Abstract
People with schizophrenia often misperceive sensations and misinterpret experiences, perhaps contributing to psychotic symptoms. These misperceptions and misinterpretations might result from an inability to make valid predictions about expected sensations and experiences. Healthy normal people take advantage of neural mechanisms that allow them to make predictions unconsciously, facilitating processing of expected sensations and distinguishing the expected from the unexpected. In this paper, we focus on two types of automatic, unconscious mechanisms that allow us to predict our perceptions. The first involves predictions made via innate mechanisms basic to all species in the animal kingdom—the efference copy and corollary discharge mechanisms. They accompany our voluntary movements and allow us to suppress sensations resulting from our actions. We study this during talking, and show that auditory cortical response to the speech sounds during talking is reduced compared to when they are played back. This suppression is reduced in schizophrenia, suggesting a failure to predict the sensations resulting from talking. The second mechanism involves implicitly learning what to expect from the current context of events. We study this by observing the brain's response to an unexpected repetition of an event, when a change would have been predicted. That patients have a reduced response suggests they failed to predict that it was time for a change. Both types of predictions should happen automatically and effortlessly, allowing for economic processing of expected events and orientation to unexpected ones. These prediction failures characterize the diagnosis of schizophrenia rather than reflecting specific symptoms.
Keywords: Schizophrenial, efference copy, corollary discharge, context, ERPs
1. Introduction
People with schizophrenia often misperceive sensations and misinterpret experiences, perhaps contributing to the cardinal symptoms of the illness, such as auditory hallucinations and delusions. These misperceptions and misinterpretations might result from a basic inability to make valid predictions about expected sensations and experiences. Healthy normal people take advantage of neural mechanisms that allow them to make predictions unconsciously, facilitating processing of sensations and distinguishing the expected from the unexpected. If predictive mechanisms are dysfunctional, sensations that should have been predicted, but were not, might take on inappropriate salience (Kapur, 2003).
Predictions can be made strategically, via conscious, executive mechanisms such as selective attention, or they can be made automatically, via unconscious and innate mechanisms. At an elemental level, restrictions on sensations happen at the peripheral sensory organs themselves: We cannot see in the infrared spectrum, nor can we hear frequencies above 20,000 Hz. In a sense, we could “predict” that we will not experience the 50,000 Hz giggles of rodents at play (Knutson, Burgdorf, & Panksepp, 1998). Other elemental, automatic, and unconscious mechanisms that provide predictions about sensations, resulting from our own actions, are the efference copy and corollary discharge systems. They allow all species in the animal kingdom to predict, and thereby suppress sensations that result from voluntary motor actions and tag these sensations as coming from “self” (see review by Crapse & Sommer, 2008). In so doing, perceptions are filtered and processed economically. Another automatic process that allows us to make predictions unconsciously is implicit contextual learning; we predict future events based on what we have learned about the past and present context.
In this paper, we discuss the roles of the efference copy and corollary discharge systems, as well as context processing, in prediction. Specifically, we discuss ERP and EEG evidence of prediction deficits in these systems in patients with schizophrenia.
2. Innate Predictions of Sensation: Efference Copy and Corollary Discharge Systems
The efference copy (Von Holst & Mittelstaedt, 1950) and corollary discharge (Sperry, 1950) systems may provide basic bottom-up restrictions on what we perceive by tagging the experiences that result from our own actions as coming from “self” and suppressing our responses to these sensations (see review by Crapse & Sommer, 2008). Although these terms are often used interchangeably or jointly as “efference copy/corollary discharge” (Feinberg, 1978), we (Ford, Roach, Faustman, & Mathalon, 2007) and others (Miall & Wolpert, 1996)(Crapse & Sommer, 2008) have distinguished between them. We use “efference copy” to refer to a copy of the motor command and the “corollary discharge” to refer to the expected sensation resulting from the action, as illustrated in Figure 1.
Figure 1.
(Left) We show a cartoon profile of a healthy control subject talking (saying “ah”) and listening to a playback of “ah”. Above the heads, we show ERPs recorded from the vertex (Cz) elicited by the onset of the speech sound (dotted vertical line) during talking (red lines) and listening (blue lines). During talking, N1 to the speech sound is suppressed relative to N1 to the same sound during listening. Amplitude (microvolts) is on the y-axis and time (milliseconds) is on the x-axis. Vertex negativity is plotted down.
The N1 of the ERP is generated in auditory cortex (colored orange during Talk, and blue during Listen). Intensity of the color in the auditory cortex denotes the strength of the response to the speech sound. The intention to say “ah” is indicated as an orange “thought bubble” over Broca's area. The orange curved arrow pointing from Broca's area to auditory cortex indicates the transmission of the efference copy of the motor plan, which produces a corollary discharge (orange burst) of the expected sensation in auditory cortex. When the expected sensation (corollary discharge) matches the actual sensation (sensory reafference) in auditory cortex (green burst), perception is suppressed.
(Right). The same is shown for schizophrenia patients, with a relative failure of the efference copy and corollary discharge being shown as faded orange. The slightly more intense orange color in auditory cortex during talking indicates relatively less suppression of the auditory cortical response to the spoken sound. The slightly less intense blue color during listening indicates an overall tendency of patients to generate a smaller N1 to sounds during passive listening.
(Permission from Schizophrenia Bulletin to reproduce elements of this figure is pending.)
Though these mechanisms are ubiquitous, and their putative actions are seen in animals from the 302 cell nematodes to humans, their neural basis has only recently been described (see review by Crapse & Sommer, 2008). Poulet and Hedwig explained how these mechanisms allow the cricket to sing at deafening intensities without deafening itself (Poulet & Hedwig, 2006). In monkeys, Sommer and Wurtz showed how these mechanisms permit the perception of a stable visual scene despite self-initiated eye movements (Sommer & Wurtz, 2006). By studying how an animal's brain adjusts perceptions according to its movements, we can see the animal as integral to its environment as it interacts with it.
Wolpert and colleagues proposed that an efference copy of the motor command is used to anticipate and cancel the sensory effects of movement (Miall, Weir, Wolpert, & Stein, 1993; Wolpert, Ghahramani, & Jordan, 1995; Wolpert, Miall, & Kawato, 1998). Transmitting an efference copy of the motor command may be an emergent property of a self-organizing system, accomplished by synchronization of oscillatory activity preceding the action (Singer, 1999). In fact, local field potential recordings from rodent somatosensory cells showed synchrony in the 7 to 12 Hz (Nicolelis, Baccala, Lin, & Chapin, 1995) and 30 to 35 Hz (Hamada, Miyashita, & Tanaka, 1999) bands preceding exploratory whisking. Hamada and colleagues suggested that the neural synchrony they observed was triggered by the transfer of an efference copy of motor preparation to somatosensory cortex, peaking 100ms before the movement.
While the efference copy and corollary discharge mechanisms are generally considered to be innate and hard-wired in lower animals, they can be affected by attention and intentions in higher animals (see review by Crapse & Sommer, 2008). Chapin and Woodward (1982) note that sensory suppression can interact with intention; when rats are walking, sensation on the paw is suppressed, but when the paw is used for exploration, it is not. That is, these mechanisms may go “off line” when needed for focusing on the sensations, as in reading Braille, learning a new language, or hitting the right note when learning a song.
2.1. Efference copy and corollary discharge actions during vocalizations
2.1.1. Invasive studies in human and non-human primates
During vocalization in human and non-human primates, efference copies from vocalization regions in frontal lobes may prepare auditory cortex for the almost simultaneous arrival of the vocalized sounds, minimizing the auditory cortical response and providing a mechanism for recognizing these sounds as self-generated. Early support for suppression during vocalization came from studies of non-human primates showing that about half of call-responsive neurons are inhibited during vocalization compared to when those sounds are recorded and played back (Muller-Preuss & Ploog, 1981). Later, Eliades and Wang recorded from single units in primary auditory cortex in marmoset monkeys and reported vocalization-induced suppression beginning before vocalization (Eliades & Wang, 2003; Eliades & Wang, 2005), with excitation of different units beginning after vocal onset (Eliades & Wang, 2003).
These findings are consistent with studies from human patients under-going pre-surgical planning for resection of epileptogenic neural tissue. Creutzfeldt and colleagues recorded from the exposed surface of the right and left temporal cortices while patients talked and listened to others talking; suppression of activity in auditory cortex was noted during talking compared to listening (Creutzfeldt, Ojeman, & Lettich, 1989). We subsequently replicated and extended this finding in a similar group of patients (Chen, et al., 2011).
2.1.2. Non-invasive studies in human and non-human primates
Data from vocalization studies in healthy human volunteers are consistent with the data described above: All report suppression of auditory cortex during vocalization in healthy controls, as seen in a reduction of the N1 amplitude of the auditory event-related brain potential (ERP), or the M100 of the event-related field, to the onset of the spoken sound as it is being spoken (Behroozmand, Karvelis, Liu, & Larson, 2009; Curio, Neuloh, Numminen, Jousmaki, & Hari, 2000; Ford, Gray, Faustman, Roach, & Mathalon, 2007; Ford, Mathalon, Heinks, Kalba, & Roth, 2001; Ford, Roach, Faustman, & Mathalon, 2007; Heinks-Maldonado, Mathalon, Gray, & Ford, 2005; Heinks-Maldonado, et al., 2007; Houde, Nagarajan, Sekihara, & Merzenich, 2002). This provides a direct test of the mechanism and is similar to methods used in non-human primates (Eliades & Wang, 2003; Eliades & Wang, 2005). The basics of our Talk/Listen paradigm are presented in Figure 1 (Ford, Gray, Faustman, Roach, & Mathalon, 2007; Ford, Mathalon, Heinks, Kalba, & Roth, 2001; Ford, Roach, Faustman, & Mathalon, 2007; Ford, Roach, & Mathalon, 2010), and typical ERPs elicited by the onset of speech sound during both the Talking and Listening conditions are shown.
Also illustrated in Figure 1 is the putative action of the efference copy of the motor command preceding the onset of speech. We found that from one trial to the next, at about 100ms before speech-sound onset, the phase of neural activity in the beta band (~16Hz) synchronized significantly more during talking than during listening. Because it was related to subsequent N1 suppression during talking, we suggested this might be a biological reflection of the efference copy (Ford, Gray, Faustman, Roach, & Mathalon, 2007).
2.2. When predictions fail
In Figure 1, we also illustrate the putative “corollary discharge”, representing the predicted sensory consequences of an action, and the “sensory reafference”, representing the actual sensory feedback. Detection of discrepancies between predicted and actual sensory consequences of self-generated acts is key to rapid recognition and correction of actions. It may underlie our ability to correct a golf swing in action and adjust voice timbre to match our culture. It may work via a subtractive comparison of the predicted with the actual sensory feedback (Angel, 1976; Jeannerod, 2003; Wolpert & Kawato, 1988), with larger mismatches being more salient. Eliades and Wang (Eliades & Wang, 2008) pointed out that this comparison process may be useful for vocal convergence, allowing monkeys to match their vocalizations to their cage-mate's (Snowdon & Elowson, 1999).
In non-human primates, Eliades and Wang found auditory neurons, which were suppressed during normal vocal feedback, showed a larger increase in firing rate to pitch-altered feedback than those neurons that were excited during normal vocal feedback (Eliades & Wang, 2008). They suggested that the vocalization-induced suppression enhanced neural sensitivity to feedback perturbation. A similar pattern has been observed in songbirds (Keller & Hahnloser, 2009).
Our data (Heinks-Maldonado, Mathalon, Gray, & Ford, 2005) and data of others (Behroozmand, Karvelis, Liu, & Larson, 2009; Curio, Neuloh, Numminen, Jousmaki, & Hari, 2000; Eliades & Wang, 2003; Eliades & Wang, 2005; Heinks-Maldonado, Nagarajan, & Houde, 2006; Houde, Nagarajan, Sekihara, & Merzenich, 2002) suggest that the closer the match between the predicted and experienced sensation, the greater the suppression of auditory cortex responsiveness in human subjects. These studies highlight the importance of valid predictions on efficient economic perception.
2.3. Manipulations of prediction
In the context of our Talk/Listen paradigm, we asked whether innate predictions are superior to learned predictions (Ford, Gray, Faustman, Roach, & Mathalon, 2007), and asked whether predictive information resulting from expectancy (i.e., warning) and from agency (i.e., self-stimulation) modulates auditory cortex in the same way and to the same degree as talking (which involves both expectancy and agency). First, we recorded N1 to the onset of a speech sound as it was being spoken, as described above and shown in Figure 1. Second, we recorded N1 to the onset of the subject's own previously recorded speech sound when it was delivered via a self-paced button press. Third, we recorded N1 to the onset of the subject's pre-recorded speech sound when its impending delivery was heralded by a count-down visual warning.
The resulting data are shown in Figure 2.
Figure 2.

ERPs from Fz, FCz, Cz, CPz, and Pz, locked to speech sound onset (0ms, dotted vertical line) are shown for each of the three experiments: Talking, Expectancy, and Agency, for the healthy controls. ERPs elicited during the two conditions for each experiment are overlaid. Amplitude (microvolts) is on the y-axis and time (milliseconds) is on the x-axis. Negativity is plotted down.
(Permission from Psychophysiology to reproduce elements of this figure is pending.)
In brief, our earlier work with the Talk/Listen paradigm was confirmed. Further, we found that talking produces more suppression of cortical responses to speech sounds than simple expectancy effects based on visual warning, or simple agency effects based on self-delivery of those sounds. To better understand the contributions of cognitive expectancy to the Talking effect, the N1 suppression effect due to talking was assessed by controlling for N1 suppression effects due to expectancy and agency with multiple regression analysis. The talking effects were only slightly reduced when expectancy and agency were controlled for. Details of the analysis appear in the original report (Ford, Gray, Faustman, Roach, & Mathalon, 2007).
Why does talking suppress auditory cortex responsiveness more than other manipulations of prediction? Is it due to superior predictions about content and timing during talking, or to the natural connections between the act and the resulting sensation? In the expectancy and agency experiments, the “ah” sounds were not produced in the moment as they were in the talking experiment, and thus exact knowledge about sound quality and timing was not as perfect as during the talking experiment. Indeed, we know that shifting the pitch of the spoken sound during talking will reduce the amount of cortical suppression of the spoken sound (Heinks-Maldonado, Mathalon, Gray, & Ford, 2005), and we know that subtle delays in timing between pressing a button and delivering a pre-recorded “ah” will reduce the amount of suppression (Whitford, et al., 2011). Perhaps superior knowledge about timing and content produces stronger suppression of auditory cortical responsiveness to the spoken sound during talking. An anonymous reviewer suggested that this could be tested by comparing the talking and self-delivery suppression effects early in the session to effects seen later in the session. If superior suppression during talking is due to natural associations, then they will not change during the session. However, if the association between pressing a button and hearing an “ah” is learned during the session, the effects would be stronger later in the session.
It is important to add that the effect of agency in our experiment was smaller than the effects reported in the literature when subjects press a button to hear a tone (Baess, Horvath, Jacobsen, & Schroger, 2011; Martikainen, Kaneko, & Hari, 2005; McCarthy & Donchin, 1976; Schafer & Marcus, 1973). To understand why the suppression we reported with self-delivery of speech sounds is not as large as suppression others have reported with self-delivery of tones, we need to directly compare cortical responses to self-delivered tones and speech sounds.
2.4. Dysfunction of the efference copy and corollary discharge mechanisms in schizophrenia
Feinberg suggested that dysfunction of the efference copy/corollary discharge mechanism may underlie the positive symptoms of schizophrenia, in particular auditory verbal hallucinations (Feinberg, 1978). This was based on the writings of Hughlings Jackson (1958) who proposed that thinking is our most complex motor act and, as such, it might conserve and utilize the computational and integrative mechanisms evolved for physical movement, in particular the efference copy and corollary discharge mechanisms. Feinberg reasoned that in the motor systems of thought, these mechanisms would act to distinguish self-produced from externally generated events. Dysfunction of these systems could explain auditory hallucinations and disruptions of the sense of self and will. Frith expanded this concept (Frith, 1987) and prompted a series of behavioral experiments confirming corollary discharge dysfunction in schizophrenia (Brebion, et al., 2000; Frith, Blakemore, & Wolpert, 2000; Lindner, Thier, Kircher, Haarmeier, & Leube, 2005; Shergill, Samson, Bays, Frith, & Wolpert, 2005; Stirling, Hellewell, & Quraishi, 1998; Turken, Vuilleumier, Mathalon, Swick, & Ford, 2003).
2.4.1. The auditory system
Subsequently, we garnered neurophysiological evidence for dysfunction of the efference copy and corollary discharge systems in schizophrenia in the auditory (Ford, Gray, Faustman, Roach, & Mathalon, 2007; Ford, Mathalon, Heinks, Kalba, & Roth, 2001; Ford, et al., 2001a; Ford, et al., 2001b; Ford, Roach, Faustman, & Mathalon, 2007; Heinks-Maldonado, et al., 2007). Using the N1 component of the ERP in our Talk/Listen paradigm, we found that the normal dampening of the auditory cortical response during talking or inner speech is less evident in patients with schizophrenia (Ford, Gray, Faustman, Roach, & Mathalon, 2007; Ford, Mathalon, Heinks, Kalba, & Roth, 2001; Ford, et al., 2001a; Ford, et al., 2001b; Ford, Roach, Faustman, & Mathalon, 2007; Heinks-Maldonado, et al., 2007). We illustrate this in Figure 1b with data taken from our Talk/Listen paradigm (Ford, Gray, Faustman, Roach, & Mathalon, 2007).
2.4.2. Auditory verbal hallucinations
In accordance with the theories laid out by both Feinberg and Frith, we hypothesized that the relative lack of suppression of N1 during talking would be related to the severity of auditory hallucinations, in a “trait-like” manner. (We use “trait-like” to convey that we did not try to assess the hallucinatory state during the EEG session, but assessed the tendency to hallucinate in the week preceding or following EEG data collection.) We were not able to find a significant relationship with hallucination severity, that is, the patients who tend to hallucinate did not have less suppression of N1 to the speech sound during talking (Ford, Gray, Faustman, Roach, & Mathalon, 2007).
In the analysis of pre-speech synchrony described above, we found that patients with schizophrenia had significantly less phase synchrony than controls, consistent with the notion of a deficient “warning” sent to auditory cortex, predicting the arrival of the sound they were about to utter. We also found a moderate, but significant, relationship between the lack of pre-speech phase synchrony and the tendency to hallucinate (Ford, Gray, Faustman, Roach, & Mathalon, 2007).
In another analysis of a different experiment and sample (Ford, Mathalon, Whitfield, Faustman, & Roth, 2002), we assessed the coherence between EEG recorded over left frontal and left temporal areas during overt sentence reading. We found that controls had greater coherence during talking than listening to a playback of it; this effect was reduced in patients with schizophrenia, especially those who tended hallucinate.
2.4.3. The somatosensory system
In order to determine whether the reduced pre-action neural synchrony observed in patients was specific to talking, we subjected EEG data recorded during a self-paced button press task to a similar time-frequency and ERP analysis (Ford, Roach, Faustman, & Mathalon, 2008). We found evidence of deficient neural synchrony, which we interpreted as deficient efference copy, preceding button presses in patients. The degree of pre-press synchrony was associated with the degree of post-press suppression of the ERP to the tactile sensation associated with the button press in controls but not in patients. Finally, deficient pre-press synchrony was associated with greater avolition and apathy in the patients, a characteristic set of symptoms that likely contribute to low functioning. This suggests that dysfunctions of the efference copy and corollary discharge mechanisms in schizophrenia are not limited to the speech-auditory system, but may affect other motor-sensory systems as well, and map onto system-specific symptoms.
3. Implicitly Learned Predictions
In the preceding section, we described ERP data recorded to sounds that were predictable by virtue of subjects actively producing them by either talking or pressing a button. Those data suggested that patients were relatively deficient in unconsciously predicting the impending result (a speech sound) of their own actions (talking). We further mentioned that this deficiency extends to other motor-sensory systems. In this section, we will discuss “passive” ERPs to experimenter-delivered sounds where predictability develops implicitly out of experience with the regularity of the sequence. Like the data described above, these data also show that patients are relatively deficient in making unconscious predictions about what is about to happen.
3.1. ERPs to deviant stimuli
There is a rich literature on ERPs to deviant sounds in a sequence of frequent, standard sounds—the oddball paradigm. If the subject is asked to respond to the deviant, it becomes a target and elicits an N2b and P300 (or P3b). If the subject is not asked to respond to the deviant, it elicits a P3a (e.g., Squires, Squires, & Hillyard, 1975) and a MMN (mismatch negativity). Näätänen and colleagues divided the N2 complex into MMN and N2b components (Naatanen, Simpson, & Loveless, 1982), distinguished on the basis of their dependence on attention and scalp topography. N2b is sensitive to attention and MMN is not. While it is difficult to rule out attention from most paradigms, these two components can also be distinguished by their scalp topographies; MMN reverses polarity below the Sylvian fissure and N2b does not. Regardless of attention, N2b/MMN and P3a elicitation are prima facie evidence that a context was learned and violated.
3.1.1. Schizophrenia and ERPs to oddball events
Roth and Cannon (1972) reported P300 amplitude reductions in patients with schizophrenia 40 years ago, and since then, more than a hundred replications and extensions of that finding have been published (see reviews by Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; Ford, 1999; Jeon & Polich, 2003). As P300 elicitation may reflect the updating of context (Donchin & Coles, 1988), abnormalities in context processing may contribute to P300 reduction in schizophrenia. There are also numerous reports of schizophrenia-related reductions of MMN (see meta-analysis, Umbricht & Krljes, 2005) and a growing number of papers are reporting reductions in P3a in schizophrenia (Alain, Bernstein, Cortese, Yu, & Zipursky, 2002; Hermens, et al., 2010; Jahshan, et al., 2011; Kiang, Braff, Sprock, & Light, 2009; Mathalon, Ford, & Pfefferbaum, 2000; Merrin & Floyd, 1994; O'Donnell, et al., 1996; Turetsky, Bilker, Siegel, Kohler, & Gur, 2009).
3.2. ERPs to standard stimuli
Using ERPs to deviant events to study pure prediction is flawed because traditional deviant tones are typically defined by both improbability and physical difference from the standard tone (e.g., pitch, intensity, duration), both of which affect N2b/MMN and P3a independent of their improbability (Polich & Kok, 1995). Alternatively, a deviant can be physically identical to the standard but be deviant by virtue of its local sequential probability. Thus, examination of otherwise identical stimuli, that derive their deviance solely from local sequential probabilities, may provide a purer means for examining prediction. Accordingly, disabilities in processing physical features often seen in schizophrenia (e.g., (Dias, Butler, Hoptman, & Javitt, 2011; Rosburg, Boutros, & Ford, 2008) will not affect the assessment of their abilities to make predictions.
While it is common to average together brain responses to all standards in an oddball paradigm, information about context processing can be derived from the brain's response to standards based on their specific position in a sequence. P3a-like responses to standard stimuli are larger when they appear later in a sequence of consecutive standards (Gilmore, Clementz, & Buckley, 2005; Stadler, Klimesch, Pouthas, & Ragot, 2006). Gilmore et al suggested that after a long series of standard tones in an oddball paradigm, subjects expect an oddball, and when it does not occur, they are surprised.
To the extent expectancies for a deviant develop with increasing number of standards, a contingent negative variation (CNV) should develop. The anticipatory component, sometimes referred to as the stimulus-preceding negativity (SPN), can be isolated from the CNV in tasks where a warning cue signals an impending stimulus to which no response is required, thereby eliminating response preparation processes (Brunia & van Boxtel, 2001). Indeed, Stadler et al. observed a CNV or SPN preceding standard stimuli when the local sequential probability indicated that a target was possible.
Thus, to the extent a standard tone can acquire “deviance” status based on its unlikely position in a sequence, a series of ERP components might be elicited by the deviant standard, including N2b/MMN and P3a, with a CNV/SPN developing in anticipation of a deviant standard.
3.2.1. Schizophrenia and ERPs to standard events
In a recent paper, we focused on ERPs associated with standard tones, as a function of expectancies generated by trial-to-trial probabilities (Ford, et al., 2011). Based on the hypothesis that patients with schizophrenia do not use context to make predictions (Barch, et al., 2001; Carter, MacDonald, Ross, & Stenger, 2001; Ford, et al., 2004; Henik, et al., 2002; Servan-Schreiber, Cohen, & Steingard, 1996; Shelley, Grochowski, Lieberman, & Javitt, 1996), we predicted that patients with schizophrenia would be less surprised by an unlikely standard in a consecutive series of standards, and would show a markedly reduced or absent P3a and N2b/MMN to the unexpected standard, and a reduced CNV/SPN preceding it.
We analyzed the ERPs to standards in a 3-stimulus auditory oddball paradigm, with infrequently occurring target tones (p=.15) and novel sounds (p=.15). Although the global probability of a standard tone was p=.70, the sequential probability of a standard varied from p=1.0 (for the 1st standard) to .16 (for the 4th standard in row).
As can be seen in Figure 3a, following the 3rd standard in a row, a CNV/SPN developed in healthy controls. This negativity suggests that healthy controls were processing the sequential probabilities of standards and saying, “It's time for a change. Get ready for something interesting or important.” A significantly smaller CNV/SPN developed in the patients, suggesting they were not anticipating a change as strongly as the controls.
Figure 3a.
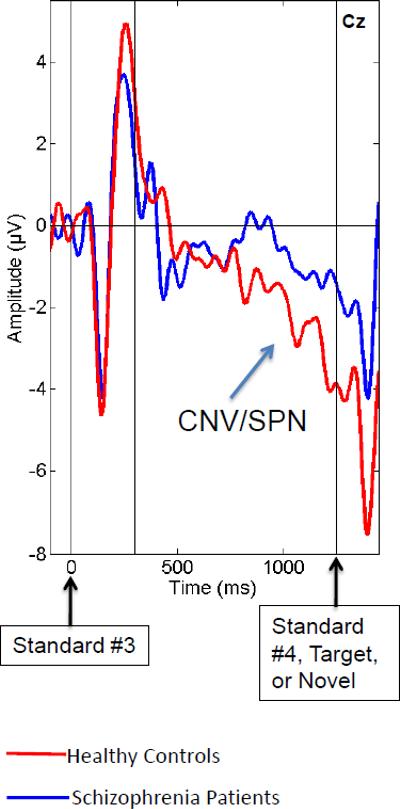
Grand average ERPs from Cz following Standard #3, regardless of whether the next stimulus was Standard #4, a Target or a Novel. Data from controls and patients are overlaid. This analysis allows the visualization and quantification of the Stimulus Preceding Negativity (SPN) or the contingent negative variation (CNV). Here and throughout Figure 3, amplitude (microvolts) is on the y-axis and time (milliseconds) is on the x-axis. Dotted lines indicate onset of Standard #3 at 0ms, 300ms, and onset of the next stimulus at 1250ms. Positivity relative to the reference electrodes is plotted up.
As can be seen in Figure 3b, when the change did not happen, the 4th standard in a row elicited a P3a in healthy controls. This positivity suggests that controls made a prediction and that the prediction was violated. The small P3a in the patients was consistent with their failure to predict that it was “time for a change”.
Figure 3b.

Grand average ERPs for 4 consecutive standards following a deviant stimulus in healthy controls (left) and patients with schizophrenia (right) for frontal, central, and parietal midline sites, Fz, Cz and Pz. ERPs are time-locked to the onset of each of the four consecutive standards, Standard #1, #2, #3, and #4, baseline corrected to the preceding 100ms. Vertical lines are at 0ms (stimulus onset), 100ms and 300ms. Note that only Standard #4 evokes a P3a.
Finally, as can be seen in Figure 3c, an N2b/MMN component was extracted by subtracting the ERP to the first standard from the ERP to the 4th standard. This negativity suggests a preconscious registration of a prediction error in the healthy controls. Again, the small N2b/MMN in the patients is consistent with their failure to register a prediction error.
Figure 3c.

Grand average ERPs from vertex (Cz) resulting from the subtraction of the ERP to Standard #1 from Standard #4 (S4–S1), overlaid for controls and patients, filtered with a 1Hz filter to remove the slow neural activity seen in Figure 3a. This subtraction allows the visualization and quantification of the N2b/MMN component elicited by Standard #4. Amplitude (microvolts) is on the y-axis and time (milliseconds) is on the x-axis. Negativity is plotted down. Dotted line indicates onset of Standard #4.
(Permission from the International Journal of Psychophysiology to reproduce elements of this figure is pending.)
It is critical to emphasize that no other features of the 4th standard made it deviant or salient other than the violation of the prediction that it was time for a change after 3 standards in a row. Thus, while the salience of targets is typically based on their different pitch, task relevance, and global probability, the salience of the 4th standard in a row is based only on the low local probability of this pattern occurring.
The failure of patients with schizophrenia to generate a large ERP to the fourth standard in a row could result from at least two abnormalities: Patients may not orient to unexpected events, or they may be unable to predict from the context that an event is unexpected. That the late positive component to the 4th standard in a row had the same scalp distribution as the P3a to a novel sound supports the contribution of orienting to the positivity elicited by Standard #4. However, arguing against a failure of the orienting response in schizophrenia are reports of normal P300s in patients to isolated targets (Roth, Goodale, & Pfefferbaum, 1991; Shelley, Grochowski, Lieberman, & Javitt, 1996) and startling noises (Ford, Roth, Menon, & Pfefferbaum, 1999) that occur at very long inter-target intervals with no intervening standards, a situation likely to elicit orienting. Instead, we suggest that patients with schizophrenia fail to use context to form predictions about pattern violations, which is consistent with their reduced CNV/SPN.
It is important to add that reaction times also reflect expectations; however, unlike the CNV/SPN preceding Standard #4, and the N2b/MMN and P3a elicited by Standard #4, the RT data suggested that patients formed normal expectancies. If we had not recorded EEG, we would conclude that patients and controls form expectancies in the same way. However, the ERP data suggest that more direct neural reflections of predictions are abnormal in schizophrenia. It is possible that the RT effects are normalized by clinical treatment and medications, while ERPs are sensitive to the underlying enduring pathophysiology of the illness.
We were unable to find any relationships between these ERP abnormalities and clinical symptoms.
4. Discussion
We constantly make predictions about future events to economically process those that match our expectations and to draw attention to those that do not. We arrive at these predictions via a variety of routes, both conscious and unconscious. In this paper, we discussed two unconscious mechanisms. Coupling the concepts of “predictions” and “unconscious” requires a loosening of how we typically think predictions. In the two instances that we discussed, predictions happen through innate systems and implicit learning. Through these systems, we become aware that we made a prediction when our prediction fails.
As discussed here, the innate, hard-wired efference copy and corollary discharge mechanisms are some of the most basic mechanisms by which we make predictions of the sensations that will result from our actions. Through the action of these mechanisms, when predictions are confirmed, sensation is suppressed. When sensation is not suppressed through a dysfunction of these mechanisms, the origin of the sensation could be ambiguous. We showed that during talking, the brain dampens its response to the sounds it produces, as reflected in a smaller auditory cortical response during talking than during listening. We showed that patients show a diminished suppression of this response during talking. In a recent essay, “Schizophrenia, Myelination, and Delayed Corollary Discharges: A Hypothesis”, together with Whitford and colleagues, we suggested that the efference copy and corollary discharge “prediction error” might lead to an increase in midbrain dopamine, causing insignificant events to become salient. This in turn could exacerbate nascent perceptual anomalies and ultimately trigger cardinal symptoms such as hallucinations and delusions (Whitford, Ford, Mathalon, Kubicki, & Shenton, 2011).
Another mechanism for narrowing our perception is implicit experience-based learning. For example, if we learn that a particular sound (e.g., a middle C on the musical scale) happens most of the time but that a different sound (e.g., C#) happens occasionally, we predict implicitly that a C is most likely to occur. But after a lot of Cs, we begin to think it is time for a change, or time for a C#. We showed that if a C# fails to occur when predicted, the brain generates a large ERP to the frequently repeated, but unexpected repetition of C. Because patients fail to generate a large brain response to the repeated stimulus, we argued that they failed to learn the context implicitly and predict that it was time for a change. In a review and synthesis of the literature “Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia”, Fletcher and Frith suggest that patients have deficits in probabilistic reasoning that affect their beliefs (Fletcher & Frith, 2009). They suggested that a normally functioning mechanism that updates context with new information is critical for accurate perception and inference. Disruption of this mechanism may result in the persistence of false beliefs in the face of incontrovertible evidence to the contrary (delusions).
Based on the writings of Whitford et al and Fletcher and Frith, we might predict that the degree of abnormality in these brain signals would map onto specific symptoms. Specifically, a failure to suppress auditory cortical responsiveness during talking might be related to a misperception of the source of self-generated inner experiences and thoughts resulting in the experience of auditory hallucinations. Further, a failure to update expectations based on context might be related to delusions. While there were some significant relationships between neurophysiological measures and symptoms, there were consistent group differences in our ERP indices of prediction. There are several reasons why we might have difficulty finding relationships with symptoms. First, our success can only be as good as our abilities to understand and quantify the patients' symptoms. Second, the preponderance of schizophrenia patients are medicated, and medication may decouple any potential relationship between symptoms and neurobiology by attenuating symptoms, while not affecting the ERP sensitivity to the propensity to experience those symptoms. Thus, we may be more successful at finding relationships with enduring features of the disease (the diagnosis itself, or its subtypes) than with current symptoms.
Thus, we suggest that patients with schizophrenia fail to correctly predict the future: They fail to predict, and thereby suppress, sensations resulting from their own actions and thoughts, and they fail to predict future events based on the current context of events. Both predictions should happen effortlessly, allowing for economic processing of expected events and orientation to unexpected ones. Although these prediction failures do not map neatly onto the expected symptoms, they do more generally reflect a propensity for psychosis, or simply the diagnosis of schizophrenia itself. As such, these same prediction failures may be seen in other diagnoses involving psychosis, like psychotic depression, mania, and some forms of dementia.
Highlights
There are behavioral data showing that patients with schizophrenia do not develop normal expectations for future events.
We provide ERP evidence from two very different paradigms indicating failures to predict the future in patients with schizophrenia.
These paradigms tap predictions that are both innate and implicitly acquired.
ACKNOWLEDGMENTS
This work was supported by grants from NIMH (R01, K02) and the VA (Merit Review), which were administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. This work was also supported by NARSAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alain C, Bernstein LJ, Cortese F, Yu H, Zipursky RB. Deficits in automatically detecting changes in conjunction of auditory features in patients with schizophrenia. Psychophysiology. 2002;39:599–606. doi: 10.1017.S0048577202394101. [DOI] [PubMed] [Google Scholar]
- Angel RW. Efference copy in the control of movement. Neurology. 1976;26:1164–1168. doi: 10.1212/wnl.26.12.1164. [DOI] [PubMed] [Google Scholar]
- Baess P, Horvath J, Jacobsen T, Schroger E. Selective suppression of self-initiated sounds in an auditory stream: An ERP study. Psychophysiology. 2011 doi: 10.1111/j.1469-8986.2011.01196.x. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, 3rd, Noll DC, Cohen JD. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Archives of General Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- Behroozmand R, Karvelis L, Liu H, Larson CR. Vocalization-induced enhancement of the auditory cortex responsiveness during voice F0 feedback perturbation. Clinical Neurophysiology. 2009;120:1303–1312. doi: 10.1016/j.clinph.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brebion G, Amador X, David AS, Malaspina D, Sharif Z, Gorman JM. Positive symptomatology and source-monitoring failure in schizophrenia--an analysis of symptom-specific effects. Psychiatry Research. 2000;95:119–131. doi: 10.1016/s0165-1781(00)00174-8. [DOI] [PubMed] [Google Scholar]
- Brunia CH, van Boxtel GJ. Wait and see. International Journal of Psychophysiology. 2001;43 doi: 10.1016/s0167-8760(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. American Journal of Psychiatry. 2001;158:1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Chen CM, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM. The Corollary Discharge in Humans Is Related to Synchronous Neural Oscillations. J Cogn Neurosci. 2011 doi: 10.1162/jocn.2010.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nature Reviews. Neuroscience. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O, Ojeman G, Lettich E. Neuronal activity in the human lateral temporal lobe. II Responses to the subject's own voice. Experimental Brain Research. 1989;77:476–489. doi: 10.1007/BF00249601. [DOI] [PubMed] [Google Scholar]
- Curio G, Neuloh G, Numminen J, Jousmaki V, Hari R. Speaking modifies voice-evoked activity in the human auditory cortex. Human Brain Mapping. 2000;9:183–191. doi: 10.1002/(SICI)1097-0193(200004)9:4<183::AID-HBM1>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early Sensory Contributions to Contextual Encoding Deficits in Schizophrenia. Archives of General Psychiatry. 2011 doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Coles M. Is the P300 component a manifestation of context updating? (Commentary on Verleger's critique of the context updating model) Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Eliades SJ, Wang X. Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations. Journal of Neurophysiology. 2003;89:2194–2207. doi: 10.1152/jn.00627.2002. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Dynamics of auditory-vocal interaction in monkey auditory cortex. Cerebral Cortex. 2005;15:1510–1523. doi: 10.1093/cercor/bhi030. [DOI] [PubMed] [Google Scholar]
- Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453:1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- Feinberg I. Efference copy and corollary discharge: implications for thinking and its disorders. Schizophrenia Bulletin. 1978;4:636–640. doi: 10.1093/schbul/4.4.636. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD. Perceiving is believing: a Bayesian approach to explaining the positive symptoms of schizophrenia. Nature Reviews. Neuroscience. 2009;10:48–58. doi: 10.1038/nrn2536. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Ford JM, Gray M, Faustman WO, Roach BJ, Mathalon DH. Dissecting corollary discharge dysfunction in schizophrenia. Psychophysiology. 2007;44:522–529. doi: 10.1111/j.1469-8986.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Gray M, Whitfield SL, Turken AU, Glover G, Faustman WO, Mathalon DH. Acquiring and inhibiting prepotent responses in schizophrenia: event-related brain potentials and functional magnetic resonance imaging. Archives of General Psychiatry. 2004;61:119–129. doi: 10.1001/archpsyc.61.2.119. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Heinks T, Kalba S, Roth WT. Neurophysiological evidence of corollary discharge dysfunction in schizophrenia. American Journal of Psychiatry. 2001;158:2069–2071. doi: 10.1176/appi.ajp.158.12.2069. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during inner speech in schizophrenia: an event-related brain potential study. American Journal of Psychiatry. 2001a;158:1914–1916. doi: 10.1176/appi.ajp.158.11.1914. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Kalba S, Whitfield S, Faustman WO, Roth WT. Cortical responsiveness during talking and listening in schizophrenia: an event-related brain potential study. Biological Psychiatry. 2001b;50:540–549. doi: 10.1016/s0006-3223(01)01166-0. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH, Whitfield S, Faustman WO, Roth WT. Reduced communication between frontal and temporal lobes during talking in schizophrenia. Biol Psychiatry. 2002;51:485–492. doi: 10.1016/s0006-3223(01)01335-x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Mathalon DH. Assessing corollary discharge in humans using noninvasive neurophysiological methods. Nat Protoc. 2010;5:1160–1168. doi: 10.1038/nprot.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Miller RM, Duncan CC, Hoffman RE, Mathalon DH. When it's time for a change: failures to track context in schizophrenia. Int J Psychophysiol. 2011;78:3–13. doi: 10.1016/j.ijpsycho.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Roth WT, Menon V, Pfefferbaum A. Failures of automatic and strategic processing in schizophrenia: Comparisons of event-related potential and startle blink modification. Schizophrenia Research. 1999;37:149–163. doi: 10.1016/s0920-9964(98)00148-0. [DOI] [PubMed] [Google Scholar]
- Frith CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychological Medicine. 1987;17:631–648. doi: 10.1017/s0033291700025873. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore SJ, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Research. Brain Research Reviews. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Clementz BA, Buckley PF. Stimulus sequence affects schizophrenia-normal differences in event processing during an auditory oddball task. Brain Research. Cognitive Brain Research. 2005;24:215–227. doi: 10.1016/j.cogbrainres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Miyashita E, Tanaka H. Gamma-band oscillations in the “barrel cortex” precede rat's exploratory whisking. Neuroscience. 1999;88:667–671. doi: 10.1016/s0306-4522(98)00468-0. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Gray M, Ford JM. Fine-tuning of auditory cortex during speech production. Psychophysiology. 2005;42:180–190. doi: 10.1111/j.1469-8986.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Arch Gen Psychiatry. 2007;64:286–296. doi: 10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Nagarajan SS, Houde JF. Magnetoencephalographic evidence for a precise forward model in speech production. Neuroreport. 2006;17:1375–1379. doi: 10.1097/01.wnr.0000233102.43526.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henik A, Carter CS, Salo R, Chaderjian M, Kraft L, Nordahl TE, Robertson LC. Attentional control and word inhibition in schizophrenia. Psychiatry Research. 2002;110:137–149. doi: 10.1016/s0165-1781(02)00100-2. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS, Sekihara K, Merzenich MM. Modulation of the auditory cortex during speech: an MEG study. Journal of Cognitive Neuroscience. 2002;14:1125–1138. doi: 10.1162/089892902760807140. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychological Medicine. 2011:1–13. doi: 10.1017/S0033291711001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannerod M. The mechanism of self-recognition in humans. Behav Brain Res. 2003;142:1–15. doi: 10.1016/s0166-4328(02)00384-4. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. American Journal of Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457:187–190. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Kiang M, Braff DL, Sprock J, Light GA. The relationship between preattentive sensory processing deficits and age in schizophrenia patients. Clinical Neurophysiology. 2009;120:1949–1957. doi: 10.1016/j.clinph.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. Journal of Comparative Psychology. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Lindner A, Thier P, Kircher TT, Haarmeier T, Leube DT. Disorders of agency in schizophrenia correlate with an inability to compensate for the sensory consequences of actions. Curr Biol. 2005;15:1119–1124. doi: 10.1016/j.cub.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Martikainen MH, Kaneko K, Hari R. Suppressed responses to self-triggered sounds in the human auditory cortex. Cerebral Cortex. 2005;15:299–302. doi: 10.1093/cercor/bhh131. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biological Psychiatry. 2000;47:434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Donchin E. The effects of temporal and event uncertainty in determining the waveforms of the auditory event related potential (ERP) Psychophysiology. 1976;13:581–590. doi: 10.1111/j.1469-8986.1976.tb00885.x. [DOI] [PubMed] [Google Scholar]
- Merrin EL, Floyd TC. P300 responses to novel auditory stimuli in hospitalized schizophrenic patients. Biological Psychiatry. 1994;36:527–542. doi: 10.1016/0006-3223(94)90617-3. [DOI] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a smith predictor? J Mot Behav. 1993;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Miall RC, Wolpert DM. Forward models for physiological motor control. Neural Networks. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- Muller-Preuss P, Ploog D. Inhibition of auditory cortical neurons during phonation. Brain Research. 1981;215:61–76. doi: 10.1016/0006-8993(81)90491-1. [DOI] [PubMed] [Google Scholar]
- Naatanen R, Simpson M, Loveless NE. Stimulus deviance and evoked potentials. Biological Psychology. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- Nicolelis MA, Baccala LA, Lin RC, Chapin JK. Sensorimotor encoding by synchronous neural ensemble activity at multiple levels of the somatosensory system. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Ohta H, McCarley RW, Hokama H, Wible CG, Law S, Nestor PG, Kikinis R, Jolesz FA, Shenton ME. The auditory P3a and P3b ERP components in schizophrenia: relationship to frontal and temporal lobe MRI volumes. In: Ogura C, Koga Y, Shimokuchi M, editors. Recent Advances in Event-Related Brain Potential Research. Elsevier Science B.V.; Amsterdam: 1996. pp. 30–35. [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biological Psychology. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Poulet JF, Hedwig B. The cellular basis of a corollary discharge. Science. 2006;311:518–522. doi: 10.1126/science.1120847. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia--a critical review. Psychiatry Res. 2008;161:259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Roth WT, Goodale J, Pfefferbaum A. Auditory event-related potentials and electrodermal activity in medicated and unmedicated schizophrenics. Biological Psychiatry. 1991;29:585–599. doi: 10.1016/0006-3223(91)90094-3. [DOI] [PubMed] [Google Scholar]
- Schafer EW, Marcus MM. Self-stimulation alters human sensory brain responses. Science. 1973;181:175–177. doi: 10.1126/science.181.4095.175. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context - A test of a theoretical model. Archives of General Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Shelley AM, Grochowski S, Lieberman JA, Javitt DC. Premature disinhibition of P3 generation in schizophrenia. Biological Psychiatry. 1996;39:714–719. doi: 10.1016/0006-3223(95)00222-7. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. American Journal of Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Elowson AM. Pygmy marmosets modify call structure when paired. Ethology. 1999;105:893–908. [Google Scholar]
- Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. Journal of Comparative and Physiological Psychology. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Stadler W, Klimesch W, Pouthas V, Ragot R. Differential effects of the stimulus sequence on CNV and P300. Brain Research. 2006;1123:157–167. doi: 10.1016/j.brainres.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Stirling JD, Hellewell JS, Quraishi N. Self-monitoring dysfunction and the schizophrenic symptoms of alien control. Psychological Medicine. 1998;28:675–683. doi: 10.1017/s0033291798006679. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Research. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Vuilleumier P, Mathalon DH, Swick D, Ford JM. Are Impairments of Action Monitoring and Executive Control Dissociable Dysfunctions in Patients With Schizophrenia? American Journal of Psychiatry. 2003;160:1881–1883. doi: 10.1176/appi.ajp.160.10.1881. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a meta-analysis. Schizophrenia Research. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Von Holst E, Mittelstaedt H. Das Reafferenzprinzip. Naturwissenschaften. 1950;37:464–476. [Google Scholar]
- Whitford TJ, Ford JM, Mathalon DH, Kubicki M, Shenton ME. Schizophrenia, Myelination, and Delayed Corollary Discharges: A Hypothesis. Schizophr Bull. 2011 doi: 10.1093/schbul/sbq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford TJ, Mathalon DH, Shenton ME, Roach BJ, Bammer R, Adcock RA, Bouix S, Kubicki M, De Siebenthal J, Rausch AC, Schneiderman JS, Ford JM. Electrophysiological and diffusion tensor imaging evidence of delayed corollary discharges in patients with schizophrenia. Psychol Med. 2011;41:959–969. doi: 10.1017/S0033291710001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert D, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw. 1988;11:1317–1329. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science. 1995;269:1880–1882. doi: 10.1126/science.7569931. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]



