Abstract
Stroke and Alzheimer’s disease (AD) are major age-related neurodegenerative diseases that may worsen the prognosis of each other. Our study was designed to delineate the prostaglandin E2 EP1 receptor role in AD and in the setting of cerebral ischemia. Genetic deletion of the prostaglandin EP1 receptor significantly attenuated the more severe neuronal damage (38.5 ± 10.6%) and memory loss induced by ischemic insult that observed in AD transgenic mice (percentage of viable hippocampal CA1 neurons: 11.2 ± 2.9%) when compared to wildtype mice (45.1 ± 9.1%). In addition, we found that the amyloid plaques were reduced in EP1 deleted AD mice. Aβ-induced toxicity (18.0 ± 7.1%) and Ca2+ response (91.8 ± 12.9%) were also reduced in EP1−/− neurons compared to control neurons in in vitro. Hence, EP1 might mediate most of the toxicity associated with COX-2 and contribute substantially to the cell death pathways in AD and stroke. Exploring potential therapeutic agent targeting EP1 receptor could potentially benefit treatments for stroke and AD patients.
Keywords: Beta-amyloid, Cyclooxygenase, Neuroinflammation, Prostaglandin E2, Stroke
1. Introduction
Accumulating evidence linking stroke and Alzheimer’s disease (AD) indicates that each exacerbates the severity of the other (Jendroska et al., 1995; Koistinaho and Koistinaho, 2005). Because there are few effective treatments for AD, especially when stroke supervenes in these patients, finding a common pathway that exacerbates neuronal loss and brain damage might provide a potential therapeutic target. Over the years, prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2), the rate-limiting enzyme responsible for prostaglandin production, have become largely discussed targets for exploration in therapy for AD and stroke patients (Ahmad et al., 2008; Bazan et al., 2002; Santovito et al., 2009). Clinical use of non-steroidal anti-inflammatory drugs or selective COX inhibitors is associated with various concerning side effects (Donnelly and Hawkey, 1997; Ray et al., 2004); thus, studying the downstream pathway may provide a unique therapeutic target which would allowed to be more selective and at the same time not simply blocking synthesis of all prostaglandins. Previously, the EP1 receptor expression in CNS neurons has been documented in the literature; for example, signals for EP1 receptor mRNA have been exposed in C57BL/6 mouse brain e.g. www.brain-map.org, and the EP1 receptor has also be documented by various independent teams at the protein level in the CNS and on neuronal cells (Kawano et al., 2006; Carlson et al., 2009). Additionally, previous studies by others and by us have provided physiological evidence that deletion of EP1 receptor significantly attenuates focal ischemic and excitotoxic brain damages and that treatment with selective receptor ligands provided significant anatomical and functional biological outcomes (Ahmad et al., 2006; Kawano et al., 2006). Thus, our study focuses on delineating the role of PGE2 EP1 receptor in AD model in the setting of cerebral ischemia. Based on this team effort, we propose that the EP1 receptor may be critical in the inflammatory and cell death pathways that are both hallmarks in the neurodegenerative conditions of AD and stroke.
We used the APPswe/PS1ΔE9 double gene mutation (APP/PS1) AD mouse model and crossed them with EP1 gene knockout (EP1−/−) mice, and explored the potential EP1 receptor role in stroke and AD-like conditions. We compared β-amyloid (Aβ) plaques, Aβ40 and Aβ42 levels, and behavioral outcomes of ischemia in APP/PS1xEP1−/− and APP/PS1 mice in together with their none-AD control mice. Furthermore, to explore the potential mechanism of the neuroprotective effect of genetic knocking out the EP1 receptor, we investigated intracellular Ca2+ response in an Aβ-enriched environment in cultured neurons.
2. Materials and Methods
2.1. Animals
All animal protocols were approved by the Animal Care and Use Committee of Johns Hopkins University. Male APP/PS1 mice, APP/PS1xEP1−/− mice, and their age-matched WT littermates were obtained from Dr. David Borchelt.
2.2. Cell Cultures, Ca2+ Imaging, Aβ toxicity, and Cell Survival
Neuronal cell cultures were prepared with the protocol described previously (Shah et al., 2010). The [Ca2+]i was measured in neuronal cultures by using Fura-2 AM (Kim et al., 2007). After treatment with freshly prepared 2.5 µM Aβ42 for 16 h, neuronal cell survival was monitored.
2.3. Induction of Global Cerebral Ischemia, Assessment of Intracranial Vascular Structure, and Neurobehavioral Tests
Global ischemia and subsequent analysis of hippocampal damage were conducted as that previously published (Zhen and Dore, 2007). Every fifth section throughout the hippocampus was sampled, and viable hippocampal CA1 neurons were counted in three serial hematoxylin and eosin-stained slides of the dorsal hippocampus (1.73 ± 0.15 mm posterior of the bregma) in each animal. The GEMINI apparatus (San Diego Instruments, San Diego, CA) was used to assess learning and memory via the step-through passive avoidance task.
2.4. Statistical Analysis
Based on the existence of PcomA, zero to two hemispheres per mouse were included in the statistical analysis. Data are expressed as mean±S.E.M. Statistical analysis was performed by one-way ANOVA followed by Bonferroni post hoc test or Student’s t-test. Significance was set at P < 0.05.
2.5. Additional Methodology
Detailed methods for Oxygen-glucose deprivation (OGD), intracellular Ca2+ imaging, the MTT assay, global cerebral ischemia, assessment of hippocampal injury, neurobehavioral testing, and immunohistochemistry are described in the Supplementary Methods.
3. Results
First, we compared Aβ plaque number, Aβ concentration and neurobehavior functions between naïve APP/PS1 and APP/PS1xEP1−/− mice. Significantly lower Aβ burden in APP/PS1xEP1−/− mice was evidenced by fewer plaques in the hippocampal area and a lower concentration of soluble Aβ42 and Aβ40 (Fig 1A). The reduced plaque load and Aβ levels may explain the benefits seen in memory function in APP/PS1xEP1−/− mice (Fig. 1A). To ascertain whether the Aβ-mediated cell death mechanism is different in the absence of EP1, we exposed hippocampal slice cultures and neuronal cell cultures to Aβ42. The CA1 regions in slice cultures and the cultured neurons from EP1−/− mice each were significantly more resistant to Aβ42 toxicity than were the corresponding WT controls (Fig. 1B). We then performed Ca2+ imaging in neuronal cell cultures to explore the potential mechanism by which EP1 receptor inhibition improves neuronal survival during Aβ-induced toxicity. The [Ca2+]i and Ca2+ transients were increased after Aβ42 treatment in WT controls; this increase was prevented by pretreatment with an EP1 receptor antagonist (SC51089) and blocked in EP1−/− neurons (Fig. 1C).
Fig. 1.
Effect of EP1 receptor on Aβ burden, Aβ42-induced toxicity and Ca2+ response in neurons. (A) The upper panel, from left to right: Representative images show Aβ staining in brain sections from APP/PS1 and APP/PS1xEP1−/− mice; quantification of Aβ plaques in hippocampus (***P < 0.001); test latency in passive avoidance test (n = 12–19, *P < 0.05 vs. WT, #P < 0.05 vs. APP/PS1). The lower panel: soluble Aβ42 and Aβ40 concentrations by ELISA assay in APP/PS1xEP1−/− and APP/PS1 mice (*P < 0.05, ***P < 0.001 vs. APP/PS1 soluble). (B) Fluorescence images of dead cells in cultured hippocampal slices. The left bar graph represents CA1 neuronal damage measured by PI fluorescence intensity in hippocampal slices culture (n = 20 slices per group, **P < 0.01). The right bar graph represents the viability of neuronal cell cultures after exposure to Aβ42 (*P < 0.05). (C) [Ca2+]i changes after Aβ42 treatment in WT cells (left trace), WT cells pretreated with EP1 receptor antagonist [SC51089 (WT+SC), center trace], and cells from EP1−/− mice (right trace). The bar graph shows amplitudes of Aβ42-induced [Ca2+]i increases in each group (number of cells = 22–25, ***P < 0.001).
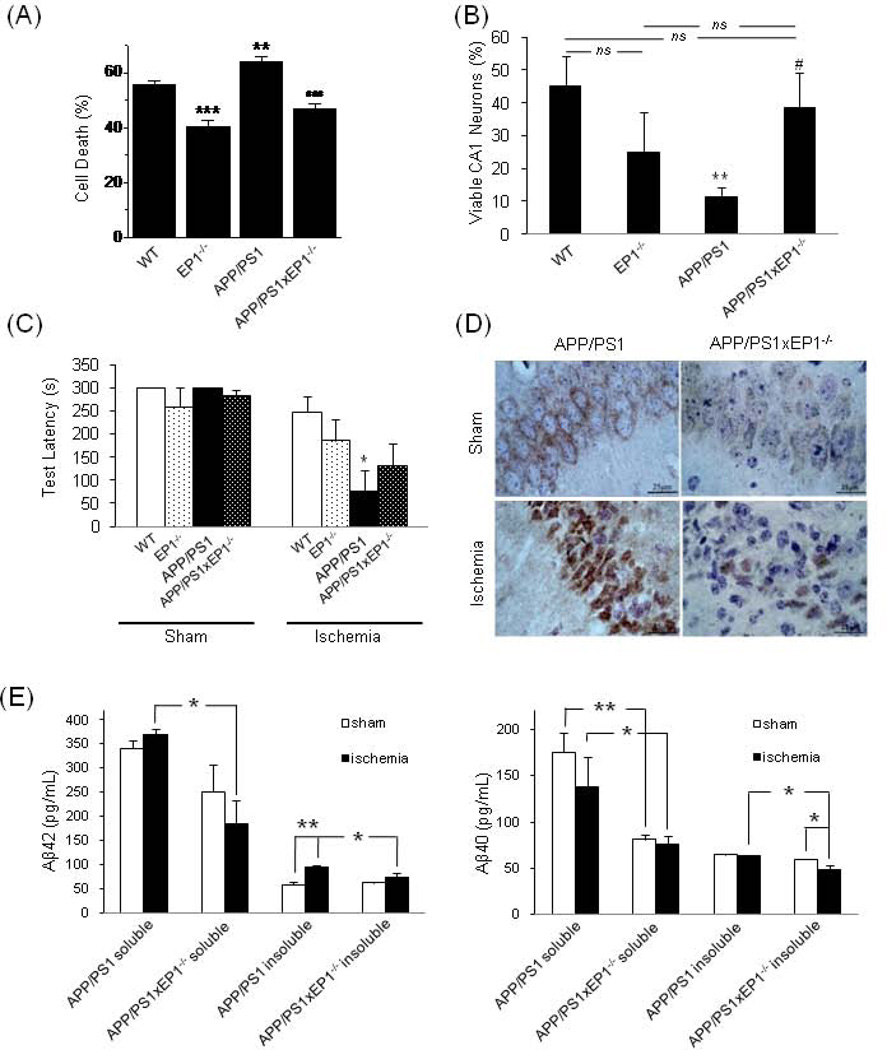
We then asked whether the reduced Aβ burden in APP/PS1xEP1−/− mice led to reduced injury development after cerebral ischemia. We approached this question in vitro by exposing cultured hippocampal slices from each of the mouse genotypes to OGD. Cell death was significantly decreased in EP1−/− tissue (P < 0.001) and increased in the APP/PS1 tissue compared to WT controls (P < 0.01) but was significantly attenuated in the APP/PS1xEP1−/− tissue compared to APP/PS1 tissue (P < 0.001; Fig. 2A).
Fig. 2.
The effect of EP1 receptor on neuronal damage and Aβ changes after ischemia in an AD model. (A) Quantification of CA1 neuronal damage measured after OGD (**P < 0.01, ***P < 0.001 compared with WT group, ###P < 0.001 compared with APP/PS1 group, n = 13–16). (B) Quantification of neuronal viability in brain sections from post-ischemic mice. The proportion of viable neurons was derived by dividing the number of viable neurons of the ischemic groups by the mean value of their gene-matched, sham-operated controls. WT sham, n = 20 hemispheres; WT ischemia, n = 17; EP1−/− sham, n = 12; EP1−/− ischemia, n = 12; APP/PS1 sham, n = 23; APP/PS1 ischemia, n = 14; APP/PS1xEP1−/− sham, n = 6; APP/PS1xEP1−/− ischemia, n = 11. **P < 0.01 compared with WT group; #P < 0.05 compared with APP/PS1 group; ns, not significant. (C) Memory latency in the passive avoidance task. WT sham, n = 9 mice; WT ischemia, n = 11; APP/PS1 sham, n = 5; APP/PS1 ischemia, n = 6; EP1−/− sham, n = 6; EP1−/− ischemia, n = 8; APP/PS1xEP1−/− sham, n = 3; APP/PS1xEP1−/− ischemia, n = 7. *P < 0.05 compared with WT ischemia group. (D) Representative images of hippocampal sections immunostained for Aβ around the neurons in the CA1 subfield. Aβ appears brown. (E) Aβ concentration in brain homogenate. The left graph shows concentration of Aβ42, and the right graph shows concentration of Aβ40. n = 3 mice per group, *P < 0.05, **P < 0.01.
To correlate our results with in vivo experiments, we assessed delayed hippocampal neuronal damage by subjecting mice to global forebrain ischemia. Similar to the in vitro findings, CA1 neuronal loss was much greater in the APP/PS1 than in the WT mice and was significantly attenuated in the APP/PS1xEP1−/− mice compared to the APP/PS1 mice (Fig. 2B). Hippocampal damage caused by ischemic insult can lead to impaired memory retention, reflected in shortened response latency in the passive avoidance task (Sakurai et al., 2008). Correlating with the anatomical differences, APP/PS1 mice had impaired memory retention after ischemic insult compared to WT mice subjected to ischemia and sham-operated APP/PS1 mice. There was no statistical difference between the APP/PS1xEP1−/− and APP/PS1 ischemia groups, but the APP/PS1xEP1−/− mice tended to show greater memory retention (Fig. 2C). We also questioned whether Aβ burden increases after ischemic insult in APP/PS1 mice and if so, whether this increase is attenuated in the absence of the EP1 receptor. We observed less intense Aβ immunostaining in the CA1 neurons of APP/PS1xEP1−/− mice after ischemia compared to that of APP/PS1 mice (Fig. 2D). the ELISA assay revealed that the insoluble Aβ42 concentration rose significantly after ischemia in APP/PS1 mice but remained unchanged in APP/PS1xEP1−/− mice. Both the soluble and insoluble Aβ concentrations were higher in APP/PS1 mice than in APP/PS1xEP1−/− mice post-ischemia (Fig. 2E). The increase of insoluble Aβ in APP/PS1 mice suggests a tendency for soluble Aβ to accumulate and potentially fibrillate after ischemia. Deletion of EP1 appeared to attenuate the increase of Aβ generation and accumulation.
4. Discussion
We observed that following transient global forebrain ischemia, APP/PS1 double mutant mice sustain more severe neuronal and functional damage than WT controls. This suggests that the Aβ burden is at least partially responsible for such outcomes. Knowing that brain levels of both COX-2 and PGE2 are increased in AD and in stroke, we questioned whether the deletion of the EP1 receptor—which has been proposed to be a key modulator of COX-2/PGE2-related neurotoxicity—would be sufficient to alleviate most of the negative outcomes. In our study, we provide the first evidence that genetic deletion of EP1 is sufficient to decrease basal and post-ischemic levels of Aβ in APP/PS1 mice. We discovered that hippocampal neuron cell death and neurobehavioral deficits after ischemic insult are attenuated in the APP/PS1−/−xEP1 as compared to APP/PS1 mice. Isolated neurons and brain slice cultures revealed that neurons derived from EP1−/− mice were more resistant to Aβ toxicity than those from WT mice. Neuronal Ca2+ signaling and Ca2+ homeostasis regulate multiple neuronal functions, including synaptic transmission, plasticity, and cell survival (Wojda et al., 2008). EP1 receptor inhibition prevented induction of Ca2+ signaling initiated by Aβ exposure by decreasing the frequency of Ca2+ transients and mitigating the increase in baseline Ca2+ levels. Prevention of Aβ-associated Ca2+ signaling in neurons by EP1 receptor inhibition is likely an additional pathway for the neuroprotective effects seen. Our study provides the rational to further explore potential therapeutic agents targeting EP1 receptor in stroke and AD-like neuropathologies.
Supplementary Material
Acknowledgements
This research was supported by grants from the National Institutes of Health, NS046400 and AG022971 (SD), and a fellowship from the Korea Research Foundation, KRF-2007-357-E00016 (YTK). The authors gratefully thank Dr. Barbara Crain for assistance with the immunological procedures, Claire Levine and all laboratory team members for their insightful comments and generous assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest statement: There are no actual or potential conflicts of interest.
References
- Ahmad AS, Saleem S, Ahmad M, Dore S. Prostaglandin EP1 receptor contributes to excitotoxicity and focal ischemic brain damage. Toxicol Sci. 2006;89:265–270. doi: 10.1093/toxsci/kfj022. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Yun YT, Ahmad M, Maruyama T, Dore S. Selective blockade of PGE2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation. Neurotox Res. 2008;14:343–351. doi: 10.1007/BF03033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Colangelo V, Lukiw WJ. Prostaglandins and other lipid mediators in Alzheimer's disease. Prostaglandins Other Lipid Mediat. 2002;68–69:197–210. doi: 10.1016/s0090-6980(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Carlson NG, Rojas MA, Black JD, Redd JW, Hille J, Hill KE, Rose JW. Microglial inhibition of neuroprotection by antagonists of the EP1 prostaglandin E2 receptor. Journal of Neuroinflammation 2009. 2009;6:5. doi: 10.1186/1742-2094-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly MT, Hawkey CJ. Review article: COX-II inhibitors--a new generation of safer NSAIDs? Aliment Pharmacol Ther. 1997;11:227–236. doi: 10.1046/j.1365-2036.1997.154330000.x. [DOI] [PubMed] [Google Scholar]
- Jendroska K, Poewe W, Daniel SE, Pluess J, Iwerssen-Schmidt H, Paulsen J, Barthel S, Schelosky L, Cervos-Navarro J, DeArmond SJ. Ischemic stress induces deposition of amyloid beta immunoreactivity in human brain. Acta Neuropathol. 1995;90:461–466. doi: 10.1007/BF00294806. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, Iadecola C. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med. 2006;12:225–229. doi: 10.1038/nm1362. [DOI] [PubMed] [Google Scholar]
- Kim YT, Namkung YL, Kwak J, Suh CK. Involvement of Na+-Ca2+ exchanger on metabotropic glutamate receptor 1-mediated [Ca2+]i transients in rat cerebellar Purkinje neurons. Neuroscience. 2007;146:170–177. doi: 10.1016/j.neuroscience.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Koistinaho J. Interactions between Alzheimer's disease and cerebral ischemia--focus on inflammation. Brain Res Brain Res Rev. 2005;48:240–250. doi: 10.1016/j.brainresrev.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Ray WA, Griffin MR, Stein CM. Cardiovascular toxicity of valdecoxib. N Engl J Med. 2004;351:2767. doi: 10.1056/NEJMc045711. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Sekiguchi M, Zushida K, Yamada K, Nagamine S, Kabuta T, Wada K. Reduction in memory in passive avoidance learning, exploratory behaviour and synaptic plasticity in mice with a spontaneous deletion in the ubiquitin C-terminal hydrolase L1 gene. Eur J Neurosci. 2008;27:691–701. doi: 10.1111/j.1460-9568.2008.06047.x. [DOI] [PubMed] [Google Scholar]
- Santovito D, Mezzetti A, Cipollone F. Cyclooxygenase and prostaglandin synthases: roles in plaque stability and instability in humans. Curr Opin Lipidol. 2009;20:402–408. doi: 10.1097/MOL.0b013e32832fa22c. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S. The flavanol (−)- epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60:575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- Zhen G, Dore S. Optimized protocol to reduce variable outcomes for the bilateral common carotid artery occlusion model in mice. J Neurosci Methods. 2007;166:73–80. doi: 10.1016/j.jneumeth.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.