Abstract
Small GTPase Rac is important regulator of endothelial cell (EC) barrier enhancement by prostacyclin characterized by increased peripheral actin cytoskeleton and increased interactions between VE-cadherin and other adherens junction (AJ) proteins. This study utilized complementary approaches including siRNA knockdown, culturing in Ca2+-free medium, and VE-cadherin blocking antibody to alter VE-cadherin extracellular interactions to investigate the role of VE-cadherin outside-in signaling in modulation of Rac activation and EC barrier regulation by prostacyclin analog iloprost. Spatial analysis of Rac activation in pulmonary EC by FRET revealed additional spike in iloprost-induced Rac activity at the sites of newly formed cell-cell junctions. In contrast, disruption of VE-cadherin extracellular trans-interactions suppressed iloprost-activated Rac signaling and attenuated EC barrier enhancement and cytoskeletal remodeling. These inhibitory effects were associated with decreased membrane accumulation and activation of Rac-specific guanine nucleotide exchange factors (GEF) Tiam1 and Vav2. Conversely, plating of pulmonary EC on surfaces coated with extracellular VE-cadherin domain further promoted iloprost-induced Rac signaling. In the model of thrombin-induced EC barrier recovery, blocking of VE-cadherin trans-interactions attenuated activation of Rac pathway during recovery phase and delayed suppression of Rho signaling and restoration of EC barrier properties. These results suggest that VE-cadherin outside-in signaling controls locally Rac activity stimulated by barrier protective agonists. This control is essential for maximal EC barrier enhancement and accelerated barrier recovery.
Keywords: endothelium, permeability, adherens junctions, cytoskeleton
Introduction
The lung endothelium forms a semi-selective barrier between circulating blood and interstitial fluid. This barrier is dynamically regulated by a balance between barrier disruptive contractile forces generated by actomyosin complex and barrier protective tethering forces developed by peripheral cytoskeletal networks and intercellular adhesive complexes. Adherens junction (AJ) protein complexes containing VE-cadherin, α,β,γ-catenins and p120-catenin play a key role in the barrier maintenance of vascular endothelial cell (EC) mononlayers (Bazzoni and Dejana, 2004; Prasain and Stevens, 2009; Vandenbroucke et al., 2008; Weber et al., 2007). AJ assembly and increased interactions with cortical cytoskeleton essential for enhancement of endothelial barrier is precisely regulated by small GTPases Rac, Cdc42 and Rap1 (Dejana et al., 2009; Vandenbroucke et al., 2008). Emerging studies uncovered an important feature of AJ complexes, which is ability to locally regulate small GTPase activities (Lampugnani et al., 2002; Noren et al., 2003; Noren et al., 2001; Wildenberg et al., 2006).
Homophilic ligation of E-cadherin in epithelial cell lines enhanced E-cadherin – p120-catenin interactions leading to recruitment of Rac to nascent E-cadherin adhesive contacts and activation of Rac1 signaling, which was necessary for further strengthening of the cadherin contact (Goodwin et al., 2003; Kovacs et al., 2002). Trans-interactions between VE-cadherins from adjacent endothelial cells during AJ assembly have been proposed to upregulate Rac activity by recruitment of Rac specific GEFs Tiam1 and Vav2 (Grosheva et al., 2001; Lampugnani et al., 2002). However, relations between Rac activation and regulation of cell junction integrity are far more complex. Expression of activated Rac disrupted functional E-cadherin – catenin complexes in pancreatic epithelial carcinoma cell lines leading to disassembly of E-cadherin-mediated adherens junctions (Hage et al., 2009). Expression of active Rac or Rho in epithelial type NBT-II cell line disrupted formation of stable cell-cell contacts (Playford et al., 2008). In endothelial cells stimulated with edemagenic agonists VEGF and IL-6, Rac activation was observed among other activated signaling pathways and led to PAK1-mediated VE-cadherin phosphorylation at S665, VE-cadherin internalization and increased barrier permeability (Gavard and Gutkind, 2006; Gavard et al., 2009). These apparently contradictory data underlie the importance of understanding the particular conditions and agonists acting on EC monolayers, which differently regulate Rho and Rac activation, VE-cadherin outside-in signaling, and the maintenance of EC barrier. It is also important to note that a role of VE-cadherin trans-interactions in modulation of Rac signaling and EC barrier enhancement induced by barrier protective agonists has not been yet explored.
Iloprost, a stable prostacyclin analog, elevates intracellular cAMP concentrations and has been implicated in the endothelial barrier enhancement, acceleration of EC monolayer recovery after stimulation with edemagenic agonists (Birukova et al., 2007b; Fukuhara et al., 2005; Wittchen et al., 2005), and lung vascular barrier protection in the animal model of ventilator-induced lung injury (Birukova et al., 2009; Birukova et al., 2010). The barrier-protective effects of iloprost are mediated by cAMP-activated protein kinase (PKA) and PKA-independent Epac-Rap1 signaling cascade (Bos, 2006), which activate Rac specific GEFs, Tiam1 and Vav2 and eventually stimulate Rac GTPase and EC barrier enhancement (Cullere et al., 2005; Fukuhara et al., 2005; Kooistra et al., 2005).
In the current study we used a model of iloprost-induced EC barrier enhancement to test the hypothesis that outside-in signaling by VE-cadherin creates a positive feedback signaling loop of Rac signaling triggered by iloprost and modulates local Rac activity at the cell periphery leading to local enforcement of cytoskeleton, adherens junctions, and improved EC barrier properties. To test this mechanism, we utilized alternative approaches to block VE-cadherin extracellular trans-interactions and examined effects of VE-cadherin disengagement on iloprost-induced Rac activation, permeability changes, and cortical actin dynamics in iloprost-stimulated human pulmonary EC monolayers, as well as during EC monolayer recovery after thrombin challenge.
Materials and Methods
Reagents and cell culture
Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO). Reagents for immunofluorescence were purchased from Molecular Probes (Eugene, OR). Antibodies to Tiam1 and p-Vav2 were from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-cortactin, cortactin, and phospho-MYPT1 from Millipore (Billerica, MA); phospho-PAK1 and di-phospho-MLC antibodies from Cell Signaling Inc (Beverly, MA); Rac1 and VE-cadherin from BD Transduction Laboratories (San Diego, CA); BV9 from Abcam (Cambridge, MA). Iloprost was purchased from Cayman (Ann Arbor, MI). Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza (Allendale, NJ), maintained in a complete culture medium according to the manufacturer’s recommendations and used for experiments at passages 5–7.
Si-RNA and DNA transfections
Pre-designed Rac1- and VE-cadherin-specific siRNAs of standard purity were ordered from Ambion (Austin, TX). Transfection of EC with siRNA was performed as previously described (Singleton et al., 2009). Nonspecific, non-targeting siRNA was used as a control treatment. After 48 hrs of transfection cells were used for experiments or harvested for western blot verification of specific protein depletion. Our preliminary testing demonstrated that nearly complete depletion of Rac1 or VE-cadherin significantly decreased basal levels of electrical resistance, led to disruption of EC barrier and even caused cell detachment in select experiments. Therefore, the experiments were performed after 48 hrs of siRNA transfection. These conditions provided significant target protein depletion verified by western blot analysis but caused only modest reduction in basal transmonolayer electrical resistance in TER studies in comparison to non-specific RNA controls (data not shown). Plasmid encoding dominant negative Rac bearing cMyc tag was kindly provided by Dr. G. Bokoch. Nucleofection of HPAEC was performed using a kit from Amaxa Biosystems (Lonza, Allendale, NJ). Optimized protocol of nucleofection is provided by manufacturer and used with minor modifications described previously (Birukova et al., 2006a). After 48 hr of transfection cells were treated with either vehicle or OxPAPC were used for permeability measurements.
Measurement of EC permeability
Measurements of transendothelial electrical resistance (TER) across confluent HPAEC monolayers were performed using the electrical cell-substrate impedance sensing system (ECIS) (Applied Biophysics, Troy, NY) as previously described (Birukov et al., 2004). Permeability of FITC-labeled dextran across endothelial monolayer was assessed in transwell assays using in Vitro Vascular Permeability Assay Kit (Chemicon International), according to the manufacturer’s instructions.
Rac activation assay
Activation of Rac GTPase in pulmonary EC culture was analyzed using Rac in vitro pulldown assay kit available from Millipore (Billerica, MA) according to the manufacturer’s protocols, as previously described (Birukova et al., 2007b).
Fluorescent resonance energy transfer (FRET)
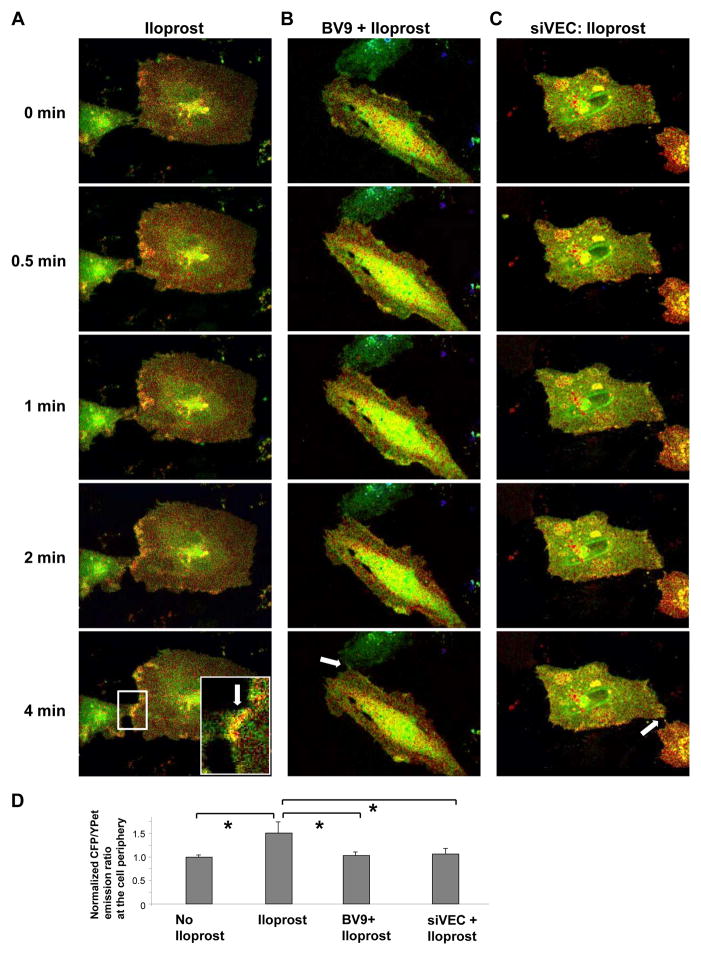
Rac-FRET biosensor was kindly provided by Yingxiao Wang (University of Illinois at Urbana-Champaign, IL). FRET analysis was performed as described elsewhere (Poh et al., 2009). Cells were seeded on glass-bottom dish coated with gelatin. 24 hrs after transfection, medium was changed to 2% FBS EBM medium for 2 hr. For detection of FRET, the cells were maintained on the microscope stage at 37 °C. To minimize the photobleaching effect, the time interval for each imaging acquisition was set to be 30 s, and images were captured for 15 min using Olympus Model IX71 Microscope System equipped with a 63X oil immerse objective and a CCD camera. Metamorph software was used to control the filter wheel and data analysis. The ratiometric images of ECFP/YPet were computed and generated by the Metamorph software to represent the spatiotemporal FRET signals. Analysis of regional Rac activation was performed using integral ECFP/YPet values in ~ 2 μm wide areas at the cell periphery and equal areas in the central parts of the cell. Increased Rac activation (ECFP/YPet emission ratio) in the areas of cell-cell contacts was normalized to Rac activation in central parts of the cell and expressed as bar graphs. Comparisons were made between un-stimulated cells and cells stimulated with iloprost (4 min) with and without BV9 pretreatment or VE-cadherin depletion.
Immunofluorescence
Endothelial monolayers plated on glass cover slips were subjected to immunofluorescence staining with appropriate antibody, as described previously (Birukova et al., 2007a). Texas Red phalloidin was used to visualize F-actin. After immunostaining, slides were analyzed using a Nikon video imaging system (Nikon Instech Co., Tokyo, Japan). Images were processed with Image J software (National Institute of Health, Washington, USA) and Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) software. Quantitative analysis of iloprost-induced VE-cadherin peripheral accumulation was performed by measurements of junctional VE-cadherin immunoreactivity normalized to square area in control and stimulated cells.
Cell adhesion assay
Plasmid encoding recombinant VE-cadherin ectodomain-Fc-6His was a gift from Dr. Mochizuki. Control human Fc fragment was purchased from EDM (La Jolla, CA). Isolation of Fc-VE-cadherin and adhesion assay were performed as described elsewhere (Fukuhara et al., 2005). In brief, HEK293 were transfected with pcDNA-VE-cadherin-Fc-6His for 24 hr followed by collection of culture medium. Fc-VE-cadherin was purified using ProBond resin (Invitrogen, Carlsbad, CA) and diluted with PBS supplemented with 2 mM of CaCl2 and MgCl2 to the final concentration 10 μg/ml. Plastic polysteren plates were coated with Fc-VE-cadherin or Fc fragment overnight, blocked with1% BSA solution for 1 hr and washed with the buffer. HPAEC were plated for 30 min followed by iloprost stimulation and determination of Rac activity or phosphorylation profile of Rac pathway readouts.
Differential protein fractionation and immunoblotting
Confluent HPAEC were stimulated with iloprost and cytosolic and membrane fractions were isolated as previously described (Birukova et al., 2011). For analysis of protein phosphorylation profile, cells were stimulated, then lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies as previously described (Birukova et al., 2007a).
Statistical analysis
Results are expressed as mean ± SD of four to six independent experiments. Experimental samples were compared to controls by unpaired Student’s t-test. For multiple-group comparisons, a one-way variance analysis (ANOVA) and post hoc multiple comparisons tests were used. P<0.05 was considered statistically significant.
Results
Iloprost enhances VE-cadherin adherence junctions in pulmonary EC monolayers
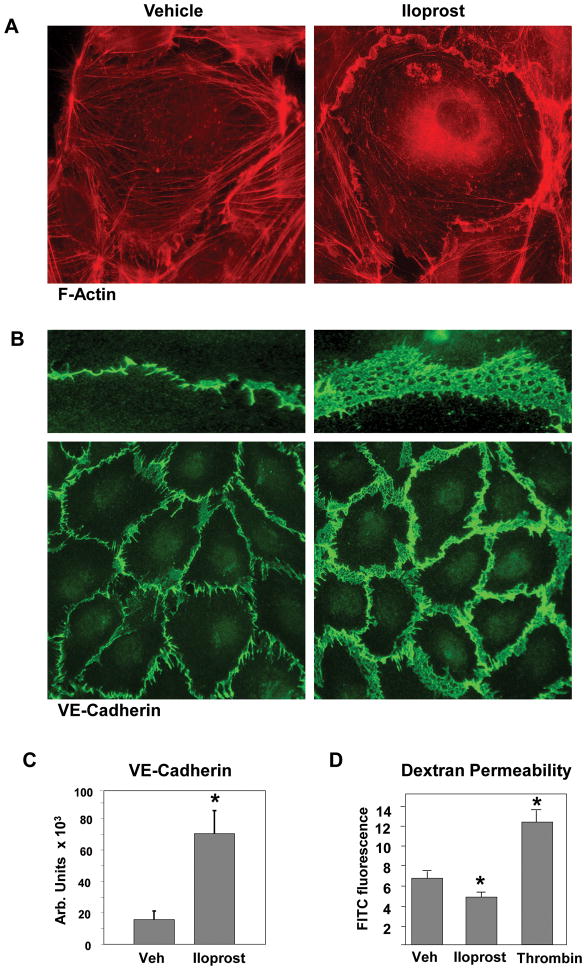
In agreement with our previous studies (Birukova et al., 2007b), EC treatment with iloprost caused robust lamellipoda formation and partial overlap of cell edges (Figure 1A) accompanied by enlargement of the area covered by VE-cadherin positive AJ (Figure 1B). Overall, iloprost challenge caused prominent increase in VE-cadherin positive areas at the regions of cell-cell interface leading to tightening of EC monolayer and enhancement of EC barrier properties, as detected by decreased permeability to FITC-labeled dextran in iloprost-treated EC monolayers assessed by transwell permeability assay (Figure 1C).
Figure 1. Effect of iloprost on F-actin remodeling, and VE-cadherin distribution in human pulmonary EC.
A and B: Endothelial cells grown on glass coverslips were stimulated with iloprost (75 nM, 10 min) followed by immunofluorescence staining with Texas Red phalloidin to detect actin filaments (A) or VE-cadherin antibody (B). C: Quantitative analysis of iloprost-induced VE-cadherin peripheral accumulation. Data are expressed as mean ± SD of five independent experiments; *p<0.05. D: HPAEC were incubated with vehicle, iloprost (75 nM), or thrombin (0.2 U/ml) for 60 min followed by measurements of permeability for FITC-labeled dextran. Permeability data are expressed as mean ± SD of four independent experiments; *p<0.05.
Inhibition of VE-cadherin cell-cell interactions by blocking antibody suppresses Iloprost-induced endothelial barrier enhancement and gap closure in iloprost-stimulated nearly-confluent EC monolayers
In the following experiments we tested the hypothesis that formation of new AJ, in particular VE-cadherin homophylic interactions, may further promote EC barrier-enhancing effects of iloprost. Previous publications described BV9 antibody which binds extracellular VE-cadherin domain and blocks extracellular VE-cadherin interactions with other VE-cadherins leading to disruption of EC monolayer barrier (Corada et al., 2001). In this study, HPAEC were pretreated with BV9 blocking antibody followed by iloprost stimulation. In agreement with published data (Corada et al., 2001), prolonged treatment with BV9 increased transendothelial permeability (Figure 2A). To examine the input of newly formed VE-cadherin trans-interactions as potential positive feedback mechanism of iloprost-induced EC barrier enhancement, in this study we used short-term BV9 treatment (30 min), when existing monolayer integrity is not affected, but formation of new VE-cadherin junctions is inhibited. BV9 treatment significantly attenuated iloprost-induced barrier enhancement in EC monolayers monitored by measurements of transendothelial electrical resistance (TER) suggesting the involvement of VE-cadherin interactions in propagation of iloprost barrier protective response (Figure 2B). Cbl is a cytosolic protein belonging to a family of E3 ubiquitin ligases. Cbl antibody has no known extracellular targets and was used as isotype-matched control to BV9 blocking antibody.
Figure 2. Effect of BV9 blocking antibody on iloprost-induced EC barrier enhancement.
A: HPAEC plated on microelectrodes were treated with vehicle (IgG) or BV9 antibody (50 μg/ml) followed by TER measurements for 15 hr. Inset depicts TER values in the time window of BV9 pretreatment (30 min) used in this study. B: Left panel - HPAEC were pretreated with BV9 antibody followed by iloprost stimulation (75 nM). TER was monitored over 2 hrs. Right panel - TER increase caused by iloprost stimulation of EC pretreated with vehicle was taken as 100%. Pretreatment with irrelevant isotype-specific antibody (Cbl) was used as negative control. Pretreatment with BV9 significantly attenuated TER increase caused by iloprost stimulation. Data are expressed as mean ± SD of five independent experiments; *p<0.05.
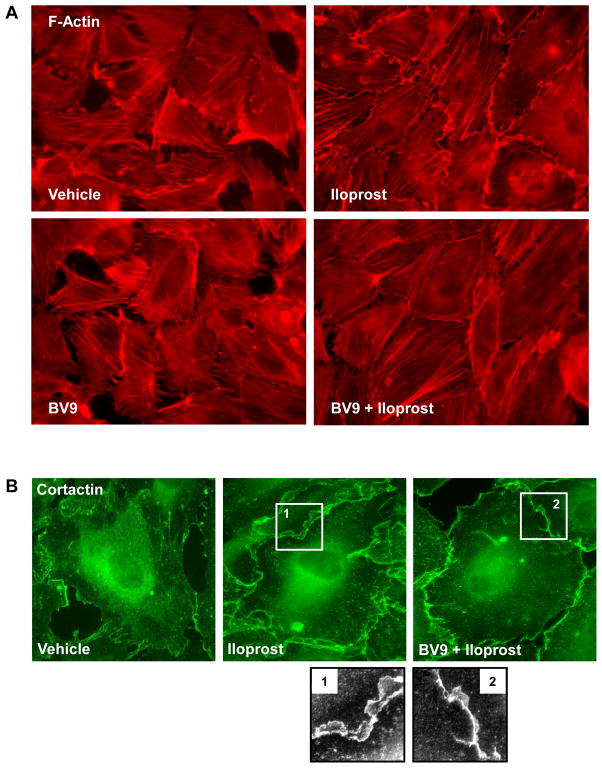
In the following experiments nearly confluent pulmonary EC were used to examine the role of VE-cadherin in remodeling of actin cytoskeleton upon iloprost treatment. Iloprost caused robust lamellipodia formation and paracellular gap closure in control EC, whereas pretreatment with BV9 antibody significantly suppressed iloprost effects, as detected by decreased lamellipodia formation and incomplete gap closure (Figure 3A). Iloprost-induced lamellipodia formation was accompanied by peripheral translocation of cortactin involved in Rac-dependent actin polymerization. Again, BV9 antibody attenuated cortactin peripheral accumulation in response to iloprost (Figure 3B). These data demonstrate that inhibition of iloprost-induced VE-cadherin extracellular trans-interactions abolished intracellular signaling which triggers peripheral cytoskeletal remodeling.
Figure 3. Effect of BV9 blocking antibody on iloprost-induced cytoskeletal remodeling.
A and B: Sub-confluent endothelial cells grown on glass coverslips were pretreated with BV9 antibody (50 μg/ml, 30 min) followed by iloprost stimulation (75 nM, 10 min). Immunofluorescence staining was performed with Texas Red phalloidin (A) or cortactin antibody (B). Insets represent high magnification images of cortactin peripheral accumulation.
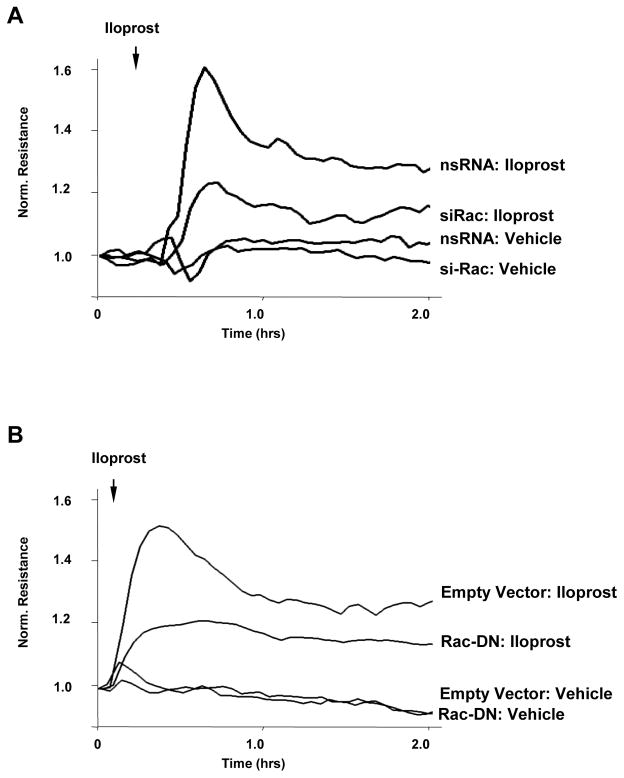
VE-cadherin engagement controls iloprost-induced activation of Rac and Rac-dependent barrier enhancement
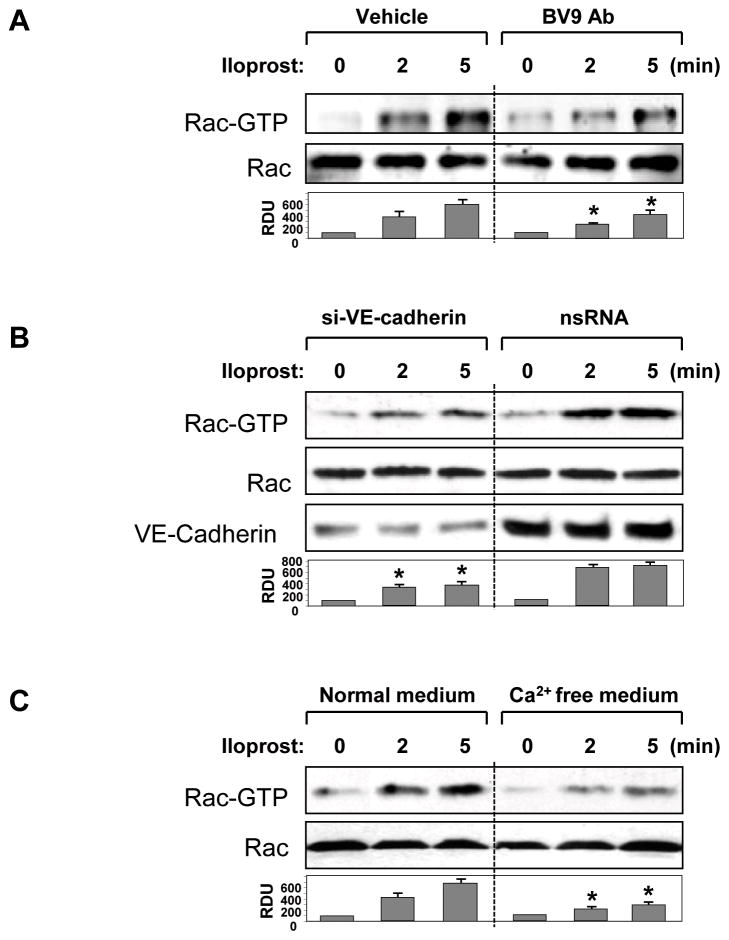
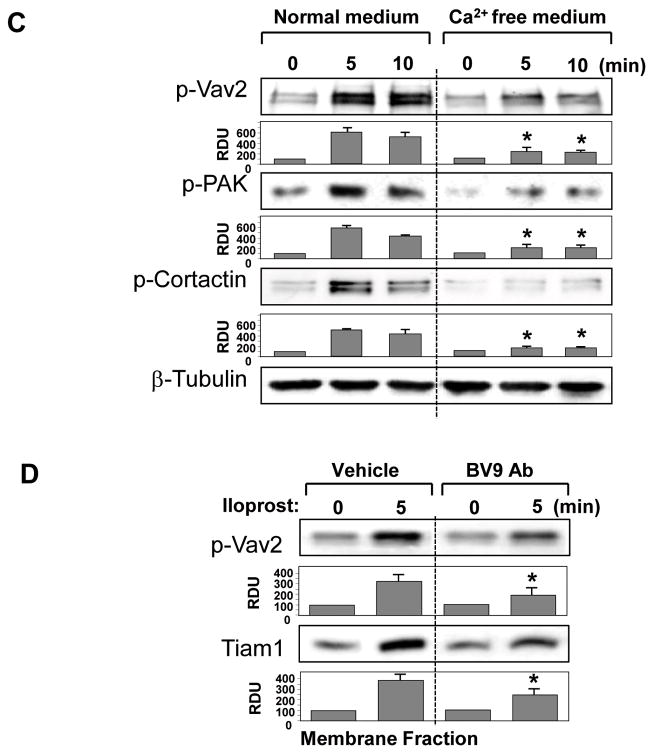
Rac GTPase plays essential role in barrier protective effects by iloprost on pulmonary endothelium (Birukova et al., 2007b; Fukuhara et al., 2005). Molecular inhibition of Rac1 by si-RNA-based knockdown (Figure 4A) or HPAEC transfection with dominant negative Rac1 mutant (Figure 4B) attenuated increases in TER induced by iloprost. To study a role of VE-cadherin trans-interactions in iloprost-induced Rac signaling, we utilized several complementary approaches. In the first approach, pulmonary EC were pretreated with BV9 antibody followed by analysis of Rac activation in response to iloprost. Results of Rac-GTP pull-down assay demonstrate that inhibition of new VE-cadherin-based cell adhesions by BV9 attenuated iloprost-induced Rac activation (Figure 5A). In the next series of experiments, endogenous VE-cadherin was depleted using siRNA approach. HPAEC were transfected with non-specific RNA or VE-cadherin-specific siRNA for 48 hr. This post-transfection time allows achieving of noticeable suppression of VE-cadherin expression, whereas EC monolayer properties were not significantly affected. Cells were treated with iloprost, and Rac activity was evaluated in pull-down assays. VE-cadherin knockdown markedly decreased Rac activation in response to iloprost as compared to non-specific RNA controls (Figure 5B). Alternatively, VE-cadherin trans-interactions were affected by removal of Ca2+, which is essential for trans-interactions between extracellular VE-cadherin domains, from culture medium. HPAEC were incubated with low serum medium followed by addition of Ca2+ chelating agent EGTA and iloprost stimulation. Similarly to the experiments described above, Ca2+ depletion of culture medium suppressed iloprost-induced Rac activation (Figure 5C).
Figure 4. Involvement of Rac in iloprost-induced EC barrier enhancement.
A: EC were transfected with Rac1-specific siRNA or non-specific RNA. EC were stimulated with iloprost (75 nM) or vehicle at the time indicated by arrow, and TER changes were monitored over 2 hr. B: EC monolayers were subjected to transfection with dominant-negative Rac (Rac-DN). Cells transfected with empty vector served as controls. After 48 hrs of transfection, cells were stimulated with iloprost. Changes in endothelial permeability were monitored by measurements of TER.
Figure 5. Effect of inhibition VE-cadherin interactions on iloprost-induced Rac activation.
A – C: HPAEC were pretreated with vehicle or BV9 (50 μg/ml, 30 min) (A), transfected with VE-cadherin-specific siRNA or non-specific RNA duplexes (B), or incubated in 2% FCS containing media with or without EGTA (5 mM, 30 min) (C). Next, EC were stimulated with iloprost (75 nM) for the indicated periods of time. Effect of iloprost on Rac activation was evaluated by Rac-GTP pulldown assay. Upper panel depicts the levels of activated, GTP-bound Rac, and the lower panel shows total Rac content in EC lysates. VE-cadherin depletion induced by specific siRNA duplexes was confirmed by western blot analysis of protein content in whole cell lysates. Result of densitometry shown as mean ± SD, * p<0.05 as compared to corresponding iloprost-stimulated controls.
In the next experiments, we performed analysis of spatial Rac activation upon iloprost treatment in live pulmonary EC using recently described Rac biosensor (Poh et al., 2009). Using this approach, local Rac activation was studied in iloprost-stimulated pulmonary EC with intact and inhibited VE-cadherin trans-interactions. Iloprost addition to non-transfected and non-specific RNA-transfected HPAEC expressing Rac sensor induced noticeable Rac activation at the cell periphery. Remarkably, iloprost-stimulated establishment of new cell-cell contacts promoted additional spike in local Rac activity at the sites of cell-cell junctions (Figure 6A, shown by arrow). In contrast, increased Rac activation at cell-cell junction areas was not observed in endothelial cells treated with BV9 antibody prior to agonist addition (Figure 6B, shown by arrow) or in cells transfected with VE-cadherin-specific siRNA (Figure 6C, shown by arrow). Collectively, these data suggest that trans-interactions between VE-cadherin extracellular domains induced by iloprost provide additional stimulation of Rac.
Figure 6. FRET analysis of iloprost-induced spatial Rac activation.
A – C: HPAEC were transfected with non-specific of VE-cadherin-specific siRNA duplexes for 24 hr followed by transfection with CFP/YPet-Rac biosensor for additional 24 hrs. FRET analysis was performed in iloprost-stimulated (75 nM) cells under control conditions (A), in HPAEC pretreated with BV9 antibody (50 μg/ml, 30 min) (B), or in VE-cadherin depleted EC (C). Images represent ratio of activated Rac to the total Rac content. Areas of Rac activation appear in yellow. Higher magnification inset in the lower frame of panel depicts increased local Rac activation at the cell junction of iloprost-stimulated cells. D: Quantitative analysis of iloprost-induced Rac activation at the areas of cell-cell contacts. Bar graphs represent normalized (cell-cell contacts/cell center) CFP/YPet emission ratio. Peripheral Rac activation in cells without iloprost stimulation was compared to Rac activation after 4 min of iloprost addition. In experiments with VE-cadherin knockdown or VE-cadherin blocking antibody Rac activation was compared to iloprost-stimulated controls (4 min). Data are expressed as mean ± SD of four independent experiments, 5–7 cells for each experiment; *p<0.05.
VE-cadherin engagement controls iloprost-induced activation of Rac signaling cascade
The following experiments further evaluated the role of VE-cadherin homophilic interactions in the modulation of iloprost-induced Rac signaling. Stimulation of EC with iloprost induced site-specific tyrosine phosphorylation of Rac-specific guanine nucleotide exchange factor (GEF) Vav2 essential for full activation of Vav2 nucleotide exchange activity (Fukuyama et al., 2006; Kawakatsu et al., 2005) (Figure 7A). Iloprost stimulation also induced autophosphorylation of Rac effector, p21-activated kinase (PAK), which is an important mediator of Rac signaling to cytoskeleton (Figure 7B). In addition, iloprost caused tyrosine phosphorylation of Rac-dependent activator of peripheral actin polymerization cortactin (Figure 7C). To further evaluate a role of iloprost-induced VE-cadherin engagement in Rac signaling mechanisms, we inhibited VE-cadherin trans-interactions induced by iloprost using the strategies described above. Cells were pretreated with BV9 blocking antibody, transfected with VE-cadherin-specific siRNA, or incubated in Ca2+-free medium. Next, cells were stimulated with iloprost and phosphorylation profile of Vav2, PAK, and cortactin was determined by western blot analysis with specific antibodies. In agreement with data presented in Figure 4, alteration of VE-cadherin interactions attenuated phosphorylation/activation of Rac-dependent Vav2, Pak1 and cortactin (Figure 7). Subcellular fractionation experiments showed iloprost-mediated accumulation of Rac-specific GEFs Tiam1 and Vav2 in the membrane fraction, which was attenuated by EC preincubation with VE-cadherin blocking antibody (Figure 7D).
Figure 7. Effect of inhibition of VE-cadherin interactions on iloprost-induced activation of Rac signaling.
A – C: HPAEC were pretreated with vehicle or BV9 (50 μg/ml, 30 min) (A); transfected with VE-cadherin-specific or non-specific RNA (B); or incubated in 2% FCS culture medium with or without EGTA (5 mM, 30 min) (C). Cells were next stimulated with iloprost (75 nM) for the indicated periods of time. Effect of iloprost on Vav2, PAK, and cortactin phosphorylation was evaluated by western blot with corresponding antibodies. VE-cadherin depletion induced by specific siRNA duplexes was confirmed by western blot analysis of protein content in whole cell lysates. Equal protein loading was confirmed by membrane probing with β-tubulin antibody. D: The membrane fraction was isolated from iloprost-treated EC with or without BV9 pretreatment. Content of phosphorylated Vav2 or Tiam1 was determined by western blot with specific antibodies. Result of densitometry shown as mean ± SD, * p<0.05 as compared to corresponding iloprost-stimulated controls.
The role of VE-cadherin homophilic interactions in regulation of Rac signaling was further addressed in cell adhesion assays. We hypothesized that Rac-mediated VE-cadherin engagement induced by iloprost may initiate a positive feedback loop of Rac activation. Extracellular domain of VE-cadherin fused with immunoglobulin Fc-fragment or control Fc-fragment were expressed and purified according to previously published study (Fukuhara et al., 2005). Human pulmonary EC were plated at low density onto dishes coated with Fc-VE-cadherin or Fc-fragment. After 30 min required for cell attachment, EC were stimulated with iloprost followed by analysis of readouts of Rac activation. Western blot analysis of Vav2 phosphorylation (Figure 8A), Rac activation (Figure 8B), or cortactin phosphorylation (Figure 8C) revealed higher activation of Rac signaling in Fc-VE-cadherin coated plates, as compared to samples collected from plates coated with Fc-fragment. These results further support the hypothesis that establishment of extracellular VEC adhesions promotes agonist-induced Rac signaling.
Figure 8. Role of VE-cadherin trans-interactions in iloprost-induced Rac signaling assessed by adhesion assay.
Plastic plates were coated with Fc-VE-cadherin or control Fc fragments as described in Methods. HPAEC were allowed to attach to the matrices during 30 min followed by stimulation with 75 nM iloprost for 5 or 10 min. A: Phosphorylated Vav2 was detected by western blot analysis with specific antibody. Equal protein loading was confirmed by membrane probing with β-tubulin antibody. B: Rac activation was determined by Rac-GTP pulldown assay. Content of activated Rac was normalized to the total Rac content in EC lysates. C: Cortactin phosphorylation in response to iloprost was assessed by western blot with corresponding antibody. Equal protein loading was confirmed by membrane probing with β-actin antibody. Result of densitometry shown as mean ± SD, * p<0.05 as compared to corresponding iloprost- stimulated controls.
VE-cadherin outside-in signaling is involved Rac-mediated EC recovery after thrombin challenge
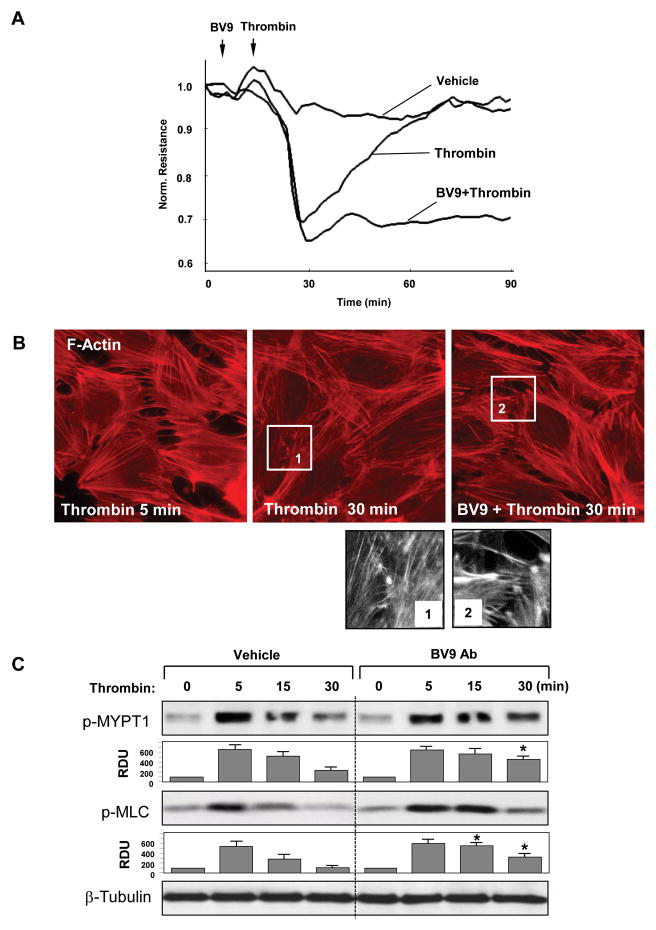
Stimulation of Rac signaling in EC monolayers during recovery phase after barrier-disruptive agonist treatment is essential for re-establishment of EC monolayer integrity (Birukova et al., 2006b; Kouklis et al., 2004; Tauseef et al., 2008). Using a model of EC barrier recovery after thrombin challenge we investigated the role of VE-cadherin ligation in the regulation of Rac signaling and reparation of EC barrier function. Pretreatment of EC monolayers with BV9 blocking antibody prior to thrombin challenge did not affect acute phase of thrombin-induced permeability increase, but markedly suppressed recovery phase after thrombin challenge (Figure 9A). Similarly to results of permeability assay, immunofluorescence staining of control and BV9-pretreated EC monolayers during recovery phase (30 min after thrombin challenge) showed that resealing of paracellular gaps observed in control cells was significantly attenuated by BV9 pretreatment (Figure 9B). Biochemical analysis revealed rapid activation of Rho signaling characterized by increased phosphorylation of Rho downstream targets, myosin light chain phosphatase (MYPT1) and myosin light chains (MLC) during acute phase of thrombin-induced EC barrier disruption, which was followed by suppression of Rho activity and de-phosphorylation of MYPT1 and MLC during recovery phase. EC pretreatment with BV9 antiboby delayed Rho inhibition during EC barrier restoration after thrombin challenge, as detected by slow dephosphorylation of MYPT1 and MLC (Figure 9C).
Figure 9. Role of VE-cadherin interactions in EC barrier recovery.
HPAEC were pretreated with BV9 antibody followed by thrombin stimulation (0.2 U/ml) for various time points. A: TER was monitored over 90 min. B: Immunofluorescence staining with Texas Red phalloidin was performed to detect actin filaments. High magnification insets depict areas of the cell-cell junctions during recovery phase in control and BV9-treated cells. C: Rho pathway activation was assessed by analysis of MYPT1 and MLC phosphorylation. Equal protein loading was confirmed by membrane probing with β-tubulin antibody. Result of densitometry shown as mean ± SD, * p<0.05 as compared to corresponding thrombin-stimulated controls.
To examine the involvement of VE-cadherin interactions in activation of Rac signaling to cytoskeleton during recovery phase, cells were subjected to immunofluorescence staining for Rac downstream target cortactin. Recovery of EC monolayer integrity after thrombin challenge was characterized by cortactin accumulation at the cell periphery and actin remodeling with accumulation of F-actin in submembrane areas. In contrast, inhibition of VE-cadherin engagement and formation of VE-cadherin-based AJ by BV9 antibody suppressed this effect (Figure 10A).
Figure 10. Role of VE-cadherin interactions in Rac pathway activation during EC barrier recovery.
HPAEC were pretreated with BV9 antibody followed by thrombin stimulation (0.2 U/ml) for 5 or 30 min. A: Immunofluorescence staining of cortactin was performed with corresponding antibody. High magnification insets depict areas of peripheral cortactin accumulation during recovery phase after thrombin challenge in control- and BV9-treated cells. B: Rac pathway activation was assessed by analysis of Vav2, PAK, and cortactin phosphorylation. Equal protein loading was confirmed by membrane probing with β-tubulin antibody. Result of densitometry shown as mean ± SD, * p<0.05 as compared to corresponding thrombin-stimulated controls.
Analysis of biochemical readouts of Rac signaling during EC monolayer recovery phase after thrombin treatment (30 min) showed increased phosphorylation/activation of Vav2, PAK, and cortactin. Phosphorylation of these proteins during EC recovery after thrombin challenge was markedly in EC monolayers pretreated with BV9 antibody (Figure 10B). Taken together, these data strongly suggest the involvement of VE-cadherin outside-in signaling in Rac activation and regulation of EC barrier properties.
Discussion
EC stimulation with prostacyclin and its synthetic analog iloprost occurs via ligation of G-protein coupled receptor IP-1 and leads to activation of cAMP-Epac-Rap1-Rac cascade (Birukova et al., 2007b; Moncada, 2006). This signaling cascade causes remodeling of peripheral cytoskeleton and re-establishment of adherens junctions. The present study emphasizes a role of VE-cadherin outside-in signaling as a critical mechanism contributing to local Rac activation at cell junctions, Rac-dependent enhancement of cortical actin cytoskeleton and VE-cadherin adhesive complexes leading to strengthening of EC monolayer barrier.
Previous reports demonstrated the involvement of cadherin ligation in control of basal Rac activity in endothelium and epithelial cell lines (Braga et al., 1999; Lampugnani et al., 2002; Noren et al., 2001). However, the role of VE-cadherin extracellular interactions in modulation of Rac signaling and EC monolayer response to barrier protective agonists has not been investigated. This study demonstrates for the first time the involvement of VE-cadherin outside-in signaling in modulation of Rac pathway of iloprost-induced EC barrier enhancement and EC monolayer recovery after challenge with barrier-disruptive agent thrombin.
This study employed several independent approaches to disengage extracellular VE-cadherin interactions, which included VE-cadherin extracellular domain blocking antibody, Ca2+ switch, and siRNA-induced VE-cadherin knockdown. Although each approach may bring about its own limitations, their combination allows for more comprehensive analysis of VE-cadherin – Rac crosstalk mechanisms. The inhibition of VE-cadherin extracellular trans-interactions by these approaches attenuated Rac activation caused by iloprost. This conclusion was further supported by experiments showing increased Rac signaling in iloprost-stimulated sparse EC cultures grown on VE-cadherin-conjugated substrate, which induces VE-cadherin trans-interactions.
We noted that Rac activity levels (both at cell junction areas and total Rac activity) in quiescent EC monolayers were lower in comparison to iloprost-stimulated EC and nearly confluent EC establishing new cell-cell contacts. Reduced levels of Rac activity in established EC monolayers are common, although the nature of Rac downregulation in “mature” EC monolayers is poorly understood. Rac downregulation in established EC monolayers may have biological significance, as it downregulates cytoskeletal motility, does not perturb existing AJ complexes, and restricts cell invasion associated with cell transformation and tumorogenesis. In turn, sustained Rac activation leads to disrupted functional E-cadherin – catenin complexes, as shown in pancreatic epithelial carcinoma cell monolayers and disassembly of E-cadherin-mediated adherens junctions (Hage et al., 2009). Expression of active Rac or Rho in epithelial type NBT-II cell line disrupted formation of stable cell-cell contacts (Playford et al., 2008). We speculate that, similarly to observed maturation of focal adhesions (Ballestrem et al., 2006), adherens junctions may undergo similar process and recruit additional proteins, some of which may negatively regulate Rac activity. The mechanisms of Rac negative regulation in “mature” EC monolayers require further investigation.
Inhibition of VE-cadherin trans-interactions by BV9 antibody, which binds extracellular VE-cadherin domain and blocks VE-cadherin homophilic interactions, markedly attenuated iloprost-induced EC barrier protective response. It is important to note that unlike previous reports which used prolonged pretreatment with BV9 antibody leading to severe disruption of cell-cell contacts and endothelial permeability (Corada et al., 2001), this study used shorter pretreatment of EC with BV9 (30 min), which did not significantly affect basal TER levels (1478+/−136 Ohm vs. 1241+/−119 Ohm in controls treated with irrelevant antibody). Thus, such treatment most likely prevented formation of novel, agonist-induced VE-cadherin interactions.
Similar reduction of iloprost-induced barrier enhancement was observed upon VE-cadherin knockdown in EC monolayers. Although VE-cadherin plays a structural role in direct control of EC permeability by adherens junctions, our results and published studies (Corada et al., 2001; Noda et al., 2010; Xu et al., 2007) show that VE-cadherin inhibition does not cause complete disruption of EC barrier. These data suggest additional mechanisms contributing to maintenance of EC monolayer integrity, which may be driven by other adhesive protein complexes such as nectins, JAMs, claudin-5, or ZO-1 (Birukova et al., 2011; Pannekoek et al., 2009; Shen et al., 2011; Tsukita et al., 2009).
Treatment of nearly-confluent EC monolayers with VE-cadherin blocking antibody suppressed closure of remaining gaps between neighboring cells caused by iloprost stimulation. Remarkably, BV9 antibody pretreatment augmented lamellopodia formation in iloprost-stimulated EC. Although both control and BV9 pretreated EC formed contacts with neighboring cells upon iloprost stimulation, no further enhancement of cortical actin network was observed in BV9-pretreated cells.
Cortical actin dynamics is a Rac-dependent process which requires activation and peripheral accumulation of regulators of actin polymerization (Burridge and Wennerberg, 2004; Kaibuchi et al., 1999; Weed and Parsons, 2001). Disengagement of VE-cadherin trans-interactions inhibited iloprost-induced membrane translocation of Rac GEFs Tiam1 and Vav2 and Vav2 phosphorylation. These events were linked to reduced Rac activation, decreased phosphorylation of Rac effectors PAK1 and cortactin, and decreased cortical cytoskeletal dynamics resulting in incomplete gap closure of iloprost-stimulated EC monolayers. Direct visualization of spatial Rac activation by FRET approach further confirmed the role of VE-cadherin trans-interactions in the additional local regulation of Rac activity in agonist-stimulated EC.
Observed inhibition of submembrane Tiam1/Vav2 localization and activation of Rac signaling caused by functional disengagement of VE-cadherin is in good agreement with published studies, which showed that reintroduction of VE-cadherin in cadherin-null EC promoted Tiam1 localization to membrane/cytoskeletal compartment (Lampugnani et al., 2002). Other study in epithelial cells suggested the mechanism by which epithelial cadherin trans-interactions lead to recruitment and activation of SRF tyrosine kinase and phosphorylation of Vav2 (Fukuyama et al., 2006). Although the present study demonstrated higher levels of Vav2 phosphorylation in iloprost-stimulated EC with increased VE-cadherin interactions, specific signaling pathways activated by iloprost and VE-cadherin ligation and contributing to Vav2 phosphorylation remain to be investigated.
EC monolayer recovery after thrombin does not involve cAMP, but engages other mechanisms leading to Rac activation and Rho downregulation, which require further investigation. For example, activation of p190RhoGAP was implicated in Rho downregulation during EC recovery after thrombin (Holinstat et al., 2006). The reported activation of Rap1 GTPase in disrupted cell monolayers promotes cytoskeletal dynamics, participates in gap resealing, and leads to activation of Rac1 in a Tiam/Vav2 –dependent manner (Arthur et al., 2004; Asuri et al., 2008). Both mechanisms promote cortical actin polymerization and associations between cortical cytoskeleton and adherens junctions. This study shows that iloprost enhances Rac signaling and recovery after thrombin by engaging additional, cAMP-Epac1-Rap1-Rac1 mechanism.
In thrombin-stimulated EC, BV9 pretreatment weakened the extracellular VE-cadherin – VE-cadherin adhesion, and it is reasonable to expect an exaggerated resistance drop after thrombin challenge and proper EC barrier restoration due to blocking appropriate VE-cadherin – VE-cadherin engagement. These are inherent limitations of this approach. However, BV9 pretreatment also prolonged MYPT1 and MLC phosphorylation during recovery phase after thrombin (30 min) thus indicating VE-cadherin-dependent modulation of Rho signaling. In turn, BV9-induced suppression of cortactin peripheral translocation and lamellopodia formation during EC recovery after thrombin indicate inhibition of Rac signaling and Rac-dependent cytoskeletal remodeling. These data provide additional support for the signaling role of extracellular VE-cadherin homophilic interactions in control of EC permeability and monolayer recovery.
Based on presented and published data we propose a hypothetical scheme of VE-cadherin – Rac positive feedback mechanism. Iloprost activates Rac GEFs (Tiam1/Vav2), induces their translocation to the membrane compartment and causes local Rac activation sufficient for initial cytoskeletal remodeling and cell spreading leading to formation of cell-cell contacts and trans-interactions between VE-cadherins from adjacent cells. These interactions trigger intracellular AJ protein complex formation, recruitment and additional activation of Rac-specific GEFs which causes prolonged local Rac activation leading to more sustained peripheral actin cytoskeleton and AJ enhancement essential for maximal barrier-enhancing effect or recovery of EC monolayer integrity.
In conclusion, the results of this study suggest that formation of extracellular VE-cadherin trans-interactions may be a key mechanism modulating local Rac activity at the cell junctions in agonist-stimulated EC monolayers. Our data also show that VE-cadherin-mediated Rac signaling plays a key role in the crosstalk between assembly of VE-cadherin containing adherens junctions and formation of underlying cytoskeletal network essential for AJ linkage to actin cytoskeleton. This mechanism is important for both, re-establishment of endothelial monolayer integrity and EC barrier enhancement. Such local activation of Rac signaling provides the basis for enhanced interactions between adherens junctions and cytoskeleton, as well as for interactions between focal adhesions, adherens junction and tight junctions described in other models of endothelial barrier enhancement.
Acknowledgments
Supported by National Heart, Lung, and Blood Institutes grants HL87823, HL76259, and HL58064 for KGB; HL89257, HL107920 and the American Heart Association Midwest Affiliate Grant-in-Aid for AAB
References
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167(1):111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asuri S, Yan J, Paranavitana NC, Quilliam LA. E-cadherin dis-engagement activates the Rap1 GTPase. J Cell Biochem. 2008;105(4):1027–1037. doi: 10.1002/jcb.21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestrem C, Erez N, Kirchner J, Kam Z, Bershadsky A, Geiger B. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J Cell Sci. 2006;119(Pt 5):866–875. doi: 10.1242/jcs.02794. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84(3):869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res. 2004;95(9):892–901. doi: 10.1161/01.RES.0000147310.18962.06. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2006a;290(3):L540–548. doi: 10.1152/ajplung.00259.2005. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol. 2006b;168(5):1749–1761. doi: 10.2353/ajpath.2006.050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Xing J, Birukov KG. Rap1 mediates protective effects of iloprost against ventilator induced lung injury. J Appl Physiol. 2009;107(6):1900–1910. doi: 10.1152/japplphysiol.00462.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res. 2010;155(1):44–54. doi: 10.1016/j.trsl.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin - {beta}-catenin interactions are involved in Rac/Cdc42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol. 2007a;293(1):L199–211. doi: 10.1152/ajplung.00020.2007. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007b;313(11):2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Zebda N, Fu P, Poroyko V, Cokic I, Birukov KG. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J Cell Physiol. 2011;226(8):2052–2062. doi: 10.1002/jcp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006 doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10(1):9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E. Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood. 2001;97(6):1679–1684. doi: 10.1182/blood.v97.6.1679. [DOI] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105(5):1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Molendini C, Baluk P, McDonald DM. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335(1):17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25(1):136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama T, Ogita H, Kawakatsu T, Inagaki M, Takai Y. Activation of Rac by cadherin through the c-Src-Rap1-phosphatidylinositol 3-kinase-Vav2 pathway. Oncogene. 2006;25(1):8–19. doi: 10.1038/sj.onc.1209010. [DOI] [PubMed] [Google Scholar]
- Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- Gavard J, Hou X, Qu Y, Masedunskas A, Martin D, Weigert R, Li X, Gutkind JS. A role for a CXCR2/phosphatidylinositol 3-kinase gamma signaling axis in acute and chronic vascular permeability. Mol Cell Biol. 2009;29(9):2469–2480. doi: 10.1128/MCB.01304-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M, Kovacs EM, Thoreson MA, Reynolds AB, Yap AS. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J Biol Chem. 2003;278(23):20533–20539. doi: 10.1074/jbc.M213171200. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Shtutman M, Elbaum M, Bershadsky AD. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J Cell Sci. 2001;114(Pt 4):695–707. doi: 10.1242/jcs.114.4.695. [DOI] [PubMed] [Google Scholar]
- Hage B, Meinel K, Baum I, Giehl K, Menke A. Rac1 activation inhibits E-cadherin-mediated adherens junctions via binding to IQGAP1 in pancreatic carcinoma cells. Cell Commun Signal. 2009;7:23. doi: 10.1186/1478-811X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of RhoA activity by focal adhesion kinase-induced activation of p190RhoGAP: role in regulation of endothelial permeability. J Biol Chem. 2006;281(4):2296–2305. doi: 10.1074/jbc.M511248200. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Ogita H, Fukuhara T, Fukuyama T, Minami Y, Shimizu K, Takai Y. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem. 2005;280(6):4940–4947. doi: 10.1074/jbc.M408710200. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579(22):4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Kouklis P, Konstantoulaki M, Vogel S, Broman M, Malik AB. Cdc42 regulates the restoration of endothelial barrier function. Circ Res. 2004;94(2):159–166. doi: 10.1161/01.RES.0000110418.38500.31. [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem. 2002;277(8):6708–6718. doi: 10.1074/jbc.M109640200. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13(4):1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. Review. Adventures in vascular biology: a tale of two mediators. Philos Trans R Soc Lond B Biol Sci. 2006;361(1469):735–759. doi: 10.1098/rstb.2005.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Zhang J, Fukuhara S, Kunimoto S, Yoshimura M, Mochizuki N. Vascular endothelial-cadherin stabilizes at cell-cell junctions by anchoring to circumferential actin bundles through alpha- and beta-catenins in cyclic AMP-Epac-Rap1 signal-activated endothelial cells. Mol Biol Cell. 2010;21(4):584–596. doi: 10.1091/mbc.E09-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Arthur WT, Burridge K. Cadherin engagement inhibits RhoA via p190RhoGAP. J Biol Chem. 2003;278(16):13615–13618. doi: 10.1074/jbc.C200657200. [DOI] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276(36):33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. Cell-cell junction formation: The role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788(4):790–796. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Playford MP, Vadali K, Cai X, Burridge K, Schaller MD. Focal adhesion kinase regulates cell-cell contact formation in epithelial cells via modulation of Rho. Exp Cell Res. 2008;314(17):3187–3197. doi: 10.1016/j.yexcr.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh YC, Na S, Chowdhury F, Ouyang M, Wang Y, Wang N. Rapid activation of Rac GTPase in living cells by force is independent of Src. PLoS One. 2009;4(11):e7886. doi: 10.1371/journal.pone.0007886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77(1):53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-beta1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90(4):323–332. doi: 10.1016/j.ejcb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res. 2009;104(8):978–986. doi: 10.1161/CIRCRESAHA.108.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103(10):1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A, Tsukita S. Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann N Y Acad Sci. 2009;1165:44–52. doi: 10.1111/j.1749-6632.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann N Y Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7(6):467–477. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- Weed SA, Parsons JT. Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene. 2001;20(44):6418–6434. doi: 10.1038/sj.onc.1204783. [DOI] [PubMed] [Google Scholar]
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127(5):1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280(12):11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am J Physiol Cell Physiol. 2007;293(4):C1309–1318. doi: 10.1152/ajpcell.00014.2007. [DOI] [PubMed] [Google Scholar]