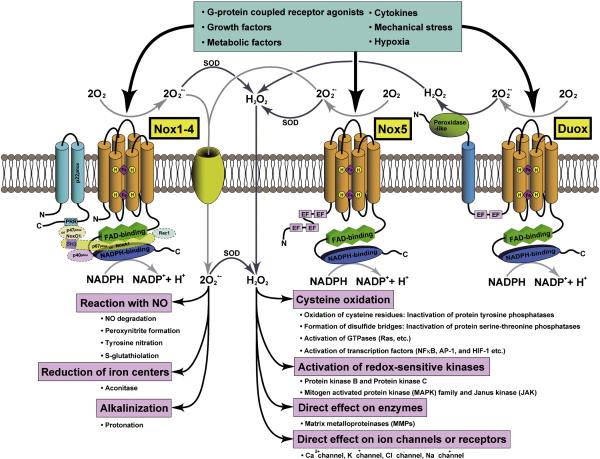
Figure 1.
Schematic representations of the structures of various subtypes of NADPH oxidase-family enzymes and their signaling pathways. Cylinders represent six transmembrane alpha-helices, PRR indicates a proline-rich region (PRR), and EF stands for Ca2+-binding EF-hand motif. Nox is either activated or upregulated by various stimuli, such as growth factors, cytokines, G-protein coupled receptor agonists, metabolic factors, mechanical stress, and hypoxia. The immediate product of NADPH oxidases is superoxide (OM2−). However, due to spontaneous and enzymatic dismutation, hydrogen peroxide (H2O2) can also be generated. O2− generation from NADPH oxidases occurs either in the extracellular or the cytosolic space. The negatively charged O2− does not permeate the lipid bilayer of biological membranes. However, it may pass through the pore of anion channels. Biological effects of Nox-derived O2− include: 1) reaction with nitric oxide (NO) leading to NO degradation, peroxynitrite formation, protein tyrosine nitration, and the addition of glutathione to thiols; 2) reduction of iron centers within enzymes; and 3) alkalinization of intracellular organelles. In most cases, however, biological effects of Nox are mediated through H2O2 after O2− is dismutated. H2O2 is a well-established signaling molecule that readily permeates biological membranes. We propose that Nox4 induces oxidation of many mitochondrial proteins due to its proximity to them.