Abstract
Although movement impairment in Parkinson’s disease includes slowness (bradykinesia), decreased amplitude (hypokinesia), and dysrhythmia, clinicians are instructed to rate them in a combined 0–4 severity scale using the Unified Parkinson’s Disease Rating Scale motor subscale. The objective was to evaluate whether bradykinesia, hypokinesia, and dysrhythmia are associated with differential motor impairment and response to dopaminergic medications in patients with Parkinson’s disease. Eighty five Parkinson’s disease patients performed finger-tapping (item 23), hand-grasping (item 24), and pronation–supination (item 25) tasks OFF and ON medication while wearing motion sensors on the most affected hand. Speed, amplitude, and rhythm were rated using the Modified Bradykinesia Rating Scale. Quantitative variables representing speed (root mean square angular velocity), amplitude (excursion angle), and rhythm (coefficient of variation) were extracted from kinematic data. Fatigue was measured as decrements in speed and amplitude during the last 5 seconds compared with the first 5 seconds of movement. Amplitude impairments were worse and more prevalent than speed or rhythm impairments across all tasks (P < .001); however, in the ON state, speed scores improved exclusively by clinical (P < 10−6) and predominantly by quantitative (P < .05) measures. Motor scores from OFF to ON improved in subjects who were strictly bradykinetic (P < .01) and both bradykinetic and hypokinetic (P < 10−6), but not in those strictly hypokinetic. Fatigue in speed and amplitude was not improved by medication. Hypokinesia is more prevalent than bradykinesia, but dopaminergic medications predominantly improve the latter. Parkinson’s disease patients may show different degrees of impairment in these movement components, which deserve separate measurement in research studies.
Keywords: Parkinson’s disease, bradykinesia, UPDRS, MBRS, KinetiSense
Clinicians must consider multiple aspects of movement including speed, amplitude, hesitations, fatiguing, and arrests in movement when assigning a single severity score in the Unified Parkinson’s Disease Rating Scale motor subscale (UPDRS-III). The modified bradykinesia rating scale (MBRS) was introduced to independently rate the movement impairments of speed, amplitude, and rhythm.1 Each of these movement components is given a score from 0 to 4 for each of tasks 23–25 of the UPDRS-III (finger taps, hand grasps, rapid alternating movements, respectively). The MBRS has demonstrated inter- and intrarater reliabilities similar to those of the UPDRS.2
Accurate assessment of movement impairment is necessary to ascertain the motor state and monitor response to standard and experimental therapeutic interventions. Several studies have shown that certain therapies may differentially improve specific components of movement impairment. For example, amplitude improves during finger taps postpallidotomy, but not speed or rhythm.3 Also, amplitude but not speed improves during bimanual compared with unimanual finger tapping.1 An example of the complexities in interpreting studies using the “bradykinesia- related” items of the UPDRS-III as an independent outcome in clinical trials is illustrated by a trial of a serotonin reuptake inhibitor antidepressant, which determined that “citalopram did not affect rigidity and tremor, but significantly improved bradykinesia and finger taps.”4 These findings seem to contradict clinical experience and several reports of potentially detrimental effects of SSRIs on motor function in patients with PD.5–7 Also, although subthalamic deep brain stimulation has been shown to be efficacious for ameliorating PD motor symptoms including “bradykinesia,” 8,9 it is unclear whether specific components of movement are improved.9–11 A recent pilot study demonstrated that levodopa normalizes bradykinesia to a greater extent than hypokinesia.12 Therefore, we sought to evaluate motor function and response to dopaminergic medication in patients with PD with various impairments in speed, amplitude, and rhythm of movement.
Patients and Methods
We recruited 85 patients with idiopathic Parkinson’s disease (PD) meeting research diagnostic criteria13 (age, 46–85 years; disease duration, 2–31 years; Hoehn & Yahr score, 1–4). Exclusion criteria included tremor severity > 1 by UPDRS-III severity score (to minimize confounding of bradykinesia assessment), previous neurosurgical procedures, cognitive impairment (Mini–Mental Score Examination < 27/30), presence of lower or upper motor neuron signs, and neurological signs suggesting a parkinsonism other than PD. All clinical testing was completed at the University of Cincinnati College of Medicine and the University of Toronto Department of Medicine under the purview of their respective institutional review boards. All subjects provided informed consent prior to their participation.
Data Collection
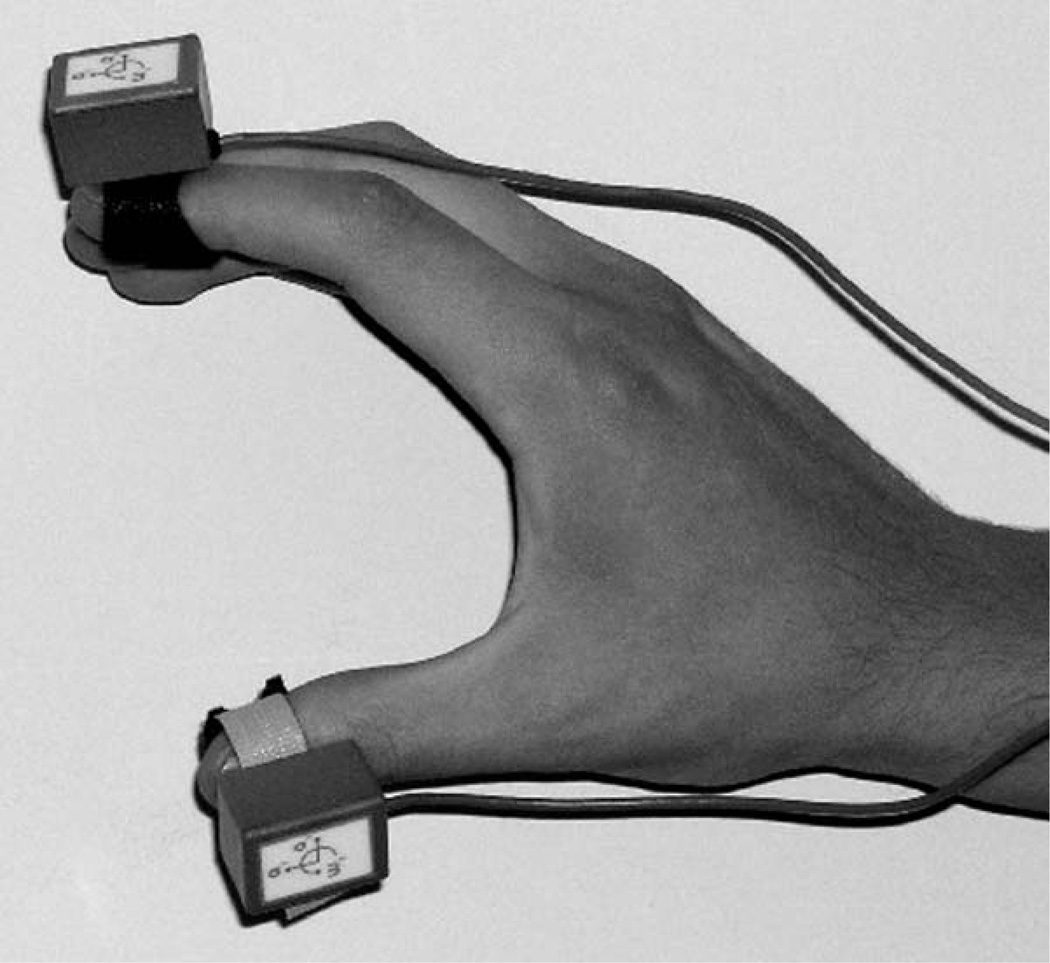
Subjects performed UPDRS-III-based finger-tapping (item 23), hand-grasping (item 24), and pronation-supination (item 25) tasks in the OFF state (12–15 hours after dopaminergic drug withdrawal) and the ON state (approximately 45–60 minutes after intake of subjects’ routine dopaminergic medications, when response was expected to be maximal). Subjects wore wireless 6-degree-of freedom motion sensors (Kineti-Sense, Great Lakes NeuroTechnologies, Inc., Cleveland, OH) on the index finger and thumb during each task (Fig. 1). Each motion sensor contained 3 orthogonal accelerometers to measure 3-D linear acceleration and 3 orthogonal gyroscopes to measure 3-D angular velocity. The units sampled motion at 128 Hz and wirelessly transmitted the data to a computer via a 2.4-GHz radio. Patients were asked to perform each of the 3 tasks using the more affected limb for 15 seconds with as large an amplitude and as fast movements as possible. Digital video was recorded of the limb-performing task for later blinded rating.
FIG. 1.
Lightweight sensor units (KinetiSense, Great Lakes Neuro-Technologies Inc.), each containing 3 orthogonal accelerometers and 3 orthogonal gyroscopes, were placed on each distal phalanx of the subject’s index finger and thumb.
Clinical Assessment
The videos were randomized and loaded onto a secure Web server for independent evaluation by 4 movement disorder neurologists unaware of the subjects’ clinical state. The raters were asked to use the UPDRS-III and MBRS for scoring each task. The MBRS was previously developed for scoring speed, amplitude, and rhythm separately on a 0–4 scale (with 0 = normal and 4 = most severe).1
Data Processing and Analysis
UPDRS-III scores and speed, amplitude, and rhythm MBRS subscores were compared ON and OFF medication for each subject. The speed, amplitude, and rhythm scores were averaged across the 3 tasks and 4 clinicians to minimize variability. The Student t test was used to determine if the MBRS subscores improved ON medication. In addition to comparisons of the PD study population as a whole, subjects were subdivided into 4 categories based on their primary impairment(s) OFF medication: strictly hypokinetic, strictly bradykinetic, both, and neither. Average amplitude and speed MBRS scores of 1 or worse were used as the hypokinetic and bradykinetic thresholds.
In addition to MBRS scores, quantitative variables were extracted and processed from the kinematic data recorded on the motion sensors and compared ON and OFF medication. The quantitative variables were extracted from the gyroscopes rather than the accelerometers because the 3 movement tasks were primarily rotational and kinematic features extracted from gyroscopes were previously found to correlate well with MBRS scores.2 First, the motion signals were band-pass-filtered from 0.3 to 5 Hz using a second order Butterworth filter. To minimize errors resulting from slight variations in the orientation of sensors on the finger and thumb, the magnitudes (Euclidean norm) of the angular velocities around the x-, y-, and z-axes were calculated. These signals from the finger and thumb sensor units were then summed and processed into quantitative variables representing speed (root mean square [RMS] angular velocity), amplitude (RMS excursion angle), and rhythm (coefficient of variation [standard deviation of a 1-second sliding window of the RMS excursion angle divided by the mean]). We have previously shown that these quantitative variables are highly correlated with clinician MBRS scores.2 The processed quantitative variables represented speed, amplitude, and rhythm, and were thus denoted “velocity,” “angle,” and “coefficient of variation,” respectively. These quantitative variables were compared ON and OFF medication using a paired t test and 1-way analysis of variance (ANOVA).
Finally, we defined fatigue as the percent decrement in angle (“angle fatigue”) or velocity (“velocity fatigue”) as measured by the motion sensors during the last 5 seconds of the task compared with the first 5 seconds. A paired t test was used to determine if significant fatigue occurred, and an ANOVA was used to determine if medication improved fatigue, whether defined as decreases in angle or decreases in velocity.
Results
MBRS Score Comparison
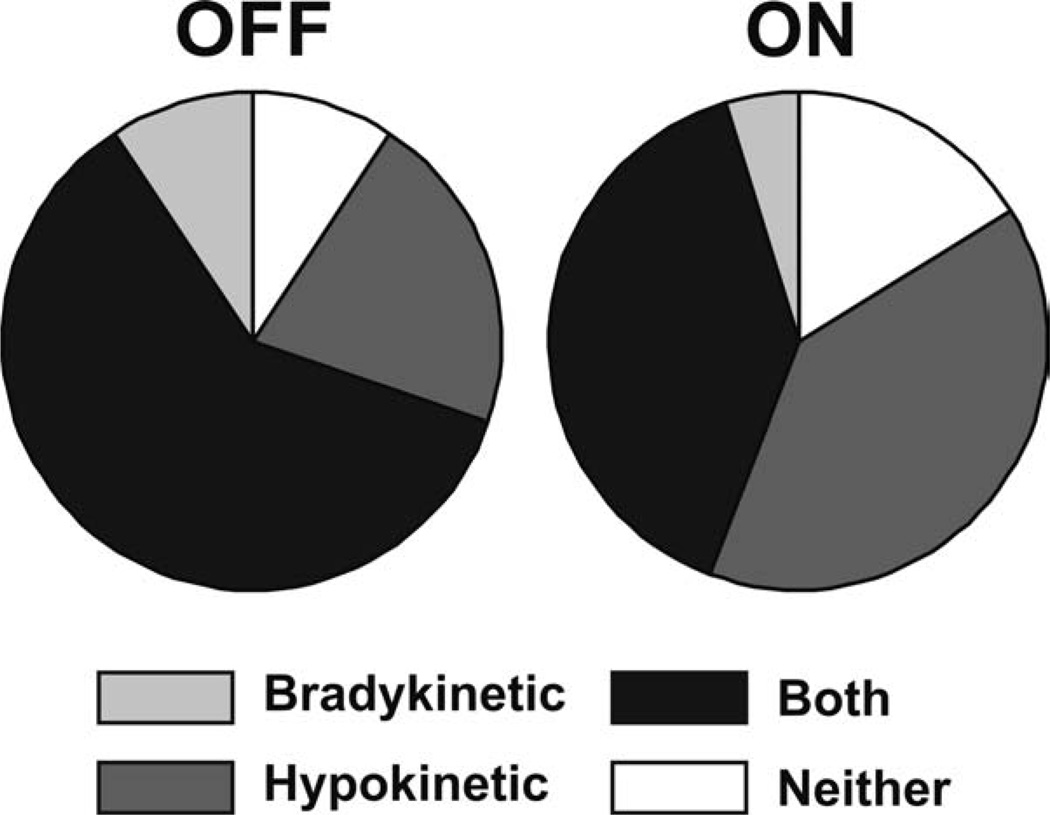
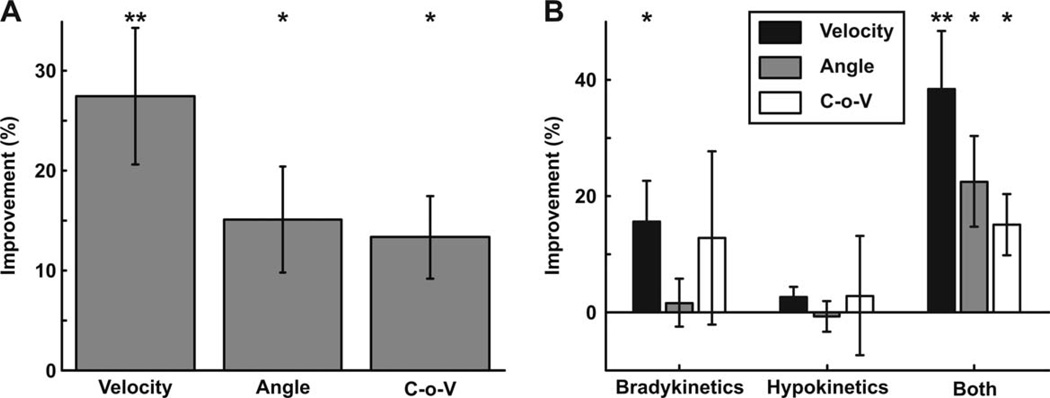
For the study population, MBRS amplitude scores were worse than speed or rhythm scores (P < 10−6). However, when comparing scores ON and OFF medication (Fig. 2A), only speed scores improved in the ON state (P < 10−6). Neither amplitude nor rhythm scores were affected by medication (P >.5). When categorizing the subjects as strictly bradykinetic, strictly hypokinetic, both bradykinetic and hypokinetic, or neither bradykinetic nor hypokinetic, UPDRS-III scores from OFF to ON improved in subjects who were strictly bradykinetic (P < .01) and both bradykinetic and hypokinetic (P < 10−6), but not in those strictly hypokinetic (Fig. 2B). In addition, there was a decrease in the percentage of strictly bradykinetic and both bradykinetic and hypokinetic subjects from the OFF to the ON state, with a relative increase in the percentage of strictly hypokinetic subjects (Fig. 3), also indicating that dopaminergic medication improved the speed but not the amplitude of movement.
FIG. 2.
A: Across all subjects, MBRS amplitude scores were worse than both speed and rhythm scores OFF medication; however, only speed scores improved ON medication. The bottom and top of the box are the first and third quartiles, respectively, and the whiskers extend to the most extreme value, up to 1.5 times the interquartile range. Points beyond the whiskers are indicated by a plus. Notches indicate the 95% confidence interval for the medians. B: Overall UPDRS-III scores OFF and ON medication for subjects who were strictly bradykinetic (“Brady”), strictly hypokinetic (“Hypo”), and both bradykinetic and hypokinetic OFF medication. Error bars equal standard error of the mean. A, B: *P < .01; **P < 10−6.
FIG. 3.
Pie charts show the percentages of subjects who were strictly bradykinetic, strictly hypokinetic, both bradykinetic and hypokinetic, and neither bradykinetic nor hypokinetic OFF (left) and ON (right) medication. The decrease in the percentage of strictly bradykinetic and both bradykinetic and hypokinetic subjects, with a relative increase in the percentage of hypokinetic subjects, reflects dopaminergic medication improving the speed but not the amplitude of movement.
Quantitative Comparison
Quantitative variables representing speed, amplitude, and rhythm showed similar changes as the MBRS scores for all subjects (Fig. 4A), with velocity improving most significantly ON medication (P < .001). Angle and coefficient of variation (unlike amplitude and rhythm MBRS scores) did improve (P < .01), although to a lesser extent than velocity (ANOVA: P < .05). When separating subjects by movement category (Fig. 4B), the same trends held; however, angle only improved significantly in subjects who exhibited impairments in both velocity and angle.
FIG. 4.
A: Percent improvement of processed quantitative variables ON medication compared with OFF for the entire study population. B: Percent improvement of processed velocity, angle, and coefficient of variation ON medication compared with OFF for test subjects who were strictly bradykinetic, strictly hypokinetic, or both bradykinetic and hypokinetic. A, B: Error bars equal the standard error of the mean; *P < 0.01, **P < .001.
Angle fatigue occurred both OFF (P < 10−14) and ON (P < 10−13) medication, as did velocity fatigue OFF (P < 10−10) and ON (P < 10−8) medication. ANOVA showed that percent fatigue did not differ when measuring speed or amplitude fatigue OFF and ON medication.
Discussion
Both the clinical MBRS scores and quantitative variables extracted from the motion sensors demonstrated that although amplitude deficits were more prevalent and more severe than speed or rhythm impairments in the OFF state, dopaminergic medication improved speed exclusively by MBRS and significantly above amplitude and rhythm by quantitative measures. UPDRS-III tasks 23–25 improved ON medication, as expected; however, speed was the only component of movement, as measured by the MBRS, that improved significantly (Fig. 2). This is further confirmed by the reclassification of subjects from the OFF to ON medication states (Fig. 3). The number of strictly bradykinetics decreased ON medication as did the number of subjects with both hypokinesia and bradykinesia. However, the number of strictly hypokinetics actually increased ON medication, indicating that dopaminergic medication preferentially improves the speed but not the amplitude of movement. In other words, many subjects with both speed and amplitude impairments OFF medication only have amplitude impairments ON medication.
We have demonstrated that clinicians differentially weigh various components of movement impairment when assigning a UPDRS score,2 which could explain why the overall UPDRS-III score improves in the ON state, although not as significantly as the speed subscore, which appears to drive the bulk of the response to dopaminergic drugs. Our results support the prior finding that speed of movement but not amplitude mirrors the response to dopaminergic medications.12 By combining multiple movement features into a single score, the UPDRS not only dilutes the power of finding true changes but may result in a differential response becoming unnoticed when evaluating the overall “bradykinesia” outcome of clinical trials.
Although the MBRS is useful in its ability to discriminate specific aspects of bradykinesia, motion sensors may provide a low-cost, objective complement to a clinical rating. The quantitative features extracted from the motion sensors have previously been shown to be highly correlated with MBRS scores2 and demonstrate similar responsiveness for measuring changes ON and OFF medication (Fig. 4). However, motion sensors were more sensitive to capturing improvements in amplitude and variability with medications (Fig. 4A) than the MBRS, which did not (Fig. 2A). Also, kinematic measures are not limited by the reliability concerns associated with clinical rating scales and can objectively process multiple movement features from a single task performance. In addition, motion sensors enable precise measurements of fatigue (decrements in speed or amplitude), which would be difficult to quantify by visual observation alone. Fatigue appears to be a property of movement impairment in PD that, although not specific to speed or amplitude impairment, helps to distinguish PD from disorders with which it may be confused, such as scans without evidence of dopaminergic deficits (SWEDDs), where a resting tremor results from focal dystonia.14 Our data showed that dopaminergic medications do not significantly improve fatiguing of either speed or amplitude.
Our findings suggest that speed, amplitude, and rhythm are differentially associated with motor impairment and response to dopaminergic medications in PD and deserve separate measurement in research studies. Separately quantifying the subcomponents of movement impairment may enable a prediction of treatment response and a more accurate assessment of novel pharmacological or DBS therapies.
Acknowledgments
Funding agencies: This study was supported by the Davis Phinney Foundation for Parkinson’s Disease and NIH/NINDS 7R43NS065554-03.
Footnotes
Relevant conflicts of interest/financial disclosures: The Davis Phinney Foundation for Parkinson’s Disease provided unrestricted support and had no role in the oversight or review of the research data or reporting. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Kishore A, Espay AJ, Marras C, et al. Unilateral versus bilateral tasks in early asymmetric Parkinson’s disease: Differential effects on bradykinesia. Mov Disord. 2007;22:328–333. doi: 10.1002/mds.21238. [DOI] [PubMed] [Google Scholar]
- 2.Heldman DA, Giuffrida JP, Chen R, et al. The modified bradykinesia rating scale for Parkinson’s disease: Reliability and comparison with kinematic measures. Mov Disord. 2011 doi: 10.1002/mds.23740. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimber TE, Tsai CS, Semmler J, Brophy BP, Thompson PD. Voluntary movement after pallidotomy in severe Parkinson’s disease. Mov Disord. 1999;122:895–906. doi: 10.1093/brain/122.5.895. [DOI] [PubMed] [Google Scholar]
- 4.Rampello L, Chiechio S, Raffaele R, Vecchio I, Nicoletti F. The SSRI, citalopram, improves bradykinesia in patients with Parkinson’s disease treated with L-dopa. Clin Neuropharmacol. 2002;25:21–24. doi: 10.1097/00002826-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Richard IH, Maughn A, Kurlan R. Do serotonin reuptake inhibitor antidepressants worsen Parkinson’s disease? A retrospective case series. Mov Disord. 1999;14:155–157. doi: 10.1002/1531-8257(199901)14:1<155::aid-mds1026>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez-Jiménez FJ, Tejeiro J, Martínez-Junquera G, Cabrera-Valdivia F, Alarcón J, García-Albea E. Parkinsonism exacerbated by paroxetine. Neurology. 1994;44:2406. doi: 10.1212/wnl.44.12.2406. [DOI] [PubMed] [Google Scholar]
- 7.Leo RJ. Movement disorders associated with the serotonin selective reuptake inhibitors. J Clin Psychiatry. 1996;57:449–454. doi: 10.4088/jcp.v57n1002. [DOI] [PubMed] [Google Scholar]
- 8.Lyons KE, Pahwa R. Deep brain stimulation in Parkinson’s disease. Curr Neurol Neurosci Rep. 2004;4:290–295. doi: 10.1007/s11910-004-0054-0. [DOI] [PubMed] [Google Scholar]
- 9.Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006;29:229–257. doi: 10.1146/annurev.neuro.29.051605.112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papapetropoulos S, Jagid JR, Sengun C, Singer C, Gallo BV. Objective monitoring of tremor and bradykinesia during DBS surgery for Parkinson disease. Neurology. 2008;70:1244–1249. doi: 10.1212/01.wnl.0000308936.27780.94. [DOI] [PubMed] [Google Scholar]
- 11.Guo X, Gao G, Wang X, et al. Effects of bilateral deep brain stimulation of the subthalamic nucleus on olfactory function in Parkinson’s disease patients. Stereotact Funct Neurosurg. 2008;86:237–244. doi: 10.1159/000131662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espay AJ, Beaton DE, Morgante F, Gunraj CA, Lang AE, Chen R. Impairments of speed and amplitude of movement in Parkinson’s disease: a pilot study. Mov Disord. 2009;24:1001–1008. doi: 10.1002/mds.22480. [DOI] [PubMed] [Google Scholar]
- 13.Ward CD, Gibb WR. Research diagnostic criteria for Parkinson’s disease. Adv Neurol. 1990;53:245–249. [PubMed] [Google Scholar]
- 14.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson’s disease: a clinical and electrophysiological study. Mov Disord. 2010;25:560–569. doi: 10.1002/mds.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]