Introduction
One of the pivotal, common pathways shared between traumatic brain injury (TBI), toxin-induced neurodegeneration, and Parkinson’s disease (PD) is the disruption of normal mitochondrial function (Ramsay et al., 1986, Vyas et al., 1986, Schapira et al., 1990, Sullivan et al., 1998, Sullivan et al., 1999, Sherer et al., 2003, Lifshitz et al., 2004, Bender et al., 2006). To date, research has shown that these three insults may interact at the mitochondrial level. Given the similar changes in mitochondrial function observed with these pathologies, exposure to a mitochondrial toxin prior to a TBI is hypothesized to produce a multifactorial injury with pathological changes greater than either insult alone.
Exposure to an environmental factor long before the onset of symptoms of PD has been shown to correlate with the future development of pathology (Veldman et al., 1998, Brown et al., 2005, Landrigan et al., 2005) and this may be the result of either a slow continuous neuronal decline or a predisposition towards increased susceptibility to future insults such as TBI. One such environmental factor which has been linked to PD is trichloroethylene (TCE), C2HCl3. TCE is a halogenated hydrocarbon that has been widely used as a solvent in the United States for: degreasing, dry cleaning, food processing, paint production, and as an anesthetic (EOHS, Bakke et al., 2007). It is estimated that as of 1997 over 400,000 workers were annually exposed to TCE in the workplace (ATSDR, 1997). TCE has been detected at 861 National Priorities List hazardous waste sites, with 213 of these sites having complete exposure pathways allowing TCE to reach the population in proximity to the site (ATSDR, 1997). Exposure to TCE has been shown to cause mitochondrial dysfunction (Gash et al., 2008) and a loss of dopaminergic neurons in the substantia nigra (Liu et al., 2010). The downstream metabolite of TCE, 1-trichloromethyl-1,2,3,4-tetrahyro-β-carboline (TaClo), has been shown to inhibit Complex I of the mitochondrial respiratory chain (Janetzky et al., 1995) and induce apoptosis through the release of mitochondrial Cytochrome C (Akundi et al., 2004). With the availability of both experimental and clinical reports of mitochondrial and dopaminergic toxicity following exposure to either TCE or its metabolite TaClo, evidence has accumulated to implicate TCE (Kochen et al., 2003, Gash et al., 2008, Liu et al., 2010) and TaClo (Bringmann et al., 1995b, Riederer et al., 2002) as relevant neurotoxins in the development of PD.
The development of PD has also been linked to TBI both in epidemiological studies (Factor and Weiner, 1991, Stern, 1991, Semchuk et al., 1993, Taylor et al., 1999, Bower et al., 2003) and in clinical evaluations (Nayernouri, 1985, Doder et al., 1999). Patients sustaining a mild head trauma with a loss of consciousness or a severe head trauma have a significantly higher probability of developing PD later in life (Bower et al., 2003). Experimentally, it has been shown that a TBI can lead to significant disruption of dopaminergic functioning (Wagner et al., 2005) and these changes are thought to play a significant role in the connection between TBI and PD. Further evidence shows that following TBI there is a reduction in tyrosine hydroxylase activity in the striatum which correlates with a reduction in evoked dopamine release (Shin et al., 2011). Dopamine and cyclic-AMP regulated phosphoprotein (DARPP-32) plays an important role in synaptic function in the striatum. Following TBI there is a reduction in the phosphorylation of DARPP-32 in the striatum (Bales et al., 2011) and this change can impart another level of deficits which could potentiate the development of PD symptoms. In human PD patients it has been shown that there are alterations in striatal dopaminergic proteins (Donnemiller et al., 2000) which reflect some of what is observed following TBI alone. It is plausible that TBI could either cause or exacerbate these changes in striatal function and result in the subsequent onset of PD.
Although there have been multiple reports linking TBI to PD (Factor and Weiner, 1991, Stern, 1991, Semchuk et al., 1993, Bower et al., 2003), current evidence only shows TBI to be a risk factor and has failed to show a causative link (Factor et al., 1988). The disruption of mitochondrial function and the initiation of cell death mechanisms may play a role in directly linking TBI and PD. Mitochondrial dysfunction after TBI can result in reductions in ATP levels (Sullivan et al., 1998), increases in lipid peroxidation (Sullivan et al., 1998), and the release of Cytochrome C which leads activation of apoptotic pathways (Sullivan et al., 2002), resulting in cell death. With its pivotal role after TBI, the mitochondrion is at a critical position to allow multiple insults to interact and produce increased pathology. Given the numerous factors linked to PD, including TCE and TBI, the concept that the development of PD involves a multifactorial injury has emerged (Semchuk et al., 1993). Experiments utilizing combinations of chemical and mechanical insults have shown that neurons can be made more susceptible to future insults following exposure to a toxin (Arundine et al., 2004, Ling et al., 2004). The maintenance of mitochondrial function is critical to preventing cell death since neuronal ATP stores can be depleted within minutes and lead to the initiation of cell death pathways (Nilsson, 2001). A toxin that is capable of inhibiting the mitochondrial respiratory chain, such as TCE (Gash et al., 2008), could produce a chronic decrease in ATP levels making neurons more susceptible to future insults since the cell would already be in a compromised state from energy depletion. The chronic inhibition of Complex I by TCE and a resulting decline in energy stores may be the link allowing TCE and TBI to interact. Given that previous studies using dual injuries have shown increased pathology (Thiruchelvam et al., 2000a, Thiruchelvam et al., 2000b, Arundine et al., 2003, Arundine et al., 2004, Ling et al., 2006, Fei and Ethell, 2008), we sought to test the hypothesis that TCE exposure and TBI can interact to produce a greater level of disease pathology. This is the first report of these two insults being studied together and provides further support that sporadic PD may be the result of a multifactorial process.
Methods
Trichloroethylene Treatment
All studies were approved by the University of Kentucky Institutional Animal Care and Usage Committee. Male Fischer 344 rats 16 weeks old (Harlan Laboratory) were orally gavaged 0.6ml of either the olive oil vehicle (Kroger Pure Olive Oil) or 1000mg/kg Trichloroethylene (Sigma Aldrich) daily for either 1 or 2 weeks. A 100mm gavage needle (Fine Science Tools) with a 16 gauge tip sized to prevent insertion of the needle into the trachea was used for the administration of TCE. One hundred and thirty two animals were utilized for the completion of these studies.
Controlled Cortical Impact Brain Injury
Following the administration of TCE for either 1 or 2 weeks, animals were anesthetized with 2% isofluorane and placed in a Kopf stereotaxic frame for positioning under a pneumatic impactor (Precision Science Instruments). A 6 mm craniotomy was performed, with a hand trephine, lateral to the central fissure on the left side of the skull, centered between lambda and bregma. Animals in injury groups received a unilateral injury directly to the surface of the brain. The injury parameters consisted of a 1.0 mm cortical compression for mild injured animals and a 2.0 mm cortical compression for moderate injured animals. Both injury severities were performed at 3.5 meters/second for 500ms. Sham animals received a craniotomy but did not receive an impact to the brain. Following the injury a piece of Surgicel (Johnson&Johnson) sized to fit into the craniotomy was placed directly on the brain. The skull cap was replaced and secured in place with dental acrylic. Once the acrylic was allowed time to harden, the scalp incision was closed with surgical staples. Animals were removed from isofluorane and placed in a clean cage and temperature was maintained at 37°C with the use of a heating pad. Five to six animals per group were utilized throughout all of the experiments.
Behavioral Analysis
Rotarod testing
Nine days after the brain injury surgeries rotarod testing was begun for all treatment groups. Animals were trained on a rotarod (Med Associates Inc.) for three days at speeds of 8rpm, 12rpm, and then 16rpm. On the fourth day (12 days post TBI) animals were tested on the rotarod using an accelerating speed starting at 3rpm and holding at 30rpm. The amount of time animals were able to stay on the rotarod on the fourth day was determined by a timer built into the rotarod, which was stopped when the animal fell off the rotarod and blocked an infrared light sensor. Animals were tested twice on the fourth day and the times for each trial were averaged for each animal. Six animals/group were utilized for rotarod testing.
Cylinder Testing
Thirty days after the brain injury animals underwent cylinder testing to assess alterations in paw placement. Animals were placed in a clear plastic cylinder with a diameter of 19cm which was mounted on a plexiglass surface with an angled mirror underneath to allow video recording of the animals for 5min. Video recordings were performed using a digital video camera, and the footage was analyzed using QuickTime software (Apple Computer Inc.) with playback set at ½actual speed. The number of contralateral and ipsilateral paw touches were quantified by an observer blinded to treatment groups. The percentage of contralateral forepaw usage was calculated using the formula . Six animals/group were utilized for cylinder testing.
Histological Analysis
Following the completion of the cylinder testing animals were sacrificed for post-injury histological analysis. Animals were anesthetized with Sodium Pentobarbital (Abbot Laboratories) and transcardially perfused with saline followed by 4% paraformaldehyde. The brains were removed and placed in 4% paraformaldehyde and 30% sucrose in PBS for 24hrs. After 24hrs the brains were transferred to a 30% sucrose/PBS buffer without paraformaldehyde. Coronal sections 30μm thick were cut with a freezing microtome throughout the rostral to caudal extent of the entire brain.
Nissl staining was performed to visualize the intact regions of the cortex for cortical tissue sparing analysis and to quantify the total number of neurons in the substantia nigra. Quantitative assessment of cortical damage employed an unbiased protocol using the Cavalieri method as previously described (Sullivan et al., 2000, Sullivan et al., 2002). All slides were assessed blindly with respect to treatment group.
For immunohistochemistry, sections containing regions of interest were incubated overnight at 4°C with a primary antibody. Slices were stained for Tyrosine Hydroxylase using a primary monoclonal antibody (Chemicom), followed by a biotinylated anti-mouse secondary antibody (Vector). Following the secondary antibody treatment sections were treated with an Avidin Biotin Peroxidase Complex, Vectastatin ABC Kit (Vector). The tissue was developed with a Diaminobenzidine tetrahydrochloride (DAB) solution with nickel intensification to allow stable long-term staining and visualization with a light microscope. Bioquant Image Analysis software (Bioquant, Nashville, TN) was used to estimate total cell number in the region of interest using the optical fractionator method (Mouton, 2002). This method represents an unbiased quantitative technique that is independent of size and shape or any conformational changes of cells. Detailed procedures regarding stereological counting are similar to our previously published studies (Liu et al., 2008). Coronal sections containing the substantia nigra (interaural 2.7-4.2) (Paxinos and Watson, 1998) were used to estimate the total number of tyrosine hydroxylase (TH) positive cells and Nissl postive cells in the SN. A set of 6 systematic sections were sampled in an independent-random manner from a total of 36-39 sections containing the entire reference space. On each stained section the area containing the SN was identified using conventional landmarks (Paxinos, 2004). An unbiased grid was overlayed on each section and a random number system embedded in the software was used to identify areas of the grid for counting. On each identified grid position an unbiased counting frame was used for the optical dissector. The counting frame was 150 × 150μm centered on the grid intersection. The height of each dissector was determined with a digital z-axis encoder (Boeckeler Instruments, Tucson, AZ). Cells were identified using a 40X objective with a high numerical aperture. The total number of cells (N) was estimated using the formula N = Q • 1/hsf • 1/asf • 1/ssf where Q is the total number of cells actually counted, hsf is the height sampling fraction, asf is the area sampling fraction, and ssf is the section sampling fraction. For analysis of TH fiber density, Image Pro software was used for the quantification of the area of the Striatum immunoreactive for TH in a blinded fashion. High resolution images throughout the entire rostral to caudal extent of the striatum using an Olympus AX80 microscope. Using Image Pro, the perimeter of the Striatum was outlined and the percentage area that was immunoreactive for TH was calculated. The average amount of immunoreactivity for a single animal was calculated separately for both the ipsilateral and contralateral sides of the brain. Six animals/group were utilized for the quantification of histological endpoints.
Western blot analysis
Quantification of dopamine transporter and type 2 dopamine receptor levels in the striatum was performed using standardized western blot protocols. Animals were asphyxiated with CO2 and rapidly decapitated. The ipsilateral Striatum and bilateral Substantia Nigra were rapidly dissected, placed into plastic tubes, and the tubes were placed into a bath of dry-ice and ethanol to rapidly freeze the tissue. Samples were frozen at −80°C until homogenized. At the time of homogenization samples were placed into lysis buffer (0.1M NaCl, 0.01M Tris-Cl, 0.001 EDTA, 1ug/ml aprotinin, 100ug/ml phenylmethylsulfonyl fluoride) and homogenized using low intensity sonication for 30 seconds. Samples were centrifuged at 13,800×G for 30min at 4°C. The protein concentrations were determined with all the samples on the same plate using the BCA protein assay kit and measuring absorbance at 560nm with a Biotek Synergy HT plate reader (Winooski, Vermont). Protein separation was performed using a 4% stacking gel followed by a 10% separation gel. Gels were run for one hour at 150 volts and 4°C with constant stirring. Following gel separation, proteins were transferred to nitrocellulose utilizing a wet transfer setup at 100 volts for one hour. Following protein transfer to the nitrocellulose, blots were washed three times with tris-buffered saline (TBS) and blocked with 5% milk in TBS for one hour at room temperature. After blocking, blots were washed three times with 5% Tween-20+TBS (TTBS) at room temperature with constant agitation. Primary antibodies towards the proteins of interest were prepared in 5% milk in TTBS. For quantification of the dopamine transporter, 40ug of protein was loaded per well and the primary antibody (AB2231, Millipore) was applied at a concentration of 1:1500 and incubated overnight. For quantification of the type 2 dopamine receptor, 60ug of protein was loaded to the gel and the primary antibody (SC-9113, Santa Cruz) was applied at a concentration of 1:800 and incubated overnight. Following incubation with the primary antibody, blots were washed with TTBS and incubated with an infrared secondary antibody (Rockland) at a concentration of 1:5000. Band intensities were determined using a Licor Odyssey infrared imager. The 50kD band representing the dopamine transporter, and the 55kD band representing the type 2 dopamine receptor were utilized for the determination of protein expression levels. Levels of each protein were expressed as a ratio of the protein of interest compared to the amount of actin for the same sample. Six animals/group were utilized for western blot analysis.
Mitochondrial isolation and oxygen consumption analysis
Mitochondria were isolated using differential centrifugation, nitrogen disruption, and a Ficoll gradient. Animals were asphyxiated with CO2 and rapidly decapitated. Tissue was rapidly removed and immediately placed in ice-cold isolation buffer (215mM Mannitol, 75mM Sucrose, 0.1% BSA, 1mM EGTA, and 20mM Hepes at pH 7.2). Due to the small size of the substantia nigra, three animals were combined for each mitochondrial sample, resulting in an n=1 for these three animals. Samples were homogenized and centrifuged at 1300×G for 3 minutes. Following the first spin the supernatant was placed in a fresh tube and the pellet was resuspended in isolation buffer and spun at 1,300×G for 3min. The supernatant from the first and second spins were collected in separate tubes and spun at 13,000×G for 10 minutes. The pellets from both tubes was combined, resuspended in 400ul isolation buffer and placed in a nitrogen bomb at 1,200psi for 10min. The pressure in the nitrogen bomb was rapidly released and the sample was placed as the top layer on a Ficoll separation column, which consisted of a 10% Ficoll layer and a 7.5% Ficoll layer. The Ficoll column with sample was centrifuged at 100,000×G for 30min at 4°C using a Beckman SW 55Ti rotor and ultra-centrifuge. The final mitochondrial pellet was resuspended in isolation buffer without EGTA to yield a final concentration of approximately 10mg/ml, and stored immediately on ice. To normalize the results, the protein concentrations were determined with all the samples on the same plate using the BCA protein assay kit and measuring absorbance at 560nm with a Biotek Synergy HT plate reader (Winooski, Vermont). Mitochondrial oxygen consumption was measured using a Clark-type electrode (Hansatech Instruments, Norfolk, England) in a continuously stirred, sealed chamber at 37°C as previously described (Sullivan et al., 2003). Isolated mitochondrial protein (100μg) was suspended in respiration buffer (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, 2 mM MgCl, 2.5 mM KH2PO4 at pH 7.2) in a final chamber volume of 0.25 ml. Mitochondrial bioenergetic analysis was measured by the sequential administration of substrates: 5mM Pyruvate, 2.5mM Malate, 150nM ADP, 1uM Oligomycin, and 1uM Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP). For the measurement of mitochondrial bioenergetic function after the 1 week exposure to TCE, purified mitochondria were analyzed using the Seahorse Biosciences XF24 Flux Analyzer as previously described (Sauerbeck et al., 2011). Five to six animals per group were utilized for the 2 week mitochondrial studies and three to four animals per group were utilized for the 1 week mitochondrial studies.
Statistical Analysis
For all experiments significance was set at p<0.05. All experiments were analyzed with a two-way analysis of variance, followed by post-hoc analysis with a Bonferroni post-test to determine which groups were significantly different. The main effects tested with the 2-way ANOVA were the effect of either TBI or TCE. If a significant difference was observed with either of the main effects or from an interaction then post-hoc analysis was performed. Data is represented as Mean+SEM.
Results
2 week exposure to TCE and TBI impair mitochondrial bioenergetic function
Mitochondrial dysfunction is a common feature of TBI, PD, and TCE exposure. The ability for both TBI and TCE exposure to independently inhibit mitochondrial function is hypothesized to be a potential mechanism allowing these insults to interact and increase pathology. Mitochondria were isolated six hours after the CCI and total (synaptic and non-synaptic) mitochondria from both the striatum and substantia nigra were analyzed to determine mitochondrial bioenergetic function. No reductions in mitochondrial function were observed in the substantia nigra six hours after the TBI or following the two week exposure to TCE (Figure 1A). At six hours post-injury exposure to TCE produced a significant main effect of mitochondrial impairment in the striatum which was observed by reductions in both the basal and maximal rates of Complex I dependent oxygen consumption by approximately 75% (Figure 1B, *=p<0.05). Exposure to the TBI alone resulted in a non-significant trend towards reduced Complex I function by approximately 30% in the striatum. The combination of TCE exposure and a TBI did not result in increased impairment compared to TCE alone, and this is likely the result of reaching a maximal amount of sustainable mitochondrial impairment.
Figure 1. 2 week exposure to TCE and TBI results in decreases in mitochondrial bioenergetics in the striatum.
Animals were exposed to TCE for 2 weeks and then subjected to a moderate TBI, and mitochondria were isolated from the striatum and substantia nigra six hours post-injury. A) Analysis of bioenergetic function in the substantia nigra revealed no significant difference between any of the treatment groups. B) Exposure to TCE for 2-weeks produced significant reductions of approximately 75% in Complex I dependent basal and maximal rates of mitochondrial oxygen consumption. The TBI alone resulted in a trend towards reduced mitochondrial function in the striatum, however, this decrease did not reach significance.
*=p<0.05, n=5-6; 2-Way ANOVA with Bonferroni post-test; Mean±SEM
1 week exposure to TCE followed by TBI results in synergistic mitochondrial dysfunction in the striatum
Given the 75% reduction in Complex I driven mitochondrial function in the striatum following a 2 week exposure to TCE (Figure 1B), and the difficulty this produces in elucidating whether an interaction between TCE exposure and TBI exists, additional animals were subsequently administered TCE for only 1 week. Similarly to the previous 2 week exposure studies, a moderate TBI resulted in a non-significant decrease in mitochondrial bioenergetic function of approximately 30%. Analysis of the main effects showed that exposure to TCE for 1 week did affect mitochondrial function, though to a much lower level than the 2 week exposure. Further post-test analysis showed that when the 1 week exposure to TCE was combined with a moderate TBI, mitochondrial bioenergetic function was reduced by approximately 50% (Figure 2, *=p<0.05). The increase in mitochondrial impairment following exposure to TCE and TBI demonstrates that the two insults are capable of interacting in an additive manner at the mitochondrial level and producing more pronounced deficits. These studies indicate that TCE and TBI are capable of interacting and supports the concept of a multifactorial injury paradigm.
Figure 2. The combined exposure of TCE for 1 week with a moderate TBI results in impaired in mitochondrial bioenergetics in the striatum.
Exposure to either TCE for 1 week or a moderate TBI did not result in significant mitochondrial dysfunction in the striatum. When the two separate insults were combined together there was a significant reduction in the Complex I driven rate of mitochondrial oxygen consumption. The dual injury paradigm resulted in synergistic mitochondrial deficits, which resulted in approximately a 50% reduction in mitochondrial function.
*=p<0.05, n=3-4; 2-Way ANOVA with Bonferroni post-test; Mean±SEM
Motor impairment occurs following exposure to TCE and a moderate TBI
Motor impairment is the major functional pathology associated with Parkinson’s disease. To investigate the potential of this multifactorial model to produce behavioral impairment, two behavioral tests were utilized: the Rotarod and Cylinder Test. Animals were tested on the Rotarod 12 days post-TBI and analysis of the main effects showed a significant difference in animals exposed to TCE. Post-test analysis revealed no differences between either the Olive Oil-TBI group or the TCE-Sham group in comparison to the Olive Oil-Sham group, which serves as the control for the individual insults. Further post-test analysis showed that following exposure to TCE and a moderate controlled cortical impact (CCI) a significant reduction of approximately 50% (Figure 3A, p<0.01) in the amount of time spent on the Rotarod was observed, indicating impairment in motor coordination and balance. The Rotarod testing shows that both exposure to TCE and a moderate TBI can interact to produce significant motor impairment. At 30 days post-TBI animals were analyzed using a cylinder test to determine longer-term deficits in forepaw usage. Analysis of the main effects showed no differences following either insult alone, however, there was a significant interaction between the two insults. Further analysis showed that with the cylinder test a significant reduction in the usage of the contralateral forepaw was observed in animals that were exposed to TCE and a moderate CCI, which decreased to 34% (Figure 3B, p<0.05) of control animals. With the decrease in forepaw usage only being present following a dual exposure to both TCE and a moderate CCI, it is again shown that there is a requirement for both insults in order to produce functional impairment. With both behavioral tests showing that the dual injury consisting of TCE exposure and a moderate TBI are required for functional deficits to appear, and that any of the single insults tested are incapable of producing functional deficits, there is again support for a dual injury mechanism. Unlike our 1 week mitochondrial data, the behavioral results indicate a synergistic effect following exposure to TCE and TBI. It should be noted that a threshold limit may be needed to pick up motor deficits following the single insults alone, which could prevent us from observing an additive effect.
Figure 3. Exposure to TCE and a moderate TBI synergistically interact resulting in functional impairment.
Exposure to both TCE for 2 weeks and a moderate controlled cortical impact TBI result in significant motor impairment. A) Twelve days after the TBI animals exhibited significant motor impairment, as exhibited by a reduced ability to stay on a rotating rod. Animals exposed to TCE and a moderate TBI have approximately a 50% reduction in the length time able to stay on the Rotarod.B) Thirty days after the TBI, forepaw usage was assessed using the cylinder test and contralateral forepaw usage decreased to 34%. These results demonstrate the necessity for both insults to be present before functional impairment occurs.
#=p<0.01, *=p<0.05; n=6; 2-Way ANOVA with Bonferroni post-test; Mean±SEM
Exposure to TCE and a moderate TBI leads to a loss of tyrosine hydroxylase positive neurons in the substantia nigra
The major histological feature of postmortem Parkinson’s disease diagnosis is the loss of dopamine neurons in the substantia nigra (SN). Following exposure to either TCE or TBI alone there was not a significant loss of TH positive neurons in the SN; however, following the dual injury with TCE and either a mild or moderate cortical impact there was a 13-17% loss of TH positive neurons (Figure 4A, p<0.05). The lack of a significant effect with either of our main effects, but the presence of a significant loss of TH+ cells only following the dual injury, indicates that exposure to both TCE and TBI can lead to a synergistic loss of dopamine neurons in the SN. This shows that exposure to both TCE and TBI can produce the major histological symptom of PD, even with injury levels which are incapable of independently producing pathology. Our analysis of TH+ neurons, like our behavioral analysis, shows a synergistic interaction between TCE and TBI. Given that previous work shows TCE alone to lead to a loss of TH+ neurons (Liu et al., 2010), different experimental parameters may result in an additive effect similar to other models (Hutson et al., 2011). To determine if the loss of TH+ cells was the result of cell death or a loss of the tyrosine hydroxylase protein, Nissl positive cells were also counted. Since there was not a reduction in the number of Nissl positive neurons, the loss of TH+ neurons was the result of a loss of the tyrosine hydroxylase protein and not a loss of cells.
Figure 4. Expose to TCE and TBI interact and result in TH-positive neuron loss in the Substantia Nigra.
Exposure to both TCE for 2 weeks and a mild or moderate controlled cortical impact TBI resulted in significant decreases in the number of TH-positive neurons in the substantia nigra at 30 days post-TBI. A) The dual injury consisting of TCE exposure and a TBI brain injury resulted in a 13-17% loss of dopamine neurons in the substantia nigra. B) Quantification of Nissl cells in the substantia nigra did not reveal any measurable amount of cell loss, indicating the loss of TH-positive neurons is due to a loss of the TH protein and not the loss of cells. C) Representative images of the substantia nigra stained for TH.
*=p<0.05; n=6; 2-Way ANOVA with Bonferroni post-test; Mean±SEM
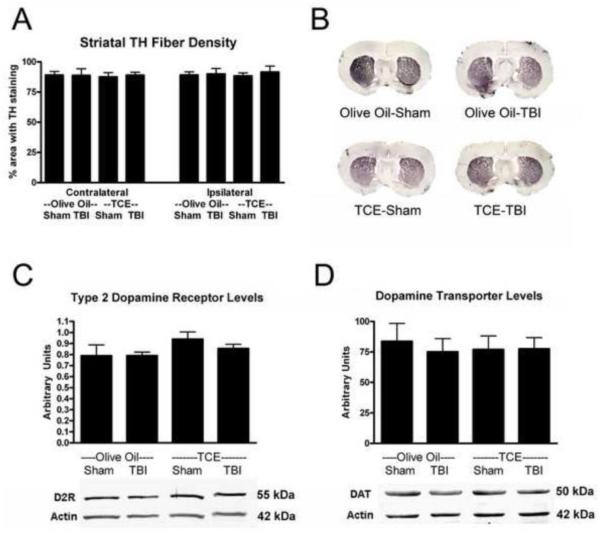
Exposure to TCE and TBI does not affect striatal tyrosine hydroxylase fiber density or levels of the dopamine transporter and dopamine receptor
Following the loss of dopamine neurons in the substantia nigra, in human PD there is a loss of both TH+ fibers and the pre-synaptic dopamine transporter (DAT) in the striatum along with an increase in the type-2 post-synaptic dopamine receptor (D2R). Following either exposure to TCE, TBI, or both, no changes were observed in the area of fibers positive for tyrosine hydroxylase in the striatum (Figure 5A). Additionally, there were no observable changes in the levels of the DAT or D2R following any of the single insults or the dual injury (Figure 5C,D). Given that the loss of dopamine neurons in the substantia nigra was between 13-17% (Figure 4A) it is not surprising that large changes in the dopamine signaling pathway were not observed in the striatum.
Figure 5. Exposure to TCE, TBI, or the dual injury does not lead to striatal changes in TH-fibers, DAT, and D2R.
Neither exposure to TCE for 2 weeks, a mild or moderate TBI, or any combination of the insults resulted in histological deficits in the striatum. A) The amount of area in the striatum immunoreactive for TH was quantified and no reduction in TH-positive fibers was measured. B) Representative images of the striatum stained for TH. C,D) Western blot analysis for levels of the dopamine transporter and type-2 dopamine receptor revealed no changes in either protein.
N=6; 2-Way ANOVA; Mean±SEM. Data are expressed as a ratio to level of β-actin.
Exposure to TCE does not affect cortical tissue loss following TBI
Following a CCI injury there is the formation of a cortical cavity which results from the loss of neuronal tissue. Given the ability for TCE to impair mitochondrial function, and the role mitochondrial dysfunction plays in post-TBI cortical tissue loss, the amount of spared cortical tissue was measured in both single and dual injured animals. Analysis of the main effects showed no differences from exposure to TCE, but as expected there was a significant effect following the TBI. With this model, following a moderate CCI, there is approximately a 30% loss of tissue, which affects both the vehicle and TCE treated groups equally (Figure 6A, *=p<0.0001). Exposure to the mitochondrial toxin TCE prior to the CCI did not lead to an increase in the size of the cortical lesion (Figure 6A). To rule out any affects which TCE may have on brain volume that could confound our analysis of tissue sparing, we compared the amount of tissue in the contralateral cortex. No differences were observed between any of the groups with respect to the amount of contralateral cortical tissue. Unlike our mitochondrial, behavioral, and histological endpoints, there was no additive effect on the cortical lesion following exposure to both TCE and TBI.
Figure 6. Exposure to TCE prior to a moderate TBI does not increase cortical tissue loss.
Animals that were exposed to TCE for 2 weeks prior to a moderate TBI did not exhibit an increase in the amount of cortical tissue loss. A) With this model a moderate TBI resulted in approximately a 30% loss of cortical tissue at 30 days post-injury. Exposure to TCE had no effect on the loss of cortical tissue. B) Representative images of Nissl stained cortex 30 days post-injury.
*=p<0.0001; n=6; 2-Way ANOVA with Bonferroni post-test; Mean±SEM
Discussion
It has been proposed that in humans the development of Parkinson’s disease (PD) may involve a multifactorial mechanism (Semchuk et al., 1993). Genetic factors (for review see(Nuytemans et al., 2010), age (Naoi and Maruyama, 1999), and the toxin MPTP (Langston et al., 1983, Langston and Ballard, 1983) have been shown to independently lead to the development of PD in humans; however, the vast majority of other insults, such as TBI, or environmental exposures have only shown a correlation for disease development (for review see(Veldman et al., 1998). Previous work from our group has shown that both in humans and rodents chronic exposure to trichloroethylene (TCE) is capable of producing pathologic features of PD such as motor impairment, loss of dopamine neurons in the substantia nigra and striatal deficits in tyrosine hydroxylase (Gash et al., 2008, Liu et al., 2010). Even though chronic high dose exposure is capable of leading to the development of Parkinsonism or complete PD in both humans and animals (Gash et al., 2008, Liu et al., 2010), it is unknown how many individuals would be exposed to high levels of TCE chronically. In the present studies we investigated the potential for a lower exposure to TCE combined with a traumatic brain injury (TBI) to produce a multifactorial model of PD. These studies provide an initial indication that TCE and TBI can interact in a multifactorial mechanism and alter several endpoints associated with PD
It has been established using various combinations of insults and toxin exposures that a multifactorial injury can produce a greater insult both in vitro (Arundine et al., 2003, Arundine et al., 2004, Fei and Ethell, 2008) and in vivo (Thiruchelvam et al., 2000a, Thiruchelvam et al., 2000b, Ling et al., 2006). In neuronal culture, cells exposed to a mechanical stretch injury prior to NMDA exposure are made more susceptible to the toxic insult (Arundine et al., 2003, Arundine et al., 2004). In rodents it has been shown that prenatal toxin exposure can make animals more susceptible to future insults, resulting in a progressive model of PD (Ling et al., 2006). Furthermore,multifactorial toxin exposure has been shown to produce a greater insult than exposure to a single toxin (Thiruchelvam et al., 2000a, Ling et al., 2006). Epidemiological studies have attempted to find a potential link between TBI and PD (Bower et al., 2003). However, a causative link between TBI and PD has been difficult to show and as a result it has been proposed that the link between TBI and PD may involve a multifactorial mechanism (Semchuk et al., 1993). Even without the addition of a secondary insult, TBI has been shown to result in impairments in the striatum (Wagner et al., 2005, Bales et al., 2011, Shin et al., 2011) which could exacerbate any additional damage to the dopaminergic system. Furthermore, in a recent experimental dual injury model it has been shown that TBI can potentiate deficits that occur following a subsequent toxin exposure and lead to changes that reflect what is observed with PD (Hutson et al., 2011). Given the changes that occur in the dopaminergic system following TBI, and that TBI can result in a higher susceptibility to toxin exposure, there is further rationale suggesting that PD may result from a multifactorial cause.
Mitochondrial dysfunction is a major pathological mechanism involved in TBI (Sullivan et al., 1998, Sullivan et al., 1999, Singh et al., 2006) as well as a common factor of many effects of toxins linked to PD (Nakamura et al., 2000, Sherer et al., 2003, Gash et al., 2008, Liu et al., 2010). It has been previously shown that either exposure to TCE (Gash et al., 2008, Liu et al., 2010) or its metabolite TaClo (Bringmann et al., 1995a, Janetzky et al., 1995) are capable of producing significant impairment in mitochondrial bioenergetic function. In order to understand if exposure to TCE is capable of leading to greater mitochondrial dysfunction following TBI, animals were exposed to TCE for two weeks and then subjected to a moderate controlled cortical impact (CCI) injury. Tissue was isolated from both the striatum and substantia nigra six hours after the brain injury since mitochondrial dysfunction has been shown to peak early after TBI (Singh et al., 2006). Samples from the substantia nigra revealed no significant impairments in mitochondrial function at six hours after the injury (Figure 1A). In contrast to the lack of an effect in the substantia nigra at six hours post-TBI, the striatum exhibited significant mitochondrial dysfunction. In these studies a significant reduction in mitochondrial function was produced as a result of exposure to TCE (Figure 1B), which made it difficult to observe a dual injury effect since it appears that mitochondrial bioenergetic function has potentially reached a floor effect of impairment. This bottoming out effect likely occurs since any further decrease in bioenergetic function would most likely lead to cell death and the mitochondria would no longer survive for analysis. Exposure to TCE resulted in approximately a 75% reduction in both basal and maximal rates of Complex I dependent mitochondrial bioenergetic function. A trend towards a reduction in mitochondrial function was observed in the striatum following exposure to TBI alone; however, this effect did not reach significance. As predicted for the other endpoints, had the TBI occurred directly over the striatum it is hypothesized that this decrease would become more pronounced.
Given the large reduction in mitochondrial function in the striatum following the 2 week exposure to TCE, it is difficult to determine whether or not the addition of a TBI is capable of making this impairment worse. To determine if TBI is capable of making the mitochondrial impairment worse after TCE exposure, further experiments were conducted using a shorter 1 week exposure of TCE. Following exposure to TCE for 1 week there was not a decrease in Complex I driven mitochondrial function in the striatum. As observed in the previous mitochondrial experiments, exposure to the TBI alone resulted in a non-significant trend towards mitochondrial impairment of approximately 30%. When the 1 week exposure to TCE was combined with the TBI, a significant reduction of approximately 50% was observed in the striatum six hours after the injury (Figure 2). The experiments utilizing the 1 week exposure of TCE show that the two insults are capable of interacting at the mitochondrial level to produce a greater amount of dysfunction than either insult alone.
The major pathological symptoms of PD are progressive impairment in motor control and loss of dopamine neurons in the substantia nigra. To investigate the potential for a dual injury model to recapitulate the motor impairments and cell loss observed in PD, both rotarod and cylinder testing were utilized to assess motor function, and tyrosine hydroxylase (TH) staining was utilized to analyze both dopamine neurons in the substantia nigra and dopamine fibers in the striatum. For these studies two different severities for the TBI were utilized, since it has been shown in humans that the severity of the injury significantly affects the link between TBI and PD (Bower et al., 2003). Following a 2 week exposure to TCE and a moderate controlled cortical impact TBI, impairments were observed both with the rotarod (Figure 3A) and cylinder test (Figure 3B), indicating not only that the dual injury paradigm in these experiments is required for motor impairment but also that a moderate and not a mild TBI is required for the presentation of motor impairment. The lack of behavioral impairments with the individual insults during the time frames utilized in these experiments resembles what we have observed previously. Though it has been shown in other models of traumatic brain injury that motor impairment can occur within the time frames we utilized (Hallam et al., 2004), multiple experiments in our lab have not shown deficits on the rotarod or cylinder test utilizing the experimental TBI parameters in these studies (unpublished data). Additionally, even though previous work utilizing TCE exposure alone has shown motor impairment on the rotarod (Liu et al., 2010), these deficits did not occur until after 5 weeks of TCE exposure, which is both a larger amount of toxin exposure and a time point that extends beyond the length of the present studies. Given our previous experience with both TCE and TBI, the lack of behavioral deficits with the single insults matches our previous observations.
To determine whether a loss of dopamine neurons in the substantia nigra was linked to the dual injury, TH-positive neurons were quantified. It was observed that in contrast to the behavioral data indicating that TCE exposure and a mild TBI did not result in dysfunction, exposure to TCE and either a mild or a moderate TBI is sufficient to produce a loss of TH-positive neurons (Figure 4A). Additionally, TH-positive cell loss was shown to be the result of a loss of the TH protein and not cell death, since quantification of Nissl cells did not result in any group differences (Figure 4B).Though the behavioral analysis and TH-positive cell counts did reach statistical significance in these studies, the deficits were not as substantial as we hypothesized they would be. The results indicate that the individual insults are insufficient to produce any impairment independently, which is desirable in this model, since the major goal was to show a dual injury effect. Different injury parameters are likely to have yielded us a more pronounced dual injury model. Specifically with regards to the TBI, had the injury been located directly over the striatum, or been more severe, we may have been able to observe more profound alterations in our endpoints.
Since a moderate TBI was required for motor impairment to occur, further experiments were conducted utilizing this level of injury. Given the loss of TH-positive neurons, the area of TH-positive fibers in the striatum was determined. As was expected for only a 13-17% loss of cells in the substantia nigra, no measurable decrease in TH-fiber density was observed in the striatum (Figure 5A). Since the levels of the dopamine transporter have been shown to be reduced in a similar model of TBI that utilizes a severe injury (Wagner et al., 2005), striatal tissue was analyzed both for levels of the dopamine transporter (DAT) and the type-2 dopamine receptor (D2R). Following either single insult or the dual injury, no reductions in the levels of DAT or D2R were observed one month following the injury (Figure 5C,D). The lack of a reduction in the DAT is likely the result of a more central and less severe TBI than previous reports (Wagner et al., 2005). Additionally, the current studies looked at changes in the non-glycosylated form of the dopamine transporter. Given that the glycosylation status of the dopamine transporter can affect dopaminergic pathology (Afonso-Oramas et al., 2009), further investigation looking at changes in the glycosylated form of the dopamine transporter will help in understanding the effects of TCE and TBI. Further tissue analysis was conducted looking at cortical tissue loss following the dual injury paradigm. A 30% loss of cortical tissue was observed following the moderate TBI, and exposure to TCE did not increase this loss (Figure 6A). Since there was not an increase in cortical tissue loss, the data suggests that the main TCE effects are not in the cortex, which supports previous findings showing that TCE significantly affects the basal ganglia (Gash et al., 2008, Liu et al., 2010). The lack of observable changes in the levels of DAT and D2R further indicate that at one month following the dual injury, this paradigm produces a very mild dual injury model.
An additional aspect of a dual injury paradigm whichthat is not tested with the current model, but likely has a significant role, is the impact that the order and timing have on outcome. The present studies focused on the effect of exposure to TCE prior to the traumatic brain injury and further experimentation is needed to understand what affect changing the order of insult would have on our endpoints. Evidence from human studies has shown that chronic exposure to TCE over many years can lead to neurological deficits (Gash et al., 2008). Our current studies were based on the hypothesis that workplace toxin exposure would be ongoing and precede exposure to TBI. However, if the toxin exposure was the result of an environmental contamination, which has been shown to occur in humans (Kilburn, 2002), then it could be equally likely that the TCE exposure could occur before or after the TBI since the toxin exposure itself could be assumed to be continuous. Furthermore, it has been shown in a similar dual injury model, which utilizes the pesticide paraquat, that a TBI can potentiate the effects of a subsequent toxin exposure (Hutson et al., 2011),m and this finding may extend to other toxins such as TCE. Importantly, it has also been shown that the timing of the insults is important for the interactions to occur. If enough time was allowed to elapse between the TBI and the exposure to paraquat then the dual injury effect was lost (Hutson et al., 2011). The timing of the insults in our studies was constructed so that the TBI occurred the day after the last day of TCE exposure. This timing was chosen to try and avoid any loss in interaction which may occur if a delay is present between the insults. More research is needed in order to better understand how the timing and order will affect the interaction of TCE and TBI, and based upon the previous research (Hutson et al., 2011) it is likely that the significant changes in outcome will occur.
The requirement for a multifactorial injury to produce the pathology shown in this study is in line with what has been seen in humans. Many insults have been determined to be risk factors for the development of human PD, but it has been difficult to show whether these same risk factors are able to independently produce pathology. This dual injury model utilizes toxin and injury levels, which are individually insufficient to produce motor impairment or a loss of TH-positive neurons - hallmarks of human PD. However, the dual injury produces significant reductions in both outcomes, indicating that sub-acute exposure to multiple insults can interact to produce a significant injury and lead to disease development. The lack of an effect with any of the single insults supports our initial study design that a single injury would not produce significant deficits. However, utilizing insults that do not independently produce pathology makes it difficult to know what levels of the insults are ideal for this dual injury model. The results presented in these studies do suggest that the dual injury hypothesis is valid; however, the lack of overwhelming changes makes determining the role of these changes in PD development difficult to prove. The lack of an effect on striatal protein expression at 30 days post-TBI suggests that the injury parameters utilized in the present study are not sufficient to elicit permanent changes in the striatum. Given the changes in striatal proteins in human PD patients (Donnemiller et al., 2000), and that other animal models recapitulate some of these changes (Wagner et al., 2005), future attempts to combine a toxic insult with TBI should focus on injury parameters more likely to elicit these changes. The mitochondrial results following the 1 week exposure to TCE do show that these two insults can interact at the mitochondrial level to produce significant dysfunction in a pivotal target mechanism of disease states.
Conclusions
These studies show that TCE and TBI can interact in an in vivo dual injury model and produce functional and histological deficits linked to the development of PD. These studies further implicate mitochondria as a central component of TCE exposure, TBI, and PD, especially in the striatum. Given the lack of increased cortical tissue loss when TCE is administered prior to the TBI, and previous evidence that more chronic exposure to TCE can lead to deficits in the substantia nigra (Gash et al., 2008, Liu et al., 2010), it is likely that the basal ganglia, especially the striatum, are more susceptible to TCE exposure. The apparent susceptibility of the basal ganglia in these studies is not surprising since previous studies have shown that mitochondrial toxins do appear to predominately affect these brain regions (Koutouzis et al., 1994, Ferrante et al., 1997), even though mitochondrial toxins are still capable of affecting all brain regions (Sauerbeck et al., 2011). The behavioral results and the loss of TH-positive neurons in the substantia nigra show that even though either insult alone is insufficient to produce changes in these outcomes, the dual injury is necessary and sufficient to result in pathology. These studies provide further evidence that TBI can play a role in the development of PD and that a multifactorial injury paradigm can produce greater pathology. Given the deficits observed in these studies, future work to incorporate TBI in a dual injury PD model should attempt to more directly target the basal ganglia in order to have a greater ability to elicit deficits. The present findings support a role for a multifactorial mechanism in the development of PD and further research will help in elucidating the complex interactions of these insults in the brain.
Research Highlights.
TCE and TBI interact synergistically and lead to neurological deficits
The interaction of TCE and TBI leads to functional and histological pathology
The mitochondrion plays a pivotal role in the interaction of TCE and TBI
Pathology can result even when the individual insults are sub-pathological
Acknowledgements
The authors would like to thank Dr. Jignesh Pandya and Andrea Sebastian for excellent technical assistance during this project. The authors would also like to thank Dr. Don Gash for assistance throughout the experiments and review of the final manuscript. This research was supported by grants from the National Institutes of Health, U.S. Public Health Service grants R01 NS48191, R01 NS062993 (P.G.S.), P30 NS051220, T32 AG000242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew Sauerbeck, 741. S Limestone St., BBSRB, Room 436, Lexington, Ky 40536, Adsaue2@uky.edu.
Randy Hunter, 800 Rose Street, Medical Center, Room MN 208, Lexington KY 40536, Randy.Hunter@Covance.com.
Guoying Bing, 800 Rose Street, Medical Center, Room MN 208, Lexington KY 40536, guoying.bing@uky.edu.
Patrick G. Sullivan, BBSRB, Room 475, 741. S Limestone St., Lexington, Ky 40536, patsull@uky.edu.
References
- Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, Abreu P, Giraldez T, Castro-Hernandez J, Salas-Hernandez J, Lanciego JL, Rodriguez M, Gonzalez-Hernandez T. (Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson’s disease. Neurobiol Dis. 2009;36:494–508. doi: 10.1016/j.nbd.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Akundi RS, Macho A, Munoz E, Lieb K, Bringmann G, Clement HW, Hull M, Fiebich BL. (1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline-induced apoptosis in the human neuroblastoma cell line SK-N-SH. J Neurochem. 2004;91:263–273. doi: 10.1111/j.1471-4159.2004.02710.x. [DOI] [PubMed] [Google Scholar]
- Arundine M, Aarts M, Lau A, Tymianski M. (Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci. 2004;24:8106–8123. doi: 10.1523/JNEUROSCI.1362-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Chopra GK, Wrong A, Lei S, Aarts MM, MacDonald JF, Tymianski M. (Enhanced vulnerability to NMDA toxicity in sublethal traumatic neuronal injury in vitro. J Neurotrauma. 2003;20:1377–1395. doi: 10.1089/089771503322686166. [DOI] [PubMed] [Google Scholar]
- ATSDR Toxicological Profile for Trichloroethylene. U.S. Department of Health and Human Services. 1997 [Google Scholar]
- Bakke B, Stewart PA, Waters MA. (Uses of and exposure to trichloroethylene in U.S. industry: a systematic literature review. Journal of occupational and environmental hygiene. 2007;4:375–390. doi: 10.1080/15459620701301763. [DOI] [PubMed] [Google Scholar]
- Bales JW, Yan HQ, Ma X, Li Y, Samarasinghe R, Dixon CE. (The dopamine and cAMP regulated phosphoprotein, 32 kDa (DARPP-32) signaling pathway: a novel therapeutic target in traumatic brain injury. Experimental neurology. 2011;229:300–307. doi: 10.1016/j.expneurol.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. (High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature genetics. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. (Head trauma preceding PD: a case-control study. Neurology. 2003;60:1610–1615. doi: 10.1212/01.wnl.0000068008.78394.2c. [DOI] [PubMed] [Google Scholar]
- Bringmann G, God R, Feineis D, Janetzky B, Reichmann H. (TaClo as a neurotoxic lead: improved synthesis, stereochemical analysis, and inhibition of the mitochondrial respiratory chain. Journal of neural transmission. 1995a;46:245–254. [PubMed] [Google Scholar]
- Bringmann G, God R, Feineis D, Wesemann W, Riederer P, Rausch WD, Reichmann H, Sontag KH. (The TaClo concept: 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline (TaClo), a new toxin for dopaminergic neurons. Journal of neural transmission. 1995b;46:235–244. [PubMed] [Google Scholar]
- Brown RC, Lockwood AH, Sonawane BR. (Neurodegenerative diseases: an overview of environmental risk factors. Environmental health perspectives. 2005;113:1250–1256. doi: 10.1289/ehp.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doder M, Jahanshahi M, Turjanski N, Moseley IF, Lees AJ. (Parkinson’s syndrome after closed head injury: a single case report. J Neurol Neurosurg Psychiatry. 1999;66:380–385. doi: 10.1136/jnnp.66.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, Wenning GK. (Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECT study using 123I-beta-CIT and 123I-IBZM. Eur J Nucl Med. 2000;27:1410–1414. doi: 10.1007/s002590000308. [DOI] [PubMed] [Google Scholar]
- EOHS Encyclopedia of Occupational Health and Saftey . 4th Edition International Labour Office; [Google Scholar]
- Factor SA, Sanchez-Ramos J, Weiner WJ. (Trauma as an etiology of parkinsonism: a historical review of the concept. Mov Disord. 1988;3:30–36. doi: 10.1002/mds.870030105. [DOI] [PubMed] [Google Scholar]
- Factor SA, Weiner WJ. (Prior history of head trauma in Parkinson’s disease. Mov Disord. 1991;6:225–229. doi: 10.1002/mds.870060306. [DOI] [PubMed] [Google Scholar]
- Fei Q, Ethell DW. (Maneb potentiates paraquat neurotoxicity by inducing key Bcl-2 family members. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05293.x. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Schulz JB, Kowall NW, Beal MF. (Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain research. 1997;753:157–162. doi: 10.1016/s0006-8993(97)00008-5. [DOI] [PubMed] [Google Scholar]
- Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS. (Trichloroethylene: Parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- Hallam TM, Floyd CL, Folkerts MM, Lee LL, Gong QZ, Lyeth BG, Muizelaar JP, Berman RF. (Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. Journal of neurotrauma. 2004;21:521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- Hutson CB, Lazo CR, Mortazavi F, Giza CC, Hovda D, Chesselet MF. (Traumatic brain injury in adult rats causes progressive nigrostriatal dopaminergic cell loss and enhanced vulnerability to the pesticide paraquat. Journal of neurotrauma. 2011 doi: 10.1089/neu.2010.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzky B, God R, Bringmann G, Reichmann H. (1-Trichloromethyl-1,2,3,4-tetrahydro-beta-carboline, a new inhibitor of complex I. Journal of neural transmission. 1995;46:265–273. [PubMed] [Google Scholar]
- Kilburn KH. (Is neurotoxicity associated with environmental trichloroethylene (TCE)? Arch Environ Health. 2002;57:113–120. doi: 10.1080/00039890209602925. [DOI] [PubMed] [Google Scholar]
- Kochen W, Kohlmuller D, De Biasi P, Ramsay R. (The endogeneous formation of highly chlorinated tetrahydro-beta-carbolines as a possible causative mechanism in idiopathic Parkinson’s disease. Advances in experimental medicine and biology. 2003;527:253–263. doi: 10.1007/978-1-4615-0135-0_29. [DOI] [PubMed] [Google Scholar]
- Koutouzis TK, Borlongan CV, Scorcia T, Creese I, Cahill DW, Freeman TB, Sanberg PR. (Systemic 3-nitropropionic acid: long-term effects on locomotor behavior. Brain research. 1994;646:242–246. doi: 10.1016/0006-8993(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. (Early environmental origins of neurodegenerative disease in later life. Environmental health perspectives. 2005;113:1230–1233. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. (Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard PA., Jr. (Parkinson’s disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N Engl J Med. 1983;309:310. doi: 10.1056/nejm198308043090511. [DOI] [PubMed] [Google Scholar]
- Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. (Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM. (Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006;199:499–512. doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. (Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G. (Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem. 2010;112:773–783. doi: 10.1111/j.1471-4159.2009.06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hunter R, Nguyen XV, Kim HC, Bing G. (Microsomal epoxide hydrolase deletion enhances tyrosine hydroxylase phosphorylation in mice after MPTP treatment. J Neurosci Res. 2008;86:2792–2801. doi: 10.1002/jnr.21725. [DOI] [PubMed] [Google Scholar]
- Mouton PR. Principles and practices of unbiased stereology. The Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- Nakamura K, Bindokas VP, Marks JD, Wright DA, Frim DM, Miller RJ, Kang UJ. (The selective toxicity of 1-methyl-4-phenylpyridinium to dopaminergic neurons: the role of mitochondrial complex I and reactive oxygen species revisited. Mol Pharmacol. 2000;58:271–278. doi: 10.1124/mol.58.2.271. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W. (Cell death of dopamine neurons in aging and Parkinson’s disease. Mech Ageing Dev. 1999;111:175–188. doi: 10.1016/s0047-6374(99)00064-0. [DOI] [PubMed] [Google Scholar]
- Nayernouri T. (Posttraumatic parkinsonism. Surgical neurology. 1985;24:263–264. doi: 10.1016/0090-3019(85)90035-7. [DOI] [PubMed] [Google Scholar]
- Nilsson GE. (Surviving anoxia with the brain turned on. News Physiol Sci. 2001;16:217–221. doi: 10.1152/physiologyonline.2001.16.5.217. [DOI] [PubMed] [Google Scholar]
- Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. (Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat. 2010 doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, editor. The rat nervous system. Elsevier Academic Press; Sydney: 2004. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sydney: 1998. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Salach JI, Singer TP. (Uptake of the neurotoxin 1-methyl-4-phenylpyridine (MPP+) by mitochondria and its relation to the inhibition of the mitochondrial oxidation of NAD+-linked substrates by MPP+ Biochemical and biophysical research communications. 1986;134:743–748. doi: 10.1016/s0006-291x(86)80483-1. [DOI] [PubMed] [Google Scholar]
- Riederer P, Foley P, Bringmann G, Feineis D, Bruckner R, Gerlach M. (Biochemical and pharmacological characterization of 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline: a biologically relevant neurotoxin? Eur J Pharmacol. 2002;442:1–16. doi: 10.1016/s0014-2999(02)01308-0. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Pandya J, Singh I, Bittman K, Readnower R, Bing G, Sullivan P. (Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. J Neurosci Methods. 2011 doi: 10.1016/j.jneumeth.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. (Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. (Parkinson’s disease: a test of the multifactorial etiologic hypothesis. Neurology. 1993;43:1173–1180. doi: 10.1212/wnl.43.6.1173. [DOI] [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Testa CM, Seo BB, Richardson JR, Kim JH, Miller GW, Yagi T, Matsuno-Yagi A, Greenamyre JT. (Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SS, Bray ER, Zhang CQ, Dixon CE. (Traumatic brain injury reduces striatal tyrosine hydroxylase activity and potassium-evoked dopamine release in rats. Brain Res. 2011;1369:208–215. doi: 10.1016/j.brainres.2010.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. (Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Stern MB. (Head trauma as a risk factor for Parkinson’s disease. Mov Disord. 1991;6:95–97. doi: 10.1002/mds.870060202. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Dube C, Dorenbos K, Steward O, Baram TZ. (Mitochondrial uncoupling protein-2 protects the immature brain from excitotoxic neuronal death. Ann Neurol. 2003;53:711–717. doi: 10.1002/ana.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Bussen WL, Scheff SW. (Cytochrome c release and caspase activation after traumatic brain injury. Brain research. 2002;949:88–96. doi: 10.1016/s0006-8993(02)02968-2. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Keller JN, Mattson MP, Scheff SW. (Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J Neurotrauma. 1998;15:789–798. doi: 10.1089/neu.1998.15.789. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson M, Scheff SW. (Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp Neurol. 2000;161:631–637. doi: 10.1006/exnr.1999.7282. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, Scheff SW. (Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Saint-Hilaire MH, Cupples LA, Thomas CA, Burchard AE, Feldman RG, Myers RH. (Environmental, medical, and family history risk factors for Parkinson’s disease: a New England-based case control study. American journal of medical genetics. 1999;88:742–749. [PubMed] [Google Scholar]
- Thiruchelvam M, Brockel BJ, Richfield EK, Baggs RB, Cory-Slechta DA. (Potentiated and preferential effects of combined paraquat and maneb on nigrostriatal dopamine systems: environmental risk factors for Parkinson’s disease? Brain research. 2000a;873:225–234. doi: 10.1016/s0006-8993(00)02496-3. [DOI] [PubMed] [Google Scholar]
- Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA. (The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson’s disease. J Neurosci. 2000b;20:9207–9214. doi: 10.1523/JNEUROSCI.20-24-09207.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman BA, Wijn AM, Knoers N, Praamstra P, Horstink MW. (Genetic and environmental risk factors in Parkinson’s disease. Clin Neurol Neurosurg. 1998;100:15–26. doi: 10.1016/s0303-8467(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Vyas I, Heikkila RE, Nicklas WJ. (Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-phenylpyridinium. J Neurochem. 1986;46:1501–1507. doi: 10.1111/j.1471-4159.1986.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Sokoloski JE, Ren D, Chen X, Khan AS, Zafonte RD, Michael AC, Dixon CE. (Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. Journal of neurochemistry. 2005;95:457–465. doi: 10.1111/j.1471-4159.2005.03382.x. [DOI] [PubMed] [Google Scholar]