Abstract
The supramolecular assembly of aquaporin-4 (AQP4) in orthogonal arrays of particles (OAPs) involves N-terminus interactions of the M23-AQP4 isoform. We found AQP4 OAPs in cell plasma membranes but not in endoplasmic reticulum (ER) or Golgi, as shown by: (i) native gel electrophoresis of brain and AQP4-transfected cells; (ii) photobleaching recovery of GFP-AQP4 chimeras in live cells; and (iii) freeze-fracture electron microscopy (FFEM). We found that AQP4 OAP formation in plasma membranes but not Golgi was not related to AQP4 density, pH, membrane lipid composition, C-terminal PDZ-domain interactions or α-syntrophin expression. Remarkably, however, fusion of AQP4-containing Golgi vesicles with (AQP4-free) plasma membrane vesicles produced OAPs, suggesting the involvement of plasma membrane factor(s) in AQP4 OAP formation. In investigating additional possible determinants of OAP assembly we discovered membrane curvature-dependent OAP assembly, in which OAPs were disrupted by extrusion of plasma membrane vesicles to ~110 nm diameter, but not to ~220 nm diameter. We conclude that AQP4 supramolecular assembly in OAPs is a post-Golgi phenomenon involving plasma membrane-specific factor(s). Post-Golgi and membrane curvature-dependent OAP assembly may be important for vesicle transport of AQP4 in the secretory pathway and AQP4-facilitated astrocyte migration, and suggests a novel therapeutic approach for neuromyelitis optica (NMO).
Keywords: AQP4, OAP, NMO, Golgi
Aquaporin-4 (AQP4) is a water channel expressed in cell plasma membranes in astrocytes and skeletal muscle, and in epithelia in kidney, lung, stomach and exocrine glands (1, 2). As found for other aquaporins, AQP4 monomers contain six membrane-spanning segments surrounding vestibules connected by a narrow aqueous pore that excludes solutes (3). The major biological functions of AQP4 relate to its expression in astrocytes in the central nervous system, which include water balance, neurosensory and excitatory phenomena, astrocyte migration and neuroinflammation (4). There has been considerable recent interest in AQP4 because of its involvement in the autoimmune neuroinflammatory disease neuromyelitis optica (NMO), where pathogenic autoantibodies directed against extracellular epitopes on AQP4 (NMO-IgG) cause transverse myelitis and optic neuritis, leading to paralysis and blindness (5).
Our lab discovered that AQP4 is the protein responsible for formation of supramolecular assemblies called orthogonal arrays of particles (OAPs) (6, 7), which are regular square arrays seen by freeze-fracture electron microscopy (FFEM) in brain, skeletal muscle, stomach and kidney (8, 9). Subsequent work delineated the molecular basis of OAP formation by AQP4. AQP4 protein is expressed as a full-length, long (M1) isoform with translation initiation at Met-1, and a shorter (M23) isoform with translation initiation at Met-23 (10). M23-AQP4 forms OAPs on its own or together with M1-AQP4 (11, 12). There is biophysical and biochemical evidence that M1 and M23 can associate in heterotetramers (13, 14). At the molecular level, OAP formation by M23 involves an intermolecular N-terminus interaction between two M23 monomers by residues just downstream of Met-23, and that the inability of M1 by itself to form OAPs is due to blocking of the N-terminal interaction by residues just upstream of Met-23 (13). A mathematical model of inter-tetrameric M23 interactions can explain and predict effects of relative M1/M23 expression on OAP size and composition (15). Though the biological functions of OAPs remain unclear, it has been speculated that OAPs are involved in AQP4 water transport function (12, 16), cell-cell adhesion (3, 17, 18) and membrane polarization (19). With regard to NMO, pathogenic AQP4 IgG autoantibodies bind preferentially to OAP-assembled AQP4 (20, 21).
Here, we investigated the cellular site and determinants of AQP4 OAP assembly. We found by biochemical, biophysical and FFEM methods that AQP4 forms OAPs in cell plasma membranes but not in endoplasmic reticulum (ER) or Golgi. Possible differences between Golgi and plasma membrane were investigated that could account for this observation, including differences in AQP4 density, cholesterol content, pH, membrane curvature and protein composition. In doing so, we discovered a novel membrane curvature-dependent phenomenon in which OAPs are reversibly disrupted in highly curved, small-diameter vesicles. We also found evidence for involvement of plasma membrane-specific factor(s) in AQP4 OAP formation.
RESULTS
AQP4 forms OAPs in plasma membrane but not in Golgi or ER
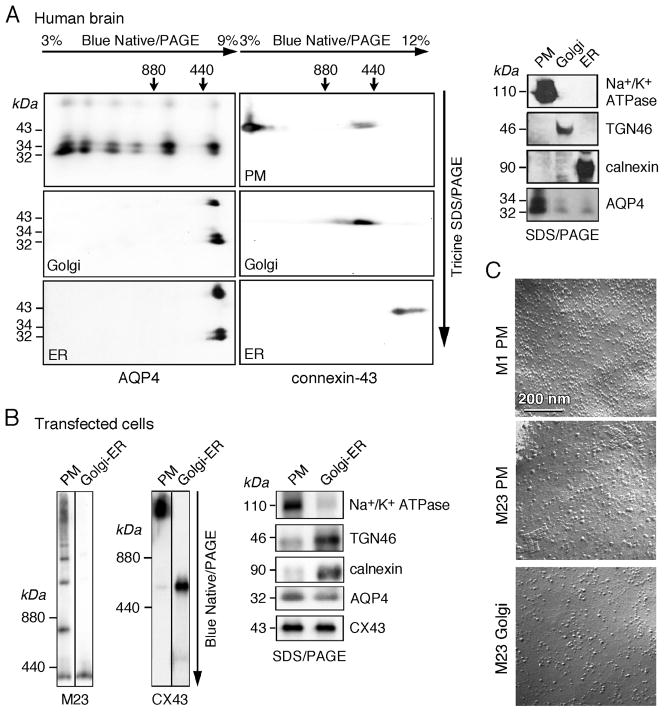
To determine the cellular compartment of OAP formation, the AQP4 oligomeric state was analyzed in membrane fractions purified from homogenates of human brain. Fractions enriched in plasma membranes, ER and Golgi were prepared by sucrose-density gradient centrifugation. By BN-SDS/PAGE and AQP4 immunoblot, AQP4 was detected as high molecular oligomer in the plasma membrane fraction, corresponding to OAPs, whereas only AQP4 tetramers were detected in the ER and Golgi fractions (Figure 1A, left). CX43 was used as a control because of its abundant expression in brain and formation of well-characterized oligomeric structures, with monomers in the ER, hexamers (connexons) in Golgi, and high molecular structures (GAPs) in the plasma membrane (8, 22) (Figure 1A, center). Fraction purity was verified by immunoblot analysis (Figure 1A, right) using as markers Na+/K+ ATPase (plasma membrane), TGN-46 (Golgi) and calnexin (ER).
Figure 1. AQP4 oligomerization in subcellular fractions of brain homogenates and transfected cells.
(A) Electrophoresis of human brain plasma membrane, Golgi and ER fractions. The first dimension is BN/PAGE, the second dimension is tricine SDS/PAGE. Immunodetection was carried out against AQP4 or CX43 (left). Immunoblot of Na+/K+ ATPase, TGN46 and calnexin (right). (B) BN/PAGE of plasma membrane and Golgi/ER fractions of U87MG cells transfected with M23-AQP4 (left) or CX43 (center). Immunoblot of Na+/K+ ATPase, TGN46 and calnexin (right). (C) Unidirectionally-shadowed freeze-fracture electron micrographs of purified subcellular fractions of M1 and M23-AQP4-transfected U87MG cells. Scale bar, 200 nm.
Studies were also done in U87MG cells after transient transfection with M23-AQP4 or CX43 (Figure 1B) in which plasma membrane and ER/Golgi fractions were isolated by differential centrifugation. BN/PAGE showed multiple bands at high molecular weight in the plasma membrane fraction, corresponding to AQP4 OAPs, and a single band at ~300 kDa in the Golgi/ER fraction, corresponding to AQP4 tetramers. CX43 native gels showed a single strong band at high molecular weight in the plasma membrane fraction, corresponding to GAPs, and two bands in the Golgi/ER fraction, corresponding to monomers and hexamers. Membrane markers confirmed fraction purity (Figure 1B, right).
FFEM showed OAPs in the plasma membrane fraction of M23-transfected cells but not M1-transfected cells (Figure 1C, top and center). The OAP density in the M23-transfected cells was ~1 per 500 μm2. OAPs were never seen in freeze-fracture micrographs of Golgi (Figure 1C, bottom) for eight different specimens in which a total of area of 11500 μm2 of Golgi membrane was examined. The FFEM data is in agreement with the conclusion from biochemical analysis that AQP4 aggregates/OAPs are absent in Golgi.
Post-Golgi assembly of AQP4 OAPs was verified in live cells by fluorescence recovery after photobleaching (FRAP). We previously generated and characterized C-terminal fusions of GFP to AQP4 (23). Figure 2A top, left shows the expected molecular sizes of GFP-tagged M1 and M23 by AQP4 immunoblot. BN/PAGE in Figure 2A, top, center shows OAP formation by M23-GFP but not by M1-GFP, as expected, which was confirmed by high-resolution clear native-polyacrylamide gel electrophoresis (Figure 2A, right). Confocal imaging of M1-GFP and M23-GFP showed plasma membrane staining by both isoforms (Figure 2A, bottom, upper panels), with M1-GFP seen in a smooth pattern and M23-GFP in a punctate pattern, reflecting their different oligomeric states on the plasma membrane. TIRFM confirmed OAP formation in the plasma membrane by M23-GFP but not M1-GFP (Figure 2A, bottom, lower panels).
Figure 2. Oligomerization of AQP4-GFP chimeras and localization in live cells.
(A) SDS/PAGE (top left), BN/PAGE (top center) and hrCN/PAGE (top right) of M1-GFP and M23-GFP. Confocal and TIRF microscopy showing plasma membrane targeting of M1-GFP and M23-GFP in transiently transfected U87MG cells (bottom). (B) Live-cell imaging of U87MG cells transiently transfected with M1-GFP and M23-GFP after low-temperature block showing Golgi localization (top), or treatment with BFA showing ER localization (bottom). Cells were co-transfected with compartment-specific marker proteins (GalT for Golgi, calnexin for ER) fused to mCherry.
To visualize AQP4 in subcellular compartments in live cells, we used BFA to block protein traffic from the ER, and low temperature to block protein exit from Golgi. Low temperature conditions were optimized to give near perfect M1 and M23 accumulation in Golgi, as confirmed by colocalization with mCherry-labeled Gal-T (Figure 2B, top). BFA treatment resulted in ER retention of M1 and M23, as confirmed by colocalization with mCherry-labeled calnexin (Figure 2B, bottom).
We previously exploited the differential diffusion dynamics of M1 vs. M23-AQP4 in the plasma membrane, as measured by single particle tracking (SPT), to characterize the determinants of OAP formation (24). Here, we used FRAP with confocal image detection to compare diffusion of M1 vs. M23-AQP4 in the Golgi, ER and plasma membrane. Figure 3A, top shows that photobleaching of plasma membrane (white arrows) produced a dark region of fluorescence that fully recovered for M1-GFP but not for M23-GFP, in agreement with OAP formation by M23-GFP but not M1-GFP at the plasma membrane. Figure 3A, bottom shows complete recovery of fluorescence in Golgi for both M1-GFP and M23-GFP. Complete recovery of fluorescence was also found for both M23-GFP and M1-GFP in the ER (not shown). Figure 3B, top shows representative recovery curves as background-subtracted fluorescence in the bleached spot, with percentage recovery summarized in Figure 3B, bottom. Fluorescence recovery was essentially complete over 2 min for M1-GFP and M23-GFP in Golgi and ER, and for M1-GFP but not M23-GFP in the plasma membrane. These results obtained in live cells support the biochemical and freeze-fracture data in Figure 1 indicating OAP assembly by AQP4 in the plasma membrane but not in Golgi.
Figure 3. Diffusion of M1 and M23-AQP4 in live cells.
(A) FRAP of M1-GFP or M23-GFP in transiently transfected U87MG cells, with plasma membrane (top) and Golgi (low-temperature treated cells, bottom) localization. Arrowheads indicate bleached area. (B) Representative kinetics of FRAP of M1-GFP and M23-GFP over 120 s (top), with summary of percentage recovery data at 120 s (bottom) (S.E., n > 10 cells, * P < 0.01).
OAP formation by AQP4 is pH-insensitive
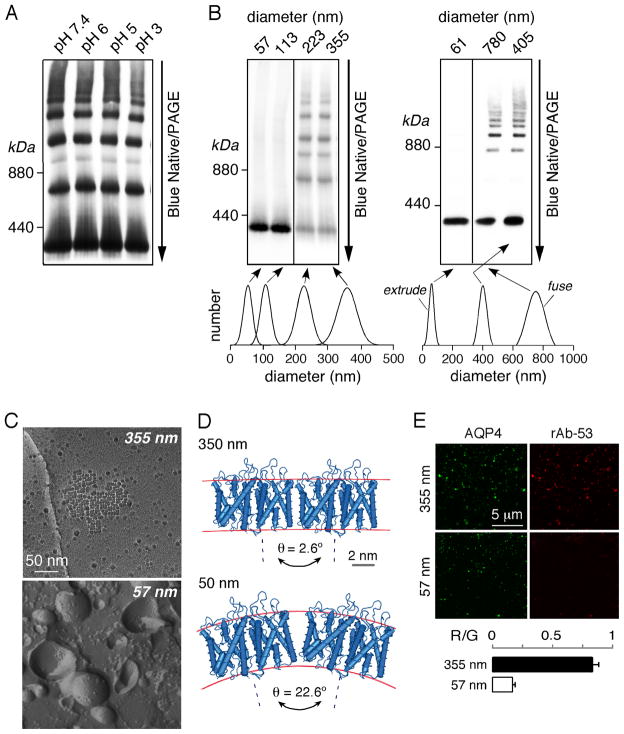
We investigated whether various differences in Golgi vs. plasma membrane could account for OAP formation in the plasma membrane but not in Golgi. Recognizing that Golgi pH is relatively acidic compared to cytoplasm, possible pH-dependent OAP assembly was studied by incubation of plasma membrane vesicles from M23-AQP4 expressing cells in buffers of different pH in the presence of digitonin to permeabilize the membrane. BN/PAGE showed, however, that AQP4 supramolecular assembly was not sensitive to pH in the wide pH range 3–7.4 (Figure 4A).
Figure 4. Membrane curvature-dependent AQP4 oligomerization.
(A) BN/PAGE of M23-AQP4 in transfected U87MG cells after incubation in buffers of indicated pH. (B) (left) BN/PAGE of M23-AQP4 in plasma membrane vesicles of different sizes generated by extrusion (top). Vesicle size distributions determined by quasi-elastic light scattering (bottom). (right) BN/PAGE of M23-AQP4 in control and extruded plasma membrane vesicles, and in extruded vesicles after fusion. (C) Freeze-fracture electron micrographs of vesicles of average diameters 355 nm (top) and 57 nm (bottom). (D) To-scale model of AQP4 tetramers in membranes of different curvatures correspond to vesicles of diameters 350 or 50 nm. (E) Fluorescence micrographs of coverslip-immobilized 355 and 57 nm diameter vesicles showing binding of NMO monoclonal antibody rAb-53 (red) and anti-AQP4 antibody (green). Red-to-green fluorescence ratios (R/G) (right) (mean ± S.E., n=3).
High membrane curvature disrupts AQP4 OAPs
Proteins in the secretory pathway are transported from the ER to the plasma membrane through the Golgi compartment in vesicles of different sizes. Because AQP4 assembly in OAPs involves the formation of crystalline planar arrays, we postulated that high membrane curvature might disrupt OAPs. To investigate this possibility, M23-containing plasma membrane vesicles were extruded through filters of different pore sizes to generate vesicles of average diameters 57, 113, 223 and 355 nm. Remarkably, BN/PAGE of vesicles of diameters 57 and 113 nm showed a single band at ~300 kDa, corresponding to AQP4 tetramers, whereas multiple bands were seen in vesicles of diameters 223 and 335 nm, corresponding to AQP4 OAPs (Figure 4B, left). In another set of experiments, 405 nm M23-containing plasma membrane vesicles were extruded to yield 61 nm vesicles, some of which were fused together using a high-Ca2+ buffer (Figure 4B, right). BN/PAGE showed a single band (AQP4 tetramers) for the extruded vesicles, but multiple bands corresponding to OAPs in the original (non-extruded) vesicles and in the fused, extruded vesicles. The disruption of AQP4 OAPs by vesicle extrusion to small diameter was confirmed by FFEM (Figure 4C). Figure 4D shows a schematic of two adjacent AQP4 tetramers in 350 vs. 50 nm diameter vesicles. In order remain aligned in a parallel orientation, adjacent AQP4 tetramers would need to tilt minimally, by 2.6°, in a 350 nm diameter vesicle, but by 22.6° in a 50 nm vesicle, which is likely to be highly energetically unfavorable.
We studied an interesting consequence of membrane curvature-dependent AQP4 OAP disruption. Autoanti-bodies against AQP4 in the disease NMO bind substantially better to AQP4 in OAPs than as separate tetramers (20). To quantify binding of a recombinant monoclonal NMO autoantibody (rAb-53), M23-AQP4 containing vesicles of diameter 355 nm or 57 nm were immobilized on a cover glass, and stained with rAb-53 antibody (and red fluorescent secondary antibody). Binding was normalized for AQP4 using an antibody against the AQP4 C-terminus and a green fluorescent secondary antibody for ratio imaging. Figure 4E shows greatly reduced red-to-green (R/G) fluorescence in the small vesicles, indicating that a consequence of OAP disruption by high membrane curvature is reduced NMO autoantibody binding.
Membrane cholesterol and SM content do not affect AQP4 OAP assembly
Golgi and plasma membranes have different lipid composition and cholesterol content, which could affect protein oligomerization, as has been found for syndecan-4, aquaporin-0 (AQP0) and glutamate transporter-1 (GLT-1) (25–27). SM-rich domains were reported to be the sites of lysenin oligomerization (28). The effect of depletion of cholesterol and SM on AQP4 OAP assembly was tested on plasma membrane vesicles from M23-expressing cells. Cholesterol was depleted by incubation of vesicles with βMCD, and SM was depleted by conversion to ceramide using SMase. BN/PAGE in Figure 5A, left (cholesterol-depletion) and right (SM-depletion) shows that these maneuvers did not affect AQP4 supramolecular assembly. Cholesterol assay showed lower cholesterol in Golgi vs. plasma membranes, and confirmed near complete cholesterol depletion with βMCD treatment (Figure 5A, right). We and others (29) have shown SM depletion of > 50% at concentrations of SMase lower than used here.
Figure 5. Determinants of AQP4 oligomerization on the plasma membrane.
(A) Effect of cholesterol depletion on M23-AQP4 oligomerization in plasma membrane vesicles from M23-AQP4-expressing U87MG cells. (left) BN/PAGE after treatment with indicated concentration of βMCD. (center) BN/PAGE after treatment with indicated concentrations of SMase to deplete SM (S.E., n=4). (right) Cholesterol content in Golgi and plasma membrane vesicles treated with βMCD (S.E., n=4). (B) BN/PAGE of M23-AQP4 oligomerization in unfused and high Ca2+-fused mixtures of plasma membrane and Golgi vesicles. As indicated, vesicles were derived from M23-AQP4-expressing or non-transfected cells. (right) Vesicle size distribution before (pre-fusion) and after incubation in high Ca2+ buffer. (C) BN/PAGE of AQP4 after α-syntrophin siRNA knock-down in human astrocyte cultures (bottom). Knock-down (α-syn) and loading (actin) controls are shown (top). (D) BN/PAGE of U87MG cells after transfection with native M23-AQP4 or a C-terminus truncation mutant lacking the PDZ-binding domain (bottom). SDS/PAGE of α-syntrophin expression is shown (top).
Plasma membranes contain factor(s) promoting AQP4 OAP assembly
Vesicle fusion experiments were done to determine whether Golgi-specific factor(s) are present that prevent OAP assembly in Golgi, and/or whether plasma membrane-factor(s) are present that facilitate OAP formation in the plasma membrane. Our strategy was to fuse M23-containing plasma membrane vesicles with non-AQP4-containing Golgi, and to fuse M23-containing Golgi with non-AQP4-containing plasma membrane vesicles. Vesicle fusion was accomplished by incubation in a high-Ca2+ buffer. BN/PAGE showed that fusion of (non-AQP4-containing) Golgi with M23-containing plasma membrane vesicles had little effect on the gel band pattern and hence AQP4 supramolecular assembly, whereas fusion of (non-AQP4-containing) plasma membrane vesicles with M23-containing Golgi resulted in appearance of higher molecular weight bands corresponding to AQP4 OAPs (Figure 5B, left). Fusion of M23-containing plasma membrane vesicles with (non-M23-containing) plasma membrane vesicles did not change the gel band pattern, nor did fusion of M23-Golgi with (non-M23-containing) Golgi (Figure 5B, center). Vesicle size analysis in Figure 5B, right confirmed vesicle fusion by the high-Ca2+ buffer method used here. We point out that vesicle sizes before and after fusion are well above those where size-dependent effects on OAP formation were observed. These findings suggest that plasma membrane factor(s) are necessary and sufficient for AQP4 OAP assembly, and that a relatively small amount of such factor(s) can promote AQP4 OAP formation. The incomplete OAP formation after fusion of M23-containing Golgi with plasma membranes may be related to the random nature of vesicle fusion in which Golgi/Golgi, Golgi/plasma membrane and plasma membrane/plasma membrane fusion can occur.
We investigated two candidate plasma membrane factors that may promote AQP4 OAP formation. It has been suggested that AQP4 may interact with α-syntrophin, a scaffold protein, through a C-terminal PDZ binding motif in AQP4 (30–32). The possible involvement of α-syntrophin in OAP formation was investigated by knock-down in human astrocytes. Immunoblot showed ~90 % reduced α-syntrophin in the siRNA-treated astrocytes (Figure 5C, top). BN/PAGE showed, however, that AQP4 supramolecular assembly was not affected by α-syntrophin knock-down (Figure 5C, bottom). To investigate the involvement of the AQP4 C-terminus PDZ-binding domain, measurements were done in U87MG cells transfected with full-length M23-AQP4 or M23-Δ6, a C-terminus truncation mutant of M23-AQP4 lacking its PDZ-binding domain (24). We found, however, that expression of full-length vs. M23-Δ6 AQP4 did not affect α-syntrophin expression (Figure 5D, top) or supramolecular assembly (Figure 5D, bottom). Therefore, neither α-syntrophin nor AQP4 PDZ-domain interactions are required for AQP4 OAP formation.
DISCUSSION
Membrane protein oligomerization generally occurs in the ER (33), though relatively little information is available about the site and mechanism(s) of membrane protein supramolecular assembly, which occurs for a few proteins including caveolins, connexins and proton pumps. The assembly of caveolins is a multistep process that involves oligomerization in the ER into ~200 kDa complexes. Caveolins are detergent soluble in the Golgi, but upon further assemble at the plasma membrane they become detergent resistant because of possible associations with cholesterol and sphingolipids (34). However, there is conflicting data that caveolin is fully assembled in the Golgi (35). For connexins, the protein chaperone ERp29 inhibits CX43 oligomerization to allow the exit of monomeric CX43 from the ER, with subsequent oligomerization into hexamers in the trans-Golgi and into GAPs at the plasma membrane. It is unclear, however, what drives/allows oligomerization of CX43 in the Golgi and why GAPs are seen in the plasma membrane but not in Golgi. Proton pumps, including the plasma membrane H+-ATPases, can form supramolecular aggregates in the plasma membrane by threonine phosphorylation (36). Supramolecular protein assembly can thus involve a variety of cellular mechanisms.
Here, we used native gel electrophoresis, confocal photobleaching and FFEM to determine the cellular compartment of AQP4 OAP assembly. BN/PAGE of fractionated membrane from human brain and transfected cells showed OAP formation by M23-AQP4 in plasma membrane but not in Golgi or ER, which was confirmed morphologically by FFEM and in live cells by photobleaching of GFP-AQP4 chimeras. Various physical and biochemical factors were considered that could account for AQP4 OAP formation in plasma membrane but not in Golgi. The microenvironment of Golgi differs from that of the plasma membrane in its pH, membrane curvature, and lipid and protein composition. Some of these factors might prevent AQP4 OAP formation in the Golgi and/or promote OAP formation in the plasma membrane.
An interesting discovery was the dependence of AQP4 OAP assembly on membrane curvature, with apparent complete OAP disruption by extrusion of OAP-containing plasma membranes to vesicles of diameter of ~110 or less. We reason that OAP formation involves AQP4 inter-tetrameric interactions having finite association (positive) free energy. High membrane curvature, which confers a large negative free energy, can overcome the favorable association free energy and hence disrupt OAPs. The negative free energy needed to disrupt AQP4 OAPs is likely to be substantial, as we found previously that OAP formation by native M23-AQP4 was not impaired by a marked reduction in AQP4 membrane density or by large changes in temperature (29); however, OAP assembly did depend on membrane density and temperature when OAP formation was weakened by introduction of certain N-terminus mutations in M23-AQP4, in which temperature changes caused rapid (< 5 s) OAP assembly and disruption (29). Our recent mathematical modeling study also suggested that the unfavorable energetics of membrane curvature can be in part responsible for the smaller-than-predicted size of OAPs formed by AQP4 at high M23-to-M1 ratio (15).
Though membrane curvature-dependent OAP assembly by AQP4 is an interesting phenomenon, it is unlikely to account for the absence of OAPs in Golgi, which consists of stacks of relatively flat membranes with much less curvature than that found to disrupt OAPs. However, there may be other processes where curvature-dependent AQP4 assembly is important. Proteins travel along the secretory pathway in transport vesicles of typical size of 40–70 nm (37). AQP4 assembly at the plasma membrane but not in upstream vesicular compartments would allow for efficient vesicular transport of AQP4 tetramers, as AQP4 OAPs cannot be accommodated in transport vesicles of 100 nm or smaller. High membrane curvature is also found at the tip of lamellipodia in migrating cells, where AQP4 has been found to polarize during astrocyte migration and facilitate the rate of migration (38). The ability of high membrane curvature to disrupt AQP4 OAPs may allow their polarization in lamellipodia and hence facilitate cell migration.
Membrane curvature-dependent assembly of AQP4 in OAPs may also have implications for the pathogenesis and therapy of NMO. In NMO autoantibodies against extracellular epitopes on AQP4 produce astrocyte damage leading to neuroinflammation and demyelination. We found that NMO autoantibodies generally have substantially higher affinity to AQP4 when assembled in OAPs compared to non-OAP-assembled tetramers (20). We found here that disruption of AQP4 OAPs in highly curved vesicles greatly reduced NMO-IgG binding. The ability of membrane curvature to disrupt OAPs formed by native, human AQP4 suggests that the energy barrier favoring OAP assembly can be overcome, as supported by mathematical modeling (15). These observations suggest the possibility of identifying small-molecule inhibitors of OAP formation by AQP4 on the astrocyte plasma membrane, which would reduce the binding and downstream pathogenicity of NMO autoantibodies.
The lipid composition of Golgi and plasma membrane differs, with relative enrichment of the plasma membrane in cholesterol and SM (37). Membrane protein binding to cholesterol or glycosphingolipids could affect its aggregation state in the plasma membrane, as has been suggested for oligomerization of a GLT-1, syndecan-4 and AQP0. We found here that depletion of cholesterol and SM did not affect the plasma membrane assembly of AQP4 in OAPs, providing evidence against effects of membrane lipid composition on AQP4 OAP assembly.
We tested whether specific factor(s) in the Golgi or plasma membrane could facilitate OAP formation at the plasma membrane and/or inhibit OAP formation in the Golgi. Vesicle fusion experiments showed that fusion of AQP4-containing Golgi vesicles with (non-AQP4-containing) plasma membrane vesicles enhanced AQP4 oligomerization, whereas fusion of (non-AQP4-containing) Golgi vesicles with AQP4-containing plasma membrane vesicles did not reduce OAP content. These results suggest the presence of plasma membrane-specific factor(s) that promote AQP4 OAP assembly.
Various plasma membrane proteins might facilitate OAP assembly.AQP4 has been proposed to interact with many membrane proteins, including the dystrophin-associated proteins dystroglycan and syntrophin. (32). AQP4 localization in brain is altered in α-syntrophin-null mice (31). The knock-down and mutagenesis data here provide evidence, however, against a functionally significant interaction between α-syntrophin and AQP4. Also, various components of the extracellular matrix have been reported to influence the aggregation and expression of AQP4, including laminin, collagen, fibronectin and agrin (9, 39). In addition to these multiple candidates, which may act in combinations, AQP4 aggregation in OAPs at the basolateral membrane of parietal cells has been reported in rat but not mouse (12, 21), suggesting species differences in AQP4-OAP promoting factors. A further complexity is the tissue-specific expression of different AQP4 anchoring proteins, such as Dp71 in astrocytes (40) and utrophin complexes in some other cell types (41). Identification of the precise factor(s) responsible for AQP4 OAP assembly in the plasma membrane will thus be quite challenging.
In summary, we found that AQP4 supramolecular assembly in OAPs is a post-Golgi phenomenon that likely involves specific, as yet unidentified, plasma membrane protein factor(s). Such factor(s) may be involved as well in plasma membrane oligomerization of other membrane proteins undergoing post-Golgi supramolecular assembly. The discovery of membrane curvature-dependent AQP4 OAP formation establishes membrane curvature as a new determinant of membrane protein supramolecular assembly with potential implications for AQP4 vesicular transport, cell migration and NMO.
Materials and Methods
DNA constructs
cDNAs encoding human AQP4-M1, AQP4-M23 and connexin-43 (CX43) were PCR-amplified using whole-brain cDNA as template. PCR fragments were ligated into the mammalian expression vector pcDNA3.1. To generate AQP4 C-terminal chimeras with enhanced green fluorescent protein (GFP) or mCherry the respective sequences were PCR amplified from their respective plasmids (Clontech, Mountain View, CA) and ligated in-frame into pcDNA3.1. An M23-AQP4 C-terminus truncation mutant was generated as described (24). Chimeras of galactose-1-phosphate uridylyl-transferase (GalT) and calnexin with mCherry were ligated into pcDNA3.1 in-frame with the fluorescent protein. All constructs were verified by sequencing.
Cell culture and transfections
U87MG (human glioblastoma-astrocytoma, ATCC HTB-14) and CHO-K1 (ATCC CCL-61) cell cultures were maintained at 37 °C in 5% CO2, 95% air in appropriate medium containing 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were transfected with cDNAs in antibiotic-free medium 12–24 h before experiments, using FuGene HD (Roche, Basel, Switzerland) at a 3:1 ratio (μl:μg nucleic acid) and 0.18 μg DNA per cm2.
Fluorescence recovery after photobleaching (FRAP)
Photobleaching of AQP4-GFP chimeras was done using a Nikon Eclipse FN1 confocal microscope equipped with Nikon 100x oil immersion lens (numerical aperture 1.49). Transfected cells grown on cover glasses were mounted in a live-cell chamber kept at 37 °C. In some experiments cells were incubated with brefeldin A (BFA) for 8 h at 37 °C to cause retention of AQP4 in the ER. In some experiments cells were incubated at 20 °C for 12–16 h to accumulate AQP4 in Golgi. GFP was excited at 488 nm using a 40 mW argon laser at 2% power, which was increased 30–50-fold for 0.5–1 s for photobleaching of a circular spot of ~10 μm diameter. Images were processed to determine background-subtracted area-integrated intensities using ImageJ (http://rsbweb.nih.gov/ij/).
Freeze-fracture electron microscopy
Pelleted cells or vesicles were fixed with 2% glutaraldehyde/0.1 M cacodylate (Electron Microscopy Sciences, Hatfield, PA) for 4 h, and then rinsed with 0.1 M cacodylate and stored in PBS. Pellets were equilibrated with cryoprotectant, 30% glycerol/0.1 M cacodylate, overnight, placed on copper freeze-fracture supports and frozen in liquid Freon 22 cooled by liquid nitrogen. Unidirectional and rotary shadowed freeze-fracturereplicas were generated as described (30). The replicas were cleaned by immersion for 2 h in concentrated sodium hypochlorite bleach, washed three timesfor 5 min each with distilled water, transferred to copper electronmicroscopy grids, and examined with a JEOL 1011 electron microscope(JEOL, Tokyo, Japan).
Total internal reflection fluorescence microscopy (TIRFM)
TIRFM was done using a Nikon Eclipse TE2000E microscope equipped with a through-objective TIRF attachment and a 100x oil immersion objective (numerical aperture 1.49) mounted on a perfect focus module (Nikon). GFP was excited using an Argon laser, Z488/10x excitation filter and Z488RDC dichroic mirror, and detected through an ET525/50m emission filter (Chroma). Images were acquired using a QuantEM 512SC deep-cooled CCD camera (Photometrics, Tucson, AZ).
Subcellular fractionations
Human brain was homogenized in homogenizing buffer [300 mM sucrose, 1 mM EDTA, protease cocktail inhibitor (Roche, Basel, Switzerland), 10 mM Tris-HCl, pH 7.2] by 20 strokes of a glass Dounce homogenizer. The homogenate was centrifuged at 500g for 10 min at 4 °C and adjusted to 1.4 M sucrose, 10 mM Tris-HCl, 0.2 mM EDTA (pH 7.4). A discontinuous sucrose gradient [2 M sucrose (1 ml), 1.6 M (2 ml), 1.4 M (4 ml, containing homogenate), 1.2 M (4 ml), 0.8 M (1 ml)] was centrifuged for 2.5 h at 25,000 rpm in an SW 27 rotor, and 1 ml fractions were collected. Protein concentration was determined by the Bradford method. Separation of plasma membrane from intracellular vesicles was performed by differential centrifugation as described (42), with minor modifications. Cells were washed three times with PBS, scraped into homogenizing buffer, and homogenized by 20 strokes in a glass Dounce homogenizer. The homogenate was spun at 4,000g for 15 min and the pellet discarded. A plasma membrane-enriched fraction was obtained by centrifugation at 17,000g for 45 min. The resulting supernatant was spun at 200,000g for 1 h to obtain cytosolic (supernatant) and the intracellular vesicle (pellet) fractions.
Electrophoresis
Cells, membrane vesicles or tissues were suspended in ten volumes of Blue Native (BN) lysis buffer (500 mM ε-aminocaproic acid, 50 mM imidazole pH 7.0, 12 mM NaCl, 10% glycerol, 1% Triton X-100, protease inhibitor cocktail). After 30 min incubation on ice, the samples were centrifuged at 22,000 g for 30 min and supernatant protein concentration determined. The supernatants for Blue Native/polyacrylamide gel electrophoresis (BN/PAGE) were supplemented with Coomassie Blue G-250 dye from a 5% suspension in 500 mM 6-aminohexanoic acid. The supernatants for high-resolution clear native/polyacrylamide gel electrophoresis (hrCN/PAGE) were supplemented with Ponceau dye 0.1%. For studies of pH effect, cells were treated with PBS containing 0.005% digitonin (with pH adjusted in the range 3–7.4), briefly washed with PBS, and lysed in BN lysis buffer. Polyacrylamide native gradient gels were prepared as described (43). Samples (20 μg protein) were mixed with 5% Coomassie Blue G-250. Ferritin was used as the molecular mass standard (440 and 880 kDa). The running buffers were: 25 mM imidazole, pH 7 (anode buffer) and 50 mM tricine, 7.5 mM imidazole, 0.02% Coomassie Blue G-250, pH 7 (cathode buffer). Proteins were blotted onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA) using a native transfer buffer (50 mM tricine, 7.5 mM imidazole). For the second dimension, lanes from the first dimension were cut into strips and equilibrated in denaturation buffer (1% SDS, 1% β-mercaptoethanol) for 1–2 h at room temperature. A single strip was then placed into a second dimension gel of the same thickness and subjected to tricine SDS-PAGE. Proteins were blotted as above. For one-dimensional Laemmli SDS-PAGE, gels consisted of a 12% running gel and 3% stacking gel. hrCNE/PAGE was performed as described (44). Samples were loaded on polyacrylamide native gradient gels 3–9%. Samples (30 μg protein) were mixed with Ponceau S 0.1% and loaded onto an identical gel to that used for BN/PAGE. The cathode buffer (50 mM tricine, 7.5 mM imidazole, pH 7.0) was supplemented with the anionic detergent sodium deoxycholate (0.05%). A VersaDoc imaging system (Biorad, Hercules, CA) was used to image fluorescent GFP-AQP4 in hrCN/PAGE gels.
Immunoblot
Proteins were blotted onto PVDF membranes at 160 mA for 1.5 h in buffer containing 1.92 M glycine, 0.25 M Tris base, 10 mM EDTA, 1% SDS. Membranes were blocked with 3% BSA and incubated with the following primary antibodies at 4 °C overnight: goat or rabbit anti-AQP4 or rabbit anti-CX43 (Santa Cruz Biotechnology, Santa Cruz, CA), calnexin, trans-Golgi network-46 (TGN-46) and Na+/K+ ATPase (ABcam, Cambridge, MA). Membranes were then rinsed, incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit IgG or donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), rinsed extensively, and labeled proteins were detected using the ECL Plus enzymatic chemiluminescence kit (Amersham Biosciences, Pittsburgh, PA).
Cholesterol and sphingomyelin (SM) depletion
Cholesterol was depleted from plasma membrane vesicles by incubation with β-methyl-cyclodextrin (βMCD) (Sigma, St Louis, MO). Vesicles (5 μg protein) in BN buffer containing different concentrations of βMCD in 100 μl total volume were incubated for 2.5 h at room temperature on a rocking platform. The treated vesicles were centrifuged at 20,000 g for 10 min at 4 °C, washed once with 100 μl BN buffer, and the pellets prepared for BN/PAGE. Cholesterol was assayed using the AmplexRed cholesterol assay kit (Invitrogen, Carlsbad, CA). SM depletion was performed on cell pellets of freshly harvested cells by addition of 0.1 U/ml sphingomyelinase (SMase, Sigma, St Louis, MO).
Vesicle fusion and extrusion
Equal amounts (5 μg/ml protein) of Golgi and plasma membrane vesicles were diluted in a final volume of 100 μl BN buffer in the presence or absence of 5 mM Ca2+, and incubated for 30 min at room temperature on a rocking platform. Samples were centrifuged at 20,000 g for 10 min at 4 °C and prepared for BN/PAGE. Aliquots (5 μl) of each fusion reaction were diluted in 50 μl BN buffer for size determination by quasi-elastic light scattering (N5 Submicron Particle Size Analyzer, Beckman, Brea, CA). Vesicles of different sizes were prepared by extrusion as described (45) using a Lipofast vesicle extruder and 50, 100 and 200 nm-pore polycarbonate filters (Avestin, Canada).
Quantitative immunofluorescence of vesicles
Vesicles of diameter 355 or 57 nm containing M23-AQP4, prepared as described above, were immobilized on poly-D-lysine-coated coverslips for 1 h, then rinsed with PBS and incubated for 30 min with human recombinant monoclonal antibody rAb-53 (46). Vesicles were then rinsed with PBS, fixed in 4 % paraformaldehyde for 15 min, and permeabilized with 0.1 % Triton X-100. Vesicles were then blocked (PBS containing 6 mM glucose, 1 mM pyruvate, 1 % bovine serum albumin) and incubated for 30 min with 0.4 μg/mL polyclonal, C-terminal specific rabbit anti-AQP4 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), then rinsed with PBS. Finally, vesicles were incubated for 30 min with 4 μg/mL goat anti-human IgG-conjugated Alexa Fluor 555 and goat anti-rabbit IgG-conjugated Alexa Fluor 488 (Invitrogen) in blocking buffer. Quantitative analysis of antibody binding was done by ratio imaging fluorescence microscopy (20) using an Nikon Eclipse FN1 confocal microscope equipped with Nikon 100x oil immersion lens.
siRNA transfections
Human fetal cortical astrocyte cultures were generated as described (47). More than 98% of the cells were positive for glial fibrillary acidic protein (GFAP). Knockdown was achieved using α-syntrophin Stealth siRNA, with a scrambled sequence used as control (Invitrogen, Carlsbad, CA). Astrocytes were seeded the day before transfection using Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Transient transfection of siRNAs was carried out using siRNA Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions.
Acknowledgments
This work was supported by grants EY13574, EB00415, DK35124, HL73856, DK86125 and DK72517 from the National Institutes of Health and grants from the Guthy-Jackson Charitable Foundation. We thank Cambier Stephanie (UCSF) for providing human astrocyte cultures.
References
- 1.Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci U S A. 1995;92:4328–4331. doi: 10.1073/pnas.92.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, Verkman AS. Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci. 1995;108 (Pt 9):2993–3002. doi: 10.1242/jcs.108.9.2993. [DOI] [PubMed] [Google Scholar]
- 3.Ho JD, Yeh R, Sandstrom A, Chorny I, Harries WE, Robbins RA, Miercke LJ, Stroud RM. Crystal structure of human aquaporin 4 at 1. 8 A and its mechanism of conductance. Proc Natl Acad Sci U S A. 2009;106:7437–7442. doi: 10.1073/pnas.0902725106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbavatz JM, Ma T, Gobin R, Verkman AS. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci. 1997;110 (Pt 22):2855–2860. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Brown D, Verkman AS. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem. 1996;271:4577–4580. [PubMed] [Google Scholar]
- 8.Rash JE, Davidson KG, Kamasawa N, Yasumura T, Kamasawa M, Zhang C, Michaels R, Restrepo D, Ottersen OP, Olson CO, Nagy JI. Ultrastructural localization of connexins (Cx36, Cx43, Cx45), glutamate receptors and aquaporin-4 in rodent olfactory mucosa, olfactory nerve and olfactory bulb. J Neurocytol. 2005;34:307–341. doi: 10.1007/s11068-005-8360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolburg H, Wolburg-Buchholz K, Fallier-Becker P, Noell S, Mack AF. Structure and functions of aquaporin-4-based orthogonal arrays of particles. Int Rev Cell Mol Biol. 2011;287:1–41. doi: 10.1016/B978-0-12-386043-9.00001-3. [DOI] [PubMed] [Google Scholar]
- 10.Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci U S A. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furman CS, Gorelick-Feldman DA, Davidson KG, Yasumura T, Neely JD, Agre P, Rash JE. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci U S A. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberstein C, Bouley R, Huang Y, Fang P, Pastor-Soler N, Brown D, Van Hoek AN. Membrane organization and function of M1 and M23 isoforms of aquaporin-4 in epithelial cells. Am J Physiol Renal Physiol. 2004;287:F501–511. doi: 10.1152/ajprenal.00439.2003. [DOI] [PubMed] [Google Scholar]
- 13.Crane JM, Verkman AS. Determinants of aquaporin-4 assembly in orthogonal arrays revealed by live-cell single-molecule fluorescence imaging. J Cell Sci. 2009;122:813–821. doi: 10.1242/jcs.042341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neely JD, Christensen BM, Nielsen S, Agre P. Heterotetrameric composition of aquaporin-4 water channels. Biochemistry. 1999;38:11156–11163. doi: 10.1021/bi990941s. [DOI] [PubMed] [Google Scholar]
- 15.Jin BJ, Rossi A, Verkman AS. Model of aquaporin-4 supramolecular assembly in orthogonal arrays based on heterotetameric association of M1/M23 isoforms. 201(100):2936–2945. doi: 10.1016/j.bpj.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenton RA, Moeller HB, Zelenina M, Snaebjornsson MT, Holen T, MacAulay N. Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol Life Sci. 2010;67:829–840. doi: 10.1007/s00018-009-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiroaki Y, Tani K, Kamegawa A, Gyobu N, Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, Mizoguchi A, Fujiyoshi Y. Implications of the aquaporin-4 structure on array formation and cell adhesion. J Mol Biol. 2006;355:628–639. doi: 10.1016/j.jmb.2005.10.081. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Verkman AS. Evidence against involvement of aquaporin-4 in cell-cell adhesion. J Mol Biol. 2008;382:1136–1143. doi: 10.1016/j.jmb.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noell S, Fallier-Becker P, Deutsch U, Mack AF, Wolburg H. Agrin defines polarized distribution of orthogonal arrays of particles in astrocytes. Cell Tissue Res. 2009;337:185–195. doi: 10.1007/s00441-009-0812-z. [DOI] [PubMed] [Google Scholar]
- 20.Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 m1/m23 isoforms and orthogonal arrays. J Biol Chem. 2011;286:16516–16524. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, Lia A, Trojano M, Frigeri A, Svelto M. Aquaporin-4 orthogonal arrays of particles are the target for neuromyelitis optica autoantibodies. Glia. 2009;57:1363–1373. doi: 10.1002/glia.20855. [DOI] [PubMed] [Google Scholar]
- 22.Das S, Smith TD, Sarma JD, Ritzenthaler JD, Maza J, Kaplan BE, Cunningham LA, Suaud L, Hubbard MJ, Rubenstein RC, Koval M. ERp29 restricts Connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell. 2009;20:2593–2604. doi: 10.1091/mbc.E08-07-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajima M, Crane JM, Verkman AS. Aquaporin-4 (AQP4) associations and array dynamics probed by photobleaching and single-molecule analysis of green fluorescent protein-AQP4 chimeras. J Biol Chem. 2010;285:8163–8170. doi: 10.1074/jbc.M109.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane JM, Van Hoek AN, Skach WR, Verkman AS. Aquaporin-4 dynamics in orthogonal arrays in live cells visualized by quantum dot single particle tracking. Mol Biol Cell. 2008;19:3369–3378. doi: 10.1091/mbc.E08-03-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raunser S, Haase W, Franke C, Eckert GP, Muller WE, Kuhlbrandt W. Heterologously expressed GLT-1 associates in approximately 200-nm protein-lipid islands. Biophys J. 2006;91:3718–3726. doi: 10.1529/biophysj.106.086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tkachenko E, Simons M. Clustering induces redistribution of syndecan-4 core protein into raft membrane domains. J Biol Chem. 2002;277:19946–19951. doi: 10.1074/jbc.M200841200. [DOI] [PubMed] [Google Scholar]
- 27.Tong J, Briggs MM, Mlaver D, Vidal A, McIntosh TJ. Sorting of lens aquaporins and connexins into raft and nonraft bilayers: role of protein homo-oligomerization. Biophys J. 2009;97:2493–2502. doi: 10.1016/j.bpj.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulma M, Herec M, Grudzinski W, Anderluh G, Gruszecki WI, Kwiatkowska K, Sobota A. Sphingomyelin-rich domains are sites of lysenin oligomerization: implications for raft studies. Biochim Biophys Acta. 2010;1798:471–481. doi: 10.1016/j.bbamem.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Crane JM, Verkman AS. Reversible, temperature-dependent supramolecular assembly of aquaporin-4 orthogonal arrays in live cell membranes. Biophys J. 2009;97:3010–3018. doi: 10.1016/j.bpj.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001;155:113–122. doi: 10.1083/jcb.200106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amiry-Moghaddam M, Xue R, Haug FM, Neely JD, Bhardwaj A, Agre P, Adams ME, Froehner SC, Mori S, Ottersen OP. Alpha-syntrophin deletion removes the perivascular but not endothelial pool of aquaporin-4 at the blood-brain barrier and delays the development of brain edema in an experimental model of acute hyponatremia. Faseb J. 2004;18:542–544. doi: 10.1096/fj.03-0869fje. [DOI] [PubMed] [Google Scholar]
- 32.Neely JD, Amiry-Moghaddam M, Ottersen OP, Froehner SC, Agre P, Adams ME. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci U S A. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- 34.Pol A, Martin S, Fernandez MA, Ingelmo-Torres M, Ferguson C, Enrich C, Parton RG. Cholesterol and fatty acids regulate dynamic caveolin trafficking through the Golgi complex and between the cell surface and lipid bodies. Mol Biol Cell. 2005;16:2091–2105. doi: 10.1091/mbc.E04-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol. 2005;170:769–779. doi: 10.1083/jcb.200506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanczewska J, Marco S, Vandermeeren C, Maudoux O, Rigaud JL, Boutry M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc Natl Acad Sci U S A. 2005;102:11675–11680. doi: 10.1073/pnas.0504498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Meer G, Vaz WL. Membrane curvature sorts lipids. Stabilized lipid rafts in membrane transport. EMBO Rep. 2005;6:418–419. doi: 10.1038/sj.embor.7400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 39.Tham DK, Moukhles H. Regulation of Kir4.1 and Aqp4 Expression and stability at the basolateral domain of epithelial MDCI cells by the extracellular matrix. Am J Physiol Renal Physiol. 2011 doi: 10.1152/ajprenal.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blake DJ, Hawkes R, Benson MA, Beesley PW. Different dystrophin-like complexes are expressed in neurons and glia. J Cell Biol. 1999;147:645–658. doi: 10.1083/jcb.147.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.James M, Nguyen TM, Wise CJ, Jones GE, Morris GE. Utrophin-dystroglycan complex in membranes of adherent cultured cells. Cell Motil Cytoskeleton. 1996;33:163–174. doi: 10.1002/(SICI)1097-0169(1996)33:3<163::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 42.Marples D, Knepper MA, Christensen EI, Nielsen S. Redistribution of aquaporin-2 water channels induced by vasopressin in rat kidney inner medullary collecting duct. Am J Physiol. 1995;269:C655–664. doi: 10.1152/ajpcell.1995.269.3.C655. [DOI] [PubMed] [Google Scholar]
- 43.Rossi A, Crane JM, Verkman AS. Aquaporin-4 Mz isoform: Brain expression, supramolecular assembly and neuromyelitis optica antibody binding. Glia. 2011;59:1056–1063. doi: 10.1002/glia.21177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wittig I, Karas M, Schagger H. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol Cell Proteomics. 2007;6:1215–1225. doi: 10.1074/mcp.M700076-MCP200. [DOI] [PubMed] [Google Scholar]
- 45.Castro BM, Silva LC, Fedorov A, de Almeida RF, Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. J Biol Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, Glogowska M, Case D, Antel JP, Owens GP, Gilden D, Nessler S, Stadelmann C, Hemmer B. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66:617–629. doi: 10.1002/ana.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]