Abstract
Objective:
To determine whether unihemispheral hemodynamic failure is independently associated with cognitive impairment among participants in the National Institute of Neurological Disorders and Stroke–sponsored, multicenter, randomized clinical trial, Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON).
Methods:
Forty-three patients were randomized into RECON after recent symptomatic carotid artery occlusion and asymmetrically increased oxygen extraction fraction (OEF) by PET (OEF ratio >1.13), indicating stage II hemodynamic failure on the side of occlusion. The PET-positive patients were compared with 28 RECON-enrolled patients who met all clinical and radiographic inclusion/exclusion criteria but had no OEF asymmetry. A multivariable regression compared patients with PET OEF >1.13 or ≤1.13, stratifying by TIA vs stroke as the qualifying event. The dependent variable was a composite neurocognitive score derived from averaging age-normalized z scores on a test battery that included global and internal carotid artery (ICA) side-relevant hemisphere-specific tests.
Results:
There were no differences in demographic, clinical, or radiologic characteristics between the PET-positive and PET-negative patients except for PET OEF asymmetry. The unadjusted average neurocognitive z score was −1.45 for the PET-positive and −1.25 for the PET-negative patients, indicating cognitive impairment in both groups but no difference between them (p = 0.641). After adjustment for age, education, side of occlusion, depression, and previous stroke, there was a significant difference between PET-positive and PET-negative patients among those with TIA as a qualifying event (average z score = −1.41 vs −0.76, p = 0.040). Older age and right ICA side were also significant in this model.
Conclusion:
Hemodynamic failure is independently associated with cognitive impairment in patients with carotid occlusion. This finding establishes the physiologic parameter upon which the extracranial-intracranial bypass will be tested. Neurology® 2012;78:250–255
Carotid artery disease has been associated with cognitive impairment,1–4 but correlations with quantitative measures of cerebral blood flow (CBF) have been rare. The Randomized Evaluation of Carotid Occlusion and Neurocognition (RECON) is an National Institute of Neurological Disorders and Stroke–sponsored, multicenter, randomized, controlled clinical trial that is an ancillary study of the Carotid Occlusion Surgery Study (COSS). RECON was designed to test the hypothesis that surgical anastomosis of the extracranial superficial temporal artery to the intracranial middle cerebral artery (EC-IC bypass) when added to best medical therapy, can improve or preserve cognitive function at 2 years in patients with symptomatic internal carotid artery (ICA) occlusion and stage II hemodynamic failure better than the best medical therapy alone. The parent study, COSS, tested the hypothesis that EC-IC bypass would significantly reduce the risk of subsequent stroke.5 Both COSS and RECON assessed hemispheral hemodynamic failure by identifying increased oxygen extraction fraction (OEF) by PET on the side of occlusion.
Reported here are the results of the baseline cognitive testing of the RECON cohort, comparing cognitive function in those who had unihemispheral cerebral hypoperfusion with a control group of clinically identical patients with carotid occlusion but without increased OEF. We took advantage of the 2-step randomization process in the study design to include this subgroup of enrolled but not randomized patients to serve as controls. Our data represent an advance over previous studies in obtaining quantitative blood flow data, measuring cognitive impairment with a disease-specific cognitive battery, and using an appropriately matched control group.
METHODS
Subjects.
Patients who met the inclusion criteria of symptomatic carotid artery occlusion (hemispheral TIA or minor stroke) <120 days before enrollment were enrolled into RECON between 2004 and 2009. Symptoms had to have been attributable to the carotid occlusion, and patients had to have a Barthel Index ≥12/20 at the time of enrollment. Nonatherosclerotic causes of carotid occlusion were excluded based on all available clinical data. TIA was defined clinically as having no residual deficit after 24 hours. MRI was not available in all patients and so was not used in the definition of TIA. All enrolled patients underwent a PET scan to measure OEF. Status of collateral arteries was not assessed as part of the protocol. Patients were eligible for randomization to one of the treatment arms if they had asymmetrically increased OEF by PET (OEF ratio >1.13), indicating stage II hemodynamic failure on the side of occlusion. Those who had an OEF ratio ≤1.13 were not randomized and went no further in the study but were included in the current analysis as control subjects. The PET threshold of 1.13 was determined by a prospective natural history study, the St. Louis Carotid Occlusion Study, which examined thresholds of OEF associated with a higher rate of subsequent stroke.6 Details of the PET protocol are published elsewhere.5 Additional exclusion criteria for the RECON study were prior clinical diagnosis of dementia due to any cause, education level less than grade 4, or any neurologic deficit that prevented acquisition of informed consent. Thus, the study group includes no subjects who would be more impaired than a level equivalent to mild cognitive impairment, and some might be normal. The PET-positive patients were compared with the 28 RECON-enrolled patients who met all clinical and radiographic inclusion and exclusion criteria but were not randomized because they had no OEF asymmetry (PET-negative patients).
Standard protocol approvals, registrations, and patient consents.
All patients provided informed consent. The study was approved by the Columbia University Institutional Review Board.
Neurocognitive testing.
Patients underwent a 1-hour neurocognitive battery consisting of 14 standardized neuropsychological tests, administered by a neuropsychologist or trained technician. The battery was designed to assess left hemisphere function, right hemisphere function, and global function. All tests had published, age-adjusted norms.7–16 All patients were administered all 14 neurocognitive tests. The order of test administration remained constant, accommodating the delays required for recall intervals and minimizing stimulus interference in memory tasks. The test battery is summarized in table 1.
Table 1.
Summary of neurocognitive tests by hemisphere specificity
Because hemodynamic failure in the hemisphere ipsilateral to the symptomatic carotid occlusion was hypothesized to affect predominantly functions associated with that hemisphere, as well as global cognitive function that would also use that hemisphere, composite scores were generated for each patient based on the hemisphere of interest: We first transformed the raw test scores for each patient into test-specific z scores for each test in the battery, derived from published norms. We then calculated a composite z score based on the average z score for the appropriate set of tests for each patient (sum of the relevant z scores divided by the number of tests included). For patients with left carotid occlusion, left hemisphere and global test scores were used; for patients with right carotid occlusion, right hemisphere and global test scores were used.
Statistical analysis.
A multivariable regression was performed comparing patients with a PET OEF ratio >1.13 with those with an OEF ratio ≤1.13, stratifying by TIA vs stroke as the qualifying event. This preplanned analysis was formulated to enable us to focus on the patients with TIA, a group in which the likely cognitive effects of stroke, per se, would not confound the hypothesized effects of hypoperfusion. Thus, for this baseline study we postulated that any potential hypoperfusion-attributable effects on cognition would be seen best in those who qualified for the study (PET-positive) with TIA only. Our dependent variable was the composite neurocognitive z score, which was the only outcome measure of cognitive function in the study. The main independent variable was PET OEF, dichotomized to >1.13 vs ≤1.13. Age, gender, education level (≤8th grade, 9th–12th grade [high school], or ≥13 years [some college]) were entered into the model as covariates, along with ICA side, depression (as measured by the Center for Epidemiologic Studies–Depression scale, and previous stroke. Patients missing any of these data elements were eliminated from the analysis.
RESULTS
A total of 71 patients, 32 with TIA as the qualifying event and 39 qualifying with mild to moderate stroke, met the enrollment criteria for this study and had complete data. There were no differences in demographic, clinical, or radiologic characteristics between the PET-positive (OEF ratio >1.13) and PET-negative (OEF ratio ≤1.13) patients, except for PET OEF asymmetry and previous stroke (table 2). The unadjusted average neurocognitive z score was −1.45 below the normative mean for the PET-positive and −1.25 for the PET-negative patients, indicating cognitive impairment among both groups (with and without hemodynamic failure) but no significant difference between them (p = 0.641).
Table 2.
Basic descriptive statistics for variables of interest, stratified by PET OEF status but not adjusted for any other variables
Abbreviations: CES-D = Center for Epidemiological Studies–Depression; ICA = internal carotid artery; IQR = interquartile range; OEF = oxygen extraction fraction.
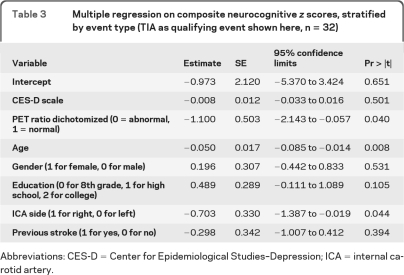
In the multivariable regression analysis, after adjustment for age, education, side of occlusion, and previous stroke, there was a significant difference between PET-positive and PET-negative patients among those with TIA as a qualifying event (average z score = −1.41 vs −0.76, p = 0.040). Older age and right ICA side were also significant in this model, as shown in table 3.
Table 3.
Multiple regression on composite neurocognitive z scores, stratified by event type (TIA as qualifying event shown here, n = 32)
Abbreviations: CES-D = Center for Epidemiological Studies–Depression; ICA = internal carotid artery.
DISCUSSION
We show in a hemodynamically well-characterized cohort that cognitive impairment was independently associated with unilateral cerebral hypoperfusion in patients with carotid artery occlusive disease. Specifically, stage II hemodynamic failure, as measured by increased OEF on the side of carotid occlusion, was associated with cognitive dysfunction. By taking advantage of the 2-step enrollment in the COSS/RECON study design, we were able to establish a well-matched control group: those who met the clinical inclusion criteria of symptomatic carotid artery occlusion but who differed only by the physiologic parameter of a lack of hemodynamic failure in the hemisphere supplied by the occluded carotid artery. Additional specificity for the independent effect of the hypoperfusion was afforded by the use of a cognitive battery that emphasized the hemisphere of interest and by our stratification by clinical subtype to focus on the subset of patients who qualified for the study without having new infarction or lasting clinical deficit (patients with TIA). In showing the association between cognitive dysfunction and hemodynamic failure in those with an absence of imaging-verified ischemic damage at the time of baseline cognitive testing, we could be more confident that the cognitive effects were attributable to the hemodynamic failure. The presence of age in the model suggests that the impact of hypoperfusion on cognition was greater with increasing age.
Demonstration of a clear correlation between cerebral hypoperfusion and cognitive impairment in the setting of carotid artery disease has been elusive, in part because those with carotid artery disease share vascular risk factors with patients who have no large vessel disease.17,18 Both sets of patients may have microinfarcts and other ischemic lesions, which are associated with cognitive decline.18–21 Several case series have shown cognitive dysfunction in patients with carotid artery stenosis or occlusion22–24 and even showed reversibility of cognitive impairment with revascularization,2–4 but an independent association with hypoperfusion has been difficult to establish.
With the refinement of PET and other hemodynamic techniques in the 1980s, it became possible to quantify cerebral hemodynamics in terms of CBF, oxygen and glucose utilization, and cerebral autoregulatory capacity.25–27 In addition, unlike methods that measured CBF alone, the PET method allowed functionally important hypoperfusion resulting from carotid occlusive disease to be distinguished from hypoperfusion due simply to reduced metabolic demands of damaged (infarcted) tissue. Using quantitative PET methods, we were able to define more precisely cerebral hemodynamic status. Current hemodynamic theory holds that as perfusion pressure falls, CBF is maintained by autoregulatory vasodilation of cerebral arterioles. As perfusion pressure falls further, CBF begins to decrease and a metabolic compensation occurs as the OEF increases to maintain tissue oxygen metabolism. This stage II hemodynamic failure, sometimes referred to as misery perfusion,28 is thought to represent a vulnerable hemodynamic state in which any further drop in perfusion will produce ischemia. Reduced cerebrovascular resistance (CVR) on the side of carotid occlusion or stenosis indicates preexisting autoregulatory vasodilation and has been associated with cognitive impairment in patients with asymptomatic carotid stenosis at baseline29 and with cognitive improvement when the impaired CVR is reversed after endarterectomy.30 Other studies have shown a lack of association between CVR and cognitive impairment.31
In an important single case study, cerebral hemodynamic measures were assessed in a 55-year-old man with bilateral ICA occlusions presenting with subacute onset of severe behavioral and cognitive changes.32 Quantitative CBF and PET studies showed a 40%–50% reduction in blood flow and metabolism. After EC-IC bypass, there were significant increases in CBF and metabolism, accompanied by neuropsychological improvement. In a larger case series, among 25 patients with mild stroke or TIA, unilateral ICA or middle cerebral artery occlusion, and increased OEF by PET, verbal and performance IQ and full-scale IQ scores were all impaired before EC-IC bypass. After intervention, there was a significant increase in IQ scores that was correlated in a multivariable analysis with measures of CBF and metabolism.33
The current study supports a mechanistic basis for what has been reported mostly as observational cases and clinical case series. The notion that reduction of CBF in a hemisphere fed by an occluded carotid artery is sufficient to produce significant cognitive dysfunction in the absence of acute infarction has until now been inadequately demonstrated. One limitation in our study is that we did not have imaging results for all our patients to know whether the topography and distribution of preexisting ischemic changes were different between those with normal vs abnormal OEF. Although increased OEF was shown to be independently associated with cognitive impairment in this study, it could be that previous strokes, which did not differ categorically between those with and without increased OEF, had different distributions or morphologies that could account for differences in cognitive function.34,35 In addition, increased OEF can cause unihemispheric neuronal loss without infarction,36 which could explain our data as well. If either differences in previous stroke topography or differences in selective neuronal loss were present in our cohort, then improvement in OEF might not result in improved cognition. The question of whether cognitive dysfunction is reversible when the hemodynamic impairment is treated will need to await the main results of the RECON trial. Nonetheless, by investigating the association between cerebral hemodynamic failure and cognitive impairment using reliable, quantitative methods for physiologic and behavioral measurements and comparing with an appropriate control group, we have been able to show the hemodynamic effects of the carotid occlusive disease on cognition, thus establishing the substrate upon which a treatment, in this case EC-IC bypass, may be meaningfully tested.
ACKNOWLEDGMENT
The authors thank our RECON coordinator Kevin Slane for his tireless assistance in gathering data, creating documents, and organizing meetings.
GLOSSARY
- CBF
cerebral blood flow
- COSS
Carotid Occlusion Surgery Study
- CVR
cerebrovascular resistance
- EC-IC
extracranial-intracranial
- ICA
internal carotid artery
- OEF
oxygen extraction fraction
- RECON
Randomized Evaluation of Carotid Occlusion and Neurocognition
AUTHOR CONTRIBUTIONS
Dr. Marshall: study conceptualization and design, data review, manuscript writing. Dr. Festa: manuscript editing, neuropsychological design. Dr. Cheung: manuscript editing, statistical design and analysis. R. Chen: manuscript review, statistical analysis. Dr. Pavol: manuscript review and revision, neuropsychological battery review. Dr. Derdeyn: PET data analysis, manuscript review and revision. Dr. Clarke: study design, data analysis, manuscript review and revision. Dr. Videen: PET study design and analysis, manuscript review and revision. Dr. Grubb: neurosurgical design input, manuscript review and revision. Dr. Adams: study design, conceptualization, manuscript review and revision. Dr. Powers: study conceptualization, manuscript review and revision. Dr. Lazar: study design, conceptualization, neuropsychological battery design, manuscript review and revision.
DISCLOSURE
Dr. Marshall has a patent pending re: a novel use of a drug for post-stroke dystonia; receives publishing royalties for OnCall Neurology (Elsevier, 2003–present); has received speaker honoraria from Ferrer International; and receives research support from the NIH/NINDS. Dr. Festa receives research support from the NIH (NINDS/NICHD/NHLBI). Dr. Cheung serves on the editorial board of Biometrics; receives publishing royalties for Dose Finding by the Continual Reassessment Method (Chapman and Hall/CRC, 2011); serves as a consultant for Remedy and Estee Lauder; and receives research from the NIH/NINDS. R. Chen receives research support from the NIH/NINDS. Dr. Pavol receives research support from Terumo Heart, Inc., EvaHeart Medical USA, Inc., and the NIH/NINDS. Dr. Derdeyn serves on a scientific advisory boards for W.L. Gore and Associates and the NIH/NINDS; serves on the editorial boards of Stroke and the American Journal of Neuroradiology; is listed on patents re: a system for surgical repair of intracranial aneurysms and a method to stimulate peripheral nerves; receives research support from the NIH/NINDS; and holds stock options in nFocus, Inc. Dr. Clarke receives publishing royalties for Statistical Methods for the Analysis of Biomedical Data, 2nd edition (Wiley-Interscience, 2002) and receives research support from the NIH (NIAMS/NINDS/NIDDK). Dr. Videen receives research support from the NIH/NINDS and the Michael J. Fox Foundation. Dr. Grubb serves on the editorial board of the Journal of Neurosurgery and receives research support from the NIH/NINDS and the Barnes-Jewish Hospital Foundation. Dr. Adams serves on scientific advisory boards for Medtronic, Inc. and Merck Serono; serves on the editorial boards of Stroke, Journal of Stroke and Cerebrovascular Diseases, and Cerebrovascular Diseases; receives publishing royalties for Handbook of Cerebrovascular Diseases, 2nd edition (Informa Healthcare, 2004) and Principles of Cerebrovascular Disease (McGraw-Hill Professional, 2006); and receives research support from the NIH/NINDS. Dr. Powers serves on a scientific advisory board for the NIH Morehouse School of Medicine; serves on the editorial boards of Stroke and Journal of Cerebral Blood Flow and Metabolism; and receives research support from the NIH (NINDS/NIAMS). Dr. Lazar serves on the editorial board of Stroke and receives research support from the NIH (NHLBI/NINDS/NICHD).
REFERENCES
- 1. Fisher C. Senile dementia: a new explanation of its causation. Arch Neurol 1951;65:1–7 [PMC free article] [PubMed] [Google Scholar]
- 2. Drinkwater JE, Thompson SK, Lumley JS. Cerebral function before and after extra-intracranial carotid bypass. J Neurol Neurosurg Psychiatry 1984;47:1041–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nielsen H, Hojer-Pedersen E, Gulliksen G, Haase J, Enevoldsen E. Reversible ischemic neurological deficit and minor strokes before and after EC/IC bypass surgery: a neuropsychological study. Acta Neurol Scand 1986;73:615–618 [DOI] [PubMed] [Google Scholar]
- 4. Binder LM, Tanabe CT, Waller FT, Wooster NE. Behavioral effects of superficial temporal artery to middle cerebral artery bypass surgery: preliminary report. Neurology 1982;32:422–424 [DOI] [PubMed] [Google Scholar]
- 5. Grubb RL, Jr, Powers WJ, Derdeyn CP, Adams HP, Clarke WR. The Carotid Occlusion Surgery Study. Neurosurg Focus 2003;14:1–7 [DOI] [PubMed] [Google Scholar]
- 6. Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion. JAMA 1998;280:1055–1060 [DOI] [PubMed] [Google Scholar]
- 7. Lezak M, Howieson DB, Loring DW, ed. Neuropsychological Assessment, 4th ed Oxford, UK: Oxford University Press; 2004 [Google Scholar]
- 8. Brandt J, Benedict RHB. Hopkins Verbal Learning Test–Revised. Lutz, FL: Psychological Assessment Resources; 1991 [Google Scholar]
- 9. Meyers JE, Meyers KR. Rey Complex Figure Test and Recognition Trial. Lutz, FL: Psychological Assessment Resources; 1995 [Google Scholar]
- 10. Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP. Distinct syndromes of hemineglect. Arch Neurol 1992;49:1187–1194 [DOI] [PubMed] [Google Scholar]
- 11. Mitrushina M, Boone KB, Razani J, D'Elia LF. Handbook of Normative Data for Neuropsychological Assessment. Oxford, UK: Oxford University Press; 2005 [Google Scholar]
- 12. Goodglass H, Kaplan E. Assessment of Aphasia and Related Disorders, 2nd ed Philadelphia: Lea & Febiger; 1983 [Google Scholar]
- 13. Zec RF, Burkett NR, Markwell SJ, Larsen DL. Normative data stratified for age, education, and gender on the Boston naming test. Clin Neuropsychol 2007;21:617–637 [DOI] [PubMed] [Google Scholar]
- 14. Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed San Antonio, TX: Harcourt Assessment; 1997 [Google Scholar]
- 15. Ruff RM, Parker SB. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: normative values for Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills 1993;76 1219–1230 [DOI] [PubMed] [Google Scholar]
- 16. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999;14:167–177 [PubMed] [Google Scholar]
- 17. Jiwa NS, Garrard P, Hainsworth AH. Experimental models of vascular dementia and vascular cognitive impairment: a systematic review. J Neurochem 2010;115:814–828 [DOI] [PubMed] [Google Scholar]
- 18. Aharon-Peretz J, Tomer R, Gabrieli I, Aharonov D, Nitecki S, Hoffman A. Cognitive performance following endarterectomy in asymptomatic severe carotid stenosis. Eur J Neurol 2003;10:525–528 [DOI] [PubMed] [Google Scholar]
- 19. Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke 2009;40:677–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marquine MJ, Attix DK, Goldstein LB, et al. Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 2010;41:1946–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bonaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromso Study. Neurology 2004;62:695–701 [DOI] [PubMed] [Google Scholar]
- 23. Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke 2009;40:1590–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bakker FC, Klijn CJ, Jennekens-Schinkel A, van der Tweel I, Tulleken CA, Kappelle LJ. Cognitive impairment in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. J Neurol 2003;250:1340–1347 [DOI] [PubMed] [Google Scholar]
- 25. Sette G, Baron JC, Mazoyer B, Levasseur M, Pappata S, Crouzel C. Local brain haemodynamics and oxygen metabolism in cerebrovascular disease: positron emission tomography. Brain 1989;112:931–951 [DOI] [PubMed] [Google Scholar]
- 26. Olsen TS, Larsen B, Herning M, Skriver EB, Lassen NA. Blood flow and vascular reactivity in collaterally perfused brain tissue: evidence of an ischemic penumbra in patients with acute stroke. Stroke 1983;14:332–341 [DOI] [PubMed] [Google Scholar]
- 27. Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 1991;29:231–240 [DOI] [PubMed] [Google Scholar]
- 28. Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P. Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia: a case study with 15O positron emission tomography. Stroke 1981;12:454–459 [DOI] [PubMed] [Google Scholar]
- 29. Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009;72:1062–1068 [DOI] [PubMed] [Google Scholar]
- 30. Fearn SJ, Hutchinson S, Riding G, Hill-Wilson G, Wesnes K, McCollum CN. Carotid endarterectomy improves cognitive function in patients with exhausted cerebrovascular reserve. Eur J Vasc Endovasc Surg 2003;26:529–536 [DOI] [PubMed] [Google Scholar]
- 31. Bakker FC, Klijn CJ, Jennekens-Schinkel A, et al. Cognitive impairment is related to cerebral lactate in patients with carotid artery occlusion and ipsilateral transient ischemic attacks. Stroke 2003;34:1419–1424 [DOI] [PubMed] [Google Scholar]
- 32. Tatemichi TK, Desmond DW, Prohovnik I, Eidelberg D. Dementia associated with bilateral carotid occlusions: neuropsychological and haemodynamic course after extracranial to intracranial bypass surgery. J Neurol Neurosurg Psychiatry 1995;58:633–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasoh M, Ogasawara K, Kuroda K, et al. Effects of EC-IC bypass surgery on cognitive impairment in patients with hemodynamic cerebral ischemia. Surg Neurol 2003;59:455–463 [DOI] [PubMed] [Google Scholar]
- 34. Derdeyn CP, Khosla A, Videen TO, et al. Severe hemodynamic impairment and border zone-region infarction. Radiology 2001;220:195–201 [DOI] [PubMed] [Google Scholar]
- 35. Yamauchi H, Kudoh T, Sugimoto K, Takahashi M, Kishibe Y, Okazawa H. Pattern of collaterals, type of infarcts, and haemodynamic impairment in carotid artery occlusion. J Neurol Neurosurg Psychiatry 2004;75:1697–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yamauchi H, Kudoh T, Kishibe Y, Iwasaki J, Kagawa S. Selective neuronal damage and chronic hemodynamic cerebral ischemia. Ann Neurol 2007;61:454–465 [DOI] [PubMed] [Google Scholar]