Abstract
The role of hypothalamic paraventricular nucleus (PVN) in cardiovascular regulation is well established. In this study, it was hypothesized that the PVN may be one of the sites of cardiovascular actions of a new angiotensin, angiotensin-(1-12). Experiments were carried out in urethane-anaesthetized, artificially ventilated, adult male Wistar rats. PVN was identified by microinjections of N-methyl-D-aspartic acid (NMDA, 10 mM). Microinjections (50 nl) of angiotensin-(1-12) (1 mM) into the PVN elicited increases in mean arterial pressure (MAP), heart rate (HR) and renal nerve activity (RSNA). The tachycardic responses to angiotensin-(1-12) were attenuated by bilateral vagotomy. The cardiovascular responses elicited by angiotensin-(1-12) were attenuated by microinjections of an angiotensin II type 1 receptor (AT1R) antagonist (losartan), but not AT2R antagonist (PD123319), into the PVN. Combined inhibition of angiotensin converting enzyme (ACE) and chymase in the PVN abolished angiotensin-(1-12)-induced responses. Angiotensin-(1-12)-immunoreactive cells and fibres were more numerous in the middle and caudal regions of the PVN. Angiotensin-(1-12) was present in many, but not all, vasopressinergic PVN cells. This peptide was also present in some non-vasopressinergic PVN cells but not in oxytocin containing PVN cells. These results indicated that: 1) microinjections of angiotensin-(1-12) into the PVN elicited increases in MAP, HR, and RSNA, 2) HR responses were mediated via both sympathetic and vagus nerves, 3) both ACE and chymase were needed to convert angiotensin-(1-12) to angiotensin II in the PVN, and 4) AT1Rs, but not AT2Rs, in the PVN mediated angiotensin-(1-12)-induced responses. It was concluded that the cardiovascular actions of angiotensin-(1-12) in the PVN are mediated via its conversion to angiotensin II.
Keywords: blood pressure, captopril, chymostatin, heart rate, losartan, sympathetic nerve activity
Introduction
The hypothalamic paraventricular nucleus (PVN) consists of different populations of neurons which include magnocellular and parvocellular neuroendocrine neurons and parvocellular pre-autonomic neurons (reviews: Armstrong et al. 1980; Badoer, 2001; Swanson & Kuypers, 1980; Swanson & Sawchenko, 1983). Vasopressin and oxytocin synthesized in the neuroendocrine neurons located in the posterior magnocellular subdivision of the PVN are transported via their axons to the posterior lobe of the pituitary where they are released at their axon terminals and carried away into the systemic circulation for regulation of fluid balance and reproductive functions (Stern, 2004). Parvocellular neuroendocrine neurons located in the periventricular region of the PVN project to the median eminence and are involved in the release of anterior pituitary hormones. The parvocellular pre-autonomic neurons are located in the posterior, dorsal and ventromedial regions of the PVN and send long descending projections to the brain stem and spinal autonomic neurons. Activation of PVN neurons projecting to the rostral ventrolateral medulla (RVLM) and intermediolateral cell column of the spinal cord (IML) results in sympathoexcitatory responses. Projections of the PVN neurons to the nucleus tractus solitarius (NTS), dorsal motor nucleus of vagus (DMNV) and nucleus ambiguus (nAmb) (Portillo et al. 1998) are involved in the modulation of NTS baroreflex neurons and parasympathetic preganglionic neurons innervating the heart (Coote, 2004; Duan et al. 1999).
Although the role of the PVN in controlling cardiovascular functions is well established (reviews: Badoer, 2001; Brookes, 1997; Coote, 2004; Dampney et al. 2005; Stern, 2004), information regarding the role of different putative neurotransmitters in this brain area in modulating these functions is incomplete. Angiotensin II (ANG II) is one of the peptides implicated as a neurotransmitter or neuromodulator in the PVN. For example, sympatho-excitatory responses elicited by cardiac receptor stimulation or hyperosmolality are attenuated after the blockade of angiotensin type 1 receptors (AT1Rs) in the PVN (Chen & Toney, 2001; Zhu et al. 2002). All components of the renin-angiotensin system (RAS), including angiotensinogen, angiotensin converting enzyme (ACE), and AT1Rs have been identified in the PVN (Ferguson, 2009; Lenkei et al. 1997; McKinley et al. 2003)
Recently a new angiotensin, angiotensin-(1-12), (ANG-(1-12), has been identified (Nagata et al. 2006). Intravenous administration of ANG-(1-12) has been reported to elicit an immediate pressor response in the rat and this effect was blocked by prior intravenous administration of an ACE inhibitor or an AT1R antagonist (Nagata et al. 2006). Because ANG-(1-12) exerted its actions via rapid conversion to ANG II in the periphery, it was named as proangiotensin-12 (Nagata et al. 2006).
It is generally accepted that angiotensinogen is the substrate for generation of ANG II. Because renin is not involved in the formation of ANG-(1-12) (Ferrario et al. 2009; Trask et al. 2008), it has been suggested that ANG-(1-12) may serve as a renin-independent alternate substrate for the immediate generation of ANG II in several organs (Trask et al. 2008). This notion is supported by a recent report in which patients treated with maximal doses of a renin inhibitor (aliskiren) elicited further reduction in blood pressure when an AT1R antagonist was administered (Oparil et al. 2007). This observation is unexpected if only renin is involved in the production of angiotensin peptides.
In the brain tissue the concentration of ANG-(1–12) is about five times greater than that of ANG II (Nagata et al. 2006). Cells immunoreactive for ANG-(1-12) have been identified in some regions of the rat brain (e.g., the NTS) (Arnold et al. 2010). Unilateral or bilateral microinjections of ANG-(1-12) into the NTS of the rat have been reported to elicit depressor responses and attenuation of baroreflex sensitivity (Arnold et al. 2010; Chitravanshi & Sapru, 2011). Microinjections of ANG-(1-12) into the hypothalamic arcuate nucleus (ARCN) of the rat have been reported to elicit pressor responses (Arakawa et al. 2011). However, little information is available regarding other central nervous system (CNS) sites where ANG-(1–12) may elicit cardiovascular responses. As pointed out above, there is increasing evidence indicating that the PVN plays a significant role in controlling cardiovascular function. The focus of this investigation was to study the cardiovascular actions of ANG-(1-12) in the PVN with the long-term goal of expanding our knowledge regarding the central cardiovascular actions of this new angiotensin because very little information is available on this topic in the literature.
Methods
General procedures
Experiments were done in adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing 320–350 g (n = 100). All animals were housed under controlled conditions with a 12:12-hr light-dark cycle. Food and water were available to the animals ad libitum. The experiments were performed according to the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (8th Edition, 2010) and with the approval of the Institutional Animal Care and Use Committee of this university.
We have previously published the details of the procedures used in this study (Chitravanshi & Sapru, 2011). Briefly, the rats were anaesthetized with inhalation of isoflurane (2–3% in 100% oxygen), one of the veins was cannulated and urethane (1.2–1.4 g/kg) was injected intravenously in 8–9 aliquots at 2-min intervals. Isoflurane inhalation was terminated as soon as urethane administration was completed. Absence of a blood pressure (BP) response and/or withdrawal of the limb in response to pinching of a hind paw indicated that the rats were properly anaesthetized. Using this procedure, administration of supplemental doses of urethane was not usually necessary. Rectal temperature was maintained at 37.0 ± 0.5°C. Femoral arterial BP and heart rate (HR) were recorded by standard techniques. The trachea was cannulated and the rats were artificially ventilated; the tidal volume and rate of respiration were adjusted so that end-tidal CO2 remained at 3.5–4.5%. All of the tracings were stored on a computer hard drive using a data acquisition system obtained from Cambridge Electronic Design Ltd (CED), Cambridge, UK. At the end of the experiment, the rats were deeply anaesthetized with a high dose of urethane (2 g/kg, i.v.), a pneumothorax was produced by an incision in one of the intercostal muscles and cessation of heart beat, which was recorded on line, indicated that euthanasia was complete.
Bilateral vagotomy
Silk sutures were placed loosely around the vagus nerves low in the neck bilaterally. For bilateral vagotomy, the silk sutures were pulled gently, one at a time, and vagus nerves were sectioned. A period of 50–60 min was allowed for stabilization of baseline BP and HR after bilateral vagotomy.
Microinjections
All microinjections into the PVN were unilateral unless indicated otherwise. The rats were placed in a prone position in a stereotaxic instrument with bite bar set at 3.3 mm below the interaural line. The dorsal medulla was exposed and microinjections were made using multi-barreled glass-micropipettes (tip size 20–40 μm). Each barrel was connected to a channel on a picospritzer. Three barrels contained N-methyl-D-aspartic acid (NMDA), artificial cerebrospinal fluid (aCSF), and ANG-(1-12). The remaining barrels contained either an AT1R and AT2R antagonist, or an ACE or chymase inhibitor. The PVN sites eliciting pressor and tachycardic responses were identified by microinjections of NMDA (10 mM). The volume of all microinjections into the PVN was 50 nl; the selection of this volume was based on our previous studies (Kawabe et al. 2008). The volumes were pressure ejected and visually confirmed by the displacement of fluid meniscus in the barrel containing the solution using a modified binocular horizontal microscope with a graduated reticule in one eye-piece. The duration of microinjection was 5–10 sec. Microinjections of aCSF (50 nl, pH 7.4) were used as controls.
Renal nerve recording
The renal nerve (RN) was identified retroperitoneally, one of the branches was sectioned distally, a few mm of the central end of the nerve were desheathed and whole nerve activity was amplified (X20,000-30,000), filtered (100–5000 Hz), digitized and stored on a computer hard drive. The digitized signals were full wave rectified and integrated using Spike2 software (CED). The baroreflex sensitivity of the renal sympathetic nerve activity (RSNA) was indicated by its inhibition in response to the pressor responses elicited by bolus injections of phenylephrine (PE; 10 μg/kg. i.v.). At the end of the experiment, the nerve was sectioned centrally and the remaining activity was considered to be the noise level which was subtracted from the whole RSNA.
Immunohistochemistry
Immunohistochemical procedures were used to identify the PVN cells containing ANG-(1-12), ANG-(1-12) colocalized with vasopressin, and ANG-(1-12) colocalized with oxytocin. In each procedure, the rats were deeply anaesthetized with urethane (1.5 g/kg, i.v.; administered in divided doses), perfused transcardially and fixed with 4% paraformaldehyde solution, the brains were removed and fixed in 4% paraformaldehyde for 36 hrs. On completion of the fixation procedure, serial sections of the hypothalamic area were cut (40 μm) in a vibratome (1000 Plus Sectioning System, The Vibratome Company, St. Louis, MO, USA), and placed in 24-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA). The sections were rinsed (rinsing of sections was always done 3 times, 10 min each) with 0.1 M phosphate buffered saline (PBS), blocked for 1 hour at room temperature with normal donkey serum containing 0.3% Triton X-100 in PBS. For ANG-(1-12)-immunostaining, the sections were placed on a shaker and incubated at 4°C for 24 hours with 3% normal donkey serum in 0.3% Triton X-100 in PBS and diluted (1: 200) primary antibody (rabbit anti-proangiotensin-12, Phoenix Pharmaceuticals Inc., Burlingame, CA, USA). Next, the sections were incubated at 4°C for 24 hrs with 2% normal donkey serum in 0.3% triton in PBS and diluted (1:200) secondary antibody (donkey anti-rabbit, conjugated with Dylight 488 (Amax = 493 nm, Emax = 518 nm; green emission) (Jackson Immunoresearch Laboratories, PA, USA). For double labeling of ANG-(1-12) and vasopressin, the sections were incubated at 4°C for 24 hours with a mixture of diluted (1: 200, each) primary antibodies (rabbit anti-proangiotensin-12, Phoenix Pharmaceuticals Inc., Burlingame, CA, USA and guinea pig anti-(Arg8)-vasopressin, Peninsula Laboratories, San Carlos, CA, USA). The sections were then incubated at 4°C for 24 hrs with a mixture of diluted (1:200, each) donkey anti-rabbit, conjugated with Dylight 594 (Amax = 593 nm, Emax = 618 nm; red emission) and goat anti-guinea pig, conjugated with Dylight 488 (Amax = 493 nm, Emax = 518 nm; green emission) (both from secondary antibodies Jackson Immunoresearch Laboratories, PA, USA). Double labeling procedure for ANG-(1-12) and oxytocin was similar except that mouse monoclonal anti-oxytocin (1:5000, Chemicon International, Temecula, CA, USA) was used as the primary antibody and the secondary antibodies consisted of goat anti-rabbit conjugated with Dylight 594 and goat anti-mouse conjugated with Dylight 488 (1:200 each, Jackson Immunoresearch Laboratories). Controls for immunostaining procedures consisted of omission of the primary antibody used for ANG-(1-12) from the protocol (negative control) and pre-absorption of the primary antibody for ANG-(1-12) with ANG-(1-12) peptide. Cross reaction of the primary antibody used for ANG-(1-12) with ANG II was determined by pre-incubation of the primary antibody with ANG II peptide. In each series of immunohistochemistry experiments, after the completion of incubation with the primary and secondary antibodies, the sections were rinsed in PBS, mounted on subbed slides, covered with Vectashield medium (Vector Laboratories, Burlingame, CA, USA) and coverslipped. The images of the sections were captured, 2 μm apart, by laser scanning confocal microscopy (AIR confocal microscope, Nikon Instruments Inc., Melville, NY, U.S.A).
Histology
Microinjection sites in the PVN were marked by microinjections of diluted (1: 42) green Lumafluor retrobeads (Lumafluor Inc, Durham, NC, USA). The animals were perfused and fixed with 4% paraformaldehyde, serial sections of the diencephalon were cut (30–40 μm), mounted on slides, covered with Vectashield mounting medium and coverslipped. The microinjection sites were identified, using a fluorescence microscope, photographed and compared with a standard atlas (Paxinos & Watson, 1986).
Drugs and chemicals
The following drugs and chemicals were used: Angiotensin-(1-12), captopril (ACE inhibitor) (Migdalof et al. 1984), chymostatin (chymase inhibitor) (He et al. 1999), isoflurane, losartan (selective AT1R antagonist), L-phenylephrine hydrochloride, [β-mercapto-β,β-cyclopentamethylene-propionyl1, o-me-Tyr2, Arg8]-vasopressin (V1a receptor antagonist), PD123319 (AT2R antagonist) (Blankley et al. 1991) and urethane. All of the solutions for the microinjections were freshly prepared in aCSF. The V1a receptor antagonist was dissolved in normal saline. Where applicable, the concentration of drugs refers to their salts. The sources of drugs were as follows: Angiotensin-(1-12) (American Peptide Company Inc., Sunnyvale, CA, USA), captopril, chymostatin, L-phenylephrine hydrochloride, V1a receptor antagonist and urethane (Sigma-Aldrich Chemicals, St. Louis, MO, USA), PD123319 (Tocris-Cookson Inc. Ellisville, MO, USA) and isoflurane (Baxter Pharmaceutical Products, Deerfield, IL, USA).
Statistical analyses
The mean and standard error of mean (SEM) were calculated for maximum changes in MAP, HR, and RSNA. In different groups of rats, comparisons of the decreases in MAP and HR in the concentration-response studies, vagotomy, microinjections of NMDA and ANG-(1-12) and blockade of MAP and HR responses by AT1R and AT2R antagonists, into the PVN were made by using a one-way analysis of variance (ANOVA) followed by Tukey-Kramer’s multiple comparison tests. For the analysis of RSNA, control value represented the average amplitude of the nerve activity during 30 sec period before the microinjections of ANG-(1-12) or NMDA into the PVN. The maximum inhibition in RSNA amplitude elicited by microinjections of NMDA or ANG-(1-12) into the PVN was expressed as percent decrease from the basal value of the RSNA amplitude. The mean values of the integrated nerve signals were compared using ANOVA followed by Tukey-Kramer’s multiple comparison tests. In all cases, the differences were considered significant at P < 0.05.
Results
Baseline values for MAP and HR in urethane-anaesthetized rats were 98.2 ± 2.1 mmHg and 386 ± 8.6 bpm, respectively (n = 100).
Concentration-response of microinjections of ANG-(1-12)
In this and other series of experiments, the PVN was always identified by microinjections of NMDA (10 mM), which elicited increases in MAP (22.8 ± 2.6 mmHg) and HR (61.3 ± 6.7 bpm). The interval between the microinjections of NMDA and other agents was at least 20 min. The increases in MAP and HR elicited by microinjections (50 nl) of different concentrations of ANG-(1-12) into the PVN are shown in Fig. 1A (n = 12). In each rat, only two concentrations of ANG-(1-12) were microinjected and different concentrations of ANG-(1-12) were microinjected in a random fashion. The interval between the microinjections of ANG-(1-12) was at least 60 min. Microinjections of 0.25, 0.50, 1.00 and 2.00 mM concentrations of ANG-(1-12) elicited 5.5 ± 0.8, 6.8 ± 0.5, 14.8 ± 1.4 and 13.2 ± 3.6 mmHg increases in MAP, respectively (Fig. 1A, top left). The same concentrations of ANG-(1-12) elicited 8.5 ± 1.4, 8.7 ± 1.2, 21.7 ± 3.2 and 16.2 ± 3.9 bpm increases in HR, respectively (Fig. 1A, top right). Cardiovascular responses elicited by microinjections of 1 mM concentration of ANG-(1-12) were greater than those elicited by 0.25 and 0.5 mM concentrations (P < 0.05). The differences in pressor and tachycardic responses elicited by 1 and 2 mM concentrations of ANG-(1-12) were not significantly different (P > 0.05). Therefore, 1 mM concentration of ANG-(1-12) was selected for other experiments. The onset and duration of cardiovascular responses to microinjections of ANG-(1-12) (1 mM) were 15–20 sec and 6–10 min, respectively. The peak effect was observed at 1–2 min. Microinjections of aCSF (50 nl) into the PVN elicited no significant changes (P > 0.05) in MAP or HR; MAP and HR values before and after microinjections of aCSF were 101.4 ± 7.4 and 97.6 ± 5.6 mmHg, respectively, and 411.8 ± 7.1 and 405.4 ± 8.4, bpm, respectively.
Fig. 1.
A: Concentration responses of ANG-(1-12) in PVN. Top left panel: MAP. Top right panel: HR. B: Effect of vagotomy. Bottom left panel: MAP. Bottom right panel: HR.
In order to compare cardiovascular responses elicited from different parts of the PVN, this nucleus was arbitrarily divided into rostral, middle and caudal regions. Cardiovascular responses elicited by ANG-(1-12) (1 mM) in these regions are shown in Table 1 (n = 9). Microinjections of ANG-(1-12) elicited greater cardiovascular responses in the middle and caudal regions of the PVN when compared to those elicited from the rostral region (Table 1).
Table 1.
Cardiovascular responses induced by microinjections of ANG-(1-12) (1 mM) in different regions of the PVN
| Regions | Coordinates (mm caudal to the bregma) | Increase in MAP (mmHg) | Increase in HR (bpm) |
|---|---|---|---|
| Rostral | 0.8–1.3 | 7.8 ± 0.5a | 11.2 ± 1.3b |
| Middle | 1.3–1.8 | 14.2 ± 0.9* | 22.6 ± 2† |
| Caudal | 1.8–2.3 | 14.4 ± 0.9* | 23.4 ± 2.6† |
There was no significant difference between the increases in MAP and HR elicited at middle and caudal regions.
Significantly greater compared to “a” (P < 0.05).
Significantly greater compared to “b” (P < 0.05).
Site-specificity of ANG-(1-12)-induced responses
In this group of rats (n = 5), microinjection of NMDA (10 mM) into a site adjacent to the PVN, anterior hypothalamic area (AHA; 1.3 – 1.6 mm caudal to bregma, 0.8 – 1 mm lateral to mid line and 8.2 – 8.6 deep from surface) elicited a decrease in MAP and HR. Microinjection of ANG-(1-12) (1 mM) at this site elicited no or small depressor and bradycardic responses which were not significant. Thus the increases in MAP and HR due to microinjection of NMDA and ANG-(1-12) into the PVN were site specific.
Reproducibility of ANG-(1-12)-induced responses
The increases in MAP in response to 3 consecutive microinjections of ANG-(1-12) (1 mM) at 40–60 min intervals were 13.6 ± 2.4, 13.3 ± 2.3, and 14.9 ± 2.1 mmHg, respectively and the increases in HR were 16.7 ± 2.2, 17.7 ± 2.4, and 19.4 ± 2.6 bpm, respectively (n = 5). A repeated measure ANOVA showed that these values were not statistically different (P > 0.05). Because no tachyphylaxis of responses was observed with repeated microinjections of ANG-(1-12) at 40–60 min intervals, the interval between different microinjections of ANG-(1-12) was at least 60 min in all experiments.
Effect of bilateral vagotomy on ANG-(1-12)-induced responses
Bilateral vagotomy did not alter increases in MAP elicited by unilateral microinjections of either NMDA (10 mM) or ANG-(1-12) (1 mM) into the PVN (n = 6). NMDA-induced increases in MAP before and after vagotomy were 20.2 ± 3.2 % and 16.3 ± 2.3 %, respectively (P > 0.05) (Fig. 1B, bottom left). Similarly, ANG-(1-12)-induced increases in MAP before and after vagotomy were 15.4 ± 2.3 % and 12.7 ± 1.5 %, respectively (P > 0.05) (Fig. 1B, bottom left). On the other hand, in the same group of rats bilateral vagotomy attenuated the increases in HR elicited by unilateral microinjections of NMDA (10 mM) as well as ANG-(1-12) (1 mM) into the PVN. NMDA-induced increases in HR before and after vagotomy were 7.5 ± 0.6 % and 1.8 ± 0.2 %, respectively (P < 0.05) (Fig. 1B, bottom right). Similarly, ANG-(1-12)-induced increases in HR before and after vagotomy were 6 ± 1.1 % and 0.7 ± 0.2 %, respectively (P < 0.05) (Fig. 1B, bottom right).
Effect of AT1R antagonist on ANG-(1-12)-induced responses
Microinjections of a smaller concentration of losartan (5 mM, n = 5) into the PVN did not significantly alter the increases in MAP and HR elicited by subsequent microinjections of ANG-(1-12) (1 mM) into the PVN. In these experiments, ANG-(1-12)-induced increases in MAP and HR before and after the microinjections of losartan were 16.4 ± 1.6 and 12.6 ± 1.4 mmHg, respectively (Fig. 2A) and 22.6 ± 2.9 and 15.6 ± 1.8 bpm, respectively (P > 0.05) (Fig. 2B). A higher concentration of losartan (10 mM; n = 5) significantly attenuated the increases in MAP and HR induced by ANG-(1-12) (1 mM) microinjections into the PVN. ANG-(1-12)-induced increases in MAP before and after the microinjection of losartan (10 mM) were 16.8 ± 1.4 and 2.2 ± 0.7 mmHg, respectively (P < 0.001) (Fig. 2A). ANG-(1-12)-induced increases in HR before and after the microinjection of losartan (10 mM) were 23.6 ± 1.2 and 4.4 ± 1 bpm, respectively (P < 0.001) (Fig. 2B). Therefore, 10 mM concentration of losartan was used for the blockade of AT1Rs in other experiments. Microinjections of aCSF (50 nl) did not alter the responses to microinjections of ANG-(1-12). Tracings showing the blockade of ANG-(1-12)-induced responses by losartan (10 mM) are presented in Figs. 2C–2F.
Fig. 2.
Effect of AT1R antagonist on ANG-(1-12)-induced responses. Group data showing the blockade of cardiovascular responses (top panels). A: MAP. B: HR. Tracings showing blockade of ANG-(1-12)-induced cardiovascular responses (bottom panels). C: The PVN site was identified by a microinjection of NMDA (10 mM); pressor and tachycardic responses were elicited. Twenty min later, microinjection of aCSF elicited no response (not shown). D: Five min later, microinjection of ANG-(1-12) (1 mM) at the same site elicited an increase in PAP, MAP and HR. E: Sixty min later, microinjection of losartan (10 mM) into the PVN elicited no responses. F: Two min later, microinjection of ANG-(1-12) (1 mM) failed to elicit a response. Five min later, microinjection of NMDA (10 mM) continued to elicit increases in PAP, MAP and HR (not shown). Top trace: pulsatile arterial pressure (PAP, mmHg), middle trace: mean arterial pressure (MAP, mmHg), bottom trace: HR (bpm)
Effect of AT2R antagonist on ANG-(1-12)-induced responses
Microinjections of a selective AT2R antagonist (PD123319) (50 mM; n = 5) into the PVN did not alter the cardiovascular responses elicited by microinjections of ANG-(1-12) (1 mM) at the same site. The increases in MAP induced by microinjections of ANG-(1-12) (1 mM) before and after the microinjection of PD123319 (50 mM) were 15.2 ± 0.9 and 14.6 ± 1.3 mmHg, respectively (P > 0.05). The increases in HR induced by the same concentration of ANG-(1-12) before and after the microinjection of the same concentration of PD123319 were 22.4 ± 2.8 and 21.6 ± 2 bpm, respectively (P > 0.05). This dose of PD123319 was selected from our earlier report (Arakawa et al. 2011). Microinjections of aCSF (50 nl) into the PVN did not alter the ANG-(1-12)-induced responses.
Effect of captopril on ANG-(1-12)-induced responses
Microinjections of captopril (100 or 200 mM; n = 5 each) into the PVN significantly reduced the increases in MAP and HR induced by microinjections of ANG-(1-12) (1 mM) at the same site. Group data for captopril-induced attenuation of cardiovascular responses elicited by microinjections of ANG-(1-12) into the PVN are shown in Table 1. The doses of captopril used for ACE inhibition was comparable to that used by others (Hocht et al, 2008). ANG-(1-12)-induced increases in MAP and HR did not completely recover to the initial values within 60 min of the microinjection of captopril (100 or 200 mM) into the PVN. Captopril did not alter the cardiovascular responses to microinjections of NMDA into the PVN. Unilateral microinjections of captopril alone (100 or 200 mM) into the PVN did not elicit any cardiovascular responses. Attenuation of pressor and tachycardic responses induced by ANG-(1-12) by 100 and 200 mM of captopril were not significantly different. Therefore, in all subsequent experiments in which the inhibition of ACE was studied, the smaller concentration of captopril (100 mM) was used. Microinjections of aCSF (50 nl) into the PVN did not alter the ANG-(1-12)-induced cardiovascular responses.
Effect of chymostatin on ANG-(1-12)-induced responses
Microinjections of chymostatin (5 or 10 mM; n = 5 each) into the PVN significantly reduced the increases in MAP and HR induced by microinjections of ANG-(1-12) (1 mM) at the same site. Group data showing the chymostatin-induced attenuation of cardiovascular responses elicited by microinjections of ANG-(1-12) into the PVN are shown in Table 1. The doses of chymostatin used for chymase inhibition were comparable to those used by others (Wei et al. 1999). ANG-(1-12)-induced increases in MAP and HR did not completely recover to the initial values within 60 min of the microinjection of chymostatin (5 or 10 mM) into the PVN. Chymostatin did not alter the cardiovascular responses to microinjections of NMDA into the PVN. Unilateral microinjections of chymostatin alone (5 or 10 mM) into the PVN did not elicit any cardiovascular responses. Attenuation of pressor and tachycardic responses induced by ANG-(1-12) by 10 mM of chymostatin was significantly greater than that induced by 5 mM concentration. Therefore, 10 mM concentration of chymostatin was selected for chymase inhibition in subsequent experiments. Microinjections of aCSF (50 nl) into the PVN did not alter ANG-(1-12)-induced cardiovascular responses.
Effect of combined microinjections of captopril and chymostatin on ANG-(1-12)-induced responses
Group data showing the attenuation of cardiovascular responses elicited by microinjections of ANG-(1-12) into the PVN by combined microinjections of captopril and chymostatin are shown in Table 3 (n = 5). In these experiments, captopril and chymostatin were microinjected into the PVN sequentially within 2 min. Combined microinjections of captopril (100 mM) and chymostatin (10 mM) into the PVN elicited significant attenuation of ANG-(1-12)-induced increases in MAP and HR (Table 3). Attenuation of ANG-(1-12)-induced cardiovascular responses by combined microinjections of captopril (100 mM) and chymostatin (10 mM) into the PVN was significantly greater than the attenuation elicited by the same concentrations of captopril and chymostatin alone (Table 1). A typical tracing showing the attenuation of ANG-(1-12)-induced responses by combined microinjections of captopril and chymostatin is presented in Fig. 3. Combined unilateral microinjections of captopril and chymostatin into the PVN did not alter baseline MAP and HR. Microinjections of aCSF (50 nl) into the PVN did not alter the ANG-(1-12)-induced cardiovascular responses.
Table 3.
Effect of combined microinjections of ACE and chymase inhibitors into the PVN on the responses elicited by microinjections of ANG-(1-12) and ANG II at the same site (n = 5 in each group)
| Agonist | Increases in MAP (mmHg) | Increases in HR (bpm) | ||
|---|---|---|---|---|
| Before* | After* | Before* | After* | |
| ANG-(1-12) (1 mM) | 17.6 ± 1.4 | 2.4 ± 0.2*** | 22.4 ±1 | 1.4 ± 0.4*** |
| ANG II (1 mM) | 20.2 ± 3.8 | 19.8 ± 5.3 | 22.6 ± 3.6 | 27.4 ± 3.9 |
The responses to ANG-(1-12) and ANG II were monitored before and after combined microinjections of captopril (100 mM) and chymostatin (10 mM) into the PVN.
P < 0.001.
HR = heart rate; MAP = mean arterial pressure; PVN = hypothalamic paraventricular nucleus.
Fig. 3.
Tracing showing attenuation of ANG-(1-12)-induced responses following ACE and chymase inhibition. Top: pulsatile arterial pressure (PAP, mmHg), middle: mean arterial pressure (MAP, mmHg), bottom: heart rate (HR, bpm). A: Microinjection of NMDA (10 mM) into the PVN elicited increases in PAP, MAP and HR. Twenty min later, microinjection of aCSF at the same site elicited no responses (not shown). B: After 5 min, microinjection of ANG-(1-12) (1 mM) at the same site elicited an increase in PAP, MAP and HR. C: Sixty min later, combined microinjection of captopril (100 mM) and chymostatin (10 mM) at the same site elicited no responses. D: Two min later, microinjection of ANG-(1-12) (1 mM) failed to elicit a response. Five min later, microinjection of NMDA (10 mM) continued to elicit increases in PAP, MAP and HR (not shown). CAPTO: captopril; CHYMO: chymostatin.
Microinjections of ANG II (1 mM) into the PVN (n = 5) elicited increases in MAP and HR which were not significantly different from those elicited by the same concentration of ANG-(1-12) (Table 3). The onset (15–20 sec) and peak (1–2 min) of cardiovascular responses to microinjections of ANG-(1-12) were delayed compared to the onset (8–11 sec) and peak (30–40 sec) of cardiovascular responses to ANG II in the PVN. The duration of the cardiovascular effects of ANG-(1-12) (6–10 min) was comparable to that of ANG II (5–10 min). Unlike the effects observed on ANG-(1-12)-induced cardiovascular responses, combined microinjections of captopril and chymostatin did not alter the cardiovascular responses elicited by ANG II in the PVN (Table 3).
Effect of microinjections of ANG-(1-12) on RSNA
Fig. 4 shows a typical recording of the effect of microinjections of ANG-(1-12) into the PVN on efferent RSNA. A bolus injection of PE (10 μg/kg, i.v.) increased MAP (30 mmHg) which, in turn, elicited reflex decrease in HR (51 bpm) (Fig. 4A). When the RSNA recovered to baseline, microinjection of NMDA (10 mM) into the PVN elicited an increase in RSNA (Fig. 4B). After 20 min, microinjection of aCSF (50 nl) into the same PVN site elicited a small decrease in RSNA which was not significant (Fig. 4C). Two min later, microinjection of ANG-(1-12) (1 mM) into PVN increased efferent RSNA (Fig. 4D). After an interval of 60 min, microinjection of losartan (10 mM) at the same site elicited a small decrease in RSNA which was not significant (Fig. 4E). Two min later, response to microinjection of ANG-(1-12) (1 mM) at the same site was blocked (Fig. 4F). Two min later, NMDA (10 mM) was microinjected again into the PVN; the responses to NMDA were not altered by losartan (Fig. 4G). Group data (n = 5) for changes in RSNA are summarized in Fig. 5. All changes in RSNA refer to comparison with the basal nerve activity just before the intravenous injections or PVN microinjections. Intravenous bolus injection of PE (10 μg/kg) decreased RSNA (74.3 ± 1.9 %; P < 0.01) which lasted for 19.2 ± 2.1 sec (Fig. 5A). Microinjections NMDA (10 mM) into the PVN elicited an increase in the RSNA (66.4 ± 7 %, P < 0.001) (Fig. 5B). Microinjection of aCSF at the same site elicited a small decrease in RSNA (1.6 ± 0.5 %) which was not significant (P > 0.05) (Fig. 5C). Microinjection of ANG-(1-12) (1 mM) into the PVN elicited a significant increase in the RSNA (23.4 ± 4.5 %, P < 0.05) (Fig. 5D). The duration of RSNA increases elicited by NMDA and ANG-(1-12) were 9.5 ± 1 and 6.8 ± 0.7 min, respectively. Microinjection of losartan (10 mM) at the same site elicited a small decrease in RSNA (1.8 ± 0.4 %) which was not significant (P > 0.05) (Fig. 5E). However, ANG-(1-12)-induced increase in RSNA was significantly (P < 0.01) attenuated by losartan; Ang-(1-12)-induced increases in RSNA before and after losartan were 23.4 ± 4.5 % (Fig. 5D) and 3.3 ± 0.4 % (Fig. 5F), respectively. On the other hand, losartan did not alter NMDA-induced increase in RSNA; increases in RSNA elicited by NMDA microinjections into the PVN before and after losartan were 66.4 ± 7 % (Fig. 5B) and 67.4 ± 6.1 % (Fig. 5G), respectively (P > 0.05).
Fig. 4.
Effect of ANG-(1-12) on renal nerve activity. 1st trace: MAP (mmHg), 2nd trace: heart rate (HR, bpm), 3rd trace: integrated RSNA (∫RSNA, μV/1s), and 4th trace: raw RSNA (μV). A: Barosensitivity of RSNA was confirmed by reflex inhibition of this activity by pressor response induced by phenylephrine (PE, 10 μg/kg, i.v.). B: When the RSNA recovered to baseline level, microinjection of NMDA (10 mM) into the PVN increased MAP, HR and RSNA. C: Twenty min later, microinjection of aCSF at the same site elicited small decreases in RSNA which were not significant. D: After 2 min, microinjection of ANG-(1-12) (1 mM) at same site increased the MAP, HR and RSNA. E: Sixty min later, losartan (10 mM) at the same site elicited small decreases in RSNA which were not significant. F: Two min later, ANG-(1-12) failed to elicit changes in MAP, HR and RSNA. G: Two min later, NMDA elicited significant increases in MAP, HR and RSNA.
Fig. 5.
Group data showing effects of ANG-(1-12) on RSNA. A: Reflex inhibition of RSNA by phenylephrine (PE, 10 μg/kg, i.v.). B: Microinjection of NMDA (10 mM) increased the RSNA. C: Microinjection of aCSF elicited a small decrease in RSNA which was not significant. D: Microinjection of ANG-(1-12) (1 mM) increased the RSNA. E: Microinjection of losartan (10 mM) elicited small decrease in RSNA which was not significant. F: ANG-(1-12)-induced increase in RSNA was significantly attenuated. G: Blockade of AT1R did not alter NMDA-induced increase in RSNA. All microinjections were made in the PVN.
Role of vasopressin in ANG-(1-12) induced responses
In this group of rats (n = 5), the pressor and tachycardic responses elicited by microinjection of NMDA (10 mM) into the PVN before and after intravenous injection of a selective V1a receptor antagonist (50 μg/kg) (see Drugs and Chemicals in Methods) were 12.5 ± 1.7 and 11.5 ± 2.1 mmHg and 55.3 ± 5.5 and 49.3 ± 5.3 bpm, respectively (P > 0.05). The pressor and tachycardic responses elicited by ANG-(1-12) (1 mM) before and after the intravenous injection of the V1a receptor antagonist were 12.3 ± 1.4 and 9.2 ± 0.8 mmHg and 23.1 ± 2.9 and 20.5 ± 3.3 bpm, respectively (P > 0.05). Thus, circulating vasopressin receptor antagonist did not significantly alter the pressor and tachycardic responses elicited by either NMDA or ANG-(1-12) into the PVN suggesting that vasopressin release into the circulation did not mediate these cardiovascular responses.
Identification of immunoreactive ANG-(1-12) cells
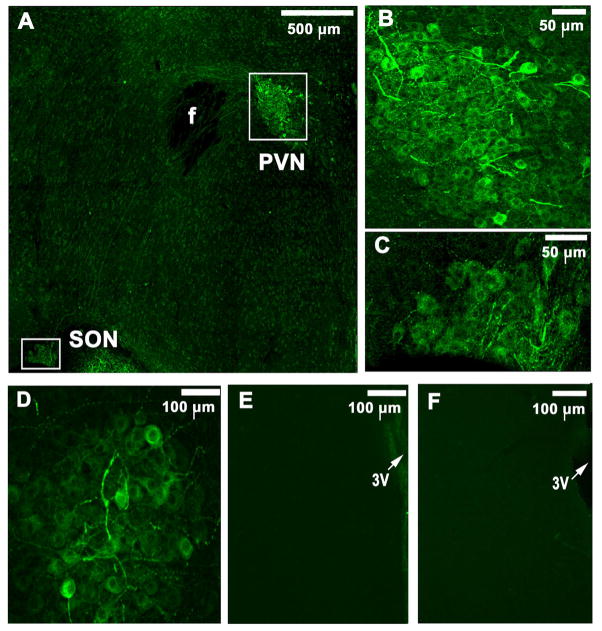
Immunohistochemical procedures (n = 8) showed the presence of ANG-(1-12)-immunostaining cells and fibres in the PVN and supraoptic nucleus (SON) (Fig. 6A). Higher magnifications of ANG-(1-12)-immunoreactive PVN and SON cells are shown in Fig. 6B and Fig. 6C, respectively. The antibody used for ANG-(1-12) did not cross react with ANG II because immunostaining for ANG-(1-12) persisted even after the pre-absorption of the antibody with ANG II peptide (Fig. 6D). Specificity of the primary antibody for ANG-(1-12) was indicated further by lack of staining for this peptide when the primary antibody was either omitted from the protocol (Fig. 6E) or pre-absorbed by incubation with ANG-(1-12) peptide (Fig. 6F). Although ANG-(1-12)-immunoreactive cells and fibres were more numerous in the middle and caudal regions of the PVN (Figs. 7C and 7F), immunoreactive cells and fibres were also present in the rostral region (Figs. 7A and 7D). In this context it may be noted that the projections to the RVLM and IML emerge from the middle and caudal portions of the PVN (Badoer, 2001; Hardy, 2001; Shafton et al. 1998). ANG-(1-12) and vasopressin containing neurons in a different PVN section are shown in Figs. 8A and 8B, respectively. Merged images of ANG-(1-12) and vasopressin containing neurons in the same section are shown in Fig. 8C; ANG-(1-12) was colocalized in many, but not all, vasopressin containing cells. Figs. 8D and 8E show ANG-(1-12) and oxytocin containing cells, respectively, in a different PVN section. Merged image in Fig. 8F indicates that ANG-(1-12) is not colocalized in oxytocin containing cells.
Fig. 6.
Identification of immunoreactive ANG-(1-12) cells. A: ANG-(1-12) containing cells and fibres were present in the PVN and SON (Dylight 488; green fluorescence). B and C: Higher magnifications of ANG-(1-12)-immunoreactive PVN and SON cells, respectively. D: Immunostaining for ANG-(1-12) persisted even after the pre-incubation of the primary antibody for ANG-(1-12) with ANG II peptide E: No staining for ANG-(1-12) was observed when the primary antibody was omitted from the protocol. F: Staining for ANG-(1-12) was absent when the primary antibody was pre-incubated with ANG-(1-12) peptide. All images were captured by confocal microscopy. f: fornix; PVN: paraventricular nucleus; SON: supraoptic nucleus; 3V: 3rd ventricle.
Fig. 7.
Immunoreactive ANG-(1-12) cells and fibres in different regions of the PVN. ANG-(1-12) immunoreactive cells and fibres (Dylight 488; green fluorescence) were present in the rostral (0.92 mm caudal to the bregma), middle (1.8 mm caudal to the bregma) and caudal (2.3 mm caudal to the bregma) regions of the PVN (panels A, B and C, respectively). Higher magnifications of ANG-(1-12) immunoreactive cells and fibres in the boxed regions of panels A, B, and C are shown in corresponding boxed areas in panels D, E and F, respectively. All images were captured by confocal microscopy.
Fig. 8.
Colocalization of ANG-(1-12) and vasopressin in PVN cells. A: ANG-(1-12) containing cells were present in the PVN (Dylight 594; red fluorescence). B: arginine vasopressin (AVP) containing cells were also present in the PVN in the same field (Dylight 488; green fluorescence). C: A merged image showing colocalization (yellow color) of ANG-(1-12) and vasopressin neurons in the same section; ANG-(1-12) was colocalized in some, but not all, vasopressin containing cells. D: ANG-(1-12) containing cells were present in the PVN cells in a different section (Dylight 594; red fluorescence). E: oxytocin containing PVN cells in the same section (Dylight 488; green fluorescence). F: Merged image indicates that ANG-(1-12) (red fluorescence) is not colocalized in oxytocin containing cells (green fluorescence). All images were captured by confocal microscopy.
Histological identification of microinjection sites
A typical PVN microinjection site marked with Lumafluor (50 nl) is shown in Fig. 9A. Figures 9B–9H represent drawings of coronal sections in different regions of the PVN. In each of these figures, each dark circle represents the centre of one microinjection site in one animal. Figures 9B and 9C (0.92 and 1.3 mm caudal to the bregma, respectively) show microinjection sites in the rostral PVN (n = 10). Figures 9D–9F (1.4–1.8 mm caudal to the bregma) show the microinjection sites (n = 35) in the middle region of the PVN. Figures 9G and 9H (1.88 and 2.12 mm caudal to the bregma, respectively) show the microinjection sites (n = 9) in the caudal region of the PVN. A majority of microinjection sites was located in the middle and caudal regions of the PVN (Paxinos and Watson,− 1986). PVN neurons in these regions have been reported to project to the RVLM and IML (Badoer, 2001; Hardy, 2001; Shafton et al. 1998). The middle and caudal regions of the PVN include both magnocellular and parvocellular neurons (Hosoya & Matsushita, 1979; Swanson & Sawchenko, 1983).
Fig. 9.
Histological identification of microinjection sites. A: A coronal section showing the microinjection site in the PVN marked with Lumaflour (50 nl; arrow); the site was located 1.6 mm caudal to the bregma, 0.3 mm lateral to midline and 8.0 mm deep from the dorsal surface of the dura (middle portion of the PVN). Panels B–H show drawings of coronal sections at different regions of the PVN (each dark circle represents the center of one microinjection site in one animal). B–C: Sections showing the microinjection sites in the rostral PVN (0.92 and 1.3 mm caudal to the bregma; n = 10). D–F: Sections representing the microinjection sites in the middle region of the PVN (1.4–1.8 mm caudal to the bregma; n = 35). G–H: Sections representing the microinjection sites in the caudal region of the PVN (1.88 and 2.12 mm caudal to the bregma; n = 9). 3V, 3rd ventricle; f, fornix; PaAP, anterior parvocellular part of the PVN; PaDC, dorsal cap of paraventricular hypothalamic nucleus; PaLM, lateral magnocellular subdivision of paraventricular hypothalamic nucleus; PaMP, medial parvocellular subdivision of paraventricular hypothalamic nucleus; PaPo, posterior portion of the PVN; Pe, periventricular hypothalamic nucleus; PVN, hypothalamic paraventricular nucleus.
Discussion
The main purpose of the present study was to determine the processing of exogenously administered ANG-(1-12) in the PVN and to identify the role of ANG II in mediating the cardiovascular responses of this new angiotensin in this nucleus. The major findings of the study were as follows. Microinjections of ANG-(1-12) into the PVN: 1) elicited increases in MAP, HR, and efferent RSNA, 2) these effects were mediated via activation of AT1Rs, but not AT2Rs, in the PVN, 3) ANG-(1-12)-induced cardiovascular responses were attenuated by prior microinjections of either captopril (ACE inhibitor) or chymostatin (chymase inhibitor) into the PVN, 4) combination of microinjections of captopril and chymostatin into the PVN elicited a greater attenuation of the cardiovascular effects of ANG-(1-12), 5) ANG-(1-12) was present in some, but not all, vasopressinergic cells, and 6) ANG-(1-12) was also present in non-vasopressinergic PVN neurons.
The concentrations of NMDA (10 mM) and ANG-(1-12) (1 mM) that elicited pressor and tachycardic responses when microinjected (50 nl each) into the PVN, did not elicit detectable cardiovascular responses when injected intravenously indicating that leakage of the drug, if any, from the microinjection site in the PVN to the peripheral circulation was not responsible for the observed responses.
Microinjections of ANG-(1-12) into the PVN showed a non-linear bell-shaped concentration-response. Similar type of concentration-response has been reported for microinjections of ANG-(1-12) into the mNTS (Chitravanshi & Sapru, 2011). This type of concentration-response has been explained by homotropic allostery in which the agonist at higher concentrations binds to a modulator site, which is different from the primary binding site, and thereby affects the function of the receptor resulting in attenuated responses (Bindslev, 2004). Another possibility is that at higher concentrations, ANG-(1-12) may activate inhibitory pathways located in the PVN causing a decrease in sympathetic activity and BP. Thus, activation of inhibitory pathways in the PVN by ANG-(1-12) at higher concentrations is likely to reduce the pressor and tachycardic effects of the peptide in the PVN. In this context, it should be noted that the presence of inhibitory GABAergic synaptic inputs to spinally projecting PVN neurons has been reported (Li et al. 2003).
Cardiovascular responses elicited by ANG-(1-12) were greater in the middle and caudal regions of the PVN. This observation is consistent with anatomical studies in which the neurons projecting to the RVLM and spinal cord were found to be more numerous in the middle and caudal regions of the PVN (Badoer, 2001; Hardy, 2001; Shafton et al. 1998). In these regions of the PVN, the parvocellular neurons projecting to the RVLM and spinal cord are located dorsomedial and ventromedial to the magnocellular neurons (Hosoya & Matsushita, 1979; Swanson & Sawchenko, 1983). Thus, our microinjections of ANG-(1-12) are expected to have reached both magnocellular and parvocellular neurons in the PVN. Because ANG-(1-12)-induced cardiovascular responses were not altered by intravenous injections of a V1a receptor antagonist, it was concluded that vasopressin release via activation of magnocellular neurons did not mediate these responses. Thus, increases in MAP and HR observed in our study were most probably due to ANG-(1-12)-induced activation of parvocellular neurons projecting to the RVLM and IML.
The presence of ANG-(1-12)-immunoreactive cells and fibres has been reported in the NTS (Arnold et al. 2010). In the present study, we have demonstrated the presence of neurons and fibres immunoreactive for ANG-(1-12) in the PVN and SON. ANG-(1-12) immunoreactivity was present in some vasopressinergic neurons. This observation is consistent with previous reports in which colocalization of another angiotensin (ANG II) with vasopressin has been observed (Imboden & Felix, 1991). ANG-(1-12) immunoreactivity was also present in non-vasopressinergic neurons. ANG-(1-12) immunoreactivity was not observed in oxytocin containing cells. The fibres emerging from the PVN neurons immunostaining for ANG-(1-12) appeared to project towards the median eminence. Distribution of neurons immunoreactive for ANG-(1-12) in the PVN was similar to ANG II-immunoreactive PVN neurons (Lind et al. 1985). In our study, we established that the antibody used for ANG-(1-12) did not cross react with ANG II because immunostaining for ANG-(1-12) persisted even after the pre-incubation of the antibody with ANG II peptide. The specificity of the antibody for ANG-(1-12) was demonstrated by lack of staining when the antibody was omitted in the protocol or when it was pre-incubated with ANG-(1-12) peptide. Although we found robust immunoreactivity of ANG-(1-12) in vasopressinergic cells, the function of ANG-(1-12) in these cells is not clear at present. The presence of ANG-(1-12)-immunoreactive cells in the PVN suggests that these cells may be the source of endogenous ANG-(1-12) in this nucleus.
In the CNS, there is wide distribution of angiotensinogen but renin levels are low (Grobe et al. 2008). This mismatch has created doubts regarding the synthesis of ANG II from angiotensinogen in the CNS. The discovery of Ang-(1–12) has prompted the speculation that this peptide may provide an alternate renin independent pathway for the generation ANG II in the CNS and it may serve as a precursor for ANG II in the central pathways regulating cardiovascular function. In this context, it may be noted that renin is not involved in the formation of ANG-(1-12) from angiotensinogen (Ferrario et al. 2009).
Previous reports from other investigators have indicated that in the myocardial tissue and plasma ANG-(1-12) is converted into ANG II (Nagata et al. 2006; Prosser et al. 2009; Trask et al. 2008). In the NTS (Arnold et al. 2010; Chitravanshi & Sapru, 2011), hypothalamic arcuate nucleus (Arakawa et al. 2011) and PVN (this study) also the responses of ANG-(1-12) are mediated via its conversion to ANG II. Angiotensin converting enzyme (ACE) is partly involved in the conversion of ANG-(1-12) to ANG II in these brain regions because microinjections of captopril attenuated ANG-(1-12)-induced responses. Chymase has been reported to be one of the alternate pathways by which ANG II is formed in various tissues (Prosser et al. 2009). In the CNS, this pathway is involved in ANG II formation in the pituitary stalk and in the pineal gland (Baltatu et al. 1997). We have previously reported that chymase may be partially involved in the conversion of ANG-(1-12) to ANG II in the ARCN and NTS (Arakawa et al. 2011; Chitravanshi & Sapru, 2011). In this study we show that in the PVN also chymase may be partially involved in the conversion of ANG-(1-12) to ANG II because microinjections of chymostatin attenuated ANG-(1-12)-induced cardiovascular responses. Greater attenuation of ANG-(1-12)-induced cardiovascular responses was observed when both ACE and chymase were inhibited simultaneously in the PVN. Thus, cardiovascular effects elicited by ANG-(1-12) in the PVN are mediated via its conversion to ANG II which has been reported to elicit increases in MAP and HR when microinjected into the PVN (Zheng et al. 2009; this study). This contention is further supported by the observation that combined microinjections of captopril and chymostatin into the PVN attenuated the cardiovascular responses to ANG-(1-12) but not ANG II. Furthermore, the onset and peak effect of ANG-(1-12) was delayed when compared to that of ANG II which is consistent with the notion that ANG-(1-12) needs to be converted to ANG II before it elicits its actions. Similar results regarding the onset of action of ANG-(1-12) and ANG II in the NTS of the rat have been reported by us earlier (Chitravanshi & Sapru, 2011).
The responses to ANG-(1-12) were mediated via AT1Rs because prior microinjections of losartan into the PVN blocked the effects of this peptide. The blockade of ANG-(1-12)-induced responses could not be attributed to desensitization because the interval between the two doses of ANG-(1-12) was at least 60 min (desensitization to repeated injections of this angiotensin did not occur at this time interval). This result is consistent with other reports indicating that the cardiovascular effects of ANG-(1-12) are mediated via AT1Rs (Arakawa et al. 2011; Arnold et al. 2010; Chitravanshi & Sapru, 2011; Nagata et al. 2006). The activation of AT1Rs can be attributed to ANG II generated from ANG-(1-12). AT2Rs in the PVN were not involved in ANG-(1-12)-induced cardiovascular responses because microinjections of PD123319 into the PVN did not alter cardiovascular responses elicited by microinjections of ANG-(1-12) in the PVN.
Based on current knowledge regarding the role of PVN in cardiovascular regulation (reviews: Badoer, 2001; Brookes, 1997; Coote, 2004; Dampney et al. 2005; Stern, 2004), the mechanism of increases in MAP, HR, and RSNA elicited by microinjections of ANG-(1-12) into the PVN can be explained as follows. Microinjections of ANG-(1-12) into the PVN result in the generation of ANG II which may excite the PVN neurons involved in cardiovascular regulation via AT1Rs. We have previously shown that extracellulary recorded activity of some neurons (e.g. barosensitive NTS neurons) is increased after direct application of ANG-(1-12) to these neurons and this effect is blocked by prior application of captopril and chymostatin indicating that the effect is mediated via formation of ANG II (Chitravanshi & Sapru, 2011). The mechanism of neuronal excitation elicited by ANG-(1-12) is not clear at this time. However, ANG II derived from ANG-(1-12) may stimulate PVN neurons via pre- and/or post-synaptic mechanisms, attenuation of synaptic GABA release in the PVN (Li et al. 2005), presynaptic disinhibition in the PVN (Li et al. 2003) and reduction in resting K+ conductance in the PVN neurons (Li & Guyenet, 1996). Direct projections from the PVN to RVLM and IML (Coote, 2004) may mediate the increases in MAP, HR, and RSNA elicited by microinjections of ANG-(1-12) into the PVN. The phenotype of the neurons mediating the cardiovascular actions to microinjections of ANG-(1-12) into the PVN is not known at present. Possible release of angiotensin at the terminals of direct and/or indirect projections of the PVN to the RVLM has been implicated in the cardiovascular responses elicited by chemical stimulation of the PVN (Tagawa & Dampney, 1999). It has been proposed that glutamate may be a co-transmitter with ANG II in some CNS pathways (e.g., projection from subfornical organ to the PVN) (Ferguson & Washburn, 1998). Because many sympathetic regulatory PVN neurons projecting to the RVLM are glutamatergic (Stocker et al. 2006), it is possible the glutamate release in the RVLM may also mediate cardiovascular effects of PVN neuronal stimulation by ANG-(1-12).
The tachycardic responses to microinjections of ANG-(1-12) may be partially mediated by inhibition of vagal outflow to the heart because bilateral vagotomy attenuated these responses. Inhibition of vagal outflow to the heart may be mediated via the projections of the PVN neurons to the nucleus tractus solitarius (NTS), dorsal vagal nucleus and nucleus ambiguus (Kawabe et al. 2009).
The chemical nature of the PVN neurons that mediate cardiovascular responses is not firmly established. As mentioned earlier in this manuscript, neurons projecting to the RVLM and spinal cord were found to be concentrated in the middle and caudal levels of the PVN (Badoer, 2001; Hardy, 2001; Shafton et al. 1998). Increases in MAP and HR elicited by chemical stimulation of the PVN are most probably mediated via these PVN projections to the RVLM and/or IML. About 85% of PVN neurons retrogradely labeled by microinjections of a tracer into the RVLM were found to be glutamatergic (Stocker et al 2006) suggesting that glutamate may be released in the RVLM in response to PVN stimulation. Consistent with this hypothesis is the report that glutamate may be a co-transmitter with ANG II in some CNS pathways (e.g., projection from subfornical organ to the PVN) (Ferguson & Washburn, 1998). However, microinjections of kynurenate (a glutamate receptor antagonist) into the RVLM had no effect on the sympathoexcitatory responses elicited by disinhibition of PVN neurons (Tagawa & Dampney, 1999) suggesting that glutamate is not the neurotransmitter released in the RVLM in response to PVN stimulation. On the other hand, microinjection of an AT1R antagonist into the RVLM significantly reduced the sympathoexcitatory responses elicited by disinhibition of PVN neurons (Tagawa & Dampney, 1999) suggesting that ANG II may be the neurotransmitter released in the RVLM in response to PVN stimulation. These reports raise the possibility that ANG-(1-12), via its conversion to ANG II, may play a role in the sympathoexcitatory responses elicited by chemical stimulation of the PVN. Consistent with this hypothesis is our observation that ANG-(1-12) immunoreactive neurons were found to be present in the parvocellular regions of the middle and caudal PVN. Further studies are needed to establish that PVN neurons retrogradely labeled from the RVLM and/or IML are immunoreactive for ANG-(1-12) and ANG II. The possibility of involvement of other putative neurotransmitters present in the PVN in mediating cardiovascular responses elicited from this nucleus cannot be excluded at the present time (Swanson & Sawchenko, 1983).
Perspectives
Central immunoneutralization of ANG-(1-12) has been reported to lower BP and improve baroreflex sensitivity and HR variability in hypertensive (mRen2)27 rats, indicating that endogenous ANG-(1-12), probably via ANG II formation, may play a role in central pathways controlling BP in hypertension (Isa et al. 2009). In this context, it may be noted that ANG-(1-12) levels are elevated in cardiac and renal tissue of hypertensive rats (Jessup et al. 2008). Up-regulation of the renin-angiotensin system in the brain has been reported in the experimental and genetic models of hypertension and heart failure (Morimoto et al. 2001; Yongue et al. 1991; Zheng et al. 2009). Signaling across blood brain barrier by ANG II has also been implicated in neurogenic hypertension (Paton et al. 2008; Tan et al. 2007). The present study provides the ground work for future investigations on the role of ANG-(1-12) in the PVN in different pathological states such as heart failure and hypertension.
Conclusion
The present study suggests that in the PVN exogenous ANG-(1-12) is converted to ANG II, which, in turn, elicits pressor and tachycardic responses via activation of AT1 receptors in this nucleus. Both ACE and chymase are involved in the conversion of ANG-(1-12) into ANG II in the PVN. ANG-(1-12) containing cells were found to be present in the PVN. These cells may be the source of endogenous ANG-(1-12) in the PVN.
Table 2.
Effect of microinjections of ACE and chymase inhibitors into the PVN on the responses elicited by microinjections of ANG-(1-12) (1 mM) at the same site (n = 5 in each group)
| Antagonist | Increases in MAP (mmHg) elicited by ANG-(1-12) | Increases in HR (bpm) elicited by ANG-(1-12) | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Captopril (100 mM) | 16 ± 1.6 | 7.2 ± 1.4*** | 21.2 ± 1.4 | 8.4 ± 1.2*** |
| Captopril † (200 mM) | 17.2 ± 2.1 | 5.8 ± 1.3*** | 21.6 ± 1.6 | 8.2 ± 1.2*** |
| Chymostatin (5 mM) | 16.6 ± 2.1 | 10.8 ± 2* | 22.2 ± 1.2 | 14 ± 0.8** |
| Chymostatin ¶ (10 mM) | 14.8 ± 1.5 | 3.4 ± 0.5*** | 23 ± 1.3 | 5.8 ± 1*** |
P < 0.05;
P < 0.01;
P < 0.001.
Attenuation of cardiovascular responses elicited by higher concentration of captopril (200 mM) was not significantly greater (P > 0.05) than that elicited by the smaller concentration of captopril (100 mM).
Attenuation of cardiovascular responses elicited by higher concentration of chymostatin (10 mM) was significantly greater (P < 0.05–0.001) than that elicited by the smaller concentration of chymostatin (5 mM).
HR = heart rate; MAP = mean arterial pressure; PVN = hypothalamic paraventricular nucleus.
Acknowledgments
This work was supported in part by N.I.H. grants HL024347 and HL076248 awarded to Dr. H. N. Sapru.
References
- Arakawa H, Chitravanshi VC, Sapru HN. The hypothalamic arcuate nucleus: a new site of cardiovascular action of angiotensin-(1-12) and angiotensin II. Am J Physiol Heart Circ Physiol. 2011;300:H951–H960. doi: 10.1152/ajpheart.01144.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Arnold AC, Isa K, Shaltout HA, Nautiyal M, Ferrario CM, Chappell MC, Diz DI. Angiotensin-(1-12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am J Physiol Heart Circ Physiol. 2010;299:H763–H771. doi: 10.1152/ajpheart.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Baltatu O, Nishimura H, Hoffmann S, Stoltenburg G, Haulica ID, Lippoldt A, Ganten D, Urata H. High levels of human chymase expression in the pineal and pituitary glands. Brain Res. 1997;752:269–278. doi: 10.1016/s0006-8993(96)01474-6. [DOI] [PubMed] [Google Scholar]
- Bindslev N. A homotropic two-state model and auto-antagonism. BMC Pharmacol. 2004;4:11–22. doi: 10.1186/1471-2210-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankley CJ, Hodges JC, Klutchko SR, Himmelsbach RJ, Chucholowski A, Connolly CJ, Neergaard SJ, Van Nieuwenhze MS, Sebastian A, Quin J, III, Essenberg AD, Cohen DM. Synthesis and structure-activity relationships of a novel series of non-peptide angiotensin II receptor binding inhibitors specific for the AT2 subtype. J Med Chem. 1991;34:3248–3260. doi: 10.1021/jm00115a014. [DOI] [PubMed] [Google Scholar]
- Brooks VL. Interactions between angiotensin II and the sympathetic nervous system in the long-term control of arterial pressure. Clin Exp Pharmacol Physiol. 1997;24:83–90. doi: 10.1111/j.1440-1681.1997.tb01788.x. [DOI] [PubMed] [Google Scholar]
- Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1844–R1853. doi: 10.1152/ajpregu.2001.281.6.R1844. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Cardiovascular responses elicited by a new endogenous angiotensin in the nucleus tractus solitarius of the rat. Am J Physiol Heart Circ Physiol. 2011;300:H230–H240. doi: 10.1152/ajpheart.00861.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote JH. The hypothalamus and cardiovascular regulation. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural Mechanisms of Cardiovascular Regulation. Kluwer Academic Publishers; Boston, MA, USA: 2004. pp. 117–146. [Google Scholar]
- Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- Duan YF, Kopin IJ, Goldstein DS. Stimulation of the paraventricular nucleus modulates firing of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol. 1999;277:R403–R411. doi: 10.1152/ajpregu.1999.277.2.R403. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DL. Angiotensin II: a peptidergic neurotransmitter in central autonomic pathways. Prog Neurobiol. 1998;54:169–192. doi: 10.1016/s0301-0082(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89:370–376. doi: 10.1159/000211202. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1-12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobe JL, Xu D, Sigmund CD. An intracellular Renin-Angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology. 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy SG. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/s0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- He S, Gaca MD, McEuen AR, Walls AF. Inhibitors of chymase as mast cell-stabilizing agents: contribution of chymase in the activation of human mast cells. J Pharmacol Exp Ther. 1999;291:517–523. [PubMed] [Google Scholar]
- Hocht C, Gironacci MM, Mayer MA, Schuman M, Bertera FM, Taira CA. Involvement of angiotensin-(1-7) in the hypothalamic hypotensive effect of captopril in sinoaortic denervated rats. Regul Pept. 2008;146:58–66. doi: 10.1016/j.regpep.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Matsushita M. Identification and distribution of the spinal and hypophyseal projection neurons in the paraventricular nucleus of the rat. A light and electron microscopic study with the horseradish peroxidase method. Exp Brain Res. 1979;35:315–331. doi: 10.1007/BF00236618. [DOI] [PubMed] [Google Scholar]
- Imboden H, Felix D. An immunocytochemical comparison of the angiotensin and vasopressin hypothalamo-neurohypophysial systems in normotensive rats. Regul Pept. 1991;36:197–218. doi: 10.1016/0167-0115(91)90057-n. [DOI] [PubMed] [Google Scholar]
- Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1-12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R111–R115. doi: 10.1152/ajpregu.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1-12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Kawabe K, Sapru HN. Cardiovascular function of a glutamatergic projection from the hypothalamic paraventricular nucleus to the nucleus tractus solitarius in the rat. Neuroscience. 2008;153:605–617. doi: 10.1016/j.neuroscience.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe T, Chitravanshi VC, Nakamura T, Kawabe K, Sapru HN. Mechanism of heart rate responses elicited by chemical stimulation of the hypothalamic paraventricular nucleus in the rat. Brain Res. 2009;1248:115–126. doi: 10.1016/j.brainres.2008.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkei Z, Palkovits M, Corvol P, Llorens-Cortès C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18:383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci. 2003;23:5041–5049. doi: 10.1523/JNEUROSCI.23-12-05041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Angiotensin II decreases a resting K+ conductance in rat bulbospinal neurons of the C1 area. Circ Res. 1996;78:274–282. doi: 10.1161/01.res.78.2.274. [DOI] [PubMed] [Google Scholar]
- Lind RW, Swanson LW, Bruhn TO, Ganten D. The distribution of angiotensin II-immunoreactive cells and fibers in the paraventriculo-hypophysial system of the rat. Brain Res. 1985;338:81–89. doi: 10.1016/0006-8993(85)90250-1. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol. 2003;35:901–918. doi: 10.1016/s1357-2725(02)00306-0. [DOI] [PubMed] [Google Scholar]
- Migdalof BH, Antonaccio MJ, McKinstry DN, Singhvi SM, Lan SJ, Egli P, Kripalani KJ. Captopril: pharmacology, metabolism and disposition. Drug Metab Rev. 1984;15:841–869. doi: 10.3109/03602538409041080. [DOI] [PubMed] [Google Scholar]
- Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res. 2001;89:365–372. doi: 10.1161/hh1601.094988. [DOI] [PubMed] [Google Scholar]
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- Oparil S, Yarows SA, Patel S, Zhang J, Satlin A. Dual inhibition of the renin system by aliskiren and valsartan. Lancet. 2007;370 (9593):1126–1127. doi: 10.1016/S0140-6736(07)61508-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Wang S, Polson JW, Kasparov S. Signalling across the blood brain barrier by angiotensin II: novel implications for neurogenic hypertension. J Mol Med. 2008;86:705–710. doi: 10.1007/s00109-008-0324-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. New York: Academic Press; 1986. [Google Scholar]
- Portillo F, Carrasco M, Vallo JJ. Separate populations of neurons within the paraventricular hypothalamic nucleus of the rat project to vagal and thoracic autonomic preganglionic levels and express c-Fos protein induced by lithium chloride. J Chem Neuroanat. 1998;14:95–102. doi: 10.1016/s0891-0618(97)10022-9. [DOI] [PubMed] [Google Scholar]
- Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat Proangiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/s0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- Stern J. Cellular properties of autonomic-related neurons in the paraventricular nucleus of the hypothalamus. In: Dun NJ, Machado BH, Pilowsky PM, editors. Neural Mechanisms of Cardiovascular Regulation. Kluwer Academic Publishers; Boston, MA, USA: 2004. pp. 147–161. [Google Scholar]
- Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol. 2006;494:673–685. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HGJM. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tagawa T, Dampney RAL. AT1 receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- Tan PS, Killinger S, Horiuchi J, Dampney RAL. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
- Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1-12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C-C, Meng QC, Palmer R, Hageman GR, Durand J, Bradley WE, Farrell DM, Hankes GH, Oparil S, Dell’Italia LJ. Evidence for angiotensin-converting enzyme- and chymase-mediated angiotensin II formation in the interstitial fluid space of the dog heart in vivo. Circulation. 1999;99:2583–2589. doi: 10.1161/01.cir.99.19.2583. [DOI] [PubMed] [Google Scholar]
- Yongue G, Angulo JA, McEwen BS, Myers MM. Brain and liver angiotensinogen messenger RNA in genetic hypertensive and normotensive rats. Hypertension. 1991;17:485–491. doi: 10.1161/01.hyp.17.4.485. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li Y, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–R1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GQ, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Physiol Heart Circ Physiol. 2002;282:H2039–H2045. doi: 10.1152/ajpheart.00854.2001. [DOI] [PubMed] [Google Scholar]