Abstract
Mammalian cells were investigated for enzymes that help correct oxidative damages in DNA. We focused on 3'-repair diesterases, which process DNA ends at oxidative strand breaks by removing 3'-blocking fragments of deoxyribose that prevent DNA repair synthesis. Two enzymes were found in a variety of mouse, bovine and human tissues and cultured cells. The two activities were purified to differing degrees from HeLa cells. One enzyme had the properties of the known HeLa AP endonuclease (Mr approximately 38,000, with identical substrate specificity and reaction requirements, and cross-reactivity with anti-HeLa AP endonuclease antiserum) and is presumed identical to that protein. The second activity did not interact with anti-HeLa AP endonuclease antibodies and had relatively less AP endonuclease activity. This second enzyme may have been detected in other studies but never characterized. In addition to the 3'-repair diesterase and AP endonuclease, this partially purified preparation also harbored DNA 3'-phosphatase and 3'-deoxyribose diesterase activities. It is unknown whether all activities detected in the second preparation are due to a single protein, although activity against undamaged DNA was not detected. The in vivo roles of these two widely distributed 3'-repair diesterase/AP endonucleases have not been determined, but with the characterizations presented here such questions may now be focused.
Full text
PDF




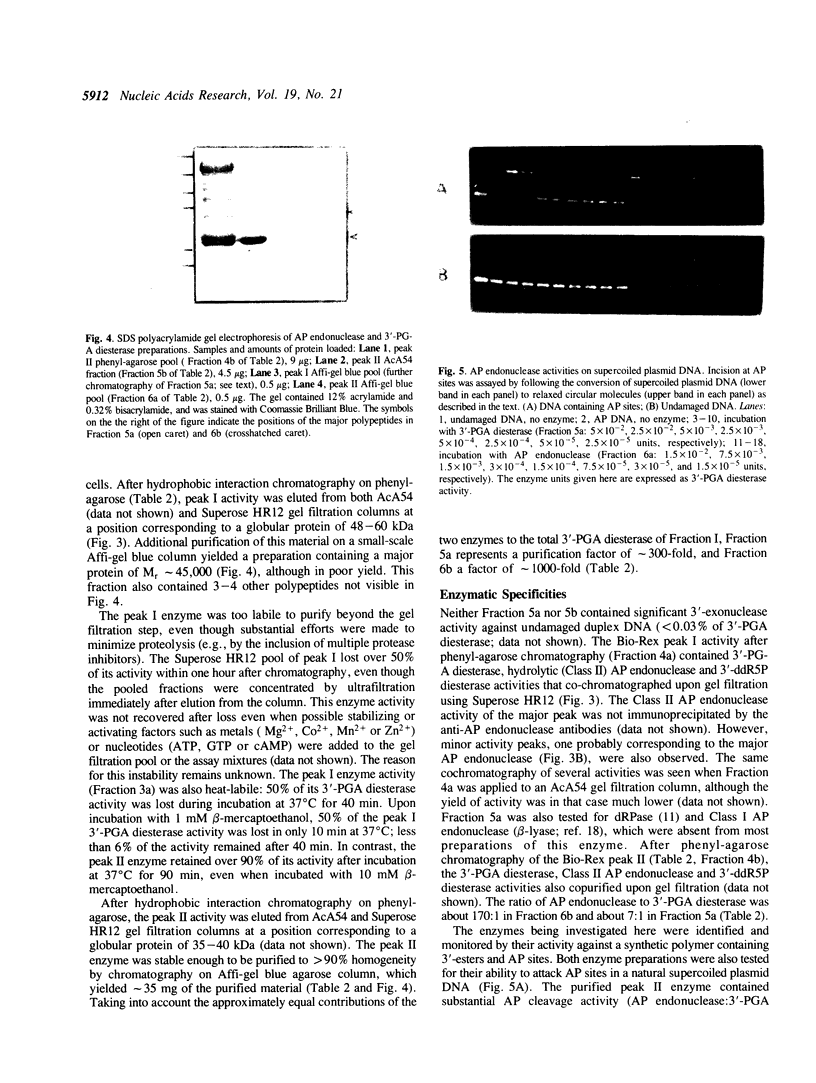
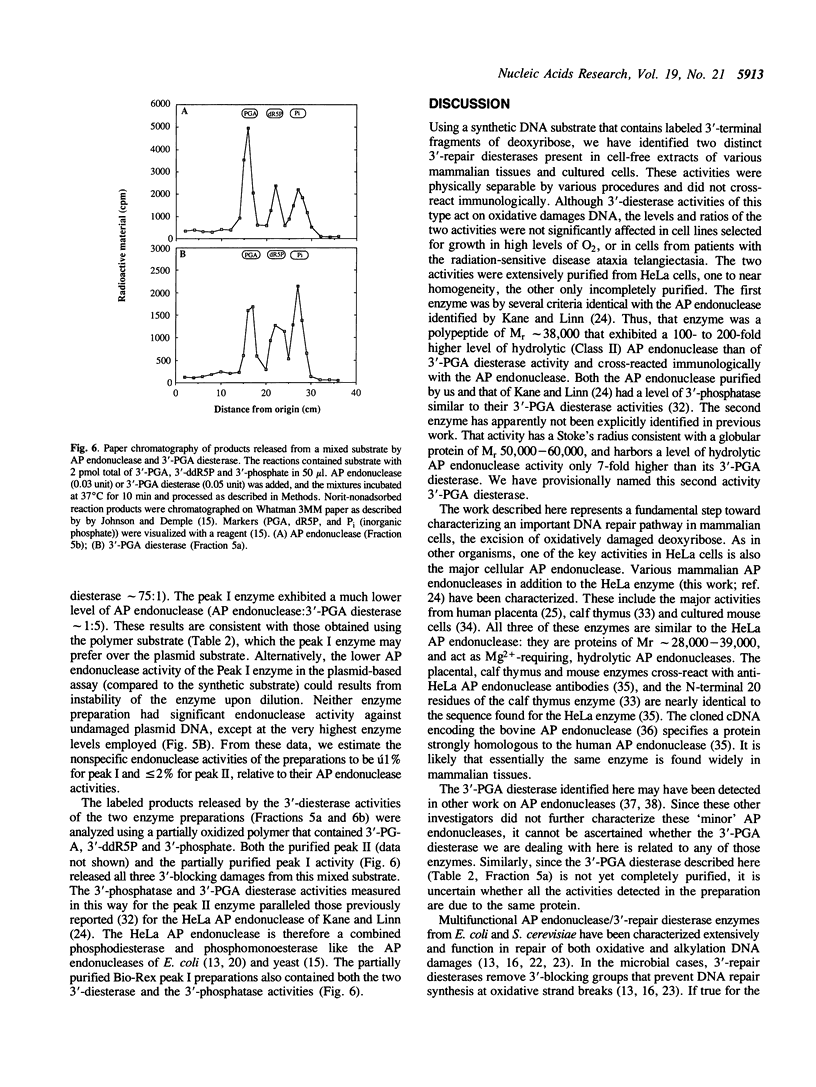

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brynolf K., Eliasson R., Reichard P. Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell. 1978 Mar;13(3):573–580. doi: 10.1016/0092-8674(78)90330-6. [DOI] [PubMed] [Google Scholar]
- Clements J. E., Rogers S. G., Weiss B. A DNase for apurinic/apyrimidinic sites associated with exonuclease III of Hemophilus influenzae. J Biol Chem. 1978 May 10;253(9):2990–2999. [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- César R., Verly W. G. The apurinic/apyrimidinic endodeoxyribonuclease of rat-liver chromatin. Eur J Biochem. 1983 Jan 1;129(3):509–517. doi: 10.1111/j.1432-1033.1983.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Demple B., Johnson A., Fung D. Exonuclease III and endonuclease IV remove 3' blocks from DNA synthesis primers in H2O2-damaged Escherichia coli. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7731–7735. doi: 10.1073/pnas.83.20.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., D'Ari R., Touati D. Oxygen-dependent mutagenesis in Escherichia coli lacking superoxide dismutase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8268–8272. doi: 10.1073/pnas.83.21.8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin W. A., Lindahl T. DNA deoxyribophosphodiesterase. EMBO J. 1988 Nov;7(11):3617–3622. doi: 10.1002/j.1460-2075.1988.tb03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille J. J., Wortelboer H. M., Joenje H. Effect of normobaric hyperoxia on antioxidant defenses of HeLa and CHO cells. Free Radic Biol Med. 1988;4(2):85–91. doi: 10.1016/0891-5849(88)90068-8. [DOI] [PubMed] [Google Scholar]
- Giloni L., Takeshita M., Johnson F., Iden C., Grollman A. P. Bleomycin-induced strand-scission of DNA. Mechanism of deoxyribose cleavage. J Biol Chem. 1981 Aug 25;256(16):8608–8615. [PubMed] [Google Scholar]
- Grafstrom R. H., Shaper N. L., Grossman L. Human placental apurinic/apyrimidinic endonuclease. Mechanism of action. J Biol Chem. 1982 Nov 25;257(22):13459–13464. [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR-mutants. EMBO J. 1988 Aug;7(8):2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Kiker N. P., Jorgensen T. J., Munck J. N. Purification and amino-terminal amino acid sequence of an apurinic/apyrimidinic endonuclease from calf thymus. Nucleic Acids Res. 1987 Jul 24;15(14):5529–5544. doi: 10.1093/nar/15.14.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner W. D., Rodriguez L. O., Hecht S. M., Haseltine W. A. gamma Ray induced deoxyribonucleic acid strand breaks. 3' Glycolate termini. J Biol Chem. 1983 Jan 25;258(2):711–713. [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Johnson A. W., Demple B. Yeast DNA 3'-repair diesterase is the major cellular apurinic/apyrimidinic endonuclease: substrate specificity and kinetics. J Biol Chem. 1988 Dec 5;263(34):18017–18022. [PubMed] [Google Scholar]
- Johnson A. W., Demple B. Yeast DNA diesterase for 3'-fragments of deoxyribose: purification and physical properties of a repair enzyme for oxidative DNA damage. J Biol Chem. 1988 Dec 5;263(34):18009–18016. [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kuhnlein U. Comparison of apurinic DNA-binding protein from an ataxia telangiectasia and a HeLa cell line. Evidence for an altered processing of apurinic/apyrimidinic endonuclease. J Biol Chem. 1985 Dec 5;260(28):14918–14924. [PubMed] [Google Scholar]
- Levin J. D., Demple B. Analysis of class II (hydrolytic) and class I (beta-lyase) apurinic/apyrimidinic endonucleases with a synthetic DNA substrate. Nucleic Acids Res. 1990 Sep 11;18(17):5069–5075. doi: 10.1093/nar/18.17.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. D., Johnson A. W., Demple B. Homogeneous Escherichia coli endonuclease IV. Characterization of an enzyme that recognizes oxidative damage in DNA. J Biol Chem. 1988 Jun 15;263(17):8066–8071. [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Mazumder A., Gerlt J. A., Absalon M. J., Stubbe J., Cunningham R. P., Withka J., Bolton P. H. Stereochemical studies of the beta-elimination reactions at aldehydic abasic sites in DNA: endonuclease III from Escherichia coli, sodium hydroxide, and Lys-Trp-Lys. Biochemistry. 1991 Jan 29;30(4):1119–1126. doi: 10.1021/bi00218a033. [DOI] [PubMed] [Google Scholar]
- Nes I. F. Purification and properties of a mouse-cell DNA-repair endonuclease, which recognizes lesions in DNA induced by ultraviolet light, depurination, gamma-rays, and OsO4 treatment. Eur J Biochem. 1980 Nov;112(1):161–168. doi: 10.1111/j.1432-1033.1980.tb04997.x. [DOI] [PubMed] [Google Scholar]
- Popoff S. C., Spira A. I., Johnson A. W., Demple B. Yeast structural gene (APN1) for the major apurinic endonuclease: homology to Escherichia coli endonuclease IV. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4193–4197. doi: 10.1073/pnas.87.11.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramotar D., Popoff S. C., Demple B. Complementation of DNA repair-deficient Escherichia coli by the yeast Apn1 apurinic/apyrimidinic endonuclease gene. Mol Microbiol. 1991 Jan;5(1):149–155. doi: 10.1111/j.1365-2958.1991.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Milne A. M., Pappin D. J., Hickson I. D. Isolation of cDNA clones encoding an enzyme from bovine cells that repairs oxidative DNA damage in vitro: homology with bacterial repair enzymes. Nucleic Acids Res. 1991 Mar 11;19(5):1087–1092. doi: 10.1093/nar/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. G., Weiss B. Exonuclease III of Escherichia coli K-12, an AP endonuclease. Methods Enzymol. 1980;65(1):201–211. doi: 10.1016/s0076-6879(80)65028-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Wallace S. S. AP endonucleases and DNA glycosylases that recognize oxidative DNA damage. Environ Mol Mutagen. 1988;12(4):431–477. doi: 10.1002/em.2860120411. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Demple B. F., Deutsch W. A., Kane C. M., Linn S. Apurinic/apyrimidinic endonucleases in repair of pyrimidine dimers and other lesions in DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4602–4606. doi: 10.1073/pnas.77.8.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]




