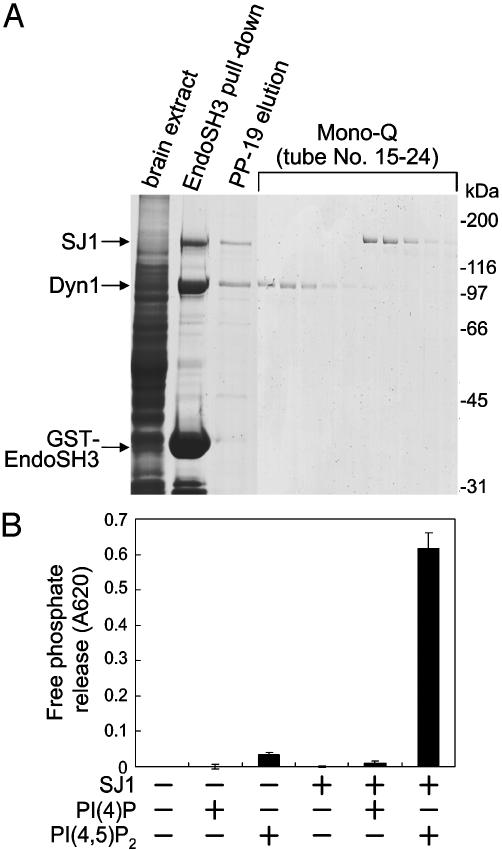
Fig. 1.
Purification of synaptojanin 1 from rat brain extract. (A) An immobilized GST fusion protein of the SH3 domain of endophilin 1 was used to affinity-purify binding proteins from a Triton X-100 rat brain extract. Bound proteins, represented primarily by synaptojanin 1 (SJ1) and dynamin 1 (Dyn1), were eluted by the PP-19 peptide (corresponding to the endophilin binding peptide of synaptojanin 1). Mono-Q chromatography of the PP-19 eluate with a linear gradient of NaCl resulted in the separation of the two proteins. Each fraction was examined by SDS/PAGE followed by protein staining. (B) Purified synaptojanin 1 has 5-phosphatase activity when tested against water-soluble phosphoinositides [diC8-PI(4)P or diC8-PI(4,5)P2] by using a malachite green-based assay for the detection of free phosphate.