Abstract
Previous studies have shown that short-term (4 weeks) or chronic (32 weeks) exposure to trichloroethylene (TCE) in drinking water of female MRL+/+ mice generated CD4+ T cells that secreted increased levels of interferon (IFN)-γ and expressed an activated (CD44hiCD62Llo) phenotype. In contrast, the current study of subchronic TCE exposure showed that midway in the disease process both of these parameters of CD4+ T cell activation were reversed. This phase of the disease process may represent an attempt by the body to counteract the inflammatory effects of TCE. The decrease in CD4+ T cell production of IFN-γ following subchronic TCE exposure could not be attributed to skewing toward a Th2 or Th17 phenotype or to an increase in Treg cells. Instead, the suppression corresponded to alterations in markers used to assess DNA methylation, namely increased expression of retrotransposons Iap (intracisternal A particle) and Muerv (murine endogenous retrovirus). Also observed was an increase in the expression of Dnmt1 (DNA methyltransferase-1) and decreased expression of several genes known to be downregulated by DNA methylation, namely Ifng, Il2, and Cdkn1a. CD4+ T cells from a second study in which MRL+/+ mice were treated for 17 weeks with TCE showed a similar increase in Iap and decrease in Cdkn1a. In addition, DNA collected from the CD4+ T cells in the second study showed TCE-decreased global DNA methylation. Thus, these results described the biphasic nature of TCE-induced alterations in CD4+ T cell function and suggested that these changes represented potentially reversible alterations in epigenetic processes.
Keywords: trichloroethylene, epigenetics, CD4+ T cells, immunotoxicity
Autoimmune diseases, such as type I diabetes, systemic lupus erythematosus (SLE), and autoimmune hepatitis (AIH), are among the most prevalent maladies in the United States, affecting approximately 8% of the population. Although the initiating events remain obscure, we know that autoimmune diseases involve self-reactive immune responses that are triggered by ill-defined environmental events in genetically susceptible individuals. There is increasing evidence that one type of environmental trigger for autoimmunity is exposure to certain toxicants.
The contribution of one common water pollutant, trichloroethylene (TCE), to autoimmunity is becoming increasingly well validated. As noted by the recent National Research Council report entitled “Assessing the Human Health Risks of Trichloroethylene: Key Scientific Issues,” the evidence on human health hazards from TCE exposure has strengthened in recent years (Committee on Human Health Risks of Trichloroethylene, National Research Council, 2006). The predominant noncancer outcome associated with TCE exposure in humans is immunotoxicity, most notably the development of autoimmune disease. Chronic TCE exposure in adults (mostly occupational but sometimes environmental) has been linked to a variety of autoimmune diseases including SLE, scleroderma, and diabetes (Czirjak et al., 1994; Dubrow and Gute, 1987; Flindt-Hansen and Isager, 1987; Hansen and Isager, 1988; Lockey et al., 1997; Yanez Diaz et al., 1992). There are also links between environmental TCE exposure and autoimmune liver diseases (Stanca et al., 2008). In addition, occupational users of TCE can develop a generalized skin hypersensitivity disease associated with nonviral, immunologically induced hepatitis, and in many cases increased levels of total IgG (Kamijima et al., 2008). Even if overt autoimmune disease is not diagnosed, signs of immune activation including increased numbers of T cells have been associated with chronic exposure to a domestic water supply contaminated with TCE (Clark et al., 1994; Kilburn and Washaw, 1992; Nietert et al., 1998). Taken together, these results provide strong circumstantial evidence that exposure to TCE can promote T cell hyperactivity and autoimmunity in humans, and this immune response can manifest itself in different types of systemic and organ-specific autoimmune disease.
Several studies have tested the immunotoxicity of TCE in an animal model by adding it to the drinking water of female MRL+/+ mice (Cai et al., 2008; Gilbert et al., 1999; Griffin et al., 2000b) The effects of TCE in mice have been primarily studied after short-term (4 weeks) exposure to identify possible initiating changes in the immune system and after long-term (32 weeks) exposure to delineate autoimmune tissue damage. Although no pathology was found after a 4-week exposure to TCE, the toxicant did impact the immune system, specifically altering the activity of CD4+ T cells with few changes noted in B cell or CD8+ T cells. Specifically, the CD4+ T cells from the MRL+/+ mice treated with TCE for 4 weeks contained a higher percentage of activated/memory cells and secreted increased levels of interferon (IFN)-γ (Griffin et al., 2000a,b), a proinflammatory cytokine that has been shown to play an important role in many types of human and experimental models of autoimmune disease. The same increase in IFN-γ production was observed in CD4+ T cells from mice exposed to TCE for 32 weeks (Griffin et al., 2000b). In addition, MRL+/+ mice exposed to occupationally relevant concentrations of TCE for 32 weeks developed a T cell–mediated liver disease commensurate with human idiopathic AIH.
Although TCE increased IFN-γ production by CD4+ T cells at the 4- and 32-week time points, it was not clear whether this effect was linear. One study of shorter duration showed that IFN-γ production by CD4+ T cells from TCE-treated mice compared with control mice was increased at 4 weeks of exposure, equivalent at 8 weeks, and decreased after 22 weeks of exposure (Griffin et al., 2000a). These kinds of fluctuations are not uncommon in autoimmunity. Idiopathic human autoimmune diseases as well as many experimental autoimmune diseases in animal models are long complex processes with stages of attenuation and disease reactivation. Delineating the mechanism by which the body at least temporarily counteracts the proinflammatory process that is required for autoimmune tissue pathology may help prolong the remission and essentially cure autoimmune disease.
In order to delineate the effects of subchronic, rather than short term or chronic, exposure to TCE, the current study examined CD4+ T cells after a 12- or 17-week exposure to TCE. The expression of multiple cytokine genes and associated regulatory components were examined in the CD4+ T cells. In addition, genes associated with DNA methylation were examined because this epigenetic process appears to regulate several aspects of CD4+ T cell function including Th1/Th2 differentiation (Winders et al., 2004), cytokine production (Bruniquel and Schwartz, 2003), and maintenance of Treg cells (Floess et al., 2007). The results obtained confirmed the biphasic nature of TCE-induced inflammation. The mechanism for the apparent attenuation was examined, and the results suggest for the first time that epigenetic mechanisms may contribute to the long-term effects of TCE on CD4+ T cells and their variability over time.
MATERIALS AND METHODS
Mouse treatment.
Eight-week-old female MRL+/+ mice (Jackson Laboratories, Bar Harbor, ME) were housed in polycarbonate-ventilated cages and provided with drinking water (ultrapure from Milli-Q Integral Water Purification System, Millipore) ad libitum. TCE (purity 99± %; Aldrich Chemical Co. Inc., Milwaukee, WI) was suspended in drinking water with 1% emulsifier Alkamuls EL-620 from Rhone-Poulenc (Cranbury, NJ). The mice (12 mice per group) received either 0, 0.02, 0.1, or 0.5 mg/ml TCE in their drinking water for 12 weeks starting at 6–8 weeks of age. Freshly made TCE-containing drinking water was provided every 2–3 days. In a second study, female MRL+/+ mice (10 mice per group) received either 0, 0.01, or 0.1 mg/ml TCE in their drinking water for 17 weeks. The mice were weighed weekly and water consumption was monitored. All studies were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences. After 12 or 17 weeks of exposure, pooled spleen cell suspensions from paired mice (n = 6 per treatment group) were examined by flow cytometry. In addition, CD4+ T cells were isolated from the pooled spleen cell suspensions of three mice (n = 4 per treatment group) using Dynabeads FlowComp Mouse CD4 (Invitrogen Corp., Carlsbad, CA) and stimulated with immobilized anti-CD3 antibody (3 μg/ml) and anti-CD28 antibody (3 μg/ml) for 24 h. Culture supernatants were then collected for cytokine evaluation, and the activated CD4+ T cells were lysed with Buffer RLT (Qiagen Sciences, Germantown, MD) and frozen (−70°C) for subsequent quantitative reverse transcriptase-PCR (qRT-PCR) analysis. In the second study, DNA was purified from half of the activated CD4+ T cells with the Epicentre Biotechnologies MasterPure DNA Purification Kit (Madison, WI) according to the manufacturers’ instructions for cell samples. Phenol and 1-bromo-3-chloropropane extraction steps were added before isopropanol precipitation of the DNA (Leakey et al., 2008). To remove residual RNA, the purified DNA was treated overnight with 0.1M NaOH. NaOH-treated single stranded DNA (ssDNA) was quantitated with the NanoDrop 2000c and neutralized with 200mM acetic acid.
Quantitative reverse transcriptase-PCR.
Fluorescence-based qRT-PCR was conducted using RNA isolated (using RNeasy; Qiagen) from activated CD4+ T cells. For each sample, complementary DNA (cDNA) was synthesized with oligo (dT) and random hexamers and 0.5 μg of total RNA using an iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA). PCR primer sequences were derived from the literature (Ramirez et al., 2006) or designed using the National Center for Biotechnology Information gene databases and subsequently synthesized by IDT, Inc. (Coralville, IA) using PrimerQuest. The primers used are listed in Table 1. All qRT-PCR reactions were carried out using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc.,) and the IQ5 iCycler (Bio-Rad Laboratories) according to manufacturer protocols. No-template controls were included to detect primer-dimer artifacts, as were negative RT controls to check for genomic DNA. DNA melting curve analysis was used as another control against primer-dimer formation and to verify the lack of contaminating DNA in the RNA preparations. Cq values > 36 were not reported because of implied low efficiency. The relative gene expression was calculated using the delta-delta Cq method based on normalization to the reference gene Eef2 (eukaryotic translation elongation factor 2). The Cq value of this reference gene did not vary more than 10% among the treatment groups and was found to be superior to β-actin, 18S ribosomal RNA, and Gapdh (glyceraldehyde-3-phosphate dehydrogenase) in terms of copy number and treatment-associated invariance in the tissues tested. Primer optimization was conducted, and the PCR amplification efficiency for the reference gene and the genes of interest was > 85% but < 110%, with the R2 value > 0.9.
TABLE 1.
Primer Sequences Used for qRT-PCR
| Gene | Primer Sequences 5′ to 3′ | Tm (°C) | |
| Bcl-2 | Sense | TGCGGCCTCTGTTTGATTTC | 56.0 |
| Antisense | CTTGTGGCCCAGATAGGCAC | 58.3 | |
| CD70 | Sense | TGCTGGTGGTGTTTATTACTGTG | 55.4 |
| Antisense | CTCTGGTCCGTGTGTGAAGG | 57.7 | |
| Cdkn1a (p21) | Sense | AATCCTGGTGATGTCCGACCTGTT | 60.2 |
| Antisense | GTGACGAAGTCAAAGTTCCACCGT | 59.4 | |
| Dnmt1 | Sense | TGATAAGGAGGACAAGGAGAATGC | 56.4 |
| Antisense | CACCGCCAAGTTAGGACACC | 58.3 | |
| Dnmt3a | Sense | CAGCACCATTCCTGGTCATGCAAA | 60.2 |
| Antisense | TCCTGTGTGGTAGGCACCTGAAAT | 60.2 | |
| Foxp3 | Sense | CCCTGCCCTTGACCTCAA | 57.4 |
| Antisense | GCCTCAGTCTCATGGTTTTGG | 56.1 | |
| IAP | Sense | GCACCCTCAAAGCCTATCTTAT | 54.6 |
| Antisense | TCCCTTGGTCAGTCTGGATTT | 55.8 | |
| Ifit1 | Sense | TGCTTTGCGAAGGCTCTGAAAGTG | 60.1 |
| Antisense | TGGATTTAACCGGACAGCCTTCCT | 60.3 | |
| Ifng | Sense | AGCTCATCCGAGTGGTCCAC | 59.1 |
| Antisense | AGCAGCGACTCCTTTTCCG | 57.8 | |
| Il17a | Sense | TGGCGGCTACAGTGAAGGC | 60.4 |
| Antisense | GGAACGGTTGAGGTAGTCTGAGG | 58.8 | |
| Il2 | Sense | CCCAAGCAGGCCACAGAATTGAAA | 60.2 |
| Antisense | AGTCAAATCCAGAACATGCCGCAG | 59.9 | |
| Il4 | Sense | AGCCATATCCACGGATGCGACAAA | 60.8 |
| Antisense | AATATGCGAAGCACCTTGGAAGCC | 60.0 | |
| Lta | Sense | TCTCTGGTGTCCGCTTCTCC | 58.8 |
| Antisense | CGATCCGTGCTTGCTCTCC | 58.5 | |
| Muerv | Sense | TGGTGGTCGAGATGGAGGTTA | 57.5 |
| Antisense | CCGTGAATGGTGGTTTTAGCA | 55.8 | |
| Myc | Sense | ACTTACAATCTGCGAGCCAGGACA | 60.3 |
| Antisense | GCCCAAAGGAAATCCAGCCTTCAA | 60.1 | |
| p53 | Sense | TGGACCCTGGCACCTACAATGAAA | 60.5 |
| Antisense | ATGCAGACAGGCTTTGCAGAATGG | 60.2 | |
| Rasa1 | Sense | AGCAACTGCCAGCACCTTTGTAAG | 60.1 |
| Antisense | AGGGTACACCAAGGCTCAGAGTTT | 60.0 | |
| Runx1 | Sense | ATGAAGCCTAACCGCTGGTTCTGA | 60.4 |
| Antisense | AAACGCTCTTCGGAGTTGACCTGA | 60.2 | |
| Tbet | Sense | TACGCATCTGTTGATACGAGTGTC | 56.5 |
| Antisense | GGTTGGATAGAAGAGGTGAGAAGG | 56.5 | |
| Tnfa | Sense | CGTGGAACTGGCAGAAGAGG | 58.0 |
| Antisense | GAGAAGAGGCTGAGACATAGGC | 56.8 |
Analysis of global DNA.
The MethylFlash Methylated DNA Quantification Kit (Epigentek) was used according to manufacturer’s instruction to quantitate global DNA methylation status. NaOH-treated ssDNA (50 ng) from each sample (CD4+ T cells from spleen cell suspensions of paired mice for n = 5 per treatment group) was used in the kit.
Phenotypic analysis of spleen cells.
Flow cytometric analysis of equal numbers of spleen cells from paired mice (n = 6 per group) was conducted. The phenotypic analysis of 30,000 events per sample was carried out using a CyFlow ML (Partec GmbH, Munster, Germany), and the data were presented as mean percentage ± SE. Nonviable cells, based on low forward scatter and side scatter, were excluded in each sample. Fluorescence minus one controls and isotype Ig controls were included. Antibodies used included PE-anti-mouse IFN-γ (clone XMG1.2, rat IgG1); APC-anti-CD4 (clone GK1.5, rat IgG2b); PECy7-anti-CD4 (clone GK1.5, rat IgG2b); Biotin-anti-CD62L (clone F344, rat IgG2a); Alexa Fluor 647-anti-IL17a (clone EBio17B7, Rat IgG2a); and FITC-anti-mouse CD44 (clone IM7, rat IgG2b), all purchased from eBioscience (San Diego, CA) or BD Biosciences (San Jose, CA). Intracellular levels of IFN-γ and interleukin (IL)-17 in CD4+ T cells were examined as previously described (Blossom and Doss, 2007).
Cytokine analysis.
The culture supernatants from the activated CD4+ T cells were examined using ELISA kits for IFN-γ (OptEIA Set Mouse IFN−γ; BD Biosciences, San Diego, CA), or READY-SET-GO ELISA kits for mouse IL-2, IL-4, IL-6, IL-17, or tumor necrosis factor (TNF)-α from eBioscience.
Statistics.
The data are presented as means and SDs. Assays were conducted using samples from 12 individual mice per treatment group or samples from equal numbers of pooled cells from six or four mice per treatment group. Comparisons between values obtained from controls and different treatment were made using a Student’s t-test. The threshold for statistical significance was set at α = 0.05.
RESULTS
TCE-Altered Lymphoid Organ Cellularity
Young female MRL+/+ mice, with their susceptibility for autoimmunity but absence of overt disease, have been used to test whether TCE alters immune function. In the first study, female MRL+/+ mice were given drinking water with or without TCE (0.02, 0.1, and 0.5 mg/ml) for 12 weeks. After 12 weeks of exposure to TCE, immune cell phenotype and function, with an emphasis on CD4+ T cells, were evaluated. None of the TCE concentrations altered weight gain in the mice during the course of the experiment (Fig. 1A). The addition of TCE did not alter water consumption (data not shown). In terms of cellularity, TCE exposure did not alter total spleen cell numbers or total number of CD4+ T cells in the spleen (Fig. 1B). Thus, this level of TCE exposure did not induce overt immunotoxicity at least in terms of growth or spleen cell numbers.
FIG. 1.
TCE did not alter growth or spleen size. (A) Body weight was monitored over time. (B) Numbers of total spleen cells and splenic CD4+ T cells were assessed by flow cytometry after 12 weeks of exposure to TCE. The data are plotted as mean ± SD.
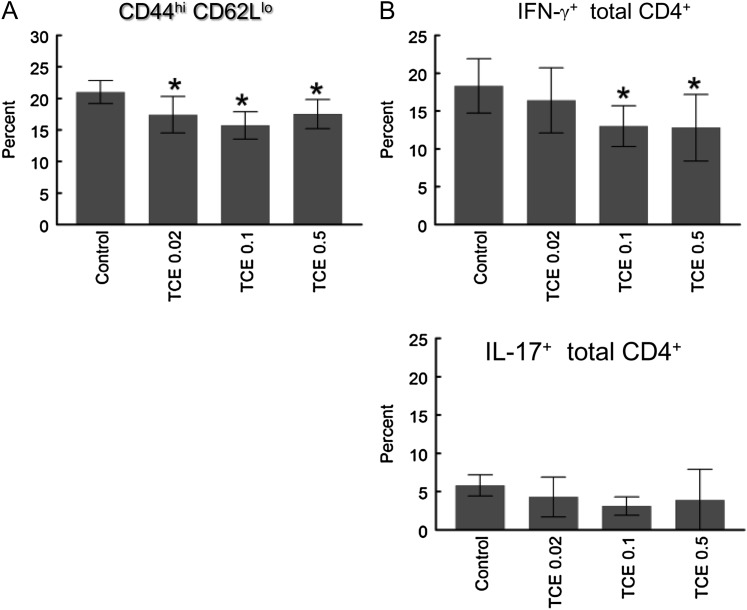
Previous studies have demonstrated that short-term and chronic exposure to TCE increased the percentage of CD4+ T cells with an activated/memory cell phenotype, i.e., CD44hi and/or CD62Llo (Gilbert et al., 2006; Griffin et al., 2000b). In contrast, the percentage of splenic CD4+ T cells expressing an activated/memory phenotype (CD44hiCD62Llo) decreased rather than increased in the mice exposed to TCE for 12 weeks (Fig. 2A).
FIG. 2.
TCE inhibited CD4+ T cell activation. (A) Percent of splenic CD4+ T cells with CD44hiCD62Llo phenotype was determined by flow cytometry after 12 weeks of TCE exposure. (B) Percent of splenic CD4+ T cells with intracellular IFN-γ or IL-17 was examined by flow cytometry after 4.5 h activation with phorbol 12-myristate 13-acetate and ionomycin. The data are plotted as mean ± SD. *Represents statistically significant differences compared with values in CD4+ T cells from control mice.
TCE-Suppressed CD4+ T Cell Cytokine Production
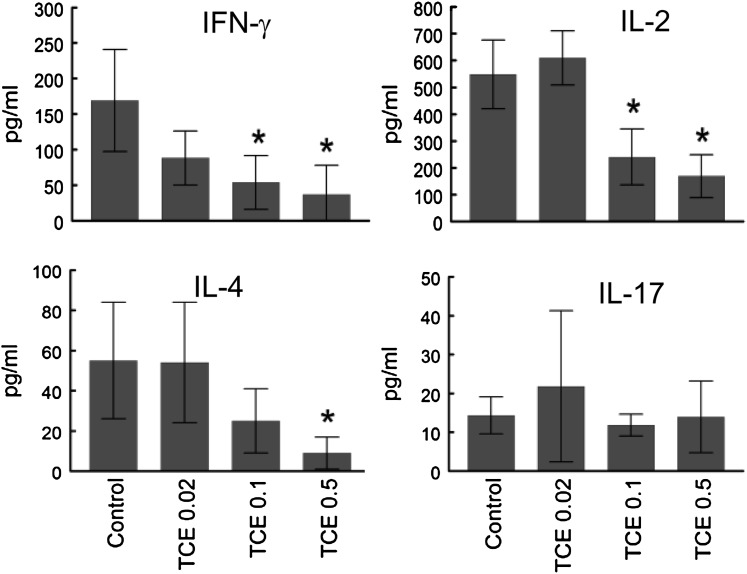
As another parameter of activation flow cytometry was used to measure IFN-γ production by individual CD4+ T cells. This analysis showed that the percentage of total CD4+ T cells producing IFN-γ was suppressed rather than enhanced by subchronic TCE exposure (Fig. 2B). The suppressive effects of TCE on cytokine production did not extend to IL-17, which was not found to be altered in total splenic CD4+ T cells (Fig. 2B). ELISAs were also used to determine total levels of cytokine secreted. Isolated splenic CD4+ T cells from the mice exposed to TCE were stimulated in vitro for 24 h with anti-CD3 and anti-CD28 antibodies. As shown in Figure 3, TCE exposure for 12 weeks inhibited IFN-γ production by total splenic CD4+ T cells. This suppression was substantial; exposure to TCE at 0.5 mg/ml suppressed IFN-γ production by 80% from CD4+ T cells. TCE exposure also induced a dramatic dose-dependent decrease in the production of IL-2 and IL-4 by the splenic CD4+ T cells. Secretion of IL-17, albeit low, was not altered in CD4+ T cells from mice exposed to TCE. Taken together, TCE exposure for 12 weeks suppressed the ability of total splenic CD4+ T cells to secrete three of the four cytokines tested.
FIG. 3.
TCE inhibited CD4+ T cell cytokine production. Splenic CD4+ T cells from mice treated for 12 weeks with TCE were stimulated for 24 h. Culture supernatants were evaluated for secreted levels of various cytokines by ELISA: The data are plotted as mean ± SD. *Represents statistically significant differences compared with controls.
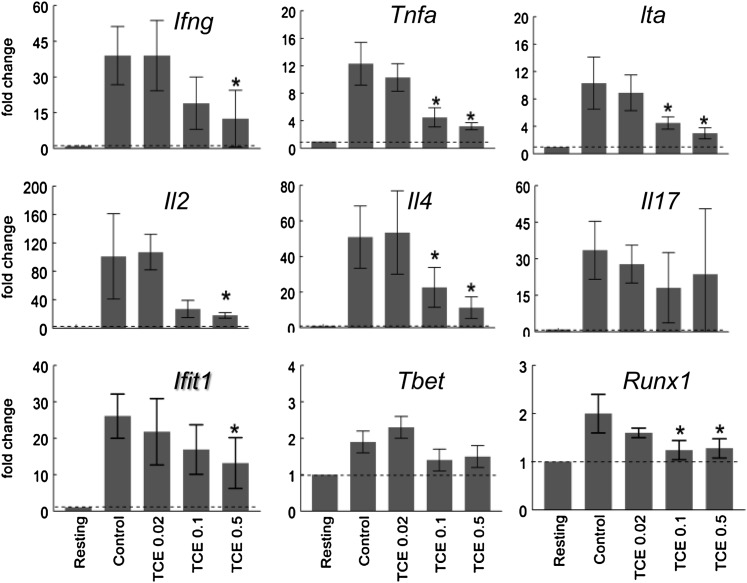
Cytokine gene expression was also examined as a correlative to secreted cytokine protein measurements. As shown in Figure 4, stimulation with anti-CD3 and anti-CD28 antibodies increased expression of Ifng (gene that encodes IFN-γ) in CD4+ T cells from the control mice. Expression of Ifng was also upregulated in stimulated CD4+ T cells from TCE-treated mice. However, compared with CD4+ T cells from control mice, Ifng expression in the CD4+ T cells from the mice exposed to 0.5 mg/ml TCE in their drinking water was suppressed by 68%. CD4+ T cells from mice treated with 0.5 or 0.1 mg/ml TCE also showed a significant decrease in expression of Tnf, Lta, and Il4, genes that encode for TNF, lymphotoxin-α, and IL-4, respectively. Only Il17 expression was unaltered in the CD4+ T cells exposed to TCE. The TCE-induced alterations in cytokine gene expression mirrored the previously described effects in protein levels measured either by ELISA or flow cytometry. Corresponding to the decrease in Ifng was a decrease in the downstream interferon-inducible gene Ifit1. In contrast, expression of Tbet, the gene that encodes the transcription factor that controls the Th1 lineage, was not altered by TCE exposure. Thus, TCE exposure at this 12-week time point and at these TCE concentrations appeared to suppress production of several cytokines, including IFN-γ, IL-2, and IL-4, and this suppression was found at the level of transcription and protein secretion. This inhibition of IFN-γ by TCE exposure could not be attributed to suppression of Tbet.
FIG. 4.
TCE inhibited CD4+ T cell expression of Ifng and other cytokine genes. Splenic CD4+ T cells from control mice or mice treated for 12 weeks with TCE were stimulated for 24 h. qRT-PCR was performed using total RNA from the activated CD4+ T cells. The values represent the mean ± SD of relative fold change comparing gene expression to that found in resting CD4+ T cells from untreated mice. *Represents statistically significant differences in fold change compared with stimulated CD4+ T cells from control mice.
TCE Did Not Increase the Percentage of Treg Cells
Although Treg cells can suppress cytokine production by effector CD4+ T cells (Sojka and Fowell, 2011), it seemed possible that a TCE-induced increase in the number of these regulatory cells could account for at least some of the suppressive effects of TCE on CD4+ T cells. Consequently, the percentage of CD4+ CD25+ FoxP3+ in the spleens was evaluated as a means of estimating effects of TCE on the prevalence of Treg cells. As shown in Figure 5A, the percentage of splenic Treg cells was unaltered by TCE exposure. Treg cell subsets within the specified phenotype have been observed. However, regardless of whether the CD4+ T cells were classified as single positive for FoxP3, CD25, or positive for both, no differences among the groups were observed (data not shown). An additional assessment by qRT-PCR showed that the CD4+ T cells from all four groups, regardless of TCE exposure, expressed similar levels of Foxp3 (Fig. 5B). These results indicate that the suppressive effects of subchronic TCE exposure could not be attributed to an increase in the number of Treg cells.
FIG. 5.
TCE did not inhibit percentage of splenic Treg cells. (A) Percent of splenic Treg cells with CD4+ CD25+ FoxP3+ phenotype was determined by flow cytometry after 12 weeks of TCE exposure. (B) Splenic CD4+ T cells from mice treated for 12 weeks with TCE were stimulated for 24 h. qRT-PCR for Foxp3 was performed using total RNA from the activated CD4+ T cells. The values represent the mean ± SD of relative fold change comparing gene expression in TCE-treated to control mice.
TCE-Induced Events Association With Epigenetic Alterations
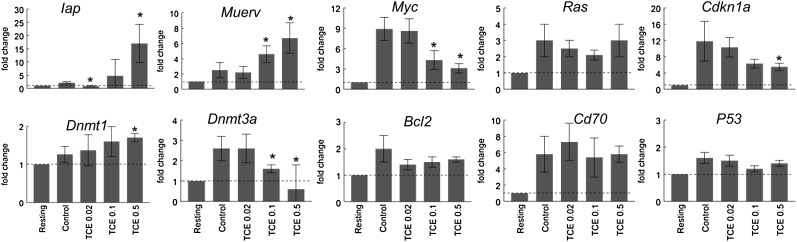
The suppressive effects of in vivo TCE exposure on the CD4+ T cells were maintained even after the cells were stimulated in vitro in the absence of further contact with TCE. To evaluate whether this persistent suppression was mediated by epigenetic alterations, the expression of several genes known to be regulated by, or associated with, DNA methylation was examined. For example, in addition to several cytokines, including IL-2 and IFN-γ, expression of the cell cycle regulatory protein Cdkn1a (p21cip1) has been shown to be negatively regulated by DNA methylation (Bruniquel and Schwartz, 2003; Zheng et al., 2006). As shown in Figure 6, Cdkn1a expression, which was low in resting CD4+ T cells, was upregulated almost 12-fold in CD4+ T cells from control mice following activation in vitro. This activation-induced increase in Cdkn1a expression was suppressed by more than 50% in CD4+ T cells from mice exposed to 0.5 mg/ml TCE. CD70 is a cell surface cytokine whose expression in CD4+ T cells is enhanced by 5-aza-2′ deoxycytidine, a drug that promotes DNA hypomethylation (Oelke et al., 2004). TCE had no effect on Cd70 expression, suggesting that TCE did not induce DNA demethylation at the 12-week time point. Instead, based on its suppressive effects on IL2, Ifng, and Cdkn1a, it seems more likely that subchronic TCE promoted rather than suppressed DNA methylation of these genes. However, this remains to be tested.
FIG. 6.
TCE inhibited CD4+ T cell expression of genes associated with DNA methylation. Splenic CD4+ T cells from control mice or from mice treated for 12 weeks with TCE were stimulated for 24 h. qRT-PCR was performed using total RNA from the activated CD4+ T cells. The values represent the mean ± SD of relative fold change comparing gene expression to that found in resting CD4+ T cells from untreated mice. *Represents statistically significant differences in fold change compared with stimulated CD4+ T cells from control mice.
We also examined expression of Iap (intracisternal A particle) and Muerv (murine endogenous retrovirus) in the CD4+ T cells from the TCE-treated mice. IAP and MuERV are retrotransposons that are normally transcriptionally silent due to DNA methylation of their long terminal repeat sequences (Walsh et al., 1998). Because DNA methylation is usually highly correlated with retrotransposon silencing, we used expression of Iap and Muerv as indicators of global changes in DNA methylation. As shown in Figure 6, expression of both Iap and Muerv was dose dependently increased in CD4+ T cells from TCE-treated mice. DNA methylation is established and partially regulated by DNA methyltransferases (Dnmts). Dnmt1 mainly methylates the daughter strands of newly replicated DNA to promote inheritance of methylation patterns (Ooi et al., 2009). Expression of Dnmt1 was increased in CD4+ T cells from mice treated with TCE (0.5 mg/ml) compared with CD4+ T cells from control mice (Fig. 6). In contrast, expression of Dnmt3a, which encodes for an enzyme that catalyzes de novo DNA methylation, was suppressed by TCE exposure. These results indicate that TCE can alter epigenetic processes in CD4+ T cell function, perhaps associated with changes in Dnmts.
In addition to alterations in DNA methylation, transcription of genes for cytokines and cell cycle proteins such as Cdkn1a can be regulated by expression of oncogenes such as c-Myc and p53 (Gartel and Radhakrishnan, 2005). Expression of p53 and Ras in CD4+ T cells was not altered by exposure to TCE (Fig. 6). In contrast, expression of Myc in CD4+ T cells underwent a dose-dependent decrease in mice exposed to TCE. Because c-Myc acts to inhibit Cdkn1a, it seems unlikely that a decrease in Myc expression was responsible for the decrease in Cdkn1a.
TCE-Induced Decrease in Global DNA Methylation
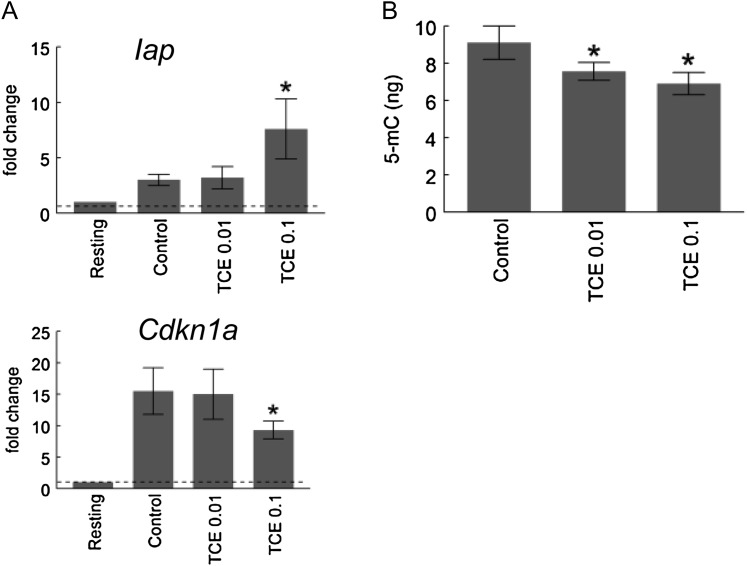
The increased expression of Iap and Muerv in the CD4+ T cells from the TCE-treated mice suggested that the toxicant decreased DNA methylation in these cells. However, because the expression of the two retrotransposons can sometimes be regulated by nonepigenetic processes, a more direct look at TCE-induced changes in DNA methylation was warranted. Because only RNA, not DNA, was collected in the 12-week TCE exposure study, an additional study was conducted to provide the necessary DNA. In this case, the MRL+/+ mice were exposed to TCE (0.01 or 0.1 mg/ml) for 17 week in drinking water. The CD4+ T cells were stimulated in vitro, and preparations of both RNA and DNA were acquired. qRT-PCR showed that similar to the earlier 12-week study, CD4+ T cells from the mice exposed to TCE for 17 weeks demonstrated increased expression of Iap and decreased expression of Cdkn1a (Fig. 7A). A methylated DNA quantification kit suitable for detecting global DNA showed that DNA isolated from the CD4+ T cells from TCE-treated mice was significantly less methylated than DNA isolated from control CD4+ T cells (Fig. 7B). This provides additional evidence that TCE alters DNA methylation in CD4+ T cells, even though gene-specific alterations still need to be identified.
FIG. 7.
TCE inhibited global DNA methylation in CD4+ T cells. Splenic CD4+ T cells from control mice or from mice treated for 17 weeks with TCE were stimulated for 24 h. (A) qRT-PCR was performed using total RNA from the activated CD4+ T cells. The values represent the mean ± SD of relative fold change comparing gene expression to that found in resting CD4+ T cells from untreated mice. *Represents statistically significant differences in fold change compared with stimulated CD4+ T cells from control mice. (B) Global DNA methylation was quantified using ssDNA from the activated CD4+ T cells. *Represents statistically significant differences in DNA methylation compared with stimulated CD4+ T cells from control mice.
DISCUSSION
This study confirmed that TCE induces complex effects on the immune system and that subchronic exposure, unlike short-term or chronic exposure, encompasses a period of immune suppression. The TCE-induced suppression was observed at concentrations commensurate with possible human exposure. Based on water consumption and TCE degradation in the water bottles, the mice given water containing TCE at 0.02, 0.1, or 0.5 mg/ml were exposed to TCE at levels of approximately 3, 14, or 64 mg/kg/day, respectively. Even the highest exposure was lower than the current 8-h permissible exposure limit (established by the Occupational Safety and Health Administration) for TCE of 100 ppm or approximately 76 mg/kg/day for humans.
Although relatively low, the level of TCE exposure tested in the current study induced several robust changes in CD4+ T cells. Although short-term exposure to TCE increases production of IFN-γ by CD4+ T cells (Griffin et al., 2000b), the current study showed that subchronic exposure to TCE suppressed IFN-γ production by CD4+ T cells. This finding was in agreement with another study using higher concentrations of TCE that showed an early TCE-induced increase in IFN-γ production by CD4+ T cells, which was followed by a decrease in IFN-γ production after 22 weeks of TCE exposure (Griffin et al., 2000a). Thus, the current study confirmed the dynamic biphasic nature of TCE’s effects on IFN-γ production by CD4+ T cells.
Several studies of idiopathic or induced autoimmune disease that provided longitudinal measurements of IFN-γ have noted time-dependent fluctuations similar to those seen in TCE-treated mice. For example, in a mouse model of proteolipid protein-induced experimental autoimmune encephalomyelitis, the number of peripheral blood mononuclear cells that secreted IFN-γ when stimulated in vitro increased after 10 days, decreased after 20 days, and increased again after 30 days (Kuerten et al., 2010). This biphasic pattern of IFN-γ production correlated with an early disease phase, followed by a temporary recovery and then clinical relapse. Such fluctuations can also be found in humans with autoimmune disease. Stimulated peripheral blood lymphocytes collected from children with a high risk of developing type 1 diabetes demonstrated a Th1 (IFN-γ) bias during the prediabetic stage followed by temporary Th3 (transforming growth factor-β) response at disease onset (Ryden et al., 2009). The U-shaped curve corresponding to the IFN-γ production by CD4+ T cells in TCE-treated mice may reflect a temporary compensatory response that is eventually overcome after long-term exposure.
There are several pieces of evidence that the function of autoreactive CD4+ T cells can be mediated by epigenetic processes. Most of this work has been done in models of lupus. Richardson et al. have shown that normal activated CD4+ T cells treated with the DNA methyltransferase inhibitor 5-aza-2′ deoxycytidine in vitro hypomethylate the Itgal gene, correspondingly upregulate lymphocyte function-associated antigen-1 (CD11a/CD18) and CD70, and become autoreactive upon adoptive transfer (Yung et al., 1996). In an analogous finding, a discreet pattern of hypermethylated and hypomethylated CpG sites has been found in CD4+ T cells from patients with SLE (Jeffries et al., 2011). Lastly, twin discordance in certain autoimmune diseases such as SLE correlates with patterns of DNA methylation in peripheral blood mononuclear cells (Javierre et al., 2010).
Unlike CD4+ T cells exposed to 5-aza-2′ deoxycytidine (Oelke et al., 2004), CD4+ T cells from mice exposed to TCE for 12 weeks in the current study did not upregulate Cd70. In addition, unlike 5-aza-2′ deoxycytidine, which has been shown to induce p21cip1 (Yang et al., 2005), TCE exposure suppressed expression of Cdkn1a (p21cip1). Similarly, decreased expression of Ifng has been linked to hypermethylation (Winders et al., 2004). Thus, if a 12- or 17-week TCE exposure affected the DNA methylation of specific genes, it appears to have done so by increasing rather than decreasing this epigenetic process. The effect of TCE on methylation-dependent retrotransposons in CD4+ T cells was also examined to further evaluate possible epigenetic alterations. Expression of both Iap and Muerv was significantly increased in the CD4+ T cells from the TCE-treated mice. The apparent contradiction of a generalized TCE-induced demethylation as indicated by increased expression of Iap and Muerv in the CD4+ T cells, but possible hypermethylation of specific cytokine genes in the same cells is not unique. Global hypomethylation accompanied by gene-specific CpG island promoter hypermethylation has been documented in several settings including cancer, atherosclerosis, and aging (Cooney, 2010; Dunn, 2003; Turunen et al., 2009). It is not clear whether expression of retrotransposons serves primarily as a marker of altered DNA methylation or instead plays a direct role in pathology.
The effects of TCE on DNA methylation are still being defined. Acute high-dose TCE (1000 mg/kg/day) decreased methylation of the promoter regions for c-jun and c-myc in liver, an effect that correlated with increased expression of these genes (Tao et al., 2000). On the other hand, a study of the cardioteratogenic effects of low-dose TCE (10 ppb) showed that exogenous folate increased rather than decreased TCE-induced gene expression alterations in embryonic hearts (Caldwell et al., 2010). This result would argue for TCE-induced hypermethylation rather than hypomethylation, and indeed, a subsequent study showed that TCE suppressed expression of the cardiac gene Serca2a in associated with methylation of its promoter (Palbykin et al., 2011). Thus, the effects of TCE on DNA methylation appear to be target cell- and concentration-specific and therefore difficult to generalize. The current study appears to be the first showing effects of TCE on epigenetic events in CD4+ T cells.
If TCE is altering epigenetic processes in CD4+ T cells that mediate cytokine production and/or other functions, this appears to be a cyclical phenomenon. This highlights the complexity of autoimmune disease development and underlines the importance of longitudinal evaluations in determining the role of epigenetic processes in the disease process. A long-term study to examine TCE-induced time-dependent changes in the methylation status of the promoters for functionally important cytokines and cell cycle proteins is now underway. It is possible that TCE-induced inflammation, and eventual autoimmune pathology, involves a process of DNA methylation–induced activation, silencing, and reactivation. Understanding the mechanism for silencing of the proinflammatory genes could enable us to prolong the attenuation part of the disease process as a therapeutic approach for the treatment of autoimmune disease.
FUNDING
Arkansas Biosciences Institute, the National Institutes of Health (1R01ES017286), and the Organic Compounds Property Contamination class action settlement (CV 1992-002603) to K.M.G.
Acknowledgments
We would like to gratefully acknowledge the excellent technical assistance of Meagan Kreps and the University of Arkansas for Medical Sciences Translational Research Institute (National Institutes of Health UL1RR029884).
References
- Blossom SJ, Doss JC. Trichloroethylene alters central and peripheral immune function in autoimmune-prone MRL(+/+) mice following continuous developmental and early life exposure. J. Immunotoxicol. 2007;4:129–141. doi: 10.1080/15476910701337035. [DOI] [PubMed] [Google Scholar]
- Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat. Immunol. 2003;4:235–240. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- Cai P, Konig R, Boor PJ, Kondraganti S, Kaphalia BS, Khan MF, Ansari GA. Chronic exposure to trichloroethene causes early onset of SLE-like disease in female MRL +/+ mice. Toxicol. Appl. Pharmacol. 2008;228:68–75. doi: 10.1016/j.taap.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell PT, Manziello A, Howard J, Palbykin B, Runyan RB, Selmin O. Gene expression profiling in the fetal cardiac tissue after folate and low-dose trichloroethylene exposure. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:111–127. doi: 10.1002/bdra.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LC, Giulano A, Walsh B, Guernsey de Zaplen J, Alberts DS, Meister J, Manson TS. The Santa Cruz County Community health Survey. Phoenix, AZ: Arizona Department of Health Services; 1994. [Google Scholar]
- Committee on Human Health Risks of Trichloroethylene, National Research Council. Assessing the Human Health Risks of Trichloroethylene: Key Scientific Issues. The National Academies Press, Washington, D.C; 2006. [Google Scholar]
- Cooney CA. Drugs and supplements that may slow the aging of the epigenome. Drug Discov. Today. 2010;7:57–64. [Google Scholar]
- Czirjak L, Pocs E, Szegedi G. Localized scleroderma after exposure to organic solvents. Dermatology. 1994;189:399–401. doi: 10.1159/000246888. [DOI] [PubMed] [Google Scholar]
- Dubrow R, Gute DM. Cause-specific mortality among Rhode Island jewelry workers. Am. J. Ind. Med. 1987;12:579–593. doi: 10.1002/ajim.4700120511. [DOI] [PubMed] [Google Scholar]
- Dunn BK. Hypomethylation: One side of a larger picture. Ann. N. Y. Acad. Sci. 2003;983:28–42. doi: 10.1111/j.1749-6632.2003.tb05960.x. [DOI] [PubMed] [Google Scholar]
- Flindt-Hansen H, Isager H. Scleroderma after occupational exposure to trichloroethylene and trichloroethane. Toxicol. Lett. 1987;95:173–181. [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Griffin JM, Pumford NR. Trichloroethylene activates CD4+ T cells: Potential role in an autoimmune response. Drug Metab. Rev. 1999;31:901–916. doi: 10.1081/dmr-100101945. [DOI] [PubMed] [Google Scholar]
- Gilbert KM, Pumford NR, Blossom SJ. Environmental contaminant trichloroethylene promotes autoimmune disease and inhibits T-cell apoptosis in MRL+/+ mice. J. Immunotoxicol. 2006;3:263–267. doi: 10.1080/15476910601023578. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Blossom SJ, Jackson SK, Gilbert KM, Pumford NR. Trichloroethylene accelerates an autoimmune response in association with Th1 T cell activation in MRL+/+ mice. Immunopharmacology. 2000a;46:123–137. doi: 10.1016/s0162-3109(99)00164-2. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Gilbert KM, Lamps LW, Pumford NR. CD4+ T cell activation and induction of autoimmune hepatitis following trichloroethylene treatment in MRL+/+ mice. Toxicol. Sci. 2000b;57:345–352. doi: 10.1093/toxsci/57.2.345. [DOI] [PubMed] [Google Scholar]
- Hansen BL, Isager H. A scleroderma-resembling disease-exposure to trichloroethylene and trichloroethane, is there a causal connection? Ugeskr. Laeg. 1988;150:805–808. [PubMed] [Google Scholar]
- Javierre BM, Fernandez AF, Richter J, Al-Shahrour F, Martin-Subero JI, Rodriguez-Ubreva J, Berdasco M, Fraga MF, O'Hanlon TP, Rider LG, et al. Changes in the pattern of DNA methylation associate with twin discordance in systemic lupus erythematosus. Genome Res. 2010;20:170–179. doi: 10.1101/gr.100289.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffries M, Dozmorov M, Tang Y, Merrill JT, Wren JD, Sawalha AH. Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics. 2011;6:593–601. doi: 10.4161/epi.6.5.15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijima M, Wang H, Huang H, Li L, Shibata E, Lin B, Sakai K, Liu H, Tsuchiyama F, Chen J, et al. Trichloroethylene causes generalized hypersensitivity skin disorders complicated by hepatitis. J. Occup. Health. 2008;50:328–338. doi: 10.1539/joh.l8013. [DOI] [PubMed] [Google Scholar]
- Kilburn KH, Washaw RW. Prevalence of symptoms of systemic lupus erythematosus (SLE) and of fluorescent antinuclear antibodies associated with chronic exposure to trichloroethylene and other chemicals in well water. Environ. Res. 1992;57:1–9. doi: 10.1016/s0013-9351(05)80014-3. [DOI] [PubMed] [Google Scholar]
- Kuerten S, Rottlaender A, Rodi M, Velasco VB, Jr, Schroeter M, Kaiser C, Addicks K, Tary-Lehmann M, Lehmann PV. The clinical course of EAE is reflected by the dynamics of the neuroantigen-specific T cell compartment in the blood. Clin. Immunol. 2010;137:422–432. doi: 10.1016/j.clim.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Leakey TI, Zielinski J, Siegfried RN, Siegel ER, Fan CY, Cooney CA. A simple algorithm for quantifying DNA methylation levels on multiple independent CpG sites in bisulfite genomic sequencing electropherograms. Nucleic Acids Res. 2008;36:e64. doi: 10.1093/nar/gkn210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockey JE, Kelly CR, Cannon GW, Colby TV, Aldrich V, Livingston GK. Progressive systemic sclerosis associated with exposure to trichloroethylene. J. Occup. Med. 1997;29:493–496. [PubMed] [Google Scholar]
- Nietert PJ, Sutherland SE, Silver RM, Pandey JP, Knapp RG, Hoel DG, Dosemeci M. Is occupational organic solvent exposure a risk factor for scleroderma? Arthritis Rheum. 1998;41:1111–1119. doi: 10.1002/1529-0131(199806)41:6<1111::AID-ART19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Oelke K, Lu Q, Richardson D, Wu A, Deng C, Hanash S, Richardson B. Overexpression of CD70 and overstimulation of IgG synthesis by lupus T cells and T cells treated with DNA methylation inhibitors. Arthritis Rheum. 2004;50:1850–1860. doi: 10.1002/art.20255. [DOI] [PubMed] [Google Scholar]
- Ooi SK, O'Donnell AH, Bestor TH. Mammalian cytosine methylation at a glance. J. Cell Sci. 2009;122:2787–2791. doi: 10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palbykin B, Borg J, Caldwell PT, Rowles J, Papoutsis AJ, Romagnolo DF, Selmin OI. Trichloroethylene induces methylation of the Serca2 promoter in H9c2 cells and embryonic heart. Cardiovasc. Toxicol. 2011;11:201–214. doi: 10.1007/s12012-011-9113-3. [DOI] [PubMed] [Google Scholar]
- Ramirez MA, Pericuesta E, Fernandez-Gonzalez R, Moreira P, Pintado B, Gutierrez-Adan A. Transcriptional and post-transcriptional regulation of retrotransposons IAP and MuERV-L affect pluripotency of mice ES cells. Reprod. Biol. Endocrinol. 2006;4:55. doi: 10.1186/1477-7827-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden A, Stechova K, Durilova M, Faresjo M. Switch from a dominant Th1-associated immune profile during the pre-diabetic phase in favour of a temporary increase of a Th3-associated and inflammatory immune profile at the onset of type 1 diabetes. Diabetes-Metab. Res. Rev. 2009;25:335–343. doi: 10.1002/dmrr.958. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Fowell DJ. Regulatory T cells inhibit acute IFN-gamma synthesis without blocking T-helper cell type 1 (Th1) differentiation via a compartmentalized requirement for IL-10. Proc. Natl. Acad. Sci. U.S.A. 2011;108:18336–18341. doi: 10.1073/pnas.1110566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanca CM, Babar J, Singal V, Ozdenerol E, Odin JA. Pathogenic role of environmental toxins in immune-mediated liver diseases. J. Immunotoxicol. 2008;5:59–68. doi: 10.1080/15476910802019086. [DOI] [PubMed] [Google Scholar]
- Tao L, Yang S, Xie M, Kramer PM, Pereira MA. Effect of trichloroethylene and its metabolites, dichloroacetic acid and trichloroacetic acid, on the methylation and expression of c-Jun and c-Myc protooncogenes in mouse liver: Prevention by methionine. Toxicol. Sci. 2000;54:399–407. doi: 10.1093/toxsci/54.2.399. [DOI] [PubMed] [Google Scholar]
- Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim. Biophys. Acta. 2009;1790:886–891. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Walsh CP, Chaillet JR, Bestor TH. Transcription of IAP endogenous retroviruses is constrained by cytosine methylation. Nat. Genet. 1998;20:116–117. doi: 10.1038/2413. [DOI] [PubMed] [Google Scholar]
- Winders BR, Schwartz RH, Bruniquel D. A distinct region of the murine IFN-gamma promoter is hypomethylated from early T cell development through mature naive and Th1 cell differentiation, but is hypermethylated in Th2 cells. J. Immunol. 2004;173:7377–7384. doi: 10.4049/jimmunol.173.12.7377. [DOI] [PubMed] [Google Scholar]
- Yanez Diaz S, Moran M, Unamuno P, Armijo M. Silica and trichloroethylene-induced progressive systemic sclerosis. Dermatology. 1992;184:98–102. doi: 10.1159/000247513. [DOI] [PubMed] [Google Scholar]
- Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G. Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk. Res. 2005;29:739–748. doi: 10.1016/j.leukres.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Yung R, Powers D, Johnson K, Amento E, Carr D, Laing T, Yang J, Chang S, Hemati N, Richardson B. Mechansims of drug-induced lupus II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J. Clin. Investig. 1996;97:2866–2871. doi: 10.1172/JCI118743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QH, Ma LW, Zhu WG, Zhang ZY, Tong TJ. p21Waf1/Cip1 plays a critical role in modulating senescence through changes of DNA methylation. J. Cell Biochem. 2006;98:1230–1248. doi: 10.1002/jcb.20838. [DOI] [PubMed] [Google Scholar]