Extranuclear sex steroid receptors require palmitoylation to traffic to the plasma membrane, where they activate signal transduction cascades. We identify DHHC-7 and -21 palmitoylacyltransferases as conserved enzymes for the three classes of sex steroid receptors.
Abstract
Classical estrogen, progesterone, and androgen receptors (ERs, PRs, and ARs) localize outside the nucleus at the plasma membrane of target cells. From the membrane, the receptors signal to activate kinase cascades that are essential for the modulation of transcription and nongenomic functions in many target cells. ER, PR, and AR trafficking to the membrane requires receptor palmitoylation by palmitoylacyltransferase (PAT) protein(s). However, the identity of the steroid receptor PAT(s) is unknown. We identified the DHHC-7 and -21 proteins as conserved PATs for the sex steroid receptors. From DHHC-7 and -21 knockdown studies, the PATs are required for endogenous ER, PR, and AR palmitoylation, membrane trafficking, and rapid signal transduction in cancer cells. Thus the DHHC-7 and -21 proteins are novel targets to selectively inhibit membrane sex steroid receptor localization and function.
INTRODUCTION
Sex steroids act through their classical receptors in the nucleus to regulate the genes that mediate many actions of these hormones in target cells (O'Malley et al., 1970; Payvar et al., 1981). However, it was demonstrated ∼40 yr ago that sex steroids, such as estrogen, signal in seconds to generate calcium flux and cyclic AMP in vitro and in vivo; this signaling occurs through plasma membrane (PM)-localized binding sites (Szego and Davis, 1967; Pietras and Szego, 1977). More recently, it was shown that activation of kinase cascade(s) primarily originates from signaling by PM-localized pools of endogenous classical sex steroid receptors, such as estrogen receptor alpha (ERα) (Razandi et al., 1999; Pedram et al., 2006). There are many mechanisms by which rapid signaling from PM-localized receptors affects gene transcription, which is modulated by the same sex steroid receptors in the nucleus (Vasudevan et al., 2001; Bjornstrom and Sjoberg, 2005; Harrington et al., 2006; Vicent et al., 2006; Bredfeldt et al., 2010; Guillermo et al., 2010). In addition, rapid signaling by PM-localized steroid receptors affects nongenomic functions, including those that prevent osteoporosis and acute vascular injury in rodent models (Kousteni et al., 2001; Chambliss et al., 2010), rescue neurons from in vivo ischemic insult (Suzuki et al., 2009), and stimulate oocyte meiosis (Finidori-Lepicard et al., 1981). Thus it is important to understand the crucial steps required for sex steroid receptor trafficking to the PM.
Many integral membrane proteins are posttranslationally modified by lipids that promote membrane localization (Mundy, 1995). These lipid modifications include acylation, palmitoylation, and myristylation. Previous studies showed that S-palmitoylation occurs on a conserved cysteine as part of a nine–amino acid motif in the ligand binding domains of all sex steroid receptors (Pedram et al., 2007). Palmitoylation promotes the physical interaction of ER with the caveolin-1 protein (Acconcia et al., 2004), and caveolin-1 transports ER from cytoplasm to caveolae rafts in the PM (Razandi et al., 2003a). At the caveolae, ER activates small G protein α- and βγ-subunit signaling in seconds, generating additional rapid signals (Razandi et al., 1999; Kumar et al., 2007). Similar signaling has been shown for membrane-localized progesterone receptors (PRs; Faivre et al., 2005) and androgen receptors (ARs; Gill and Hammes, 2007). Some of these studies indicate rapid ER signaling from the membrane of breast cancer cells promotes cell proliferation, migration, and survival (Levin and Pietras, 2008). Similarly, rapid signaling from membrane ARs to Src activation in prostate cancer cells stimulates cell proliferation (Migliaccio et al., 2000).
Identification of the enzyme(s) that causes this posttranslational modification of the sex steroid receptors is needed to understand the dynamics of receptor palmitoylation and trafficking. Fukata and colleagues identified 23 mammalian proteins as palmitoylacyltransferases (PATs), and all contain an Asp-His-His-Cys (DHHC) amino acid signature sequence (Fukata et al., 2004; Hou et al., 2009). In this paper, we report that DHHC-7 and -21 proteins serve as PATs for ER, PR, and AR, driving membrane localization and rapid signaling by these receptors to important functions in hormone-responsive cancer cells.
RESULTS
DHHC-7 and -21 are ER PATs
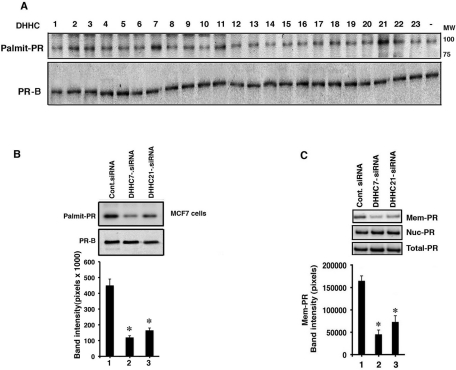
To identify potential PATs for ERα, we expressed single plasmids coding for each of the known 23 mammalian DHHC proteins in MCF-7 breast cancer cells. Only expression of DHHC-7 or -21 significantly enhanced endogenous ERα palmitoylation (Figure 1A). All DHHC proteins were detected using hemagglutinin (HA) antibody, indicating that the unbiased screen probably detected all candidate PATs.
FIGURE 1:
Identification of ER PATs. (A) Transfection of MCF-7 cells was carried out with each of 23 separate plasmids from a cDNA library in a separate well of cells, which was followed by [3H]palmitic acid labeling, immunoprecipitation of endogenous ERα from cell lysates, separation by SDS–PAGE, and fluorography. Immunoblots of ER total protein and HA-tagged DHHC PAT proteins are also shown in this representative experiment (n = 3 experiments). (B) Distribution of ERα in MCF-7 cells. Top, cells were transfected with the various siRNAs and analyzed by immunofluorescence microscopy. Arrows indicate membrane ERα. Bar graph represents the mean ± SEM densities of ERα blots in membrane or nuclear cell fractions from three experiments, density-analyzed by ANOVA plus Schefe's test. *, p < 0.05 for control vs. DHHC siRNA. Bottom, validation of the siRNA for endogenous DHHC PATs proteins and RT-PCR for the DHHC-22 transcript. (C) DHHC-7 or -21 siRNA(s) significantly reduces [3H]palmitate incorporation into endogenous ERα in MCF-7 cells. Bar graph shows combined data from three experiments. *, p < 0.05 for control siRNA vs. DHHC-7 or -21 siRNA.
We then used small interfering RNAs (siRNAs) to candidate DHHC PATs endogenously expressed in MCF-7 cells to further support ERα as substrate. Protein knockdown of DHHC-7 and -21 was specific (Figure 1B, left), and DHHC-22 transcripts were determined, since no commercial antibody is available. Endogenous ERα's at the PM in MCF-7 cells were only decreased from DHHC-7 or -21 knockdowns (Figure 1B, right). We counted 200 cells in each condition and found PM ERα in 92% of the control siRNA-transfected cells (white arrows). Membrane localization of ER was reduced to 24 and 33%, respectively, from DHHC-7 or -21 knockdown, but was insignificantly affected by DHHC-22 knockdown (85%; Table 1). The selective effects for PM localization were confirmed by Western blotting (Figure 1B, far right), also indicating that localization of nuclear ERα was unaffected. The latter result reflects the fact that nuclear ERα is not palmitoylated (Pedram et al., 2007). Knockdown of either DHHC-7 or -21 (but not DHHC-22) significantly decreased endogenous ERα palmitoylation (Figure 1C), and the residual acylation probably reflects incomplete knockdown and redundancy of the function of the two DHHC proteins.
TABLE 1:
Localization of ERα in MCF-7 cells.
| Cells showing receptor (%) | ||
|---|---|---|
| Condition | Nucleus | Plasma membrane |
| Control siRNA | 100 ± 2 | 92 ± 7 |
| DHHC-7 siRNA | 100 ± 3 | 24 ± 3a |
| DHHC-21 siRNA | 100 ± 1 | 33 ± 2a |
| DHHC-22 siRNA | 100 ± 3 | 86 ± 4 |
Data are mean ± SEM from three experiments, derived from counting 200 MCF-7 cells per condition in each of the separate experiments.
a p < 0.05 by ANOVA plus Schefe's test for nucleus vs. plasma membrane.
Rapid signaling by 17-β-estradiol (E2) occurs through PM and not nuclear ER (Pedram et al., 2006, 2009b). We therefore determined the impact of the PATs on these functions. Only the knockdown of DHHC-7 or -21 significantly impaired the ability of E2 to stimulate extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/AKT kinases (Figure 2A), and to generate cAMP (Figure 2B). In contrast, knockdown of DHHC-22 did not affect E2 activation of ERK and PI3K/AKT (Figure 2C). Importantly, reduction of either putative ER PAT significantly, but incompletely, impaired E2 signaling that enhanced cell proliferation/viability, as shown by the MTT assay (Figure 2D). This finding is consistent with previous work that showed that both nuclear and PM ER pools contribute to breast cancer cell proliferation (Razandi et al., 2003a). Interestingly, knockdown of both DHHC-7 and -21 was not significantly more potent than the individual PAT protein knockdowns in inhibiting rapid signaling by E2 (Figure 2A). This finding may be due to cooperation between DHHC-7 and -21 proteins to palmitoylate ER, and the knockdown of one protein significantly disrupted the steroid receptor localization and function.
FIGURE 2:
Signaling activity of ER is diminished by DHHC-7 and -21 protein knockdown. (A) Rapid signaling by E2 to ERK and PI3/AKT kinase activation. Bar graph data are mean ± SEM densities from three experiments. *, p < 0.05 for control vs. E2; +, p < 0.05 for E2 vs. E2 plus either siRNA to DHHC-7, DHHC-21, or both DHHC siRNAs. (B) cAMP generation is prevented by DHHC-7 or -21 siRNA. Bar graph represents the mean ± SEM results from three experiments. *, p < 0.05 for control vs. E2; +, p < 0.05 for E2 vs. E2 plus either DHHC-7 or -21 siRNA. (C) DHHC-22 siRNA does not affect E2 signaling. Bar graphs are from three experiments. *, p < 0.05 for control or DHHC-22 siRNA vs. either siRNA plus E2. (D) E2-induced cell proliferation/viability determined by the MTT assay (n = 3 experiments). (E) DHHC-7 and -21 have no effect on EGF signaling. MCF-7 cells were incubated with DHHC-7 or -21 siRNAs, then incubated with EGF (10 ng/ml) for 15 min (ERK or PI3K/AKT activation; left) or for 24 h for MMT proliferation/viability studies (right). The studies were repeated three times. *, p < 0.05 for condition vs. condition plus EGF.
We also determined whether DHHC-7 or -21 influenced epidermal growth factor (EGF) signaling, since transactivation of the EGF receptor is required for membrane ER signaling in hormone-responsive cancers (Razandi et al., 2003b). EGF stimulated ERK and PI3K/AKT activity and increased the viability of MCF-7 cells. These actions were unaffected by DHHC knockdown, indicating specificity of the PATs for ER (Figure 2E). The results also suggest that EGF receptor signaling does not require cross-talk to membrane-localized ERα.
Structure–function interactions and localization of DHHC proteins and ERα
We previously determined that a nine–amino acid palmitoylation motif in the ligand-binding (E) domains of all sex steroid receptors is important for the acylation that drives the receptors to the PM (Pedram et al., 2007). The cysteine at amino acid 451 is the site of palmitic acid attachment to ERα. We therefore expressed wild-type (WT) or a C451A mutant ERα and DHHC-7 or DHHC-21 in ER-null CHO cells and determined protein interactions. Expressed DHHC-7 and -21 proteins each strongly associated with WT ERα but failed to significantly interact with C451A ERα (Figure 3A). The results indicate that an intact palmitoylation site is required for PAT binding to ERα. Importantly, only WT, but not C451A ERα, was robustly palmitoylated upon DHHC-7 or -21 expression (Figure 3B). In contrast, DHHC-22 expression did not stimulate ER palmitoylation beyond the basal palmitoylation seen from endogenous PAT(s) (Figure 3B, lanes 1 and 7). We conclude that the PATs promote palmitoylation of ERα specifically at cysteine 451, consistent with the inability of C451A ERα to traffic to the PM (Pedram et al., 2007).
FIGURE 3:
Structural basis for interaction of ER and DHHC proteins. (A) DHHC-7 and -21 proteins physically interact with WT but not mutant ERα. HA-tagged, plasmids encoding DHHC-7 or -21 were expressed in CHO cells with WT or C451A palmitoylation-site mutant ERα. The cells were lysed and immunoprecipitation (IP) of ER was followed by Western blotting with HA antibody, or by first doing HA immunoprecipitation, which was followed by blotting for ER. A representative study from three experiments is shown. (B) CHO cells were transfected to express either ER construct with DHHC constructs and then labeled with palmitic acid to determine ER palmitoylation. Bar graph is data from three experiments. *, p < 0.05 for pcDNA3 plus ER WT (lane 1) vs. DHHC-7 (lane 3) or DHHC-21 (lane 5) plus ER WT; +, p < 0.05 for pcDNA3 plus ER WT (lane 1) vs. pcDNA3 plus ER C451A (lane 2), DHHC-7 plus ER WT (lane 3) vs. DHHC-7 plus ERC451A (lane 4), DHHC-21 plus ER WT (lane 5) vs. DHHC-21 plus ER C451A (lane 6). (C) CHO cells were transfected with WT ER, DHHC (control, as empty vector), and/or DHHC-7 or -21, then labeled with palmitic acid and exposed to 10 mM hydroxylamine for 4 h. Bar graph is data from three experiments. *, p < 0.05 for ER WT (lane 1) vs. DHHC-7 (lane 3) or DHHC-21 (lane 5) plus ER WT; +, p < 0.05 for ER WT (lane 1) vs. ER WT plus hydroxylamine (lane 2) or ER WT plus DHHC control (lane 7) vs. ER WT plus DHHC control plus hydroxylamine (lane 8), DHHC-7 plus ER WT (lane 3) vs. DHHC-7 plus ER WT plus hydroxylamine (lane 4), DHHC-21 plus ER WT (lane 5) vs. DHHC-21 plus ER WT plus hydroxylamine (lane 6). (D) Localization of endogenous ERα and DHHC-7 and -21 proteins in MCF-7 cells. MCF-7 cells were fixed and exposed to ERα rabbit anti-human antibody, which was followed by FITC-conjugated goat-anti rabbit second antibody. For DHHC proteins, cells were incubated with mouse monoclonal antibodies, and then with Texas red–conjugated, anti–mouse IgG; cells were examined by fluorescence microscopy. The study shown was repeated two more times (E) Colocalization of DHHC-7 or -21 and ERα in the Golgi organelle. MCF-7 cells were incubated with primary antibodies for DHHC-7, DHHC-21, or ERα, and with antibody to human Golgi protein, and then with FITC-conjugated secondary antibodies; cells were examined by fluorescence microscopy. White arrows indicate membrane ERα. A representative study of three is shown.
Palmitoylation of ERα appears to occur by a reversible thioester linkage of palmitic acid at the internal C451 site (Pedram et al., 2007). We examined this by expressing WT ERα in CHO cells with DHHC control vector or DHHC-7 or -21 protein-encoding plasmids. Palmitoylation was determined in the absence or presence of hydroxylamine, which significantly cleaves thioester bonding of fatty acyl groups (Drisdel et al., 2006; Pedram et al., 2007). Hydroxylamine inhibited both basal ERα palmitoylation that resulted from the endogenous PATs and the additional palmitoylation promoted by expressing DHHC-7 or -21 (Figure 3C).
Membrane-bound, but not nuclear, ERα is palmitoylated. Palmitoylation likely occurs at the Golgi (Pedram et al., 2007), where many proteins are palmitoylated by DHHC proteins (Fukata and Fukata, 2010). We determined where endogenous DHHC-7 and -21 proteins colocalize with ER. In MCF-7 cells, ERα is densely localized to the nucleus and is seen at extranuclear sites (Figure 3D). DHHC-7 and -21 proteins were found to be exclusively extranuclear and colocalized with some ERα in cytoplasm. We also determined that endogenous DHHC-7 and -21 colocalize with a Golgi marker (Figure 2E, top panels) and that some ERα also localizes to the Golgi (Figure 3E, bottom panels). These results support the idea that the ERα–PAT protein interaction (Figure 3A) occurs in cytoplasm (Figure 3D), at least in part at the Golgi organelle (Figure 3E).
Signaling by membrane ER affects epigenetic transcription
Palmitoylation and the resulting trafficking of ERα are relevant only for the membrane-localized pool (Pedram et al., 2006, 2009b). Thus the loss of palmitoylation should not significantly affect the transcription of genes regulated by nuclear ERα binding to promoter/enhancer estrogen-response elements (EREs). To test this, we knocked down DHHC-7 or -21 and determined endogenous pS2 gene abundance in MCF-7 cells. pS2 transcription is ERE-mediated (Harrington et al., 2006), and E2 comparably stimulated endogenous gene expression under control or DHHC-specific siRNA expression conditions (Figure 4A). Thus E2 modulation of the pS2 gene does not require membrane ERα signaling.
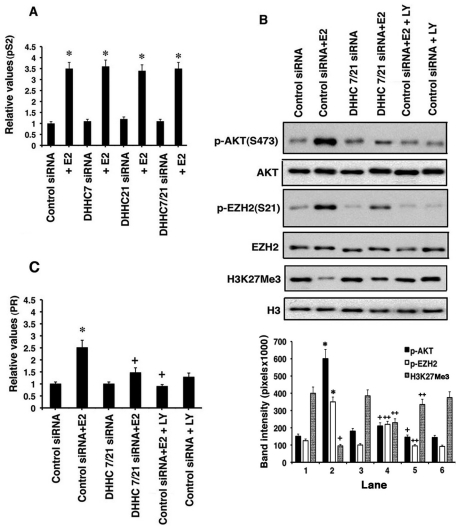
FIGURE 4:
DHHC regulates signaling to the epigenetic modulation of the PR gene. (A) pS2 gene expression (qRT-PCR) induced by E2 is not affected by DHHC-7 or -21 knockdowns. DHHC siRNAs alone had no effects. The bar graph is from three experiments; *, p < 0.05 for any siRNA alone condition vs. siRNA(s) plus E2. (B) DHHC knockdown inhibits E2 signaling through PI3K/AKT (top panel) to the phosphorylation of EZH2 (middle panel), altering histone 3 trimethylation at Lys-27 (bottom panel). The soluble PI3K inhibitor LY 290042 (10 μM) was also used. Total AKT, EZH2, and histone 3 proteins are shown. Bar graphs are from three experiments. *, p < 0.05 for control siRNA vs. control siRNA plus E2 (p-AKT and p-EZH2); +, p < 0.05 for control siRNA plus E2 vs. DHHC siRNA plus E2, or control siRNA plus E2 plus LY 290442 (p-AKT), or for control siRNA vs. control siRNA plus E2 (H3L27Me3); ++, p < 0.05 for control siRNA plus E2 vs. DHHC-7/21 siRNA plus E2 or control siRNA plus E2 plus LY (p-EZH2, H3K27Me3). (C) PR gene expression is stimulated by E2 through PI3K/AKT in MCF-7 cells but is inhibited by DHHC siRNA. The data are normalized for GAPDH expression in the same samples. Bar graph represents combined data from three experiments. *, p < 0.05 for control siRNA vs. same plus E2; +, p < 0.05 for control siRNA plus E2 vs. DHHC siRNA plus E2 or control siRNA plus E2 plus LY 290442.
In contrast, ERK activation by membrane PR or ER collaborates with nuclear-localized sex steroid receptors to enhance transcription of some genes (Madak-Erdogan et al., 2008; Subtil-Rodriguez et al., 2008) Interestingly, several estrogenic compounds stimulate PR gene expression, in part by signaling through AKT to phosphorylate Ser-21 of EZH2. Phosphorylation of EZH2 at this site inhibits the histone trimethyltransferase activity of this protein (Bredfeldt et al., 2010). EZH2 is the primary histone 3 trimethyltransferase for Lys-27 (repressive mark; Margueron et al., 2008), and the PR gene is epigenetically derepressed as a result of estrogen signaling (Bredfeldt et al., 2010). AKT signaling by ER in the study by Bredfeldt et al. presumably occurred through PM-localized receptors, as we have shown in MCF-7 cells (Pedram et al., 2009a).
To investigate the impact of DHHC proteins on this important function, we determined that E2-stimluated AKT activity was diminished from DHHC knockdown or by a soluble inhibitor of PI3K, LY 290042 (Pedram et al., 2009a; Figure 4B, top panel). Inhibition of E2-induced AKT activity by either approach prevented sex steroid–induced phosphorylation of EZH2 at the inhibitory site (Ser-21) (middle panel). E2 also inhibited the H3K27Me3 (trimethylation) repressive mark (bottom panel), which was reversed by DHHC protein knockdown or LY290042. This signaling pathway importantly contributed to E2-stimulated endogenous PR gene expression, since either inhibiting the DHHC proteins or blocking AKT activation significantly reversed this steroid effect (Figure 4C). In this way, DHHC-induced trafficking of ER to the PM results in rapid signaling that induces epigenetic modulation of gene expression.
DHHC-7 and -21 are PATs for PRs and ARs
All sex steroid receptors must be palmitoylated for localization to the PM (Pedram et al., 2007). We therefore sought to identify the PATs that promote PR and AR trafficking to and functioning at the PM in hormone-responsive cancer cells. Expression in MCF-7 cells of the individual DHHC-encoding plasmids from a cDNA library is seen in Figure 1A, an experiment that allowed us to also probe the endogenous PR palmitoylation shown here. Only expression of DHHC-7 and -21 clearly promoted the enhanced palmitoylation of endogenous PRB (Figure 5A). We previously showed that both PRA and PRB are found at the PM of MCF-7 cells (Pedram et al., 2007), but PRB is dominant. Therefore we used an antibody that only identifies PRB. Knockdown of DHHC-7 and -21 in the MCF-7 cells significantly prevented endogenous PR palmitoylation (Figure 5B) and PM localization (Figure 5C). As a result, progesterone signaling through ERK and PI3K was inhibited, and the enhanced cell viability/proliferation seen in response to the steroid ligand decreased significantly (Figure 6, A and B).
FIGURE 5:
DHHC-7 and -21 are progesterone receptor PATS. (A) Transfection of individual plasmids into MCF-7 cells encoding one DHHC protein each from a cDNA library of 23 plasmids was followed by [3H]palmitic acid labeling, immunoprecipitation of endogenous PRB from cell lysates, and separation by SDS–PAGE and fluorography. Immunoblots for PR total protein are shown. A representative experiment from three experiments is shown. DHHC protein expression is from the same experiment(s) that is also used for ER PAT screening and is shown in Figure 1A. (B) DHHC-7 or -21 siRNA significantly reduced [3H]palmitate incorporation into endogenous PR. Total PR protein serves as loading/normalization control from a representative experiment of three experiments total. Bar graphs are mean ± SEM densities from three experiments. *, p < 0.05 for control siRNA vs. DHHC-7 or -21 siRNA. (C) Distribution of PR in MCF-7 cells. Cells were transfected with siRNAs and Western blots of cell fractions were performed. Bar graph is membrane PR density data from three experiments. *, p < 0.05 for siRNA control vs. DHHC siRNA.
FIGURE 6:
Signaling functions of PR are decreased by DHHC-7 and -21protein knockdown. (A) Rapid signaling by P to ERK and PI3/AKT kinase activation is prevented by DHHC-7 or -21 siRNA. Bar graphs represent the mean ± SEM from data combined from three experiments. *, p < 0.05 for control vs. P; +, p < 0.05 for P vs. P plus DHHC-7, DHHC-21, or both siRNA(s). (B) E2-induced cell viability is decreased upon DHHC-7 or -21 knockdown. Viability is determined by the MTT assay. The bar graph represents data combined from three experiments. *, p < 0.05 for control vs. P; +, p < 0.05 for P vs. P plus DHHC-7 or DHHC-21 siRNA.
Similarly, expression of the individual plasmids in C4-2 prostate cancer cells resulted in enhanced palmitoylation of endogenous AR only by the same PATs for palmitoylating ER and PR (Figure 7A). Knockdown of DHHC-7 or -21 with siRNA diminished AR localization only at the PM (Figure 7B) and diminished AR palmitoylation (Figure 7C). PAT knockdown inhibited dihydrotestosterone (DHT) signaling to ERK and PI3K/AKT (Figure 8A) and decreased cell proliferation and viability (Figure 8B). These latter results indicate that membrane AR signaling in prostate cancer cells contributes to the hormone-induced biology. Thus the DHHC-7 and -21 proteins are conserved as PATs for all classes of sex steroid receptors.
FIGURE 7:
ARs are substrates for DHHC-7 and -21. (A) Transfection of individual DHHC-encoding plasmids into C4-2 prostate cancer cells was followed by [3H]palmitic acid labeling. Immunoblots for AR total protein and HA-tagged DHHC PAT proteins are shown. A representative experiment repeated two more times is shown. (B) Distribution of AR in C4-2 cells. Left, cells were transfected with the siRNAs and Western blots of cell fractions were performed. Knockdown of DHHC-7 or -21 decreased membrane, but not nuclear, localization of the steroid receptor. Bar graphs are combined mean ± SEM membrane AR densities from three experiments. *, p < 0.05 for control siRNA vs. DHHC siRNA. (C) DHHC-7 or -21 siRNA significantly reduced [3H]palmitate incorporation into endogenous AR. Total AR protein is shown. Bar graphs are data from three experiments. *, p < 0.05 for control siRNA vs. DHHC siRNA.
FIGURE 8:
Signaling functions of AR are decreased by DHHC-7 and -21 protein knockdown. (A) Rapid signaling by DHT to ERK and PI3/AKT kinase activation is inhibited by DHHC-7 or -21 knockdown. Bar graph data represent the combined mean ± SEM from three experiments. *, p < 0.05 for control vs. DHT; +, p < 0.05 for DHT vs. DHT plus DHHC-7 or -21 siRNA. (B) DHT-induced cell viability is decreased upon DHHC-7 or -21 knockdown and is determined by the MTT assay. The bar graph represents combined data from three experiments. *, p < 0.05 for control vs. DHT; +, p < 0.05 for DHT vs. DHT plus DHHC-7 or -21 siRNA.
DISCUSSION
Ongoing research continues to reveal the important roles of extranuclear steroid receptors (Chambliss et al., 2010; Wong et al., 2010; Levin, 2011). Rapid signaling from PM-localized ER, PR, and AR affects genomic and nongenomic functions (Faivre et al., 2005), for example, in hormone-responsive cancers (Migliaccio et al., 2000; Hammes and Levin, 2007). Therefore understanding receptor trafficking creates targets to use for selective interference with rapid signaling by sex steroids.
Trafficking of all sex steroid receptors to the PM requires palmitoylation at a highly conserved nine–amino acid motif within the ligand-binding/E domain (Acconcia et al., 2004; Pedram et al., 2007). Palmitoylation promotes the physical association of ER with caveolin-1, thus facilitating ER translocation to the PM (Acconcia et al., 2004; Pedram et al., 2007). Endogenous ER is highly enriched in PM caveolae rafts (Chambliss et al., 2002; Razandi et al., 2002), in which caveolin-1 is a coat protein that scaffolds a localized signaling complex to interact with sex steroid receptors. Interactions of signaling molecules in proximity to membrane ER results in the generation of calcium flux, cyclic nucleotides, and kinase(s) activation (Pedram et al., 2006; Hammes and Levin, 2007). Interestingly, many classical G protein–coupled receptors (GPCRs), including dopaminergic and adrenergic receptors, contain a very similar nine–amino acid sequence (F(X6)LL, where “X” is any amino acid) found in conserved intracellular loops (Duvernay et al., 2006). Although this sequence contributes to the membrane localization of these receptors, there is neither a cysteine in this motif nor does palmitoylation occur on classical GPCRs, so it is unclear how this sequence promotes trafficking to the membrane.
A key to understanding sex steroid receptor PM trafficking is identification of the PAT(s) that cause receptor acylation. In this study, we identify the DHHC-7 and -21 proteins as PATs for ER, PR, and AR. Selective knockdown of endogenous DHHC-7 and -21 prevents PM localization and rapid signaling, and impairs both nongenomic and genomic functions, including epigenetic up-regulation of the PR gene by ER. These findings indicate that DHHC PATs for ER may be potential targets to limit the epigenetic effects of xenoestrogens (Bredfeldt et al., 2010), environmental sex steroid mimics/disrupters (Portier, 2002), or endogenous sex steroid actions in hormone-responsive cancers. Up-regulation of PR by ER significantly promotes mammary cancer in mice (Pool et al., 2006) and breast cancer from sex hormone replacement after menopause (Rossouw et al., 2009). We suggest that additional rapid signaling by steroid receptors at the PM may affect other epigenetic functions.
The physical complex formed between the DHHC PATs and the palmitoylation motif of ERα creates a functional interaction. Because mutation of the cysteine amino acid at 451 of ERα prevents the association with PATs, the PATs cannot palmitoylate the receptor. As a result, the S451A ERα protein fails to significantly traffic to the PM (Pedram et al., 2007) and does not significantly activate multiple rapid signals. We report that the identified, endogenous PATs colocalize with ERα in cytoplasm, especially in the Golgi. Based upon our previous studies (Pedram et al., 2007) and the current data, we suggest that receptor palmitoylation takes place at this organelle, a common site for plasma membrane–bound protein palmitoylation (Fukata and Fukata, 2010). This idea is supported by the finding that palmitoylation of ER prior to its transport to the PM caveolae is required for its cytoplasmic interaction with caveolin-1 (Acconcia et al., 2004; Pedram et al., 2007).
Based on our results, we propose a conserved mechanism of trafficking for all sex steroid receptors. Interestingly, both identified PATs contribute to receptor palmitoylation, suggesting redundancy of function. This is not unusual, since some proteins undergo palmitoylation by several PATs, while individual PATs acylate multiple substrates (Fukata et al., 2004). DHHC-7 palmitoylates the SNAP25 protein that is essential for neuronal exocytosis (Greaves et al., 2010), and both DHHC-7 and -21 PATs palmitoylate the membrane endothelial nitric oxide synthase enzyme that produces nitric oxide in the vasculature (Fernandez-Hernando et al., 2006). Also, cooperation between two PATs to palmitoylate a single substrate site has been demonstrated but is poorly understood (Fukata et al., 2004). This may be applicable to sex steroid receptor palmitoylation.
Receptor localization to discrete sites of the cell is critical for proper function, and redundancy of PATs may ensure proper trafficking. In epithelial cells, mislocalization of tumor suppressor or oncogenic proteins underlies the development of malignancy (Calnan and Brunet, 2008; Jones, 2008; Lee and Kim, 2009; Neufeld, 2009). Aggressive breast cancer or breast cancer that is resistant to endocrine therapy is sometimes associated with significantly increased ERα localization and function outside the nucleus, including at the PM (Kumar et al., 2002; Fan et al., 2007). Our search of the Oncomine database revealed that the DHHC-21 gene is significantly overexpressed in human breast cancer compared with normal breast epithelium. It is possible that alterations in steroid receptor PAT abundance or function contributes to increased ER at the PM in some situations.
However, a more important limiting factor for PM trafficking is that E2 rapidly induces the dimerization of ∼85% of ERα (Razandi et al., 2004). Since palmitoylation preferentially occurs on ERα monomers (Razandi et al., 2010), the steroid ligand controls the amount of receptor available for palmitoylation by controlling dimerization. Additionally, all ERs contain a nuclear localization signal (NLS) that drives ∼80% of the receptors to the nucleus (Pedram et al., 2006). Perhaps the abundance of the nuclear receptor is required to bind the many promoters and enhancer elements throughout the genome. However, significant pools of endogenous mitochondrial sex steroid receptor (∼15% of total ERα) and PM-localized ER exist. To overcome the strong influence of the NLS in driving receptors exclusively to the nucleus, all sex steroid receptors require palmitoylation for trafficking to the PM (Pedram et al., 2007). In normal cells, the abundance of PATs is likely to be a minor contributing factor, because the amount/availability of enzyme substrate (steroid receptor) is more important. As mentioned above, however, increased PAT abundance may contribute to the enhanced extranuclear ER localization and kinase activation seen in some breast cancers (Kumar et al., 2002; Fan et al., 2007).
Heat-shock protein 27 (Hsp27) facilitates palmitoylation of all sex steroid receptor classes upon binding to their palmitoylation motifs (Razandi et al., 2010). The palmitoylation motif is localized deep within the receptor structure, even as a monomer. We propose that Hsp27 binds the receptor and thereby changes its conformation, thus allowing the now-identified PATs to bind at the same site and palmitoylate the receptors. The dynamics of these protein interactions will require complicated nuclear magnetic resonance structural studies.
Finally, diminishing DHHC function inhibits both ER and PR localization at the PM of breast cancer cells. Preventing PM localization of DHHC substrates, such as Ras and Src kinases, by PAT knockdown may further contribute to the loss of rapid signal transduction in these settings. Hence, DHHC PATs may be a therapeutic target in aggressive breast cancers that require ER, PR, and growth factor tyrosine kinase receptor signal transduction for proliferation and survival (Dowsett et al., 2006). Targeting PATs may also enable repression of AR function at the membrane of aggressive prostate cancers. Selective antagonists for membrane sex steroid receptors and enhanced small-interfering RNA (siRNA) delivery techniques directed against PATs in vivo may be useful to inhibit multiple signaling pathways in these tumors.
MATERIALS AND METHODS
Cell culture and reagents
MCF-7 and CHO-K cells were from ATCC and C4-2 prostate cancer cells (AR positive) were a gift from Ganesh Raj (University of Texas, Southwestern, Dallas, TX). Cells were cultured in phenol red–free DMEM-F12, 10% charcoal-stripped fetal bovine serum (FBS), 1% antibiotic–antimycotic (15240–062; Life Technologies, Carlsbad, CA) at 37°C to 75% confluency in 100-mm dishes or six-well plates and exposed to 10 nM E2, 100 nM progesterone acetate (P) or 10 nM DHT (Steraloids, Newport, RI) for the times indicated in the experiments. ERα (MC-20), PRB (C-19), and AR (C-19) antibodies (all polyclonal, raised in rabbits); green fluorescent protein antibody (GFP; polyclonal, rabbit); and HA antibodies (monoclonal, mouse) were from Santa Cruz Biotechnology (Santa Cruz, CA). Golgi marker antibody (AE-6, mouse monoclonal) from Santa Cruz is directed against the isolated Golgi cisternae functional regions composed of various enzymes and is derived from a human lymphoma cell line, per the manufacturer. Antibodies to DHHC-7 and -21 (monoclonal, mouse), phospho-EZH2 Ser-21, histone H3, and H3K27Me3 (polyclonal, rabbit) antibodies were from Abcam (Cambridge, MA), and were used for immunoblots in subcellular fractions and for immunofluorescence microscopy. We also used a DHHC-7 polyclonal antibody raised in rabbits (Novus Biologicals, Littleton, CO) for the Golgi colocalization study. Phospho-AKT Ser-473 antibodies (monoclonal, rabbit) were from Cell Signaling Biotechnology (Danvers, MA). Double-stranded control and RNAs for the DHHC-7, -21 and -22 proteins (two siRNAs each) were obtained from Santa Cruz. Each siRNA was used for all key experiments, and the data shown are from experiments using either of the two siRNAs. Construction and transfection/expression of all ER, AR, PR, and GFP (pEGFP vector; Clontech, Mountain View, CA) plasmids were previously described (Pedram et al., 2007). The DHHC cDNA plasmid library used the pEF-BOS-HA backbone vector and was provided by Y. Fukata and M. Fukata (Fukata et al., 2004). PD 98059, a MEK inhibitor, was a gift from Alan Saltiel (Parke-Davis, Ann Arbor, MI), and the LY290042 (PI3K inhibitor) was from Calbiochem (San Diego, CA).
Cell fraction isolation
Cells were homogenized using 15–20 strokes of a tight-fitting Dounce homogenizer until ∼ 90% of the cells were broken. Homogenates were centrifuged at 4000 rpm for 10 min to pellet the nuclear fractions. Supernatants were layered onto a discontinuous sucrose gradient (1.0–2.5 M) made up in buffer containing 10 mM Tris (pH 7.6), 2 mM EDTA, 2 mM dithiothreitol, protease inhibitor cocktail, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The mixtures were centrifuged at 2000 rpm for 30 min at 4°C to produce a top layer (cytosol). Cytosolic fractions were centrifuged at 100,000 × g for 1 h. The pellet was centrifuged again to isolate the membrane fraction, which was separated by SDS–PAGE on a 10% gel, transferred to nitrocellulose, and then immunoblotted with the indicated antibodies (Santa Cruz), which were detected using the ECL kit (Amersham/GE Healthcare, Waukesha, WI). Western blots were also used to normalize total receptor or other proteins.
Steroid receptor palmitoylation
Palmitoylation of sex steroid receptors was carried out as previously described (Pedram et al., 2007). MCF-7, CHO, or C4-2 cells were labeled with [3H]palmitic acid (0.5 mCi/ml) for 4 h in the absence of sex steroids. Cytoplasmic extracts were immunoprecipitated with antibodies to ERα, PR, or AR, each of which was conjugated to protein A Sepharose. Separation of samples by SDS–PAGE was followed by autoradiography with emulsifying enhancer.
ERK and PI3K/AKT activity
ERK was immunoprecipitated from lysates of MCF-7 cells exposed to various steroids or EGF (10 ng/ml). Equal ERK protein amounts from each experimental condition were added to separate tubes containing [32P]ATP and substrate peptide (myelin basic protein; Sigma-Aldrich, St. Louis, MO) in buffer to determine kinase activity. Activity was determined by autoradiography of phosphorylation of substrate protein. (Pedram et al., 2006). PI3K activity was determined as phosphorylation of AKT at Ser-473 (p-AKT) by Western blotting (Pedram et al., 2006) after 15 min of exposure of the synchronized cells to steroid or EGF. Total ERK or AKT protein, as identified by Western blotting, was used for sample activity normalization. In control DHHC signaling experiments, MCF-7 cells were incubated with DHHC-22 siRNA, recovered overnight in DMEM-F12 medium without serum or phenol red, and then incubated with 10 nM E2 for 15 min, with ERK or PI3K/AKT activation determined at that time.
cAMP generation
Adenylate cyclase activity in the membrane suspension was determined by measuring cAMP generation in the presence of IBMX (phosphodiesterase inhibitor) after cells were incubated for 5 min with 10 nm E2 in DMEM-F12 medium. After incubation, the cells were washed and 0.1N HCl was added to the cell monolayers for 20 min at room temperature to extract cAMP. The supernatants were collected and recentrifuged for use in the cAMP radioimmunoassay according to the manufacturer's protocol (Perkin Elmer-Cetus, Waltham, MA; Pedram et al., 2006).
MTT assay
Cell proliferation/viability was quantified by a colorimetric assay according to the manufacturer's instructions (Sigma Chemical Corporation). The kit measures mitochondrial dehydrogenase activity in viable cells based on the conversion of the MTT to MTT–formazan crystal by mitochondrial enzyme. In brief, synchronized MCF-7 and C4-2 cells were exposed to various treatments in DMEM-F12 medium for 24 h. At the end of incubation, the cells were washed and then incubated with medium containing 1 mg/ml MTT for 4 h at 37°C. MTT–formazan was extracted by overnight incubation at 37°C with 100 μl extraction buffer (20% SDS, 50% formamide adjusted to pH 4.7 with 0.02% acetic acid, and 0.025 N HCl]. Optical densities at 570 nm were measured using extraction buffer as a blank.
Cell localization of steroid receptor proteins
Steroid receptor localization was determined by immunofluorescent cell microscopy (Drisdel et al., 2006). After MCF-7 or C4-2 cells were cultured on glass bottom dishes (MatTek, Ashland, MA), the cells underwent siRNA transfection or other treatments, and then they were recovered over 24 h, fixed with 3.5% paraformaldehyde, permeabilized with 0.2% Triton X-100, and then incubated with sex steroid receptor antibody overnight at 4°C. Nonspecific immunoglobulin G (IgG) antibody served as control. Incubation with the first antibody was followed by incubation with fluorescein isothiocyanate (FITC)-conjugated second antibody (green color) or IgG antibody (no visualization) for 2 h at room temperature. For colocalization studies, human-specific antibodies to Golgi markers (Santa Cruz Biotechnology) were used for separate red and green fluorescence. A similar method was used for fluorescence colocalization of endogenous ERα with DHHC proteins or Golgi in MCF-7 cells using monoclonal antibodies against each protein. Identically treated MCF-7 cells were also used for plasma membrane and nuclear fraction isolation. The fractions were separated by SDS–PAGE on a 10% gel, transferred to nitrocellulose, immunoblotted with the indicated antibodies (Santa Cruz Biotechnology), and detected using the ECL kit.
Epigenetic modulation studies
MCF-7 cells were cultured in DMEM-F12 medium and transfected with DHHC siRNAs (3 μg/well of cells in six-well plates), which was followed by 24 h of recovery and overnight synchronization. The cells were incubated with 10 nM E2 ± 10 μM LY294002 for 30 min to determine AKT and EZH2 phosphorylation, and for 24 h to determine PR gene expression using real-time PCR (RT-PCR). MCF-7 cell monolayers were washed in ice-cold phosphate-buffered saline supplemented with 5 mM sodium butyrate to retain histone acetylation. After centrifugation at 2000 rpm for 5 min in the cold, cell pellets were resuspended in lysis buffer (10 mM Tris-HCl, pH 7–7.4, 10 mM NaCl, 3 mM MgCl2, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF, and complete protease inhibitor cocktail) with 0.5% NP-40 on ice. Cell lysates were centrifuged for 5 min at 6000 rpm at 4°C. Phospho-AKT (Ser-473) was determined from supernatants by immunoblotting as readout for PI3K activity (Pedram et al., 2006). The activity-inhibiting phosphorylation at Ser-21 of the histone methyltransferase EZH2 was also investigated. Total AKT and EZH2 served as loading controls. Pellets were resuspended in media with 5 mM MgCl2 and 0.8M HCl, sonicated at low speed for 20 s, and incubated on ice for 1 h. The solution of histone proteins was centrifuged at 14,000 rpm for 10 min at 4°C. Supernatants were transferred to a new tube, and histones were precipitated with a 50% solution of trichloroacetic acid dissolved in ddH2O. Precipitated histones were collected by centrifugation for 20 min at 14,000 rpm at 4°C. The resultant pellet was washed with ice-cold acetone, dried, and subsequently resuspended with ddH2O (150 μl) and 1.5 M Tris-HCl (pH 8.8; 2 μl). Histone proteins (10 μl) were resolved by SDS–PAGE on a 10–20% Tris-tricine gel (Bio-Rad, Hercules, CA), and changes in trimethylation of histone 3 at Lys-27 relative to total histone 3 were investigated by immunoblotting with antibodies from Abcam.
Quantitative RT-PCR
MCF7 cells were grown in phenol red–free DMEM with 10% charcoal dextran–treated FBS for greater than 24 h before transfection. Cells were transiently transfected with control or DHHC siRNAs plasmids for 24 h, then washed and incubated for 24 h in either 10 nm E2 ± LY290042 or an ethanol vehicle. Total RNA was extracted using the RNeasy kit from Qiagen (Valencia, CA). cDNA was synthesized using the Improm-II reverse transcription system (Promega, Madison, WI). Quantitative RT-PCR (qRT-PCR) was used to determine pS2 and PR gene expression, and RT-PCR was used to determine DHHC-22 expression, normalized to the housekeeping gene, GAPDH. Primers were designed with Primer3 (http://frodo.wi.mit.edu) and were blasted for specificity.
pS2:F5′-ATACCATCGACGTCCCTCCA-3′,R5′-AAGCGTGTCTGAGGTGTCCG-3′; PR:F5′-CGCGCTCTACCCTGCACTC-3′,R5′-TGAATCCGGCCTCAGGTAGTT-3′; ZDHHC22:F5′-GCCTACATCTCCGCTGTCCTTT-3′, R5′ATGGCGAACCAGAGGTAGAGCA-3′; GAPDH:F 5′-CCACAGTCCATGCCATCA-3′, R5′-GGATGACCTTGCCCACAG-3′ primers were designed for annealing temperature of 60°C and to amplify regions of ∼95–120 base pairs. PCR amplicon sizes were confirmed by agarose gel electrophoresis prior to qRT-PCR analysis. For qRT-PCR, 500 ng of cDNA was used in a 50-μl reaction consisting of 25 μl of SYBR Green, qPCR Supermix (Invitrogen, Carlsbad, CA), 1 μl of 10 μM forward/reverse primer stocks, and nuclease-free water. Thermocycling was carried out using the iCycler (Bio-Rad) with a melting-curve temperature of 60°C. Relative mRNA levels were calculated using the Ct method.
Image acquisition
The microscopic images were obtained using a Nikon Eclipse TE-200 microscope with magnification from 200–400×, at room temperature. Imaging medium (Vectashield) with antifade properties was from Vector Laboratories (Burlingame, CA). A Nikon Diagnostic Instruments camera (Model 3.2.0) was used in conjunction with Spot Advance software (Sterling Heights, MI) to capture and transfer images to the computer. Rhodamine-conjugated (red) and FITC-conjugated (green) secondary antibodies (Vector Laboratories) were used for fluorescence visualization.
Data analysis
Data from multiple experiments were used to calculate a mean ± SEM and were analyzed by analysis of variance (ANOVA) plus Schefe's test at a p < 0.05 level of significance.
Acknowledgments
The work was supported by grants from the Research Service of the Department of Veteran's Affairs and the National Institutes of Health CA-10036.
Abbreviations used:
- ANOVA
analysis of variance
- AR
androgen receptor
- DHHC
Asp-His-His-Cys
- DHT
dihydrotestosterone
- E
ligand binding
- E2
17-β-estradiol
- EGF
epidermal growth factor
- ER
estrogen receptor
- ERE
estrogen-response element
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- GFP
green fluorescent protein
- GPCR
G protein–coupled receptors
- HA
hemagglutinin
- NLS
nuclear localization signal
- P
progesterone acetate
- PAT
palmitoylacyltransferase
- PI3K
phosphatidylinositol 3-kinase
- PM
plasma membrane
- PMSF
phenylmethylsulfonyl fluoride
- PR
progesterone receptor
- qRT-PCR
quantitative RT-PCR
- RT-PCR
real-time PCR
- siRNA
small interfering RNA
- WT
wild-type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-07-0638) on October 26, 2011.
A.P. and M.R. performed the research; R.J.D. and E.L. designed the research and analyzed the data.
The authors report no conflicts of interest and have nothing to disclose.
REFERENCES
- Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor α membrane localization regulation by 17β-estradiol. Mol Biol Cell. 2004;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan DR, Brunet A. The FoxO code. Oncogene. 2008;27:2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Chambliss KL,, et al. Non-nuclear estrogen receptor α signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Dowsett M, Houghton J, Iden C, Salter J, Farndon J, A'Hern R, Sainsbury R, Baum M. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor, and HER2 status. Ann Oncol. 2006;17:818–826. doi: 10.1093/annonc/mdl016. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Alexander JK, Sayeed A, Green WG. Assays of protein palmitoylation. Methods. 2006;40:127–134. doi: 10.1016/j.ymeth.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem. 2006;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor rapid signaling and nuclear actions in breast cancer models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67:1352–1360. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:369–377. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finidori-Lepicard J, Schorderet-Slatkine S, Hanoune J, Baulieu EE. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981;292:255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–996. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Fukata M. Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev. 2010;11:161–175. doi: 10.1038/nrn2788. [DOI] [PubMed] [Google Scholar]
- Gill A, Hammes SR. G βγ signaling reduces intracellular cAMP to promote meiotic progression in mouse oocytes. Steroids. 2007;72:117–123. doi: 10.1016/j.steroids.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves J, Gorleku OA, Salaun C, Chamberlain LH. Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases. J Biol Chem. 2010;285:24629–24638. doi: 10.1074/jbc.M110.119289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillermo P, Vicent A, Silvina N, Roser Z, Ballare C, Clausell J, Beato M. Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol Endocrinol. 2010;24:2088–2098. doi: 10.1210/me.2010-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes SR, Levin ER. Extra-nuclear steroid receptors: nature and function. Endo Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20:491–502. doi: 10.1210/me.2005-0186. [DOI] [PubMed] [Google Scholar]
- Hou H, John Peter AT, Meiringer C, Subramanian K, Ungermann C. Analysis of DHHC acyltransferases implies overlapping substrate specificity and a two-step reaction mechanism. Traffic. 2009;10:1061–1073. doi: 10.1111/j.1600-0854.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- Jones FE. HER4 intracellular domain (4ICD) activity in the developing mammary gland and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:247–258. doi: 10.1007/s10911-008-9076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousteni S, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct interactions with Gαi and Gβ mediate nongenomic signaling by estrogen receptor β. Mol Endocrinol. 2007;12:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- Kumar R, et al. A naturally occurring MTA1 variant sequesters oestrogen receptor-α in the cytoplasm. Nature. 2002;418:654–657. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim SS. The function of p27 KIP1 during tumor development. Exp Mol Med. 2009;41:765–771. doi: 10.3858/emm.2009.41.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER. Extra-nuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25:377–384. doi: 10.1210/me.2010-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER, Pietras RJ. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res Treat. 2008;108:351–361. doi: 10.1007/s10549-007-9618-4. [DOI] [PubMed] [Google Scholar]
- Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol. 2008;22:2116–2127. doi: 10.1210/me.2008-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D. EZH1 and EZH2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A, et al. Steroid-induced androgen receptor-oestradiol receptorβ-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy DI. Protein palmitoylation in membrane trafficking. Biochem Soc Trans. 1995;23:572–576. doi: 10.1042/bst0230572. [DOI] [PubMed] [Google Scholar]
- Neufeld KL. Nuclear APC. Adv Exp Med Biol. 2009;656:13–29. doi: 10.1007/978-1-4419-1145-2_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley BW, Sherman MR, Toft DO. Progesterone receptors in the cytoplasm and nucleus of chick oviduct target tissue. Proc Natl Acad Sci USA. 1970;67:501–508. doi: 10.1073/pnas.67.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F, Wrange O, Carlstedt-Duke J, Okret S, Gustafsson JA, Yamamoto KR. Purified glucocorticoid receptors bind selectively in vitro to a cloned DNA fragment whose transcription is regulated by glucocorticoids in vivo. Proc Natl Acad Sci USA. 1981;78:6628–6632. doi: 10.1073/pnas.78.11.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Evinger AJ, Kim JK, Lee EY, Levin ER. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell. 2009a;20:3374–3389. doi: 10.1091/mbc.E09-01-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EYHP, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor a (MOER) mouse. J Biol Chem. 2009b;284:3488–3495. doi: 10.1074/jbc.M806249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- Pool AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science. 2006;314:1467–1470. doi: 10.1126/science.1130471. [DOI] [PubMed] [Google Scholar]
- Portier CJ. Endocrine dismodulation and cancer. Neuro Endocrinol Lett. 2002;23((suppl 2)):43–47. [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb D, Levin ER. Identification of a structural determinant for the membrane localization of ERα. Mol Cell Biol. 2003a;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. Estrogen receptors associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors derive from a single transcript: studies of ERα and ERβ expressed in CHO cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. 2010;30:3249–3261. doi: 10.1128/MCB.01354-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and function as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Parks S, Levin ER. Proximal events in ER signaling from the plasma membrane. J Biol Chem. 2003b;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, et al. Risks and benefits of estrogen plus progesterone in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. J Am Med Assoc. 2009;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Subtil-Rodriguez A, Millan-Arino L, Quiles I, Ballare C, Beato M, Jordan A. Progesterone induction of the 11β-hydroxysteroid dehydrogenase type 2 promoter in breast cancer cells involves coordinated recruitment of STAT5A and progesterone receptor to a distal enhancer and polymerase tracking. Mol Cell Biol. 2008;28:3830–3849. doi: 10.1128/MCB.01217-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego CM, Davis JS. Adenosine 3′, 5′-monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DM. Early membrane estrogenic effects required for full expression of slower genomic actions in a nerve cell line. Proc Natl Acad Sci USA. 2001;98:12267–12271. doi: 10.1073/pnas.221449798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent GP, Ballare C, Nacht AS, Clausell J, Subtil-Rodriguez A, Quiles I, Jordan A, Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Wong WP, Tiano JP, Liu S, Hewitt SC, Le May C, Katzenellenbogen JA, Katzenellenbogen BS, Korach KS, Mauvais-Jarvis F. Extranuclear estrogen receptor-α stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci USA. 2010;107:13057–13062. doi: 10.1073/pnas.0914501107. [DOI] [PMC free article] [PubMed] [Google Scholar]