Abstract
Estrogen signaling plays an important role in breast carcinogenesis. An increased understanding of estrogen gene targets and their effects will allow for more directed and effective therapies for individuals with breast cancer, particularly those with estrogen receptor positive tumors resistant to tamoxifen therapy. Here, we identify YPEL3 as a growth suppressive protein down-regulated by estrogen in estrogen receptor positive breast cancer cell lines. Estrogen repression of YPEL3 expression was found to be independent of p53 but dependent on estrogen receptor alpha expression. Importantly, YPEL3 expression, which is induced by the removal of estrogen or treatment with tamoxifen triggers cellular senescence in MCF-7 cells while loss of YPEL3 increases the growth rate of MCF-7 cells. Taken together these findings suggest that YPEL3 may represent a potential target for directed hormonal therapy for estrogen receptor positive breast cancer patients.
Keywords: YPEL3, cellular senescence, tamoxifen, estrogen receptor, p53
Introduction
Breast cancer is the second most common cancer occurring in women with nearly 200,000 women diagnosed annually 1. Of those women who develop breast cancer, approximately two-thirds are estrogen receptor positive 2. Estrogen is a steroid hormone which binds to its receptor in the cytoplasm and is then translocated into the nucleus to induce its many pro-growth actions. One of the available therapies for individuals with estrogen receptor positive tumors is tamoxifen, a selective estrogen receptor modulator (SERM). Tamoxifen competitively binds the estrogen receptor and can have either pro-estrogenic or anti-estrogenic effects. In mammary epithelium, tamoxifen forms a nuclear estrogen receptor complex that inhibits DNA synthesis and pro-estrogenic effects. Recently it was reported that in several ER+ breast cancer cell lines, tamoxifen can trigger a senescent phenotype 3 suggesting a mechanism by which it may induce growth repression in breast epithelium 4. When successful, five years of tamoxifen therapy has been shown to decrease the incidence of local recurrence by 50% 5. However, approximately 40% of women with estrogen receptor positive tumors demonstrate tamoxifen resistance and develop local or distant disease while on chemo-preventative therapy, and this significantly limits the treatment options for these patients. The current prognosis and disease free survival for women who develop tamoxifen-resistant tumors is significantly less than their tamoxifen-sensitive counterparts highlighting the need for a better understanding of the molecular events leading to this effect 6.
Estrogen receptor signaling causes a plethora of effects. While estrogen receptor alpha has been studied extensively, estrogen receptor beta has been discovered more recently and its effects and interactions are still largely unknown. It is thought, in some cases, that estrogen receptor beta may antagonize the alpha receptor and ultimately have anti-proliferative effects 7. In the classic view of estrogen signaling, the bound estrogen receptor interacts with estrogen response elements (EREs) in genomic DNA. Estrogen receptor binding can lead to the recruitment of other transcription factors or be recruited through association with other transcription factors resulting in either induction or inhibition of transcription. Additionally, some components of estrogen signaling are activated independent of DNA binding, the so-called non-genomic regulation. For example, estrogen signaling can cause rapid phosphorylation of ERK and activation of its downstream effects independent of transcriptional activity 8. In addition, the estrogen receptor has been shown to modulate microRNA expression in MCF-7 cells and microRNA expression patterns may correlate with tamoxifen sensitivity 9. In general, estrogen increases the expression of genes involved in pro-growth pathways and has been shown to decrease expression and downstream effects of growth inhibitory genes 7. It has been reported that estrogen can inhibit the functional activity of p53, one of the most studied tumor suppressor proteins. Estrogen signaling causes the intracellular redistribution of p53 confining it to the cytoplasm in ER+ breast cancer cells 10.
We recently reported that the YPEL3 gene is a novel p53 target 11. p53 activation of YPEL3 leads to its growth suppression through a pathway of cellular senescence. We have previously demonstrated YPEL3 to be down-regulated in human colon tumors 12. In a recent gene expression profiling experiment examining how estrogen impacts the transcriptional activity of breast cancer cells, YPEL3 was reported as an estrogen down-regulated gene 13. Here we report that YPEL3 expression is decreased in the presence of estrogen in estrogen receptor positive breast tumor cell lines. Estrogen repression of YPEL3 is independent of p53 and dependent on estrogen receptor alpha. When estrogen receptor positive tumor cells are treated with tamoxifen, an increase in YPEL3 expression is observed. Consistent with our recent report that YPEL3 induction triggers cellular senescence in tumor cells 11, we observed that blocking estrogen signaling triggered a YPEL3-dependent cellular senescence, while loss of YPEL3 resulted in increased cell growth. Thus we believe that YPEL3 deregulation may play a significant role in tamoxifen resistance in estrogen receptor positive breast tumors.
Materials and Methods
Reverse transcription-PCR
Total RNA was isolated using the e.Z.N.A. Total RNA kit (Omega Bio-Tek) according to the manufacturer’s instructions. RNA quantity was assessed using the Nanodrop with 260/280 ratios ranging from 2 to 2.2. One microgram of total RNA was used as a template for cDNA synthesis using the qScript cDNA SuperMix (Quanta Biosciences). Taqman-based PCR was performed in triplicate using Assay on Demand probe sets (Applied Biosystems) and an Applied Biosystems 7900 Sequence Detection System. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an endogenous control. All Assay on Demand (AOD) probes used in these experiments (YPEL3, pS2, p21, p53, ERα and GAPDH) were validated gene targets. Error bars in each Figure represent 95% confidence interval from a single experiment. All experiments were completed in biological triplicate with similar results.
Cell lines and reagents
MCF-7 and ZR-75.1 cells were derived from breast carcinomas and express wild-type estrogen receptor alpha and p53. T-47D cells were derived from a ductal carcinoma and are wild-type estrogen receptor alpha and express mutant p53. SkBr-3 cells were derived from breast carcinoma and are devoid of estrogen receptor alpha and express mutant p53. All cell lines were purchased from American Type Culture Collection. All cell lines passages in these experiments were grown for no longer than 3 months. MCF-7 and SKBr3 cells were grown in DMEM with 10% fetal bovine serum. ZR-75.1 and T-47D cells were grown in RMPI-1640 with 10% fetal bovine serum. Charcoal/Dextran stripped fetal bovine serum was obtained from Invitrogen. Estrogen and Tamoxifen were obtained from Sigma and MP Biomedicals, LLC, respectively. Chemical resuspension and storage was per manufacturer’s recommendations.
Immunoblotting
Whole-cell extracts and Western blotting were performed as previously described with the following modifications 14. Cell pellets were lysed in three freeze-thaws using RIPA buffer [25 mmol/L Tris-HCl (pH 7.6), 150 mmol/L NaCl, 1% NP40, 1% sodium deoxycholate] containing a protease inhibitor cocktail (Sigma). For endogenous YPEL3 Western, 10% to 20% gradient Tricine gels were used to separate protein extracts. For p53 and ER-alpha Westerns, 12% sodium dodecyl sulfate polyacrylamide gels were used to separate protein extracts. Proteins were transferred to polyvinylidene difluoride using a Bio-Rad transfer system. Antibodies were obtained from Proteintech (YPEL3), Neomarkers (ER alpha, Ab-10), and Calbiochem (p53, Ab-4 and Ab-6).
Lentiviral production
Lentivirus was produced by the cotransfection of 293FT cells with a pLenti vector and lentiviral packaging mix (Invitrogen) according to manufacturer’s instructions. Lentivirus-containing supernatant was harvested at 48 hours posttransfection, purified by centrifugation, and stored at −80°C. Viral transductions were carried out overnight in the presence of 6 μg/mL Polybrene (Sigma). Following transductions, cells were selected with 750 μg/mL Zeocin or 1μg/mL Puromycin. Plasmids were obtained from Addgene (shCON, shp53), Sigma (shERα, shYPEL3(B)) and Open Biosystems (shYPEL3(A)).
Senescence-associated β-galactosidase staining
Cells were processed with the Senescence β-galactosidase Staining kit (Cell Signaling Technology, Millipore) according to the manufacturer’s instructions and were visualized on an Olympus 1 × 70 fluorescence microscope. Experiments were completed in biological triplicate counting at least 100 cells per plate. Error bars represent the standard error of the means.
ViaCell Counting/MTT Assays
MCF-7 cells or MCF-7 cells transduced with lentivirus expressing a shYPEL3 target were plated in 35 mM dishes. At each time point, three plates of cells were separately trypsinized and subjected to ViCell counting following manufacturer’s recommendations. Each time point represents the average of three biological samples with error bars representing the standard deviation. For MTT assays the cells were seeded (1000 cells/well) into black 96 well tissue culture plates. At the indicated time point, 6 wells per cell type were treated with Cell Quant-Blue MTT reagent (BioAssay) for 2 hours following manufacturer’s dilutions. Sample fluorescence was determined using a Tecan Safine2 with an excitation wavelength of 530 nm and emission wavelength of 590 nm.
Results
YPEL3 expression increases in ER positive cells grown in the absence of estrogen
We initially grew MCF-7 (ER+, p53 wt) cells in charcoal stripped serum (CSS) to determine if estrogen was playing a regulatory role in YPEL3 mRNA expression. MCF-7 cells grown in CSS containing media showed a 4.8-fold increase in relative YPEL3 mRNA expression when compared to cells grown in media with 10% fetal bovine serum (Figure 1A). Similarly, we saw an increase in YPEL3 protein in MCF-7 cells grown in CSS (Figure 1B). The induction of YPEL3 was also observed in ZR-75.1 (ER+, p53 wt) and T-47D (ER+, p53 mutant) when these cells were grown in charcoal stripped serum (Supplemental Figure 1). To determine if the elevation in YPEL3 mRNA expression was a result of interactions with the estrogen receptor, we performed a similar experiment using SkBr-3 cells (ER-, p53 mutant). In these cells, growth in charcoal stripped serum did not modulate YPEL3 mRNA expression (Figure 1C).
Figure 1.
YPEL3 expression increases in ER positive breast cancer cells grown in the absence of estrogen. RT-PCR analysis demonstrating relative YPEL3 mRNA expression in (A) MCF-7 (breast, ER+, p53 wt) and (C) SkBr-3 (breast, ER-, p53 mutant) cell lines grown for 48 hours in complete media or charcoal stripped serum. (B) Western blot analysis of MCF-7 cells grown in complete media (CM) or charcoal stripped serum (CSS) with the addition of MG132 for 1 hour prior to lysis. RT-PCR error bars represent 95% confidence interval of a single experiment. Experiments were completed in triplicate with similar results.
Estrogen represses YPEL3 expression of endogenous gene
Charcoal:dextran stripping reduces the serum concentration of many hormones and certain growth factors, such as estradiol, cortisol, corticosterone, the B vitamins, T3, T4 and prostaglandins. To determine if the elevation of YPEL3 mRNA expression seen with growth in charcoal stripped serum was specific to estrogen, we added back beta-estradiol to the experimental conditions. pS2, also known as TIFF1, was used as a positive control. pS2 expression has previously been shown to be increased in the presence of estrogen, and this effect is regulated by the estrogen receptor 15. MCF-7 cells were grown in the presence or absence of estrogen. When beta-estradiol was added back to the growth media at a concentration of 1 nM, YPEL3 mRNA expression was repressed to 30% of that seen in charcoal stripped serum (Figure 2A). As expected, the addition of beta-estradiol to the growth media caused a greater than 2-fold elevation in pS2 mRNA expression (Figure 2A). Addition of ten times more beta-estradiol (10 nM) did not result in a statistically significant further reduction in YPEL3 or increase in pS2 mRNA expression suggesting that estrogen signaling was saturated at 1 nM.
Figure 2.
Estrogen represses YPEL3 expression of endogenous gene. (A) RT-PCR analysis demonstrating relative YPEL3 and pS2 mRNA expression in MCF-7 (ER+) cells grown in charcoal stripped serum (CSS) with the addition of increasing doses of beta-estradiol for 24 hours. (B) RT-PCR analysis demonstrating relative YPEL3 and pS2 mRNA expression in MCF-7 cells grown in complete media (CM) or charcoal stripped serum (CSS) with the addition of beta-estradiol (E2) or beta-estradiol and tamoxifen (Tam) for 24 hours. Error bars represent 95% confidence interval of a single experiment. Experiments were completed in triplicate with similar results.
As additional support of estrogen’s repressive role on YPEL3 gene expression, we utilized the selective estrogen receptor modulator, tamoxifen. MCF-7 cells were cultured in the absence of estrogen, with the addition of 1 nM beta-estradiol or with the addition of 1 nM beta-estradiol and 1 μM tamoxifen 16. A 3-fold increase in YPEL3 mRNA expression was seen when MCF-7 cells were cultured in charcoal stripped serum when compared to YPEL3 expression in complete media (Figure 2B). As expected, this increase was blocked by the addition of beta-estradiol. However, with the simultaneous addition of tamoxifen and beta-estradiol, YPEL3 mRNA expression increased by 185% compared to beta-estradiol alone consistent with the model where YPEL3 expression is regulated through the estrogen receptor (Figure 2B). With growth in charcoal stripped serum, pS2 expression was decreased to 55% of that seen in complete media (Figure 2B). The addition of 1 nM beta-estradiol allowed for the level of expression to return back to that seen in complete media (Figure 2B). The addition of tamoxifen caused a 20% reduction in pS2 mRNA expression (Figure 2B), as expected given its inhibitory effects on the estrogen receptor alpha.
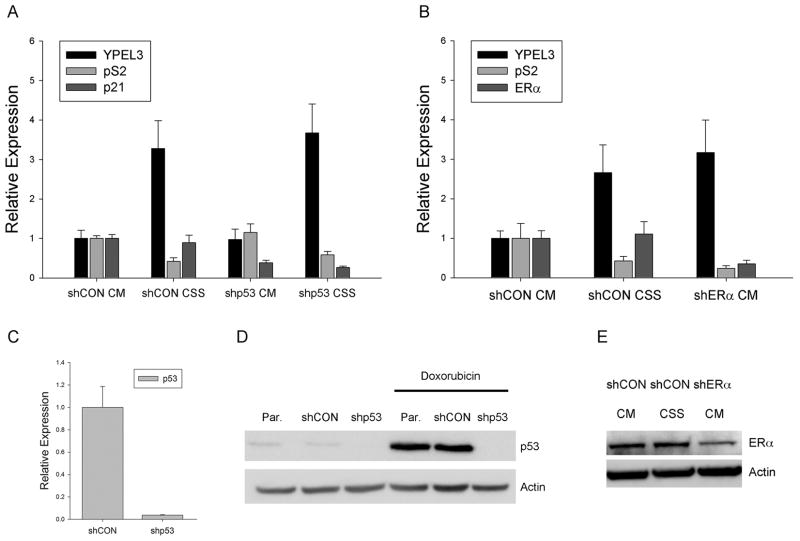
Elevation in YPEL3 mRNA expression upon removal of estrogen in ER+ breast cancer cells is p53 independent and ERα dependent
Towards the goal of determining if YPEL3 mRNA induction following estrogen removal in breast cancer cells was dependent on p53 and estrogen receptor alpha, we used lentiviral transduction of shRNAs targeting each gene. shCON, a scramble shRNA vector that does not target any human genes 17, was used as a short hairpin control. p53 and estrogen receptor alpha knockdown was confirmed by both RNA and Western blot analysis (Figures 3B, 3C, 3D and 3E). In the case of p53, since it is normally expressed at low levels in MCF-7 cells 18, shp53 knockdown was confirmed following treatment for 24 hours with 0.5 μg/mL doxorubicin (Figure 3C). MCF-7 cells stably selected for the loss of p53 were grown in complete media or charcoal stripped serum. There was a similar elevation (3-fold) in YPEL3 mRNA expression in control cells and shp53 cells when grown in charcoal stripped serum (Figure 3A). Furthermore, p21 expression was monitored to assess whether growth in charcoal stripped serum induced a p53 response. p21 expression was not modulated by growth in charcoal stripped serum for 48 hours in either shCon or shp53 cells (Figure 3A). Taken with the increase in YPEL3 mRNA seen when T-47D cells, which harbor mutant p53, were grown in charcoal stripped serum (Supplementary Figure 1), these findings demonstrate that wild-type p53 is not playing a significant role in the estrogen repression of YPEL3 gene expression.
Figure 3.
Estrogen repression of YPEL3 mRNA expression is p53 independent and ERα dependent. (A) RT-PCR analysis demonstrating relative YPEL3, pS2 and p21 mRNA expression in pooled MCF-7 cells stably selected for expression of shp53 or control (shCON) grown in complete media (CM) or charcoal stripped serum (CSS), (C) RT-PCR analysis demonstrating relative p53 mRNA expression and (D) Western blot analysis in pooled MCF-7 cells stably selected for expression of shp53 or control (shCON) confirming RNA and protein knockdown of p53 expression. Doxorubicin treatment was at 0.5 μg/mL for 24 hours. (B) RT-PCR analysis demonstrating relative YPEL3, pS2 and ERα mRNA expression in MCF-7 cells infected at an MOI=5 with control (shCON) or shERα viral constructs and placed in complete media (CM) or charcoal stripped serum (CSS) for 48 hours. (E) Western blot analysis of MCF-7 cells infected at an MOI=5 with control (shCON) or shERα viral constructs and placed in complete media (CM) or charcoal stripped serum (CSS). Error bars for RT-PCR represent 95% confidence interval for a single experiment. Experiment was completed in triplicate with similar results.
Next, we tested if estrogen repression of YPEL3 mRNA expression was dependent upon expression of ER alpha. Attempts to create stable MCF-7 cell lines selected for the loss of estrogen receptor alpha using lentiviral shERα were unsuccessful likely due to the growth dependency of estrogen signaling in MCF-7 cells (K. Miller, personal communication). Instead MCF-7 cells were super-infected (MOI=5) with shERα or shCON and grown in either complete media or charcoal stripped serum. MCF-7 cells infected with shCON virus and grown in the absence of estrogen showed a 2.6-fold elevation in YPEL3 mRNA expression (Figure 3B). Interestingly, MCF-7 cells infected with shERα grown in complete media also demonstrated an elevation (3.1-fold) in YPEL3 mRNA expression (Figure 3D). Thus, cells grown in the absence of estrogen or the absence of estrogen receptor alpha both demonstrated an elevation of YPEL3 mRNA expression suggesting it is the cooperation of estrogen with its receptor, alpha subtype, that is allowing for the observed modulation of YPEL3 mRNA expression. Knockdown of estrogen receptor alpha expression in MCF-7-shERα cells was 35% of MCF-7-shCon cells based on relative mRNA expression (Figure 3B), and confirmed at the protein level by western blot (Figure 3E).
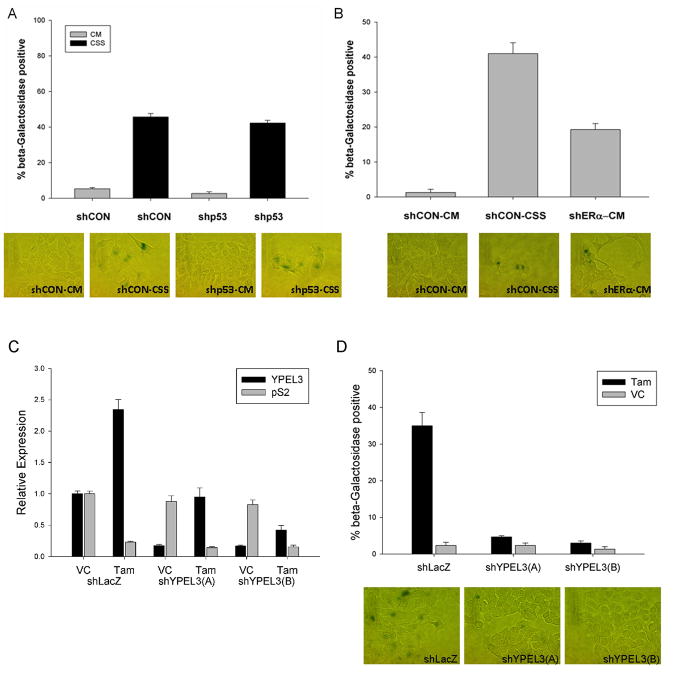
Estrogen removal causes YPEL3 dependent cellular senescence
Elevated expression of YPEL3 causes an induction of cellular senescence in both primary and tumor cell lines 11. We questioned whether the elevated YPEL3 expression seen with growth in charcoal stripped serum was correlated with an elevation in cellular senescence. To test this, MCF-7 cells were grown in DMEM media with 10% fetal bovine serum or in DMEM media containing 10% charcoal stripped fetal bovine serum in the presence or absence of 1 nM beta-estradiol. Cells grown in charcoal stripped serum had a significant induction of cellular senescence as measured by SA-beta-galactosidase activity at a pH of 6 19 (Figure 4A). Again, induction of senescence after 6 days of treatment in media containing charcoal strip serum was seen in T-47D cells. Here we monitored senescence based on increases in SA-beta-galactosidase positive cells and the induction of p21, an essential senescence activator in breast cancer cells 3 (Supplementary Figure 2). The addition of 1 nM beta-estradiol to MCF-7 cells grown in charcoal stripped serum brought the level of cellular senescence down to 35% beta-galactosidase positive cells (Figure 4B). Increasing doses of beta-estradiol to 100 nM resulted in an inhibition of beta-galactosidase activity back to levels seen in cells grown in complete media (Supplementary Figure 3). To determine if the cellular senescence seen in MCF-7 cells grown in charcoal stripped serum was dependent upon YPEL3, MCF-7 cells were infected with lentivirus shRNA vectors targeting YPEL3 (shYPEL3(A) and shYPEL3(B)) or an shLacZ vector as a short hairpin control. MCF-7 cells stably selected for the loss of YPEL3 grown in charcoal stripped serum did not demonstrate an elevation in cellular senescence. In contrast, shLacZ expressing MCF-7 cells did show the expected induction of cellular senescence when grown in charcoal stripped serum (Figure 4B). Knockdown of YPEL3 expression was confirmed with relative mRNA expression (Figure 4C). Similar findings were seen with T-47D and ZR-75.1 cell lines (Supplementary Figures 4 and 5). These results point to an essential role for YPEL3 in estrogen-dependent cellular senescence in ER+ breast cancer cells.
Figure 4.
Estrogen removal causes a YPEL3 dependent cellular senescence. (A) Beta- galactosidase assay of MCF-7 cells grown in complete media (CM) or charcoal stripped serum (CSS) with the addition of 1 nM beta-estradiol (E2) for 6 days. (B) Beta-galactosidase assay of pooled MCF-7 cells stably selected for expression of shLacZ (control) or shYPEL3 constructs (A) and (B) grown in complete media (CM) or charcoal stripped serum (CSS). Images represent cells grown in charcoal stripped serum. (C) RT-PCR analysis demonstrating relative YPEL3 expression in MCF-7 parental cells and pooled MCF-7 cells stably selected for expression of shYPEL3 or shLacZ confirming RNA knockdown of expression. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate. Error bars for RT-PCR represent 95% confidence interval for a single experiment. Experiment was completed in triplicate with similar results.
Loss of YPEL3 triggers increased cell proliferation
Given that induction of YPEL3 triggers a growth suppression in MCF-7 and U2OS cells 11 we next sought to determine the rate of cell growth in MCF-7 cells expressing shYPEL3. When compared to MCF-7 cells, shYPEL3-expressing MCF-7 cells showed a statistically significant increase in cell numbers when grown over 10 days (Figure 5A). Using an MTT based assay, YPEL3 knockdown for 6 days led to an increase in cell numbers while YPEL3 induction over the same period resulted in a decrease in cell number (Figure 5B). These findings suggest that the while YPEL3 induction triggers growth inhibition through cellular senescence 11, decreased YPEL3 expression enhances cell proliferation.
Figure 5.
YPEL3 knockdown leads to increased cell proliferation. (A) MCF-7 cells or cells infected with a lentivirus expressing a shYPEL3 RNA were plated in 6 well cells (10,000 pe well). At the indicated days three wells for each cell type were counted and averaged using a ViCell counter. Inset: RT-PCR analysis of YPEL3 expression in MCF-7 or MCF-7-shYPEL3 cells. (B) MCF-7-shYPEL3, MCF-7 and MCF-7TetRYPEL3 cells were plated at 500 cells per well in a Costar 96 well tissue culture dish. MCF-7TetR-YPEL3 cells were treated with 10uM tetracycline. At one or six days post plating, the relative numbers of cells were determined by using a fluorescence based MTT assay (CellQuanti-Blue, BioAssay Systems). Relative Cell Number represents the average MTT values (after background subtraction) of the day 6 wells normalized to the average MTT values taken on day 1. Error bars represent the standard error of the mean from three separate ViCell counts (A) or from four wells (B).
Estrogen regulated senescence seen with removal of estrogen in ER+ breast cancer cells is p53 independent and ERα dependent
While the increase in YPEL3 mRNA expression seen with the removal of estrogen was not dependent upon the expression of p53, we wanted to determine if the elevation of cellular senescence seen with the removal of estrogen was dependent on p53. In previous studies, we have demonstrated that over expression of YPEL3 alone could induce cellular senescence independent of p53 11. Again, MCF-7 cells stably selected for the loss of p53 or a control siRNA (shCON) were cultured in complete media or charcoal stripped serum for 6 days. At the completion of 6 days, beta-galactosidase activity at a pH of 6 was measured. MCF-7 shp53 cells grown in the absence of estrogen had a similar increase in cellular senescence when compared to control cells (Figure 6A). This corresponds with the resulting increase in YPEL3 mRNA expression (Figure 3A). These results suggest that both the elevation in YPEL3 mRNA expression and the elevation in cellular senescence seen with estrogen removal are independent of p53 expression.
Figure 6.
Estrogen regulated senescence seen with removal of estrogen in ER+ breast cancer cells is p53 independent and ERα dependent. (A) Beta-galactosidase assay of pooled MCF-7 cells stably selected for expression of shp53 or control (shCON) and grown in complete media (CM) or charcoal stripped serum (CSS). (B) Beta-galactosidase assay of MCF-7 cells infected at an MOI=5 with control (shCON) or shERα viral constructs and placed in complete media (CM) or charcoal stripped serum (CSS) for 6 days. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate. Tamoxifen treatment of ER+ breast cancer cells causes induction of YPEL3-dependent cellular senescence. (C) RT-PCR analysis demonstrating relative YPEL3 and pS2 mRNA expression or (D) beta-galactosidase assay in pooled MCF-7 cells stably selected for expression of shLacZ (control) or shYPEL3 constructs (A) and (B) treated with 0.5 mM tamoxifen (Tam) or vehicle control (VC). Images represent cells treated with tamoxifen. Error bars for RT-PCR represent 95% confidence interval for a single experiment. Experiment was completed in triplicate with similar results. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate.
We also tested whether the expression of estrogen receptor alpha was required for the observed induction of cellular senescence. MCF-7 cells were infected at an MOI=5 with shERα and shCON viral constructs. Cells were then grown in the presence or absence of estrogen. Again, MCF-7 cells cultured in the absence of estrogen had a robust increase in beta-galactosidase activity. Similar to the mRNA expression (Figure 3C), MCF-7 cells transduced with lentivirus expressing shERα and grown in the presence of estrogen had an elevation of cellular senescence, although not to the level seen in control cells grown in the absence of estrogen (Figure 6B). The incomplete response is likely due to the incomplete knockdown of estrogen receptor alpha resulting from our inability to create stable pools of shERα expressing MCF-7 cells (Figure 3D).
Tamoxifen treatment of ER+ breast cancer cells causes induction of YPEL3-dependent cellular senescence
Tamoxifen treatment of ER+ breast tumors has previously been shown to induce a G0/G1 phase cell cycle growth arrest 4. MCF-7 cells were exposed to tamoxifen treatment to assess if YPEL3 expression was elevated under these conditions, and further, if YPEL3 was required for the growth arrest seen with tamoxifen treatment. MCF-7 cells stably selected for the loss of YPEL3 (shYPEL3(A) and shYPEL3(B)) or control (shLacZ) were grown in complete media and treated with 0.5 μM tamoxifen or vehicle control (100% ethanol) for 6 days. There was a 2.3- fold induction of YPEL3 mRNA expression in control cells (shLacZ) treated with tamoxifen verses vehicle control (Figure 6C). Similarly, there was a 35% induction of cellular senescence in tamoxifen-treated shLacZ cells that was not observed in either pool of MCF-7 cells expressing shYPEL3 (Figure 6D). Interestingly, while there was some induction of YPEL3 mRNA expression in the shYPEL3 cell lines with tamoxifen treatment, levels did not exceed those observed in shLacZ cells grown in complete media and thus were insufficient to induce cellular senescence (Figures 6C and 6D).
Discussion
YPEL3 was initially identified as SUAP, small unstable apoptotic protein where it was noted to be induced upon interleukin-3 removal in a myeloid precursor cell line 20. It was later renamed YPEL3 due to its inclusion in a 5 member family with homology to the Drosophila Yippee protein 21. We initially became interested in YPEL3 following a microarray experiment using MCF-7 cells in which p53 was reactivated using RNAi approaches to target Hdm2 and HdmX 18. We subsequently identified YPEL3 as a novel p53-regulated gene capable of triggering cellular senescence 11.
In the present study, we provide data demonstrating that the elevation in YPEL3 mRNA expression and cellular senescence seen when ER+ breast cancer cells are grown in the absence of estrogen is independent of p53 expression (Figures 3A and 5A, Supplemental Figures 2, 4 and 5). We believe estrogen regulation of YPEL3 expression and its inhibition of YPEL3 dependent senescence represents a novel signaling pathway distinct from our recent findings that p53 transactivates the YPEL3 gene 11. Consistent with that model, preliminary studies showed that YPEL3 knockdown was unable to block doxorubicin-induced senescence in MCF-7 cells 22 (Supplemental Figure 6).
While we were unable to identify an estrogen response element within 5 kb of the transcriptional start site, YPEL3 was reported in a gene expression profiling study as a gene down-regulated in the presence of estrogen in both MCF-7 and ZR-75.1 breast cancer cells 13. In fact, an earlier chromatin immunoprecipitation-paired end diTag cloning and sequencing study reported YPEL3 as a downregulated gene harboring an estrogen response element 30kb upstream of the YPEL3 promoter 12. Studies to examine if estrogen binds to this putative ERE are ongoing. It is possible that estrogen repression of YPEL3 is occurring through an indirect mechanism such as activation of microRNAs 9 or other estrogen response genes. However, we do not favor such a model because when MCF-7 cells were grown in charcoal stripped serum with beta-estradiol, repression of YPEL3 was seen within 8 hours of treatment, the same timepoint where pS2 started to show significant induction (Supplemental Figure 7). Studies are ongoing to examine estrogen receptor binding surrounding the YPEL3 gene.
One aspect of estrogen repression of YPEL3 and senescence that appears to be disconnected is that while 1 nM beta-estradiol was sufficient to repress YPEL3 mRNA levels back to levels seen in cells grown in normal serum (Figure 2B), higher doses of estradiol (10–100 nM) were required to inhibit cellular senescence to baseline levels (Supplemental Figure 3). We are currently assessing how YPEL3 proteins levels are impacted by estrogen regulation. Nevertheless, we believe the modulation of YPEL3 by estrogen is a significant finding given that the cellular senescence observed following removal of estrogen, knockdown of the estrogen receptor alpha or addition of tamoxifen are all dependent on the presence of YPEL3. From a clinical perspective it is possible that deregulated YPEL3 signaling plays an important role in tamoxifen resistance in patients with estrogen receptor positive breast tumors. Forty percent of women with estrogen receptor positive breast cancers are resistant to tamoxifen chemoprevention 6. We are currently testing whether the lack of response to tamoxifen therapy may be, in part, due to YPEL3 deregulation. Further, if wild-type YPEL3 can be directly targeted to tamoxifen-resistant breast tumors, it might provide an alternative treatment pathway. However, prior to any targeted YPEL3 therapy, an examination of how estrogen regulation of YPEL3 functions in normal mammary gland development and differentiation would be warranted. Nevertheless the present study not only highlights the complexities of estrogen receptor signaling in breast cancer cells but suggests that understanding YPEL3 signaling may provide a novel targeted breast cancer therapy.
Supplementary Material
YPEL3 mRNA induction in estrogen receptor positive mammary breast cancer cells grown in charcoal stripped serum. RT-PCR analysis demonstrating relative YPEL3 mRNA expression in ZR-75.1 (ER+, p53 wt) and T-47D (ER+, p53 mutant) breast cancer cell lines grown for 6 days in either complete media or charcoal stripped serum. Error bars represent 95% confidence interval of the fold change (RQ). Experiment was completed in triplicate with similar results.
Estrogen removal in T-47D cells results in increased SA-beta galactosidase positive cells, YPEL3 mRNA and p21 mRNA. (A) Beta-galactosidase assay of T-47D cells grown in complete media (CM) or charcoal stripped serum (CSS) for 6 days. Error bars represent standard error of the means for at least 100 cells counted per field of at least three representative fields. (B) RT-PCR analysis demonstrating increases in YPEL3 and p21 mRNA expression in T-47D cells grown in complete media (CM) or charcoal stripped serum (CSS) for 6 days. Error bars represent 95% confidence interval of the fold change (RQ).
Increasing doses of estrogen enable return of background senescence to baseline. Beta-galactosidase assay of MCF-7 cells grown in complete media (CM) or charcoal stripped serum (CSS) with increase doses of beta-estradiol (E2). Vehicle control (VC) has highest amount of 100% ethanol added with addition of beta-estradiol. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate.
T-47D cells demonstrate a YPEL3-dependent cellular senescence following estrogen depletion. (A) Beta-galactosidase assay of pooled T-47D cells stably selected for expression of shCon (control) or shYPEL3 construct (B) were grown for 6 days in complete media (CM), charcoal stripped serum (CSS) or CSS containing 1nM beta-estradiol (CSS+E2). Images show representative SA-beta-galactosidase stained cells. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate. (B) RT-PCR analysis demonstrating relative YPEL3 and p21 expression in T-47D shRNA cell lines treated as described in (A). Error bars for RT-PCR represent 95% confidence interval for a single experiment.
ZR-75.1 cells demonstrate a YPEL3-dependent cellular senescence following estrogen depletion. (A) Beta-galactosidase assay of pooled ZR-75.1 cells stably selected for expression of shCon (control) or shYPEL3 construct (B) were grown for 6 days in complete media (CM), charcoal stripped serum (CSS) or CSS containing 1 nM beta-estradiol (CSS+E2). Images show representative SA-beta-galactosidase stained cells. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate. (B) RT-PCR analysis demonstrating relative YPEL3 and p21 expression in ZR-75.1 shRNA cell lines treated as described in (A). Error bars for RT-PCR represent 95% confidence interval for a single experiment. Experiment was completed in triplicate with similar results.
Time course of YPEL3 repression following addition of estrogen. MCF-7 cells were grown for 48 hours in DMEM (phenol-free) containing 10% charcoal-stripped serum. Beta-estradiol (1nM) was added and RNA isolated from cells exposed for the indicated time (0–28 hours). RT-PCR analysis demonstrating relative YPEL3 (light grey bars) and pS2 (dark grey bars) mRNA expression was performed as previously described. Error bars represent 95% confidence interval for a single experiment.
Doxorubin treatment triggers senescence in MCF7 cells that is independent of YPEL3. MCF7 cells expressing shRNAs targeting no human gene (shCon), YPEL3 (shYPEL3) or p53 (shp53) were grown in treated with doxorubicin (0.6 μg/mL) for 2 hours (Dox) or untreated (No dox) and then grown for an additional six days before subjected to the SA-beta-galactosidase assay. Images show representative SA-beta-galactosidase stained cells. Based on RNA isolated from the cells treated as described above MCF7-shp53 and MCF7-shYPEL3 each showed a 80% reduction in expression of their respective genes (data not shown).
Acknowledgments
This work was supported by National Cancer Institute grant no. CA64430 (to SJB).
List of Abbreviations
- YPEL3
Yippee like 3
- SUAP
Small unstable apoptotic protein
- CM
Complete media
- CSS
Charcoal stripped serum
- VC
Vehicle control
- E2
beta-estradiol
- Tam
Tamoxifen
- ERE
Estrogen response element
- ERα
Estrogen receptor alpha
- SERM
Selective estrogen receptor modulator
- MOI
Multiplicity of infection
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Clark GM, Osborne CK, McGuire WL. Correlations between estrogen receptor, progesterone receptor, and patient characteristics in human breast cancer. J Clin Oncol. 1984;2:1102–9. doi: 10.1200/JCO.1984.2.10.1102. [DOI] [PubMed] [Google Scholar]
- 3.Mumcuoglu M, Bagislar S, Yuzugullu H, Alotaibi H, Senturk S, Telkoparan P, Gur-Dedeoglu B, Cingoz B, Bozkurt B, Tazebay UH, Yulug IG, Akcali KC, et al. The ability to generate senescent progeny as a mechanism underlying breast cancer cell heterogeneity. PLoS One. 2010;5:e11288. doi: 10.1371/journal.pone.0011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–18. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 6.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–58. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 7.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–31. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 8.Coleman KM, Smith CL. Intracellular signaling pathways: nongenomic actions of estrogens and ligand-independent activation of estrogen receptors. Front Biosci. 2001;6:D1379–91. doi: 10.2741/coleman. [DOI] [PubMed] [Google Scholar]
- 9.Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, Vagner S. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–40. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- 10.Molinari AM, Bontempo P, Schiavone EM, Tortora V, Verdicchio MA, Napolitano M, Nola E, Moncharmont B, Medici N, Nigro V, Armetta I, Abbondanza C, et al. Estradiol induces functional inactivation of p53 by intracellular redistribution. Cancer Res. 2000;60:2594–7. [PubMed] [Google Scholar]
- 11.Kelley KD, Miller KR, Todd A, Kelley AR, Tuttle R, Berberich SJ. YPEL3, a p53- Regulated Gene that Induces Cellular Senescence. Cancer Res. 2010;70:3566–75. doi: 10.1158/0008-5472.CAN-09-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C-Y, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, et al. Whole-Genome Cartography of Estrogen Receptor Alpha Binding Sites. PLoS Genet. 2007;3:e87. doi: 10.1371/journal.pgen.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicatiello L, Mutarelli M, Grober OM, Paris O, Ferraro L, Ravo M, Tarallo R, Luo S, Schroth GP, Seifert M, Zinser C, Chiusano ML, et al. Estrogen Receptor {alpha} Controls a Gene Network in Luminal-Like Breast Cancer Cells Comprising Multiple Transcription Factors and MicroRNAs. Am J Pathol. 2010;176:2113–30. doi: 10.2353/ajpath.2010.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markey M, Berberich SJ. Full-length hdmX transcripts decrease following genotoxic stress. Oncogene. 2008;27:6657–66. doi: 10.1038/onc.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown AM, Jeltsch JM, Roberts M, Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984;81:6344–8. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowell LN, Graham JD, Bouton AH, Clarke CL, O’Neill GM. Tamoxifen treatment promotes phosphorylation of the adhesion molecules, p130Cas/BCAR1, FAK and Src, via an adhesion-dependent pathway. Oncogene. 2006;25:7597–607. doi: 10.1038/sj.onc.1209747. [DOI] [PubMed] [Google Scholar]
- 17.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science (New York, NY) 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 18.Heminger K, Markey M, Mpagi M, Berberich SJ. Alterations in gene expression and sensitivity to genotoxic stress following HdmX or Hdm2 knockdown in human tumor cells harboring wild-type p53. Aging. 2009;1:89–108. doi: 10.18632/aging.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92:9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker SJ. Small unstable apoptotic protein, an apoptosis-associated protein, suppresses proliferation of myeloid cells. Cancer Res. 2003;63:705–12. [PubMed] [Google Scholar]
- 21.Hosono K, Sasaki T, Minoshima S, Shimizu N. Identification and characterization of a novel gene family YPEL in a wide spectrum of eukaryotic species. Gene. 2004;340:31–43. doi: 10.1016/j.gene.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Elmore LW. Adriamycin-induced Senescence in Breast Tumor Cells Involves Functional p53 and Telomere Dysfunction. Journal of Biological Chemistry. 2002;277:35509–15. doi: 10.1074/jbc.M205477200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
YPEL3 mRNA induction in estrogen receptor positive mammary breast cancer cells grown in charcoal stripped serum. RT-PCR analysis demonstrating relative YPEL3 mRNA expression in ZR-75.1 (ER+, p53 wt) and T-47D (ER+, p53 mutant) breast cancer cell lines grown for 6 days in either complete media or charcoal stripped serum. Error bars represent 95% confidence interval of the fold change (RQ). Experiment was completed in triplicate with similar results.
Estrogen removal in T-47D cells results in increased SA-beta galactosidase positive cells, YPEL3 mRNA and p21 mRNA. (A) Beta-galactosidase assay of T-47D cells grown in complete media (CM) or charcoal stripped serum (CSS) for 6 days. Error bars represent standard error of the means for at least 100 cells counted per field of at least three representative fields. (B) RT-PCR analysis demonstrating increases in YPEL3 and p21 mRNA expression in T-47D cells grown in complete media (CM) or charcoal stripped serum (CSS) for 6 days. Error bars represent 95% confidence interval of the fold change (RQ).
Increasing doses of estrogen enable return of background senescence to baseline. Beta-galactosidase assay of MCF-7 cells grown in complete media (CM) or charcoal stripped serum (CSS) with increase doses of beta-estradiol (E2). Vehicle control (VC) has highest amount of 100% ethanol added with addition of beta-estradiol. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate.
T-47D cells demonstrate a YPEL3-dependent cellular senescence following estrogen depletion. (A) Beta-galactosidase assay of pooled T-47D cells stably selected for expression of shCon (control) or shYPEL3 construct (B) were grown for 6 days in complete media (CM), charcoal stripped serum (CSS) or CSS containing 1nM beta-estradiol (CSS+E2). Images show representative SA-beta-galactosidase stained cells. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate. (B) RT-PCR analysis demonstrating relative YPEL3 and p21 expression in T-47D shRNA cell lines treated as described in (A). Error bars for RT-PCR represent 95% confidence interval for a single experiment.
ZR-75.1 cells demonstrate a YPEL3-dependent cellular senescence following estrogen depletion. (A) Beta-galactosidase assay of pooled ZR-75.1 cells stably selected for expression of shCon (control) or shYPEL3 construct (B) were grown for 6 days in complete media (CM), charcoal stripped serum (CSS) or CSS containing 1 nM beta-estradiol (CSS+E2). Images show representative SA-beta-galactosidase stained cells. Error bars for beta-galactosidase experiments represent standard error of the means for assays completed in biologic triplicate. (B) RT-PCR analysis demonstrating relative YPEL3 and p21 expression in ZR-75.1 shRNA cell lines treated as described in (A). Error bars for RT-PCR represent 95% confidence interval for a single experiment. Experiment was completed in triplicate with similar results.
Time course of YPEL3 repression following addition of estrogen. MCF-7 cells were grown for 48 hours in DMEM (phenol-free) containing 10% charcoal-stripped serum. Beta-estradiol (1nM) was added and RNA isolated from cells exposed for the indicated time (0–28 hours). RT-PCR analysis demonstrating relative YPEL3 (light grey bars) and pS2 (dark grey bars) mRNA expression was performed as previously described. Error bars represent 95% confidence interval for a single experiment.
Doxorubin treatment triggers senescence in MCF7 cells that is independent of YPEL3. MCF7 cells expressing shRNAs targeting no human gene (shCon), YPEL3 (shYPEL3) or p53 (shp53) were grown in treated with doxorubicin (0.6 μg/mL) for 2 hours (Dox) or untreated (No dox) and then grown for an additional six days before subjected to the SA-beta-galactosidase assay. Images show representative SA-beta-galactosidase stained cells. Based on RNA isolated from the cells treated as described above MCF7-shp53 and MCF7-shYPEL3 each showed a 80% reduction in expression of their respective genes (data not shown).