Abstract
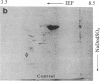
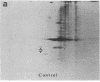
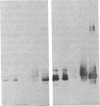
A 37-kDa glycoprotein has been described recently, whose synthesis is dramatically increased after injury of the rat sciatic and optic nerves. Cells in the nerve sheath, distal to the site of injury, produce and secrete large amounts of this protein, so that by 3 weeks after injury, it represents 2-5% of the total soluble extracellular protein in the regenerating sciatic nerve sheath, although it fails to accumulate in damaged optic nerve. Results presented here reveal extensive homology between the 37-kDa nerve injury-induced protein and a well-studied serum protein, apolipoprotein E (apoE), that is involved in lipid and cholesterol metabolism and that has been shown recently to be present in adult and developing rat astroglia. Both proteins have identical isoelectric focusing points and similar molecular masses. Antibodies raised against the 37-kDa protein recognize apoE and anti-apoE serum crossreacts with the 37-kDa protein. Sequence data for two 14 amino acid stretches of the 37-kDa protein match identical regions of apoE. These data suggest that the 37-kDa protein is identical to serum apoE and that it could have similar functions to the latter. In the nervous system, for example, it may be involved in the mobilization and reutilization of lipid in the repair, growth, and maintenance of myelin and axonal membranes, both during development and after injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila E. M., Holdsworth G., Sasaki N., Jackson R. L., Harmony J. A. Apoprotein E suppresses phytohemagglutinin-activated phospholipid turnover in peripheral blood mononuclear cells. J Biol Chem. 1982 May 25;257(10):5900–5909. [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Benfey M., Aguayo A. J. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982 Mar 11;296(5853):150–152. doi: 10.1038/296150a0. [DOI] [PubMed] [Google Scholar]
- Bignami A., Dahl D., Nguyen B. T., Crosby C. J. The fate of axonal debris in Wallerian degeneration of rat optic and sciatic nerves. Electron microscopy and immunofluorescence studies with neurofilament antisera. J Neuropathol Exp Neurol. 1981 Sep;40(5):537–550. doi: 10.1097/00005072-198109000-00005. [DOI] [PubMed] [Google Scholar]
- Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985 Oct;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Eto Y., Suzuki K., Suzuki K. Lipid composition of rat brain myelin in triethyl tin-induced edema. J Lipid Res. 1971 Sep;12(5):570–579. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Franson P., Ronnevi L. O. Myelin breakdown and elimination in the posterior funiculus of the adult cat after dorsal rhizotomy: a light and electron microscopic qualitative and quantitative study. J Comp Neurol. 1984 Feb 10;223(1):138–151. doi: 10.1002/cne.902230111. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gordon V., Innerarity T. L., Mahley R. W. Formation of cholesterol- and apoprotein E-enriched high density lipoproteins in vitro. J Biol Chem. 1983 May 25;258(10):6202–6212. [PubMed] [Google Scholar]
- Hallpike J. F., Adams C. W. Proteolysis and myelin breakdown: a review of recent histochemical and biochemical studies. Histochem J. 1969 Nov;1(6):559–578. doi: 10.1007/BF01012862. [DOI] [PubMed] [Google Scholar]
- Hui D. Y., Harmony J. A., Innerarity T. L., Mahley R. W. Immunoregulatory plasma lipoproteins. Role of apoprotein E and apoprotein B. J Biol Chem. 1980 Dec 25;255(24):11775–11781. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Jen G., Thach R. E. Inhibition of host translation in encephalomyocarditis virus-infected L cells: a novel mechanism. J Virol. 1982 Jul;43(1):250–261. doi: 10.1128/jvi.43.1.250-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo C., Innerarity T. L., Mahley R. W. Obligatory role of cholesterol and apolipoprotein E in the formation of large cholesterol-enriched and receptor-active high density lipoproteins. J Biol Chem. 1985 Oct 5;260(22):11934–11943. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Lipoprotein receptors and cholesterol homeostasis. Biochim Biophys Acta. 1983 May 24;737(2):197–222. doi: 10.1016/0304-4157(83)90001-1. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- McLean J. W., Fukazawa C., Taylor J. M. Rat apolipoprotein E mRNA. Cloning and sequencing of double-stranded cDNA. J Biol Chem. 1983 Jul 25;258(14):8993–9000. [PubMed] [Google Scholar]
- Müller H. W., Gebicke-Härter P. J., Hangen D. H., Shooter E. M. A specific 37,000-dalton protein that accumulates in regenerating but not in nonregenerating mammalian nerves. Science. 1985 Apr 26;228(4698):499–501. doi: 10.1126/science.3983637. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Politis M. J., Pellegrino R. G., Oaklander A. L., Ritchie J. M. Reactive glial protein synthesis and early disappearance of saxitoxin binding in degenerating rat optic nerve. Brain Res. 1983 Aug 29;273(2):392–395. doi: 10.1016/0006-8993(83)90870-3. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Fields K. L., Hakomori S. I., Mirsky R., Pruss R. M., Winter J. Cell-type-specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res. 1979 Oct 5;174(2):283–308. doi: 10.1016/0006-8993(79)90851-5. [DOI] [PubMed] [Google Scholar]
- Rawlins F. A., Hedley-Whyte E. T., Villegas G., Uzman B. G. Reutilization of cholesterol-1,2-H3 in the regeneration of peripheral nerve. An autoradiographic study. Lab Invest. 1970 Mar;22(3):237–240. [PubMed] [Google Scholar]
- Rawlins F. A., Villegas G. M., Hedley-Whyte E. T., Uzman B. G. Fine structural localization of cholesterol-1,2- 3 H in degenerating and regenerating mouse sciatic nerve. J Cell Biol. 1972 Mar;52(3):615–625. doi: 10.1083/jcb.52.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene J. H., Shooter E. M. Denervated sheath cells secrete a new protein after nerve injury. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4169–4173. doi: 10.1073/pnas.80.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes G. J., McGuire C. B., Norden J. J., Freeman J. A. Nerve injury stimulates the secretion of apolipoprotein E by nonneuronal cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1130–1134. doi: 10.1073/pnas.83.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. L., Hall S. M. Chronic Wallerian degeneration--an in vivo and ultrastructural study. J Anat. 1971 Sep;109(Pt 3):487–503. [PMC free article] [PubMed] [Google Scholar]
- Yao J. K., Dyck P. J. Cholesterol esterifying enzyme in normal and degenerating peripheral nerve. J Neurochem. 1981 Jul;37(1):156–163. doi: 10.1111/j.1471-4159.1981.tb05303.x. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., Utermann G., Mahley R. W., Weisgraber K. H., Havel R. J., Goldstein J. L., Brown M. S., Schonfeld G., Hazzard W. R. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res. 1982 Aug;23(6):911–914. [PubMed] [Google Scholar]