479. doi: 10.1146/annurev.bi.47.070178.002313. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Strand breakage by the DNA untwisting enzyme results in covalent attachment of the enzyme to DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3800–3804. doi: 10.1073/pnas.74.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Dean F. B., Cozzarelli N. R. Mechanism of strand passage by Escherichia coli topoisomerase I. The role of the required nick in catenation and knotting of duplex DNA. J Biol Chem. 1985 Apr 25;260(8):4984–4994. [PubMed] [Google Scholar]
- Dean F., Krasnow M. A., Otter R., Matzuk M. M., Spengler S. J., Cozzarelli N. R. Escherichia coli type-1 topoisomerases: identification, mechanism, and role in recombination. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):769–777. doi: 10.1101/sqb.1983.047.01.088. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Durban E., Mills J. S., Roll D., Busch H. Phosphorylation of purified Novikoff hepatoma topoisomerase I. Biochem Biophys Res Commun. 1983 Mar 29;111(3):897–905. doi: 10.1016/0006-291x(83)91384-0. [DOI] [PubMed] [Google Scholar]
- Edwards K. A., Halligan B. D., Davis J. L., Nivera N. L., Liu L. F. Recognition sites of eukaryotic DNA topoisomerase I: DNA nucleotide sequencing analysis of topo I cleavage sites on SV40 DNA. Nucleic Acids Res. 1982 Apr 24;10(8):2565–2576. doi: 10.1093/nar/10.8.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro A. M., Olivera B. M. Poly(ADP-ribosylation) of DNA topoisomerase I from calf thymus. J Biol Chem. 1984 Jan 10;259(1):547–554. [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Jongstra-Bilen J., Ittel M. E., Niedergang C., Vosberg H. P., Mandel P. DNA topoisomerase I from calf thymus is inhibited in vitro by poly(ADP-ribosylation). Eur J Biochem. 1983 Nov 2;136(2):391–396. doi: 10.1111/j.1432-1033.1983.tb07754.x. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Pflugfelder G., Wang J. C. The cleavage of DNA by type-I DNA topoisomerases. Cold Spring Harb Symp Quant Biol. 1984;49:411–419. doi: 10.1101/sqb.1984.049.01.047. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15(1):1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- Liu L. F. HeLa toposiomerase I. Methods Enzymol. 1983;100:133–137. [PubMed] [Google Scholar]
- Liu L. F., Miller K. G. Eukaryotic DNA topoisomerases: two forms of type I DNA topoisomerases from HeLa cell nuclei. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3487–3491. doi: 10.1073/pnas.78.6.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Micrococcus luteus DNA gyrase: active components and a model for its supercoiling of DNA. Proc Natl Acad Sci U S A. 1978 May;75(5):2098–2102. doi: 10.1073/pnas.75.5.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low R. L., Kaguni J. M., Kornberg A. Potent catenation of supercoiled and gapped DNA circles by topoisomerase I in the presence of a hydrophilic polymer. J Biol Chem. 1984 Apr 10;259(7):4576–4581. [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Prell B., Vosberg H. P. Analysis of covalent complexes formed between calf thymus DNA topoisomerase and single-stranded DNA. Eur J Biochem. 1980 Jul;108(2):389–398. doi: 10.1111/j.1432-1033.1980.tb04734.x. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Rowe T. C., Rusche J. R., Brougham M. J., Holloman W. K. Purification and properties of a topoisomerase from Ustilago maydis. J Biol Chem. 1981 Oct 25;256(20):10354–10361. [PubMed] [Google Scholar]
- Sander M., Nolan J. M., Hsieh T. A protein kinase activity tightly associated with Drosophila type II DNA topoisomerase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6938–6942. doi: 10.1073/pnas.81.22.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse-Dinh Y. C., Wong T. W., Goldberg A. R. Virus- and cell-encoded tyrosine protein kinases inactivate DNA topoisomerases in vitro. Nature. 1984 Dec 20;312(5996):785–786. doi: 10.1038/312785a0. [DOI] [PubMed] [Google Scholar]
- Tse Y. C., Kirkegaard K., Wang J. C. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J Biol Chem. 1980 Jun 25;255(12):5560–5565. [PubMed] [Google Scholar]
- Wang J. C., Becherer K. Cloning of the gene topA encoding for DNA topoisomerase I and the physical mapping of the cysB-topA-trp region of Escherichia coli. Nucleic Acids Res. 1983 Mar 25;11(6):1773–1790. doi: 10.1093/nar/11.6.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. Interaction between DNA and an Escherichia coli protein omega. J Mol Biol. 1971 Feb 14;55(3):523–533. doi: 10.1016/0022-2836(71)90334-2. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]