Abstract
Recent work suggests that IL-2 and IL-15 induce distinctive levels of signaling through common receptor subunits and that such varied signaling directs the fate of antigen-activated CD8+ T cells. Here we directly examined proximal signaling by IL-2 and IL-15 and CD8+ T cell primary and memory responses as a consequence of varied CD122-dependent signaling. Initially, IL-2 and IL-15 induced similar pStat5 and pS6 activation, but these activities were only sustained by IL-2. Transient IL-15-dependent signaling is due to limited expression of IL-15Rα. To investigate the outcome of varied CD122 signaling for CD8+ T cell responses in vivo, OT-I T cells were utilized from mouse models where CD122 signals were attenuated by mutations within the cytoplasmic tail of CD122 or intrinsic survival function was provided in the absence of CD122 expression by transgenic Bcl-2. In the absence of CD122 signaling, generally normal primary response occurred, but the primed CD8+ T cells were not maintained. In marked contrast, weak CD122 signaling supported development and survival of TCM but not TEM cells. Transgenic expression of Bcl-2 in CD122−/− CD8+ T cells also supported the survival and persistence of TCM cells but did not rescue TEM development. These data indicate that weak CD122 signals readily support TCM development largely through providing survival signals. However, stronger signals, independent of Bcl-2, are required for TEM development. Our findings are consistent with a model whereby low, intermediate, and high CD122 signaling support TCM memory survival, TEM programming, and terminal TEFF differentiation, respectively.
Introduction
IL-2 and IL-15 are two important cytokines that regulate a number of key aspects of the immune system. The receptors for these cytokines utilize unique α subunits (IL-2Rα or CD25 and IL-15Rα), which are primarily responsible for ligand binding specificity, but use common β (IL-2Rβ or CD122) and γ subunits (γc or CD132), which not only participate in ligand binding but dictate signal transduction (1). Correspondingly, the IL-2R and IL-15R activate identical signal transduction pathways, the Jak1, 3/Stat5, MAPK and PI-3K/Akt pathways (2, 3), implying that both cytokines activate identical downstream effector molecules.
Interestingly, the biological responses mediated by IL-2 and IL-15 are highly distinct as illustrated by the unique role of IL-2 for Treg cell development and homeostasis (4) and IL-15 for NK and NK-T cell development and CD8 T memory survival (2, 5–7). A simple explanation for such distinct biological outcomes by cytokines that utilize common receptor signaling subunits might be context dependent, i.e. unique cellular niches that segregate cells secreting IL-2 and IL-15 or expressing IL-2R and IL-15R. However, work with activated CD8+ T cells in vitro demonstrates that these two cytokines direct distinct cell fates as IL-2 readily promotes effector development while IL-15 supports memory-like cells (8–10). As neither the cytokines nor their receptors are segregated in this setting, receptor proximal signal transduction by these two cytokines must be distinct, but there remains minimal data how such initial proximal signaling varies.
CD122-dependent signaling is critically involved in CD8+ T cell immunity and memory. IL-2 supports development of short-lived TEFF cells characterized by high expression of Klrg1 in part by transcriptional regulation of T-bet and Blimp-1 (10–13). IL-15 is essential for the survival and homeostasis of memory cells in part by maintaining expression of pro-survival Bcl-2 (14, 15). CD8+ memory programming is another function dependent upon CD122 signaling, but the nature of this activity is more controversial. Initially, memory programming was described as an IL-2-dependent activity where there was relatively normal memory development but the recall response by the memory pool was impaired due to failed IL-2R signaling during the primary response (16, 17). These studies relied on CD8+ T cells from CD25−/− mice, which respond to IL-15 but not IL-2. Using a similar experimental strategy, other studies reported somewhat divergent results. In one case in the absence of IL-2R signaling, CD8+ T cells poorly developed into short-lived and long-lived T effector-memory (TEM) cells, and upon re-challenging, the remaining long-lived memory cells exhibited poor recall expansion and altered secondary effector activity (18). This work suggests a more complex role for IL-2 with potential survival and programming functions. Alternatively, and in marked contrast, largely normal memory recall responses were readily elicited to several infectious agents (19), raising the possibility that IL-2 and IL-15 may substantially overlap in the regulation of CD8+ T cell memory (15, 20). Based on these results and the lack of molecular correlates of memory programming, it remains ambiguous if memory programming is unique to IL-2, reflects a specific contribution to TEM, or simply reflects redundant pro-survival function by these two cytokines.
An additional potentially important factor in memory development, programming, and survival is that the strength of signal through CD122 might control these distinct outcomes. Varied signaling thresholds regulate IL-2-dependent events for Treg and TEFF cells (10, 21, 22). With respect to the primary response by CD8+ T cells, virus-activated CD8+ T cells that expressed high levels of CD25 exhibited increased phospho-Stat5 (pStat5) activation ex vivo and preferentially developed into Klrg1+ terminally differentiated short-lived TEFF cells (21). Such terminal differentiation was further promoted by exogenous IL-2. In contrast, CD25lo cells preferentially developed into long-lived memory cells. However, whether this simply reflected enrichment in memory precursor cells or also is directly related to a requirement for distinctive IL-2R signaling remains unknown.
In the present study we directly studied three key issues related to IL-2 and IL-15 signaling by CD8+ T cells. First, we established the basis by which proximal signaling varies between IL-2 and IL-15. Second, we examined the extent the primary and memory responses varied as a direct consequence of varying CD122 signaling strength. Lastly, we addressed the extent CD122 signaling supports memory development solely through promoting cellular survival.
Material and Methods
Mice
CD122−/− mice (23) and CD122−/− mice expressing T cell-targeted transgenic CD122 with mutations (Y1 and Y3) in cytoplasmic tyrosine residues (22) have been previously described. These mice were crossed to OT-I mice to yield OT-I CD122−/− and OT-I CD122−/− IL-2Rβ mutant mice. CD45.1-congenic C57BL/6, OT-I and IL-15−/− mice were obtained from Taconic Farms. C57BL/6, human Bcl-2 transgenic mice (C57BL/6Tg (Bcl-2)36Wehi), and IL-15Rα−/− mice were obtained from the Jackson Laboratory. OT-I CD122−/− mice were crossed to Bcl-2 transgenic mice to yield OT-I WTBcl-2Tg and OTI CD122−/−Bcl2Tg mice. CD45.1 was also crossed onto OT-I background and provide by E. Gilboa (University of Miami). Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami.
Adoptive Transfer and immunization of OT-I
OT-I T cells were purified (typically ≥90%) by magnetic based positive selection using anti-CD8 beads (Miltenyi Biotec). For adoptive transfer, naïve CD45.2 OT-I T cells (0.5 × 106) from the indicated mouse models were injected i.v. into naïve CD45.1 C57BL/6 recipient mice. 24 hr after adoptive transfer, mice were immunized by i.v. injection of OVA257–264 (10 µg) (synthesized by AnaSpec, San Jose, CA) and LPS (10 µg) (from E.coli 055:B5, Sigma, Saint Louis, Missouri). At day 12 or day 27 after priming, some mice were re-challenged with OVA 257–264 (10 µg) and LPS (10 µg) i.v. and the resulting secondary TEFF cells were analyzed three days later.
In vitro culture
OT-I spleen cells (1 × 106 cells/well) were cultured in 24 well flat bottom plates in RPMI 1640 complete medium (CM) with OVA257–264 peptide (0.1nM) for 72 hours (24). Normal C57BL/6 spleen cells were cultured (2 × 106 cells/well) with anti-CD3 (5% supernatant) or LPS (1ug/ml) in 24-well flat bottom plates in CM for 48 hr (25) to generate T or B cell blasts, respectively. For intracellular signaling experiments, in vitro-derived OT-I and anti-CD3 activated T cells were washed 3 times, and re-cultured in CM at (1 × 106 cells/well) in 24 well plates without cytokines for 4 hours. For ex vivo derived OT-I cells from immunized mice, they were cultured in CM for 30 min. Cells were stimulated with mouse IL-2 (10 ng/ml) and IL-15 (10 ng/ml) for 15–180 min unless otherwise indicated. For growth and survival experiments, anti-CD3 activated T cells were washed 3 times, and re-cultured in 5 ml of CM at (1 × 105 cells/ml) in T25 flasks in absence or presence of IL-2 (10 ng/ml) or IL-15 (10 ng/ml) for 48 hrs.
FACS analysis
Antibodies used in flow cytometry for cell surface or intracellular cytokine staining were purchased from Biolegend (San Diego, CA), eBioscience (San Diego, CA) or BD Bioscience (San Diego, CA). Other purchased antibodies are IL-15Rα (R&D Systems Minneapolis, MN), Granzyme B (Invitrogen, Carlsbad, CA), pStat5 (pY694, BD Bioscience) or pS6 kinase (Cell Signaling, Denver, MA). Intracellular staining for mouse Bcl-2 (clone 3F11) and human Bcl-2 (clone 100) was performed according to manufacturer’s instructions (BD Biosience). Cells were analyzed with a Becton Dickson LSR2 and FACS Diva or FlowJO software. Typically 100,000–300,000 events were collected per sample.
Cell surface staining was performed as previously described (26). Intracellular staining for pStat5 or pS6 kinase was performed as previously described (27, 28). To measure intracellular cytokine expression, spleen cells from the different mouse models were cultured (2.5 × 106 cells/well) in 24 well plates in 1 ml of CM for 5 hrs with OVA257–264 (0.1 nM), in the presence of brefeldin A (GolgiPlug, BD-Biosciences). Granzyme B was determined after 24 hr ex vivo stimulation with OVA257–264 where brefaldin A was added during the last 4 hrs of culture. The cells were subjected to surface staining with the markers of interest and then permeabilized using Cytofix Cytoperm (BD Pharmingen) prior to staining with mAbs to IFNγ, IL-2, TNF-α or granzyme B. For intracellular staining for Ki67 and Bcl-2, cells were permeabilized using Fixation/Permeabilization buffer from Foxp3 staining kit (eBiosience).
Statistical Analysis
Data were analyzed by unpaired Student-T-test or by one-way ANOVA with Tukey’s multiple comparison test. Statistically significant differences (p<0.05) for comparison to WT controls are designated by an asterisk in the graphs.
Results
IL-2 and IL-15 induce quantitative distinct receptor proximal signaling
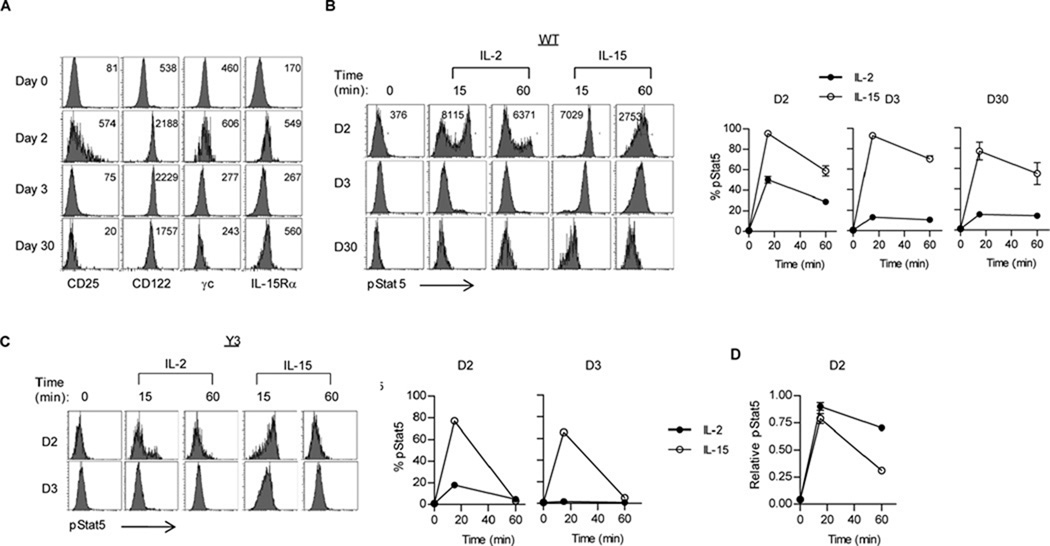
Both IL-2 and IL-15 activate Stat5 and the PI-3K/Akt signaling pathways (2, 3). To study receptor proximal signal transduction by these two cytokines, we examined the activation of Stat5 and the S6 kinase, the latter is a downstream target of the PI-3K/Akt/mTORC1 pathway (29), by flow cytometry using mAbs to their activation intermediates, pStat5 and pS6. Antigen-activated OT-I T cells were cultured in medium for 4 hr to return activation of these pathways to basal levels and then were stimulated with IL-2 or IL-15 for the indicated times. Representative FACS profiles for time course (Fig. 1A) and results from multiple time-course (Fig. 1B, left) and dose-response experiments (Fig. 1B, right) revealed that IL-2 induced more sustained signaling than IL-15. Both cytokines initially induced comparable and maximal levels of pStat5 by 15 min. However, IL-2-dependent pStat5 activation remained relatively high for 2 hr whereas IL-15-dependent pStat5 was more transient. As expected, the peak of pS6 activation occurred later. Notably, IL-15 supported much lower levels of activated pS6. As previously noted (22), IL-2 induced an obviously bimodal distribution of pS6hi and pS6lo cells (Fig. 1A) and these were strikingly reduced for the IL-15 stimulated CD8+ T cells. As the Stat5 and PI-3K pathways cooperate to promote T cell proliferation, this result might explain the capacity to drive more extensive T cell expansion by IL-2 when compared to IL-15 (Fig. 3B and (22, 30, 31). Dose-response curves at the initiation of pStat5 and pS6 activation (15 min) for IL-2 and IL-15 were nearly identical (Fig. 1B, right), consistent with effective ligand capture by IL-2Rα and IL-15Rα and the similar high affinity (Kd~10−11M) ligand binding by the IL-2R and IL-15R.
Fig. 1. IL-2- and IL-15-induced signaling by activated OT-I T cells.
Activated OT-I T cells were cultured for 4 hr in CM prior to stimulation with IL-2 or IL-15. (A) Shown are representative FACS analysis and (B) time-course and dose-response studies for activation of pStat5 and pS6. pStat5 activation was determined after activated OT-I T cells were co-cultured with mouse IL-2 and IL-15 at 10 ng/ml unless otherwise indicated (C, D) or replenished with IL-15 (E). (F) pStat5 activation was determined for OT-I T blasts pre-treated with IL-2 or IL-15 at 4° C prior to culture at 37° C. Data were normalized to maximal pStat5 or pS6 induction by WT OT-I cells, which was that observed for IL-2 at 15 or 120 min, respectively. Data in (B) represent at least 3 independent and (C–F) each an independent experiment.
Fig. 3. IL-2- and IL-15-induced signaling and function by anti-CD3 activated T cells expressing mutant CD122.
Anti-CD3 activated T cells were cultured for 4 hr in CM prior to stimulation with mouse IL-2 or IL-15 (10 ng/ml). Analyses were on CD8+ gated T cells. (A) Time-course analyses for activation of pStat5 or pS6. Data were normalized to maximal pStat5 or pS6 induction by WT T cells, which was that observed for IL-2 at 15 or 180 min, respectively. (B–E) The anti-CD3 activated T cells were washed and re-cultured in the presence or absence of IL-2 or IL-15, as indicated, for 48 hr. (B) The fold expansion by IL-2 and IL-15 was determined relative to the input cell number by cell counting. For the recovered cells, FACS analysis was performed for CD8+ gated cells to assess the expression (MFI) of CD25 (C), cytokine protection from cell death (% 7-AAD+ cells in medium minus % 7-AAD+ cells in IL-2 or IL-15) (D), or expression (MFI) of Bcl-2 (E). Data in (A–E) represent at least 2 independent experiments, except for pS6 activation (A, lower set of graphs), which represents a single experiments.
IL-2Rα has a short cytoplasmic tail (11 amino acids) with no known signaling kinase activity but TRAF and Syk associates with IL-15Rα and induces signaling in fibroblasts and mast cells (32). One possible explanation, therefore, for the lower signal transduction by IL-15 is that activity associated with IL-15Rα down-regulates signal transduction through CD122 and γc. However, this possibility is highly unlikely for CD8+ T cells because domain swapping of the IL-2Rα cytoplasmic tail with that of IL-15Rα in knock-in mice revealed that IL-15Rα acts to enhance activation of Stat5 rather than attenuate the signaling (33).
Alternatively, the inability for IL-15 to sustain signaling might reflect exhaustion of key signaling intermediates, either through the capacity of IL-15Rα to enhance signaling or through distinctive orientation of the cytoplasmic tails of CD122 and γc in the context of the IL-15R. These possibilities were functionally tested by evaluating the extent IL-15 dampens IL-2-dependent signaling. When activated OT-I T cells were stimulated by a mixture of IL-2 and IL-15 or the cells were first stimulated with IL-15 before adding IL-2, the activation of pStat5 resembles cells solely stimulated with IL-2, rather than less sustained IL-15-dependent pStat5 activation (Fig. 1C). Similar results were obtained for pS6 (Fig. S1A). Furthermore, if activated T cells were stimulated with IL-15 for 60 min, when pStat5 returned to a low level, the addition of lL-2 rapidly led to substantial sustained pStat5 activation (Fig. 1D). Thus, IL-15 does not deplete or desensitize signal transduction dependent upon CD122 and γc.
Another possibility for the lower signaling through the IL-15R is that the IL-15R depleted IL-15. However, when IL-15 was re-added to activated OT-I T cells 45 min after the initial induction of pStat5 (Fig. 1E) or pS6 (Fig. S1B), these signaling molecules were not reactivated. This finding shows that transient IL-15-dependent signaling is not due to inadequate cytokine levels during the test period. Notably, when activated OT-I T cells were pretreated with IL-2 or IL-15 and washed prior to culture at 37° C, activation of pStat5 (Fig. 1F) and pS6 (Fig. S1C) by IL-2 and IL-15 were very similar and resembled the transient activation of these signaling molecules by continuous stimulation with IL-15. This finding suggests that quantitatively lower levels of signaling associated with the IL-15R may be due to a limiting component at the initiation of signaling, perhaps cytokine receptor subunits themselves.
IL-15Rα is rapidly depleted by IL-15
Both IL-2 and IL-15 cause rapid receptor-mediated endocytosis of the cytokine complexed with its high affinity receptor (34–36). Thus, signal transduction is potentially limited by depletion of any one of the receptor subunits. To investigate this issue, the levels of CD25, IL-15Rα, CD122, and γc were followed after anti-CD3 activated CD8 T cells were stimulated with IL-2 or IL-15. First, we confirmed for these activated T cells that IL-2 and IL-15 induced pStat5 in a manner very similar to OVA257–264-activated OT-I T cells, including more transient pStat5 activation by IL-15 (Fig. 2A). FACS analyses of the surface levels of cytokine receptor subunits for anti-CD3 activated CD8+ T cells prior to stimulation by IL-2 or IL-15 revealed very high levels of CD25, but relatively low levels of IL-15Rα, CD122, and γc (Fig. 2B, left). Upon stimulation with IL-2, levels of CD25 and IL-15Rα were at best minimally reduced, but the levels of CD122 and γc markedly decreased (Fig. 2B, right). For IL-2R-dependent signaling, CD122 is the limiting component, but these levels are sufficient for sustained signal transduction.
Fig. 2. IL-2R and IL-15 expression and transpresentation by anti-CD3 activated CD8+ T cell blasts.
Activated T cells were cultured for 4 hr in CM prior to stimulation with mouse IL-2 or IL-15 (10 ng/ml) or as indicated. Analyses were on CD8+ gated T cells. (A) Activation of pStat5 by IL-2 and IL-15. Data in (A) and (C) were normalized to maximal pStat5 induction by WT T cells which was that observed for IL-2 at 15 min. (B) Expression of the indicated cytokine receptor subunits after culture in IL-2 or IL-15. To the left are representative FACS histograms where the open histograms (dark line) represent WT cells and the shaded historgrams represent IL-15R−/− cells. Data to the right represent the mean fluorescent intensity (MFI) normalize to receptor subunit expression in the absence of IL-2 or IL-15. (C) IL-15-dependent pStat5 induction is dominated by cis-presentation as assessed by co-cultures of CD45.1 congenic WT and IL-15Rα−/− activated T cells stimulated with soluble IL-15. (D) WT activated T or B cells were treated with IL-15 (10ng/ml) at 4° C and each individually were mixed at an 1:1 ratio with IL-15Rα−/− T blasts and cultured for the indicated times at 37° C. Shown is pStat5 activation by the WT T blasts, representing cis presentation, or by the IL-15Rα−/− T blasts co-cultured with WT T or B blasts to promote trans-presentation, as indicated. Data in (A) and (B–D) represent 3 and 2 independent experiments, respectively. (E) pStat5 induction by anti-CD3 activated CD8+ T cells 15 min after the addition of the indicated concentrations of IL-2 or IL-15. Data are representative of 3 experiments.
After stimulation with IL-15, no reduction was noted for CD25, while CD122 and γc levels remained at ≥75% of that found for unstimulated cells. Thus, reduced IL-15-dependent signaling cannot be due to limiting levels of CD122 and γc. However, the levels of IL-15Rα were reduced by approximately 90% (Fig. 2B, right). Thus, for IL-15R-dependent signaling, IL-15Rα is the limiting subunit. This rapid loss of IL-15Rα prevents high affinity binding of IL-15 and provides a ready explanation for the more transient signal transduction associated with the IL-15R.
IL-15 induces signaling by both cis- and trans-presentation of IL-15/IL-15Rα to CD122 and γc (37). We assessed the extent cis versus trans-presentation accounts for activation of pStat5 by CD8+ T cells. First, when activated CD45.1-congenic wild-type (WT) and IL-15Rα−/− T cells were co-cultured with soluble IL-15, pStat5 was only activated in the WT T cells (Fig. 2C). Thus, cis presentation of IL-15 to CD122 and γc dominates the response to exogenous IL-15. Second, when WT T and B blasts were pre-treated with IL-15 and used to trans-present IL-15 to IL-15Rα −/− T blasts, only a minimal increase in pStat5 was noted for the IL-15Rα−/− T cells whereas pStat5 activation was readily noted by cis presentation for the pre-treated WT T blasts (Fig. 2D). These experiments demonstrate little trans-presentation occurs in vitro even when using activated B cells that express IL-15Rα, but do not respond to this cytokine due to the absence of CD122. In addition, increased levels of IL-15 supported strong pStat5 induction by WT, but not IL-15Rα−/−, CD8+ activated T cells, indicating that high IL-15 does not overcome the loss of cis-expressed IL-15Rα (Fig. 2E). Thus, trans-presentation of IL-15 to the IL-15R does not support strong sustained signal transduction as seen with IL-2R. Collectively, when compared to the IL-2/IL-2R interaction, our results indicate that IL-15R deliver quantitatively lower and distinguishable signal transduction in activated CD8+ T cells due to the limited expression of IL-15Rα.
Signal transduction by TEFF cells bearing mutant CD122
To further explore the consequences of varied CD122-dependent signaling, pStat5 and pS6 activation by anti-CD3 activated T cells bearing defined mutations within the cytoplasmic tail of CD122 were examined. These experiments utilized three lines of previously characterized transgenic mice on the CD122−/− genetic background whose T cells expressed CD122 where key tyrosine residues were mutated to phenylalanine. These mutations were in only Y341, (Y1), required for association of the adaptor protein Shc, in both Y395 and Y498 (Y2), required for optimal association of STAT5, or in all three of these tyrosine residues Y341, Y395, and Y498 (Y3). Past work showed that transgenic expression was restricted to T lineage cells, with levels similar to that expressed by control mice (22). In response to IL-2, signal transduction was attenuated, but not abrogated, with the least signaling associated with the Y3 mutations, leading to increasingly impaired IL-2-dependent T cell proliferation (22). Additionally, T cells from CD122−/− mice that expressed WT transgenic CD122 developed IL-2-dependent signaling, biological responses, and gene expression profiles that closely paralleled those by control mice (22) (and unpublished data). Therefore, normal B6 mice (designated as WT) served as the controls for these and all subsequent studies.
First, IL-2- versus IL-15-dependent signal transduction was compared for activated CD8+ T cells bearing these mutant CD122 molecules (Fig. 3A). Each CD122 mutant activated pStat5. For the Y1 mutation, initial pStat5 activation 15 min post-stimulation by IL-2 and IL-15 were very similar to each other and to that induced in activated CD8+ WT T cells whereas pStat5 activation by CD122 with the Y2 and Y3 mutations was similarly lower. Moreover, all 3 mutant CD122 molecules failed to sustain IL-2-dependent pStat5 activation and poorly activated pS6 to IL-2 or IL-15. This latter result is predicted for the Y1 and Y3 mutations, as Y341 is required for Shc association to CD122 that leads to activation of PI-3K/Akt and downstream activation of S6 (38). The poor activation of pS6 by the Y2 mutation is consistent with a requirement for optimal activation of Stat5 for induction of the PI-3K/Akt pathway, as previously reported (39). Overall, the Y1 mutation leads to signal transduction by IL-2 and IL-15 that approximates that induced in activated CD8+ T cells by IL-15. In contrast, signal transduction by the Y2 and Y3 mutations were similar, but lower than supported by IL-2 or IL-15.
Given the roles of IL-2 and IL-15 in promoting T cell growth and survival, we tested the extent these mutant CD122 molecules supported these activities in activated CD8+ T cells. Anti-CD3-activated T cells were prepared from mice expressing WT and mutant CD122 molecules and these T cell blasts were re-cultured with IL-2 or IL-15 for 48 hr. Cell recovery (Fig. 3B) and CD25 expression (Fig. 3C) decreased with increased number of tyrosine mutations. For the recovered cells, only the Y1 mutation supported protection from apoptosis (Fig. 3D) and increased levels of Bcl-2 (Fig. 3E) that were comparable to that found for WT T cells. Thus, survival, but not clonal expansion, of activated CD8+ T cells is readily supported by weak IL-15-like signal transduction associated with the Y1 mutation of CD122.
Primary and recall responses by CD122-deficient CD8+ T cells
The distinctive activation of pSTAT5 and pS6 by CD8+ T cells bearing mutant CD122 provides a direct approach to assess the contribution of varied CD122-dependent signaling strength for a CD8-dependent immune response in vivo. However, we first established the requirement for CD122 signaling for primary and recall responses to OVA257–264 by CD122−/− OT-I T cells. To prevent autoimmunity that is associated with CD122−/− mice, the OT-I CD122−/− mice received WT Treg cells at birth (40). WT or CD122−/− OT-I T cells were adoptively transferred to syngeneic CD45.1 B6 mice, to facilitate identification of the donor cells, and 1 day later the mice were immunized with OVA257–264 and LPS.
The CD122−/− OT-I T cells expanded and contracted (Fig. 4A) and developed into IFNγ- and IL-2-secreting TEFF cells (Fig. 4B) comparable to WT OT-I T cells. However, granzyme B expression was markedly lower (Fig. 4C). In addition, WT and CD122−/− OT-I T cells exhibited a largely similar cell surface phenotype on day 2, 3 and 7 post-challenge, including similar proliferative status, as assessed by Ki67, and expression of pro-survival Bcl-2 (Fig. 4D). This analysis also showed that CD25 was detected at a low level only on day 2 post-immunization, suggesting that the WT OT-I T cells can only respond to IL-2 at this time. In addition, on day 3 WT OT-I T cells were characterized by increased CD27lo cells, consistent with somewhat greater activation.
Fig. 4. Primary responses by CD122-deficient OT-I T cells.
WT or CD122−/− OT-I T cells were adoptively transferred to CD45.1-congenic mice and immunized with OVA257–264 and LPS. OT-I T cells were enumerated based on expression of CD45.2 and CD8. (A) Time-course of the primary response by FACS evaluation the % donor CD8+ CD45-2+ T cells as a fraction of the total number of CD8+ T cells in the spleen at the indicated time post-immunization. At day 3 post-immunization, production of IFNγ and IL-2 (B) and granzyme B (C) by the indicated OT-I T cells was assessed ex vivo by intracellular FACS analysis after stimulating spleen cells with OVA257–264. (D) At the time post-immunization, the OT-I T cells were analyzed for the indicated markers by FACS. Data are representative of 3 experiments.
Although CD122−/− CD8 T cells readily generated a largely normal primary response, they were not maintained long-term (Fig. 4A), most likely because they did not receive survival and homeostatic signals through IL-15 (7). By day 7 post-challenge after initial contraction, several phenotypic changes were apparent between WT and CD122−/− OT-I T cells, where the latter cells were more prominently CD62Lhi, Ly-6Clo, and Ki67neg (Fig. 4D). The lower percentage of Ki67+ CD122−/− OT-I T cells is consistent with a lower proliferative rate with time post-challenge and represents a contributory factor for the sharper reduction in these antigen-activated T cells. These findings suggest potential intrinsic problems with CD122−/− OT-I T cells.
Since CD122−/− T cells were not maintained long-term, i.e. day 30, properties of WT and CD122−/− OT-I cells were assessed at an intermediate time point after initial contraction. By day 15 post-challenge, the relative numbers of CD122−/− OT-I T cells were severely reduced and barely detectable, but after re-challenge, these few CD122−/− OT-I T cells readily expanded (Fig. 5A). Although the absolute relative numbers remained lower than WT cells, the overall extent of expansion by CD122−/− OT-I T cells was significantly greater than WT OT-I T cells (Fig. 5B). However, the function and phenotype of the recalled CD122−/− OT-I T cells were not entirely normal. WT OT-I T cells were characterized by increased percentage of IFNγ producing cells (Fig. 5C) and CD62Llo, CD127lo, and Klrg1+ cells (Fig. 5D), markers of highly activated TEFF cells, and. Overall, these results demonstrate that CD122−/− OT-I T cells are not impaired in their capacity to elicit recall responses characterized by extensive expansion, but may harbor intrinsic defects related to development of highly activated TEFF cells. The expression of Ly-6Chi is a marker of memory CD8+ T cells (41). CD122−/− OT-I T cells were distinctively characterized by a higher proportion of Ly6Clo cells (Fig. 5D). This result raises the possibility that expression of memory cell properties require CD122 signaling.
Fig. 5. Recall responses by CD122-deficient OT-I T cells.
(A) At day 15 post-immunization, the engraftment of persistent primary or secondary recalled OT-I evaluated by FACS as the % donor CD8+ CD45-2+ T cells within the total number of CD8+ T cells in the spleen. (B) The fold expansion of the secondary recalled OT-I T cells in relationship to the persistent primary cells was calculated. (C) Production of IFNγ and IL-2 by the recalled OT-I T cells was determined by intracellular cytokine staining. (D) FACS analysis of the recalled WT and CD122−/− OT-I T cells. The bar graphs display the percent of cells with effector phenotype (CD62Llo, CD127lo, LY6Clo, and KLRG1+). Data represent the mean ± SEM of 3 to 5 mice per group derived from at least three independent experiments.
CD122 signaling thresholds and the survival of CD8+ T memory cells
Two immediate questions arise concerning the lack of CD122−/− T cells by 30 day post primary antigenic challenge. First, to what extent is this abnormality simply due to the lack of a CD122-dependent survival signals? Since Bcl-2 is an important target of IL-2 and IL-15 signaling (14, 42), this issue was directly examined by evaluating the extent transgenic Bcl-2 expression in CD122−/− OT-I T cells promoted persistent T cells. The second question is to what extent does varied CD122 signaling support CD8+ memory development/survival? This was approached by determining the extent that OT-I T cells that bear mutant CD122 supported persistent OT-I memory cells. Here we utilized the transgenic Y1 and Y3 CD122 mutants on the CD122−/− genetic background as these varied in Bcl-2 expression and CD8 T cell survival as well as the levels of IL-2- and IL-15-driven signaling in vitro (Fig. 3). We confirmed that at the peak of the primary response, the expansion each of these types of OT-I T cells was similar to each other and to WT and CD122−/− OT-I T cells (Fig. 6A).
Fig. 6. Low CD122 signaling supports the survival of CD8+ memory cells.
The indicated OT-I T cells were adoptively transferred to CD45.1-congenic mice and immunized with OVA257–264 and LPS. OT-I T cells were enumerated based on expression of CD45.2 and CD8. (A) Expansion of OT-I T cells day 3 post primary immunization. (B) The persistence of OT-I T cells 30 days post primary response. Recalled secondary OT-I T cell numbers (C) and fold-expansion (D) 30 days after the primary immunization that were re-challenged with OVA257–264 and LPS 3 days previously, i.e. on day 27. Ki67 expression was determined by FACS analysis of the persistent (E) and recalled (F) OT-I T cells. (G) Expression of endogenous mouse Bcl-2 by FACS analysis. The MFI from OT-I T cells was normalized to the MFI from the recipient CD8 T cells and displayed as percent Bcl-2 expression levels. Data are the mean ± SEM ≥ 4 mice/group derived from at least three independent experiments with the exception of Fig. 6E, F and G for mutant Y1 where 1–2 mice were used.
30 days after the primary challenge, similar relative numbers of persistent Y1, Y3, and CD122−/−Bcl2Tg OT-I T cells were found that were much higher (p<0.05, ANOVA) than detected for CD122−/− OT-I T cells. These numbers, however, for Y3 and CD122−/−Bcl2Tg OT-I T cells were significantly lower (p<0.05, ANOVA) than detected for WT OT-I T cells (Fig. 6B). Thus, either weak CD122-dependent signaling or pro-survival Bcl-2 in the absence of IL-2 or IL-15 signaling supported retention of antigen-activated OT-I T cells, although it was not always entirely normal. Upon secondary recall, the relative numbers of WT, Y1, Y3, and CD122−/−Bcl2Tg OT-I T cells each increased by 5 to 10 fold (Fig. 6C, D). Only CD122−/− OT-I T cells showed significantly lower overall expansion. The lower expansion by CD122−/−Bcl2Tg OT-I T cells was not secondary to transgenic human Bcl-2 because expansion by WTBcl2Tg OT-I T cells was comparable to non-transgenic control WT OT-I T cells.
To evaluate the basis by which mutant CD122 and ectopic Bcl-2 increased the persistence and recall of OT-I T cells when compared to CD122−/− OT-I T cells, the fraction of cells in cell cycle was assessed by determining the expression of the proliferative marker Ki67. For the persistent cells prior to recall, only a small fraction (1–3%) of the cell were Ki67+ (Fig. 6E). However, this homeostatic proliferation was significantly lower for Y3 and CD122−/−Bcl2Tg OT-I T cells and a comparable low percentage of Ki67+ cells was noted for one Y1 mouse that was similarly analyzed. The detection sometimes of fewer persistent cells from OT-I T cells with impaired or absent CD122 signaling may in part be related to lower homeostatic proliferation. In marked contrast, all groups of OT-I T cells exhibited substantial and similar proliferation upon re-challenge (Fig. 6F). Collectively, these results indicate that CD122 signaling importantly contributes to homeostatic proliferation but is not required to drive proliferation of memory cells after secondary antigen encounter.
Given the importance of CD122 signaling in contributing to expression of Bcl-2 (14, 42), we also evaluated the levels of expression of endogenous mouse Bcl-2 by the persistent OT-I T cells 30 days post-primary immunization. Y1 and Y3 OT-I T cells expressed endogenous Bcl-2 similar to WT cells (Fig. 6G). It was not possible to directly assess reliably Bcl-2 expression by the persistent CD122−/− OT-I T cells due to their few numbers. However, mouse-Bcl-2 expression is reduced by 50% in CD122−/−Bcl-2Tg OT-I T cells. These results confirm the requirement for CD122 signaling for optimal expression of pro-survival Bcl-2. In CD122−/−Bcl-2Tg OT-I T cells, the human transgenic Bcl-2 was readily detected in these cells (Fig. S2). This finding and the normal levels of mouse Bcl-2 in Y1 and Y3 OT-I T cells demonstrate another critical role for CD122 signaling for memory T cells is to support proper expression of Bcl-2, which is achieved by relatively weak CD122 signaling.
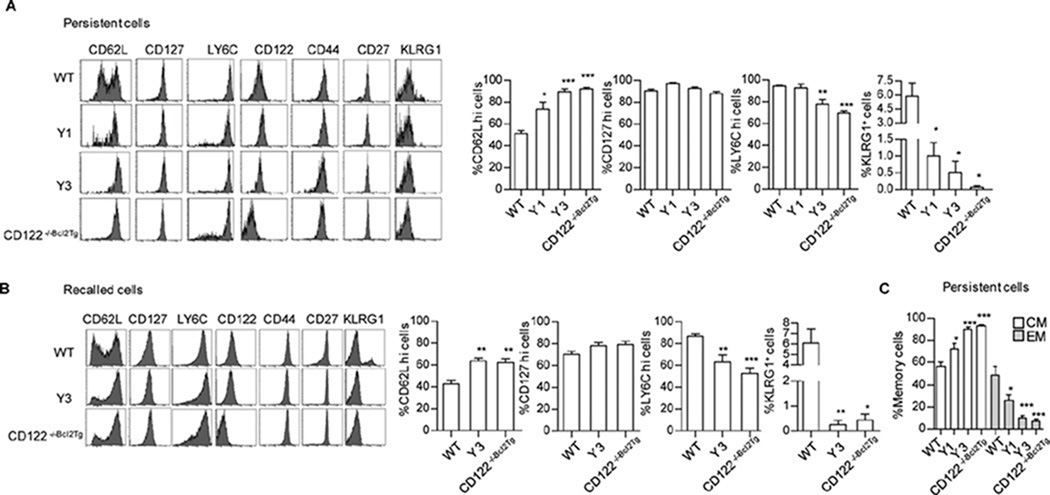
The development of TCM and TEM depends on varied CD122 signaling
To determine whether the lack of proper CD122 signaling affected other activities associated with the persistent cells, memory phenotype and function were tested before and after secondary recall. Representative FACS histograms used to quantify the persistent and recalled cells are shown for these phenotypic and functional analyses. For the persistent cells, in comparison to WT OT-I T cells, Y1, Y3, and CD122−/−Bcl2Tg OT-I T cells all showed a significantly higher and dominant percentage of CD62Lhi cells and these were essentially all CD127hi, a phenotype characteristic of TCM cells (Fig. 7A). Therefore, using these markers, WT OT-I cells were a near equal mix of TCM and TEM cells whereas with no (CD122−/−Bcl2Tg) or the weak (Y3) CD122 signaling, nearly all the persistent cells were TCM cells. The Y1 mutant, which exhibits CD122-dependent signaling similar to that induced by IL-15, still favor TCM cells (Fig. 7C). These results indicate that strong CD122 signaling is required for full TEM development while low to no CD122 signaling support TCM traits.
Fig. 7. TEM development requires greater CD122-dependent signaling than TCM survival.
30 day after antigen priming, phenotype was analyzed for the indicated persistent and secondary recalled (3 days after recall) from the indicated OT-I memory T cells. OT-I T cells were enumerated based on expression of CD45.2 and CD8. Cell surface expression of the indicated markers by FACS analysis was determined for the persistent (A) and recalled (B) OT-I memory cells. Shown are representative histograms and mean ± SEM for selected markers. (C) The distribution of persistent TEM (CD62Llo CD127hi) and TCM (CD62Lhi CD127hi). Data represent the ≥ 4 mice per group derived from at least three independent experiments.
The Ly-6Chi memory marker was also somewhat lower on persistent CD122−/−Bcl2Tg and Y3 OT-I T cells, suggesting some defect in full memory development (Fig. 7A). There were very few Klrg1+ Y1, Y3, and CD122−/−Bcl2Tg OT-I T cells, associating a requirement for strong CD122 signaling for this subpopulation of antigen-experienced cells, where low expression of Klrg1 is related to memory precursor cells (11). The secondary recalled TEFF cells mirrored these phenotypic properties as WT OT-I T cells were characterized by a greater fractions of CD62Llo, Ly-6Chi, Klrg1+ cells (Fig. 7B). These differences likely reflect some intrinsic defects in memory development as a consequence of impaired CD122 signaling.
When examined 5 hr after ex vivo re-stimulation, the persistent Y1, Y3, CD122−/−Bcl2Tg OT-I T cells contained fractions of cytokine producing cells that were largely similar to WT OT-I T cells (Fig. 8A), including the levels of IFNγ as assessed by the MFI. However, when compared to WT OT-I T cells, the secondary recalled response was somewhat attenuated, especially for TNFα by Y3 and CD122−/−Bcl2Tg OT-I T cells (Fig. 8B). Moreover, although the % IFNγ+ cells were not significantly different, all recalled OT-I T cells with impaired CD122 signaling produced lower levels of IFNγ based on the MFI of the IFNγ+ cells. Thus, in the absence of full CD122 signaling, the persistent cells contain some defects in the expression of surface markers and functional activities.
Fig. 8. Cytokine production by persistent and recalled memory OT-I cells.
30 day after antigen priming, the functional activity was determined for the persistent (A) and the secondary recalled (B) from the indicated OT-I memory cells. Shown are representative FACS contour plots (left), mean ± SEM ≥ 4 mice per group derived from at least three independent experiments (middle), and the MFI of the IFNγ+ cells.
IL-2 and IL-15 induced signaling by primary and memory OT-I T cells
To relate the developmental differences in TEM and TCM upon varied CD122 signaling, we examined the expression of IL-2R and IL-15R subunits and pStat5 activation upon stimulation with IL-2 or IL-15 at various times after antigen challenge. At 2 days post challenge, OT-I T cells exhibited increased levels of CD25, CD122, γc and IL-15Rα (Fig. 9A). However, only a fraction (approximately 40%) of the OT-I cells expressed CD25 while the other subunits were uniformly increased on all cells. At 3 days post-challenge, this induction return to baseline for CD25 and γc and was reduced for IL-15Rα, while high levels of CD122 remained. For the persistent cells 30 days post-challenge, increased levels of CD122 and IL-15Rα were observed.
Fig. 9. Cytokine receptor expression and pStat5 activation by antigen-primed OT-I T cells directly ex vivo.
At the day indicated post challenge, WT or Y3 CD122 mutant OT-I T cells were analyzed for expression of IL-2R and IL-15R subunits (A) or induction of pStat5 (B–D) by mouse IL-2 or IL-15 (10 ng/ml). OT-I T cells were enumerated based on expression of CD45.2 and CD8. (A) Representative FACS histograms of the indicated receptor subunit. The number in the histogram represents the MFI of the total cell population. Representative histograms (left) and the %Stat5+ cells (right) for WT (B) and Y3 (C) OT-I T cells stimulated with IL-2 or IL-15. (D) The MFI of Stat5 expression was assessed for only the Stat5+ OT-I cells 2 day post antigen challenge. Representative MFI values are shown in the histograms in Fig. 10B. All data are representative of 6 mice/group from 2 independent experiments, except analysis of IL-15Rα expression (A) and pStat5 activation by Y3 OT-I T cells (D), which are representative of 3 mice/group.
When these same cells were activated with IL-2 or IL-15 directly ex vivo, substantial IL-2 induced pStat5 activation was only noted at day 2 post challenge whereas IL-15 activated pStat5 at all time points (Fig. 9B). For IL-2-induced signaling at day 2, bimodal IL-2-dependent pStat5lo and Stat5hi activation was observed, which corresponded to the CD25neg/lo and CD25hi cells. These results directly correlate pStat5 activation with the level of expression of the high affinity IL-2R and IL-15R. When comparing total pStat5 activation by IL-2 and IL-15 on day 2, IL-15 supports greater signaling. However, if only pStat5hi signaling by IL-2 is considered, these cells show more sustained pStat5 than induced by IL-15 (Fig. 9D). These latter finding are consistent with our in vitro findings that showed that the initiation of pStat5 activation (15min) for IL-2 and IL-15 were almost identical whereas after more prolonger cytokine signaling (60 min) IL-2 dependent pStat5 activation remained high but L-15 dependent pStat5 was more transient. Furthermore, similar to responses in vitro (Fig. 3A), at day 2 post-challenge, OT-I T cells from the CD122 Y3 mutant showed reduced capacity to sustain IL-2 and IL-15-dependent pStat5 activation (Fig. 9C) when compared to WT OT-I T cells (Fig. 9B). Collectively, these data demonstrate that in vivo activated OT-I T cells have a restricted period of time to respond to IL-2, but not IL-15. For cells expressing readily detectable CD25, IL-2-induced pStat5 activation is more sustained than IL-15-induced pStat5. Moreover, the minimal pStat5 activation by cells expressing undetectable CD25 further indicates the strength of IL-2- and IL-15-dependent signaling is regulated by levels of a limiting receptor subunit. These latter points are in agreement with results shown for IL-2R and IL-15R on in vitro activated CD8+ T cells.
TEM development is supported by IL-2
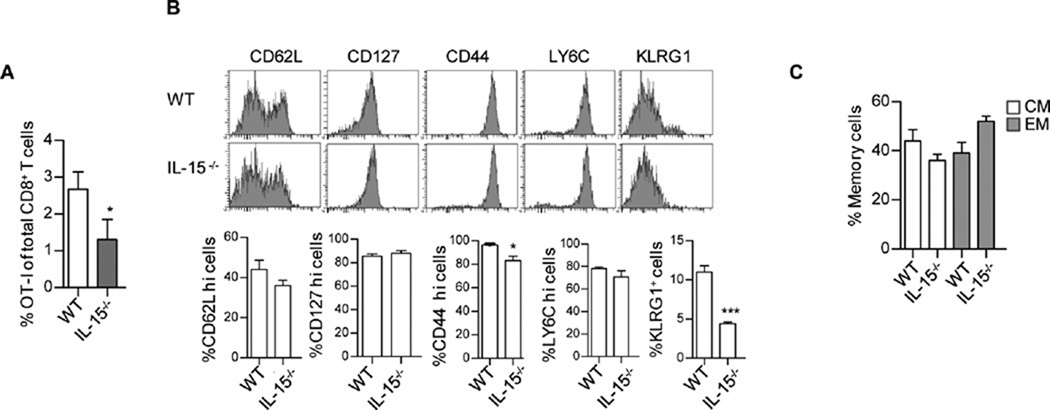
The sustained expression of high affinity IL-15R and induction of pStat5 by IL-15 at all times post-antigen challenge is in line with the role in supporting CD8 memory survival and homeostasis. However, this observation raises the point that the development of TEM might also depend on IL-15 rather than IL-2 signaling. To directly test this point, we examined OT-I memory development in IL-15-deficient recipients. At 17 day post challenge, fewer OT-I T cells were detected in IL-15-deficient hosts, as expected, due to the requirement for IL-15 in CD8 memory survival (Fig. 10A). However, for those cells detected, a normal distribution of TEM and TCM were seen as assessed by expression of CD62L and CD127 (Fig. 10B, C). However, there was a significant decrease in the persistence of KLRG1+ cells (Fig. 10 B and C) which were also CD62Llo CD127lo cells (not shown) in the absence of IL-15. These findings demonstrate that IL-2 is sufficient for the development of TEM cells, whereas IL-15 is required for production of persistent Klrg1+ OT-I TEFF cells after challenge with OVA257–264 and LPS.
Fig. 10. IL-15 is not required for optimal TEM development.
CD45.1-OT-I T cells were adoptively transferred into WT or IL-15−/− recipient mice and challenged with OVA257–264 and LPS 24 hr later. 17 days post challenge, the level and phenotype of the persistent cells was determined. (A) The representation of OT-I T cells in the total CD8+ T cell compartment was determined for the spleen. OT-I T cells were enumerated based on expression of CD45.1 and CD8. (B) Representative histograms and mean ± SEM are shown for the indicated markers after gating on OT-I T cells. (C) The distribution of TCM and TEM by enumerating the proportions of CD62Lhi CD127hi and CD62Llo CD127hi OT-I cells, respectively. Data are representative of 4 mice/group.
Discussion
Previous reports have documented that IL-2 and IL-15 distinctively affect signaling, biosynthetic pathways, proliferation, and function of antigen-activated CD8+ T cells in vitro (8, 30). These cytokines also play important roles for CD8+ T cell immune responses in vivo (1–3, 43). How IL-2 and IL-15 result in these distinct activities in CD8+ T cells while depending on identical CD122 and γc receptor subunits to initiate signal transduction has remained elusive. Varied signaling strength has been suggested to discriminate some activities ascribed to IL-2 versus IL-15 (9, 10, 21). However, there is also a lack of direct data that examines the outcome of CD8 T cell responses in vivo as a consequence of varied signaling through CD122 and γc.
One main point of our study, therefore, is that we show that initial proximal signaling induced by IL-2 and IL-15 varies for antigen-activated CD8+ T cells. We found that initial Stat5 and PI-3K/Akt activation, the latter assessed by pS6, by IL-2 and IL-15 was similar. Importantly, only IL-2 sustained activation of these two pathways. The weaker signaling by IL-15 was not due to more rapid uptake of cytokine because increasing IL-15 did not restore signaling or to selective depletion, exhaustion, or desensitization of signaling intermediates because these pathways in IL-15-treated cells were readily activated by IL-2. Rather, expression of IL-15Rα was found to be limiting, providing a mechanism to quickly temper IL-15R-signaling induced by physiological concentration of IL-15.
Similar to another study (33), IL-15-dependent signal transduction by activated CD8+ T cells in vitro was most evident after cis presentation of IL-15/IL-15Rα to CD122 and γc. However, in vivo trans-presentation is the main means by which IL-15 induces signaling in target populations, including CD8+ T cells (43, 44). There are several reasons to suggest that trans-presented IL-15 also mediates weak signaling through CD122 and γc. First, our attempts to induce IL-15-dependent signaling through trans-presentation in vitro yielded much lower Stat5 activation than cis presented IL-15. Second, although expression of IL-15Rα is widely distributed in vivo, the level of expression is low. Thus, limiting IL-15Rα leading to transient weak IL-15-induced signaling after cis presentation is also a likely result from trans-presented IL-15. Lastly, the cytoplasmic tail of IL-15Rα enhances signaling by CD122 and γc when IL-15 is presented in cis but not in trans (33). Our in vitro experiments, therefore, if anything may have somewhat over-estimated IL-15R signaling by activated polyclonal or OT-I CD8+ T cells.
Considering these points in the context of IL-2- and IL-15-dependent signaling under physiological conditions, the lack of a cytoplasmic domain for IL-2Rα and trans-presentation of IL-15 by IL-15Rα to CD122 and γc results in a qualitative identical utilization of signaling pathways associated with CD122 and γc. High levels of IL-2Rα provide a mean for continual capture of IL-2 to sustain signaling whereas limiting IL-15Rα tempers engagement of CD122 and γc, limiting signal transduction. Thus, varied levels of IL-2Rα and IL-15Rα provide a simple, yet powerful, mechanism to quantify and tune signaling through common intermediates leading to distinctive biological responses.
Our study also showed that these basic tenets hold for the potential of antigen-activated CD8+ T cells to response to IL-2 and IL-15 in vivo, but with some important caveats. First, using peptide and LPS as an immunogen, only a fraction of the activated OT-I cells expressed a relative high level of CD25 that was very transient, i.e. only observed on day 2 post primary challenge, and was considerably lower than seen for in vitro activated T cells. In contrast, IL-15Rα was induced uniformly at a relatively low level that persisted for at least 30 days. Thus, antigen activated CD8+ T cell have a very restricted period of time when they can respond to IL-2 whereas they are poised for an extent period of time to response to either cis or trans-presented IL-15 that likely facilitates signaling for homeostatic maintenance. When CD8+ T cells are stimulated with infectious agents in vivo, CD25 levels are also expressed transiently, but for a somewhat more extended time (19, 21). Similar to the in vitro responses, for those OT-I T cell that expressed high CD25 in vivo, IL-2 induced more sustained pStat5 activation than IL-15. However, IL-2-induced pStat5 was weaker than that supported by IL-15. Thus, the level of IL-2R and IL-15R signaling strength is also predicted by the levels of their respective α-chains.
A second important point of our study is that we directly investigate the role of CD122 signaling strength and pro-survival signaling for antigen-driven immune response in vivo. First, we established the baseline requirement for CD122-dependent signaling. These experiments further emphasize that primary immune responses are readily generated in the absence of both IL-2 and IL-15 as assessed for CD122-deficent OT-I T cells. However, in the absence of CD122, primary TEFF cells that produce granzyme B or express Klrg1 were impaired and the primary activated OT-I T cells did not persist. The former activities have been shown to primarily depend on IL-2 and the latter on IL-15 (10, 14, 21).
Given the largely normal primary response, we stressed examining the role of CD122 signaling for memory cells. Providing a pro-survival signal through transgenic Bcl-2 in CD122−/− OT-I T cells or weak CD122-dependent Stat5 activation through expression CD122 with the Y3 mutation in OT-I T cells substantially improved the development of memory cells. These memory cells were nearly exclusively TCM cells. This finding provides direct data that weak CD122-dependent signaling is sufficient to drive development of TCM cells and that this is largely explained by induction of pro-survival Bcl-2. However, evaluation of Ki67 expression indicates that homeostatic proliferation by these persistent cells requires more extensive signaling. This might represent a defect in IL-15-driven homeostatic proliferation by the mutant CD122s or a requirement for IL-2 to program this aspect of a TCM response. As the Y1 CD122 mutation approximates signal transduction induced by IL-15, the development of a substantial TCM response appears only to require survival signals through CD122 supported by IL-15. Moreover, these mutants are poor activators of the PI-3K/Akt pathway consistent with Stat5 signaling as the main driver for TCM survival. Indeed, TCM development is favored when CD8+ immune responses are generated in vivo in the presence of rapamycin (45), which blocks TORC1, an important down-stream mediator of the PI-3K/Akt pathway.
In the complete absence of IL-2 and IL-15 signaling, a few CD122−/− TCM phenotypic cells are detected that readily generate substantial recall expansion and secondary TEFF cells at 15 and 30 days post-priming, although expansion at day 30 was more limited. Thus, for the most part, our data support the idea that TCM do not require an IL-2 programming for functional responses but rather require IL-15 for their maintenance. However, we did note some phenotypic alterations in CD62L, Ly-6C, and Klrg1 expression and in cytokine responses by secondary TEFF cells, consistent with some intrinsic problem in these cells without normal CD122 signaling. Similar phenotypic difference as well as altered quality of the resulting cytokine responses has also been noted in secondary CD8+ TEFF response to LCMV in the absence of IL-2R signaling (18). In addition, not only was the survival, but the composition of the secondary CD8+ memory pool to Listeria monocytogenes altered, with fewer TEM cells in the absence of IL-15 (15). These results suggest that some signaling through CD122 optimizes aspects of T memory responses independent of survival/homeostasis.
Another important conclusion from our work is that TEM development depends on CD122 signaling greater than required for TCM cells and this primarily depends on IL-2R signaling during the primary response. The development of TEM was significantly impaired in both the Y1 and Y3 IL-2Rβ mutations, the former which supports IL-2R signaling similar to that by WT IL-15R. Furthermore, antigen-activated OT-I T cells readily developed into normal proportions of TEM and TCM in the absence of IL-15, directly demonstrating that IL-2R signaling is sufficient to promote TEM production that only readily occurred during the initial two days after antigen challenge. The proportion of TEM found in WT and Y3 OT-I T cells also nicely correlated with the fraction of OT-I T cells on day two that supported high IL-2-induced pStat5 activation. Past studies that implicated IL-2 in CD8+ memory suggested that IL-2 signaling during priming was not required for memory development but for programming secondary recall responses (16). More recent studies, including ours, challenge and refine this view. In one study recalled expansion of memory cells was noted without IL-2R signaling (19). As noted herein, this effect may be due to the production of TCM due to weak IL-15-like CD122 signaling. In addition, mostly CD62Lhi TCM cells were readily generated when antigen and IL-2 signals were limited (46). Furthermore, in the absence of IL-2 signaling, the main effect was impaired development of TEM cells (18).
Although it is relatively easy to study the requirements for IL-2 or IL-15 for T cell immunity, it is much more difficult to investigate role IL-2 or IL-15 signaling strength in mediating such responses in vivo. Recent work addressing this question has suggested that weak IL-2 signaling favors persistent memory cells while stronger IL-2R signals favor terminal differential of Klrg1+ short lived effector cells (10, 21). These conclusions were based on the in vivo behavior of T cells that were first primed in vitro in the presence of low versus high IL-2 or in vivo primed T cells that were isolated and fractionated based on low versus high IL-2Rα expression and then transferring these cells into a secondary recipient primed with antigen in parallel to the donor cells. Important limitations of these studies are the extent that in vitro priming recapitulated the in vivo situation and the extent IL-2Rαhi and IL-2Rαlow expression actually represents cell development as a function of varied IL-2R signaling strength rather than IL-2-independent differences marked by varied levels of IL-2Rα. Thus, our study establishes that varied signaling strength controls distinct aspects of the CD8+ T memory response and extends previous work by separating signaling strength requirements for memory survival/homeostasis and development of TEM cells.
In conclusion, based on our study and that of others (10, 21), there appears to be three critical ranges of CD122-dependent signaling for a normal CD8+ T cell immune response. Weak IL-15-like signals support TCM development and homeostasis. Stronger CD122 signaling mediated primarily by IL-2 is required to support TEM cells. This signaling most likely represents an IL-2-dependent developmental trigger for TEM cells rather than a requirement for homeostasis because persistent TEM cells do not express IL-2Rα and cannot use IL-2 for their long-term survival. Lastly, high IL-2R signaling is required for generation of short-lived terminally differentiated TEFF cells. An important question, therefore, is how IL-2 and IL-15 levels are coordinately regulated in vivo to normally allow the production and maintenance of these key cell populations during and after a primary immune response. The availability of cytokines and overall levels of receptors subunit represent critical determinants that influence the strength of IL-2 and IL-15 signaling.
Supplementary Material
Acknowledgments
We thank Dr. Jing Yang for expert technical assistance and Dr. Oliver Umland of University of Miami Diabetes Research Institute Flow Cytometry Core for skilled assistance with FACS analysis and flow panel design for 9 color staining.
This work was supported by National Institutes of Health Grants AI040114 and CA109094.
Abbreviations used in this paper
- TCM
Central memory T cells
- TEff
Effector T cells
- TEM
Effector memory T cells
- MFI
Mean Fluorescence Intensity
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 2.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 4.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 7.Boyman O, Letourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naive and memory T cells. Eur J Immunol. 2009;39:2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 8.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 9.Manjunath N, Shankar P, Wan J, Weninger W, Crowley MA, Hieshima K, Springer TA, Fan X, Shen H, Lieberman J, von Andrian UH. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J Clin Invest. 2001;108:871–878. doi: 10.1172/JCI13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 15.Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 184:35–44. doi: 10.4049/jimmunol.0803355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci U S A. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 21.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Yu A, Zhu L, Altman NH, Malek TR. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity. 2009;30:204–217. doi: 10.1016/j.immuni.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268::1472–-1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 24.Dalyot-Herman N, Bathe OF, Malek TR. Reversal of CD8+ T cell ignorance and induction of anti-tumor immunity by peptide-pulsed APC. J Immunol. 2000;165:6731–6737. doi: 10.4049/jimmunol.165.12.6731. [DOI] [PubMed] [Google Scholar]
- 25.Malek TR, Yu A, Scibelli P, Lichtenheld MG, Codias EK. Broad programming by IL-2 receptor signaling for extended growth to multiple cytokines and functional maturation of antigen-activated T cells. J Immunol. 2001;166:1675–1683. doi: 10.4049/jimmunol.166.3.1675. [DOI] [PubMed] [Google Scholar]
- 26.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer Res. 2008;68:2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 27.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 28.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 29.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 30.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 31.Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays. 2006;28:362–377. doi: 10.1002/bies.20380. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z, Xue HH, Bernard J, Zeng R, Issakov D, Bollenbacher-Reilley J, Belyakov IM, Oh S, Berzofsky JA, Leonard WJ. The IL-15 receptor {alpha} chain cytoplasmic domain is critical for normal IL-15Rα function but is not required for trans-presentation. Blood. 2008;112:4411–4419. doi: 10.1182/blood-2007-03-080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemar A, Subtil A, Lieb M, Morelon E, Hellio R, Dautry-Varsat A. Endocytosis of interleukin 2 receptors in human T lymphocytes: distinct intracellular localization and fate of the receptor α, β, and γc chains. J Cell Biol. 1995;129:55–64. doi: 10.1083/jcb.129.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumaki S, Ochs HD, Timour M, Schooley K, Ahdieh M, Hill H, Sugamura K, Anderson D, Zhu Q, Cosman D, et al. Characterization of B-cell lines established from two X-linked severe combined immunodeficiency patients: interleukin-15 binds to the B cells but is not internalized efficiently. Blood. 1995;86:1428–1436. [PubMed] [Google Scholar]
- 36.Yu A, Malek TR. The proteasome regulates receptor-mediated endocytosis of interleukin-2. J Biol Chem. 2001;276:381–385. doi: 10.1074/jbc.M007991200. [DOI] [PubMed] [Google Scholar]
- 37.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 38.Hunt AE, Lali FV, Lord JD, Nelson BH, Miyazaki T, Tracey KJ, Foxwell BM. Role of interleukin (IL)-2 receptor β-chain subdomains and Shc in p38 mitogen-activated protein (MAP) kinase and p54 MAP kinase (stress-activated protein Kinase/c-Jun N-terminal kinase) activation. IL-2-driven proliferation is independent of p38 and p54 MAP kinase activation. J Biol Chem. 1999;274:7591–7597. doi: 10.1074/jbc.274.11.7591. [DOI] [PubMed] [Google Scholar]
- 39.Lockyer HM, Tran E, Nelson BH. STAT5 is essential for Akt/p70S6 kinase activity during IL-2-induced lymphocyte proliferation. J Immunol. 2007;179:5301–5308. doi: 10.4049/jimmunol.179.8.5301. [DOI] [PubMed] [Google Scholar]
- 40.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 41.Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C−) to TCR/CD8 signaling in response to antigen. J Immunol. 1998;160:3236–3243. [PubMed] [Google Scholar]
- 42.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 43.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 44.Mortier E, Advincula R, Kim L, Chmura S, Barrera J, Reizis B, Malynn BA, Ma A. Macrophage- and dendritic-cell-derived interleukin-15 receptor α supports homeostasis of distinct CD8+ T cell subsets. Immunity. 2009;31:811–822. doi: 10.1016/j.immuni.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 45.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obar JJ, Lefrancois L. Early signals during CD8 T cell priming regulate the generation of central memory cells. J Immunol. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.