At present, the most well developed Alzheimer disease biomarkers are the cerebrospinal fluid analytes amyloid-β?42 residue and tau and the brain imaging measures amyloid PET, fluorodeoxyglucose PET, and MR imaging.
Abstract
Alzheimer disease (AD) is one of, if not the most, feared diseases associated with aging. The prevalence of AD increases exponentially with age after 60 years. Increasing life expectancy coupled with the absence of any approved disease-modifying therapies at present position AD as a dominant public health problem. Major advances have occurred in the development of disease biomarkers for AD in the past 2 decades. At present, the most well-developed AD biomarkers are the cerebrospinal fluid analytes amyloid-β 42 and tau and the brain imaging measures amyloid positron emission tomography (PET), fluorodeoxyglucose PET, and magnetic resonance imaging. CSF and imaging biomarkers are incorporated into revised diagnostic guidelines for AD, which have recently been updated for the first time since their original formulation in 1984. Results of recent studies suggest the possibility of an ordered evolution of AD biomarker abnormalities that can be used to stage the typical 20–30-year course of the disease. When compared with biomarkers in other areas of medicine, however, the absence of standardized quantitative metrics for AD imaging biomarkers constitutes a major deficiency. Failure to move toward a standardized system of quantitative metrics has substantially limited potential diagnostic usefulness of imaging in AD. This presents an important opportunity that, if widely embraced, could greatly expand the application of imaging to improve clinical diagnosis and the quality and efficiency of clinical trials.
© RSNA, 2012
Learning Objectives:
After reading the article and taking the test, the reader will be able to:
• List the relationships among pathologic features, biomarkers, and clinical expression of Alzheimer disease
• Describe the characteristic signature of Alzheimer disease on images obtained with different modalities
• Explain how imaging biomarkers are used in Alzheimer disease clinical trials
Accreditation and Designation Statement
The RSNA is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The RSNA designates this journal-based activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicans should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Statement
The ACCME requires that the RSNA, as an accredited provider of CME, obtain signed disclosure statements from the authors, editors, and reviewers for this activity. For this journal-based CME activity, author disclosures are listed at the end of this article.
Introduction
The clinical manifestation of Alzheimer disease (AD) and its associated brain patholophysiology, senile plaques and neurofibrillary tangles, was described more than 100 years ago (1). For roughly the next three-fourths of a century, the disease was to some extent ignored; it was believed to be an uncommon condition and thus not of great public health concern. This view has changed substantially in recent years, and AD is now recognized as a potentially unprecedented public health problem.
This is driven by three forces: (a) the inexorable aging of the population in all industrialized societies, (b) the fact that the incidence of AD increases exponentially with age in people older than 60 years, and (c) the current absence of any disease-modifying interventions.
Demographics and Public Health Impact
AD is a progressive neurodegenerative condition that primarily affects cognition. AD is not a normal part of aging; however, old age is its single greatest risk factor. Dementia is defined as disease-related loss of memory and other cognitive abilities of sufficient severity to interfere with activities of daily living. While there are a number of possible causes of dementia, the most common cause by far in elderly persons is AD (2). The current estimated prevalence in the United States is 5.3 million individuals, 5.1 million of whom are over the age of 65 years (2). Approximately one-eighth (13%) of all individuals in United States over the age of 65 years and more than half of all persons older than 85 carry a clinical diagnosis of AD dementia. The incidence doubles every 5 years after the age of 60. The prevalence of AD is greater in women than men; however, this is due to the greater life expectancy of women. Because of population aging, the estimated prevalence in the United States alone will be 7.7 million by 2030 and 14 million by 2050. AD is the fifth leading cause of death for those aged 65 years and older (3).
In addition to the personal toll on patients and families, the disease has substantial economic consequences. AD is the most common illness leading to nursing home placement (2). Total payments for health care and nursing home expenses for 2010 were $172 billion, including $123 billion for Medicare and Medicaid. By the year 2050, the estimated cost of AD in the United States will exceed $1 trillion per year. The cumulative cost from 2011 through 2050 is estimated at $20 trillion (2). The public health problem is not limited to the United States. Estimates of global costs of the disease in 2009 were $604 billion per year, or 1% of the world’s gross domestic product. About 70% of people with dementia live at home. Most of these patients receive unpaid help from family members and friends. In 2009 in the United States alone, in addition to formally counted health care costs described above 10.9 million family and other unpaid caregivers provided an estimated 12.5 billion hours of care, valued at $144 billion (2).
Clinical Course of the Disease
Longitudinal studies performed over the past 20 years have demonstrated that in individuals who eventually develop AD dementia, the cognitive symptoms begin insidiously and, in most cases, progress gradually. A large literature on mild cognitive impairment (MCI) has arisen since the mid-1990s that documents the gradual impairments of cognitive function (4). MCI refers to the transitional phase between normality and clinically evident dementia (5). The critical clinical distinction between the MCI and dementia phase of AD is loss of functional independence as the defining feature of dementia. Other clinical rating scales likewise reflect gradual development of clinical symptoms (6). The gradual onset and prolonged time course of AD stands in sharp contrast to many other medical conditions that have a clearly definable clinical onset, and often resolution.
Longitudinal studies show that a clinical diagnosis of MCI is a significant risk factor for the subsequent development of AD dementia. The rate of conversion from MCI to AD dementia is approximately 12% per year, which stands in contrast to the 1%–2% per year rate for cognitively normal elderly persons (7). Within 5 years, approximately 80% of individuals diagnosed with MCI will have converted to dementia (5).
Genetics and Molecular Pathways: The Amyloid Cascade Hypothesis
AD is commonly categorized as either early onset or late onset. Late onset sporadic disease (after age 65 years) accounts for over 95% of cases. The much rarer early onset cases manifest in persons younger than age 65, some as young as their 30s. Fewer than 1% of cases are found in a small number of families worldwide with autosomal dominant mutations in one of three genes: the amyloid precursor protein gene on chromosome 21, the presenilin-1 gene on chromosome 14, or the presenilin-2 gene on chromosome 1 (8–12). These known autosomal dominant mutations are all involved in production or metabolism of amyloid protein, which then implicates amyloid-β (Aβ) peptide, in the causal pathway leading to AD (13).
The vast majority of prevalent AD cases are sporadic with onset after age 65 years. Deterministic genetic mutations for late onset AD have not been found, but the ε4 allele of the apolipoprotein E (APOE) gene is the major genetic risk factor (14). The APOE gene encodes a protein with a key role in cholesterol metabolism. Three normally occurring alleles of APOE exist, ε2, ε3, and ε4. APOE ε3 is the most prevalent allele in the general population, with a frequency of roughly 80%. The ε4 allele of APOE increases the risk of developing AD and also lowers the mean age at onset of the disease (15–18). APOE ε4 contributes to AD pathogenesis by modulating the metabolism, aggregation, and clearance of Aβ peptide and also perhaps by directly regulating brain lipid metabolism and synaptic functions (19,20). From a biomarker perspective, APOE ε4 seems to act primarily as a risk factor for brain Aβ deposition (21,22). The ε2 allele decreases the risk of developing AD; however, this protective effect is not as strong as the risk conferred to carriers of the ε4 allele (17).
The available genetic evidence in both autosomal dominant and sporadic late onset AD points to the Aβ pathway. This evidence led to the amyloid cascade hypothesis, which asserts that dysfunction in the Aβ pathway is the initiating, or at least a very early, pathophysiologic event in the disease (23). Familial AD is sometimes referred to as a disease of overproduction of Aβ, and late onset sporadic AD is a disease of inadequate Aβ clearance. The majority of clinical trials on disease modification attempted to date have targeted Aβ mechanisms on the basis of the amyloid cascade hypothesis (24).
Pathologic Features and Their Relationship to Clinical Symptoms
Two abnormal protein aggregates characterize AD pathologically. These proteinopathies are the basis of the well-known amyloid plaques and neurofibrillary tangles (Fig 1). Neurofibrillary tangles are intracellular aggregates, while amyloid plaques form in the extracellular space. Other important pathologic features are periplaque inflammatory reaction and neurodegeneration, which is characterized by synapse loss, neuron loss, and gross cerebral atrophy (25–28).
Figure 1:

Plaques and tangles. Photomicrograph shows a neuritic plaque (upper arrow) and a neurofibrillary tangle (lower arrow) in an 85-year-old individual with autopsy-verified AD. (Beilchowsky silver stain.)
The neurofibrillary pathologic process of AD begins in the transentorhinal area and progresses to the hippocampus, then to paralimbic and adjacent medial-basal temporal cortex, to neocortical association areas, and lastly to primary sensorimotor and visual areas (29). Tau protein is a component of the cytoskeletal microtubule system. In AD, tau becomes hyperphosphorylated, disassociates from microtubules, assumes a paired helical filament configuration, and forms insoluble neurofibrillary tangles inside neurons (30).
The second major proteinopathy associated with AD is Aβ deposition. The hallmark Aβ peptide deposit in AD is the neuritic plaque, which consists of a dense central Aβ core with inflammatory cells and dystrophic neurites in its periphery. The core is made up of Aβ, which has aggregated into β pleated sheets (31). Amyloid precursor protein is a normal transmembrane protein. Normal proteolytic metabolism of amyloid precursor protein by secretases results in several peptide fragments (32). The most important of these are the Aβ1–40 and Aβ1–42 fragments (33). Of the two, Aβ1–40 is more abundant, while Aβ1–42 is more prone to aggregate (34). Soluble oligomeric Aβ fibrils are generally thought to be the neurotoxic Aβ species.
The field has seen intense debate on the question of which of the two hallmark proteinopathies is causative, Aβ or tau. Clinical examination–autopsy correlation studies (30,35–37) have demonstrated a much tighter correlation between neurofibrillary pathologic processes and cognitive impairment than between amyloid pathologic processes and cognitive impairment. Conversely, the available genetic evidence cited above strongly implicates a derangement in Aβ metabolism as the primary instigating factor in AD. Autopsy findings in cognitively normal subjects are informative. Approximately 30% of cognitively normal elderly subjects have AD pathologic features at autopsy (38–40). In most cases, cognitively normal subjects who meet autopsy criteria for AD have neocortical diffuse amyloid plaques but not extensive neocortical neurofibrillary tangles. These observations have been interpreted to support the position that amyloid develops first, while neocortical neurofibrillary tangles appear later (37).
AD Biomarkers
A biomarker is a physiologic, biochemical, or anatomic parameter that can be objectively measured as an indicator of normal biologic processes, pathologic processes, or responses to a therapeutic intervention. The term is used in the AD field to describe both biofluid analytes and imaging measures. Biofluid analytes in this context can refer to proteins in any biofluid; however, cerebrospinal fluid (CSF) biomarkers are presently the most well developed. At present, there are five AD biomarkers that have been widely studied and are well enough validated to be commonly considered for use in clinical trials. Two of these are CSF analytes and three are brain imaging measures (Table). These five biomarkers fall into two major mechanistic categories: (a) biomarkers of Aβ accumulation, which are represented by abnormal radiotracer retention on amyloid PET images and low levels of CSF Aβ42, and (b) biomarkers of neuronal degeneration or injury, which are indicated by elevated levels of CSF tau (both total and phosphorylated); decreased FDG uptake on PET images in the temporoparietal cortex; and atrophy on structural MR images in the medial, basal, and lateral temporal lobes and the medial and lateral parietal cortices. Other potential AD biomarkers have been summarized in recent reviews (41,42); some of these are measurements from imaging methods that will be discussed later as potential future applications.
The Five Major Biomarkers of AD

FDG = fluorodeoxyglucose, MR = magnetic resonance, PET = positron emission tomography.
Both CSF Aβ42 and amyloid PET findings are biomarkers of brain Aβ plaque deposition (Fig 2). Nearly all subjects who have received a clinical diagnosis of AD have positive amyloid imaging studies (43–45). Excellent correspondence has been seen between antemortem amyloid PET imaging findings and Aβ deposition in the brain (or cerebral vasculature) at autopsy (46,47). A consistent finding across different studies is that around 30% of cognitively normal elderly subjects have abnormal findings from PET amyloid imaging studies, which matches quite well the proportion of cognitively normal elderly subjects with an autopsy diagnosis of AD, as described above (48–52). Older age, APOE*E4 genotype, and a family history of AD are all associated with greater amyloid PET binding in cognitively normal late-middle-aged to elderly subjects (22,53,54). Measurable increases in amyloid load over time are seen in some cognitively normal elderly subjects (55–57). Although most amyloid imaging investigations published to date have used carbon 11 Pittsburgh compound B (PiB) as the tracer, newer fluorine 18 (18F)-labeled compounds (58–61) will have a higher clinical effect owing to the greater availability of 18F-labeled ligands. Depressed levels of CSF Aβ42 correlate with both the clinical diagnosis of AD and Aβ pathophysiology at autopsy (62–64). Extremely high concordance is present between an abnormally low level of CSF Aβ42 and positive PiB PET amyloid imaging findings in subjects who have undergone both tests (50,65–69). This is taken as evidence that these two biomarkers are, to a substantial extent, equivalent measures of brain Aβ pathophysiology.
Figure 2:
Amyloid imaging spectrum. Illustration of the spectrum found on PET amyloid images with Pittsburgh compound B. Top row: Negative amyloid imaging scans with “low” Pittsburgh compound B retention in an elderly cognitively normal control subject and in a subject with MCI. Bottom: Positive amyloid imaging scans with “high” Pittsburgh compound B retention in an elderly cognitively normal control subject, a subject with MCI, and a subject with AD. Around 70% of cognitively normal elderly people have negative amyloid imaging scans, while 30% have clearly abnormal scans. Around 60% of MCI subjects have positive scans, while 40% have negative scans. Most AD dementia subjects have positive scans.
CSF tau is an indicator of tau pathophysiology and associated neuronal injury. While phosphorylated tau may be a more specific indicator of AD, both phosphorylated tau and total tau levels increase in AD (70). Elevated CSF tau can be seen in other conditions, such as stroke and head trauma, and therefore is not specific for AD, but it does map to clinical disease severity (71,72). In AD, the cause of elevated tau level in the CSF is speculated to be a direct result of a tau pathophysiology that disrupts neurons, particularly axons, thereby releasing cytoskeletal elements, including tau, into the extracellular space, which appear in the CSF (73,74). Elevated CSF tau correlates with neurofibrillary pathophysiology at autopsy (75).
FDG PET measures of glucose metabolism reflect net brain metabolism, which predominantly reflects synaptic activity (76,77). In the context of AD, decreased FDG uptake is an indicator of impaired synaptic function. FDG PET studies in AD subjects demonstrate a specific topographic pattern of decreased glucose uptake in a lateral temporoparietal and a posterior cingulate-precuneus distribution (78,79) (Fig 3). Imaging-autopsy correlation studies have demonstrated good correlation between the antemortem FDG PET diagnosis of AD and postmortem confirmation (80). Clinically asymptomatic middle-aged carriers of the APOE*E4 gene, who are at increased risk for developing AD dementia later in life, have reduced FDG uptake in an AD-like pattern (81–83).
Figure 3:
Typical FDG PET uptake pattern in AD. Decreased FDG uptake in lateral temporoparietal cortex (arrows) and posterior cingulate–precuneus area is seen in the AD subject and more mildly in the MCI subject.
The structural MR imaging findings in AD are a measure of cerebral atrophy, which reflects microscopic neurodegeneration (loss of synapses, dendritic processes, and neurons) (84). Volumetric or voxel-based measures of brain atrophy display tight correlation between the severity of atrophy and the severity of cognitive impairment (72,85–88). Rates of brain atrophy correlate with rates of concurrent cognitive decline (89,90) (Fig 4). Atrophy on MR images is not specific for AD, but the degree of hippocampal atrophy correlates well with Braak staging at autopsy (29,91–93). The topographic distribution of atrophy on MR images (medial, basal, and lateral temporal lobe and medial parietal cortex) maps well with Braak staging of neurofibrillary tangles in subjects who have undergone antemortem MR imaging and postmortem AD staging (94,95).
Figure 4:
Atrophy and clinical stage of AD. Coronal three-dimensional T1-weighted volume MR images (repetition time msec/echo time msec/inversion time msec, 7/900/2.8/900) in three individuals in their 70s are shown. The cognitively normal control subject shows little atrophy; the AD subject, considerable atrophy; and the MCI subject, an intermediate level of atrophy.
Temporal Ordering of AD Biomarkers
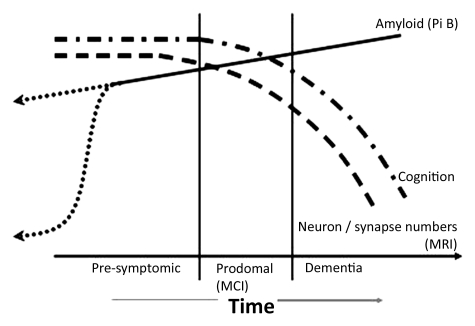
On the basis of the observations that AD biomarkers provide information about different AD-related pathophysiologic processes and the idea that these processes may be temporally ordered, great interest has arisen recently in the idea of staging disease by using biomarkers. Several biomarker-based models of disease have been proposed (57,96–98). One biomarker-based disease model (57) was based on empiric observations from serial MR imaging studies and Pittsburgh compound B PET studies (Fig 5). This model (57) embodies the following data-derived (51,57) principles: (a) The presence of brain amyloidosis is necessary but not sufficient to produce cognitive decline; (b) the neurodegenerative component of AD, rather than the amyloid component, is the direct substrate of cognitive impairment, and the rate of cognitive decline is driven by the rate of neurodegeneration; and (c) the rates of amyloid deposition and of neurodegeneration are dissociated: Amyloid deposition is an early event, and neurodegeneration is a later occurring event. These principles are supported by biomarker data (51,57), as well as autopsy data, which indicate that the aspect of the AD pathologic process that is most closely coupled with cognitive impairment is neurodegeneration, particularly synapse loss (26,40,99). In this amyloid and neurodegeneration model (57), which was based on longitudinal and cross-sectional MR imaging and amyloid PET imaging data, clinical symptoms do not appear until fairly late in the disease process (Fig 5).
Figure 5:
Graph shows amyloid and neurodegeneration model, relating imaging, pathologic, and clinical manifestation over an individual’s adult lifetime. The lifetime clinical course of the disease (horizontal axis) is divided into presymptomatic, prodromal (MCI), and dementia phases. The vertical axis illustrates increasing amyoid deposition, decreasing brain volume, and decreasing cognitive ability as the disease progresses from left to right. Dashed line = neurodegeneration detected on MR images, dotted-and-dashed line = cognitive function, solid line = amyloid deposition detected on Pittsburgh compound B (Pi B) PET. The time course of amyloid deposition in late middle age is represented as two possible theoretic trajectories (dotted lines), reflecting uncertainty about the time course of early amyloid deposition. (Reprinted, with permission, from reference 57.)
An expanded version of this disease biomarker model (96), illustrated in Figure 6, incorporates all five of the most well-validated AD biomarkers into a hypothetical comprehensive sequence of biomarker events as subjects progress from cognitively normal in middle age to dementia in old age. This hypothetical model rests on the assumption that these five AD biomarkers become dynamic in a sequential manner. The hypothesis is that amyloid biomarkers—PET amyloid imaging findings and CSF Aβ42 measures—depart markedly from normality 15 or more years before the first clinical symptoms appear. Biomarkers of neuronal injury—CSF tau and FDG PET findings—become dynamic later. Structural MR imaging is the most dynamic of the five major biomarkers in the clinically symptomatic phase of the disease, where the MR findings correlate best with clinical symptom severity. Preliminary empiric evidence supports this concept of temporal ordering of AD biomarkers that lies at the heart of the model (100–104). This model follows the principle that none of the biomarkers are static; rates of change in each biomarker vary over time and follow a nonlinear time course, which is hypothesized to be sigmoid shaped. The hypothetical sigmoid-shaped trajectory was based in part on the observation that many measurements have floor and ceiling effects with a roughly linear response in the midregion. It was also based in part on biologic considerations. A sigmoid shape as a function of time implies that the biomarker experiences an initial acceleration phase and a later deceleration phase, with the inflection midpoint in the sigmoid curve representing the onset of deceleration (105).
Figure 6:
Hypothetical model of the dynamic biomarkers of the AD cascade. Horizontal axis indicates clinical stages of AD: cognitively normal, MCI, and dementia. Vertical axis indicates changing values of each biomarker—from maximally normal (bottom) to maximally abnormal (top). Aβ in the brain is identified by presence of CSF Aβ42 or PET amyloid imaging findings (red line). Tau-mediated neuronal injury and dysfunction are identified by means of CSF tau level or FDG PET findings (blue line). Brain atrophy is measured with structural MR imaging (light green line). Onset and worsening of cognitive function are indicated by a purple line. Onset and worsening impairment in functional activities of daily living are indicated by dark green line. (Reprinted, with permission, from reference 96.)
Modifiers of Clinical Expression: Cognitive Reserve and Genetics
A feature of the biomarker model presented in Figure 6 is the lag between Aβ plaque formation and the neurodegenerative cascade followed by clinical symptoms. Intersubject variation in these lag periods is likely due to a number of factors. One factor is person-to-person differences in Aβ processing or in the effects of soluble oligomeric Aβ on neuronal injury. Many of these are likely under genetic control. Recent genome-wide association studies have identified new susceptibility loci for late onset AD (other than APOE) that implicate pathways related to brain cholesterol metabolism and inflammation (106–109).
A major modifier of the clinical expression of underlying AD pathophysiology is brain resiliency, or cognitive reserve (110). Cognitive reserve is an inclusive term that encompasses different mechanisms, including the numbers of neurons and synapses, the sensitivity of neurons and glia to pathologic processes, neuroplasticity, and the ability of a person to engage alternative cognitive processing strategies in the face of pathologic brain insult (110). Reserve can be expressed as the difference between the predicted and the observed cognitive performance of an individual for a given level of brain pathophysiology (111). The principle that subjects with higher reserve have a greater capacity to cope with pathologic insults than do those with low reserve is supported by data from several sources. Epidemiologic studies consistently show that higher educational and occupational attainment is protective against dementia (112–116). Clinical-autopsy studies have shown that at a given level of AD pathologic findings at autopsy, highly educated individuals are less likely to manifest clinical symptoms of dementia than are less-educated individuals (117,118). Similar findings have been reported in vivo, where among individuals with elevated Pittsburgh compound B uptake on PET amyloid imaging studies, cognitive performance was better with increasing education (119–121). Authors of a recent report (122) also suggest that lifetime cognitive enrichment may reduce amyloid deposition in later life.
Modifiers of Clinical Expression: Coexistent Age-related Brain Abnormality
A core concept essential to understanding relationships between brain pathophysiology and cognitive impairment in elderly persons is the frequency of comorbid brain pathologic conditions in this age group. Community-based clinical-autopsy studies have consistently demonstrated that a substantial majority of elderly subjects who received a diagnosis of AD dementia in life have pathologic conditions in addition to AD at autopsy. The most common of these are cerebrovascular disease (CVD) and Lewy body disease (123,124). The reason mixed conditions are so common is straightforward. As is the case with AD, processes such as Lewy body disease and CVD increase in prevalence with age. The older an individual is, the more likely he or she is to have accumulated different independent brain pathologic conditions.
While pure vascular dementia is uncommon, vascular contributions to cognitive impairment are very common (125,126). CVD is generally regarded to be the second most common pathologic contributor, after AD, to dementia in elderly persons (123,127,128). The most common vascular lesion found in community autopsy studies is microinfarction (124,127,129,130). Evidence to date indicates that the effects of AD abnormalities and vascular brain injury on cognitive expression are additive (131–133). Vascular risk factors likewise increase the risk of developing dementia (134–137). This has led to the recommendation to engage in a “heart healthy” lifestyle as a means of avoiding or delaying the onset of dementia.
The defining neuropathologic feature of dementia with Lewy bodies is pathologic aggregation of α-synuclein protein in neurites to form Lewy bodies (138). Lewy body disease is the proteinopathy underlying both Parkinson disease and dementia with Lewy bodies (139). Lewy body disease is commonly found as a comorbid condition, along with AD and CVD, in demented subjects examined in community autopsy studies (127,130). Hippocampal sclerosis, grain disease, and TDP-43 proteinopathy are also common coexistent conditions at autopsy, although not as common as AD, CVD, and Lewy body disease (140).
One condition that may seem to merit greater coverage in a review on dementia is frontotemporal lobar dementia (FTLD) (141). FTLD describes a family of neurodegenerative disorders characterized by focal lobar degeneration of the frontal and/or temporal lobes. FTLD is actually as common as AD in subjects under the age of 60 years (142,143); however, in individuals older than 65, AD and Lewy body disease account for the majority of neurodegenerative dementias, while FTLD is far less common.
Diffusion, Perfusion, Spectroscopy, and Functional MR Imaging
It might seem odd to find discussion of diffusion, perfusion, spectroscopy, and functional MR imaging toward the end of a review on AD biomarkers. However, although these MR imaging techniques have received substantial attention from investigators, none is well enough established to be included as a biomarker in newly formulated diagnostic criteria for AD (144–149), nor are any of these techniques routinely used in AD clinical trials. These techniques might best be regarded presently as of academic interest and awaiting sufficient validation to be accorded wider clinical use. Each of these techniques will be briefly described below.
Diffusion Imaging
A reasonable explanation for the relationship between AD pathologic features and abnormalities in diffusion is as follows (150,151): Cell membranes and intracellular structures act to impede the diffusion of water molecules. The pathologic disruption of cell membranes that occurs in AD increases the mean diffusivity of water. The coherence of directional water diffusion—fractional anisotropy—is also abnormal in AD and MCI (152–163). While mean diffusivity is increased, fractional anisotropy values are decreased due to loss of tract integrity (154,164,165). Of the two measures, fractional anisotropy has received greater attention because it provides unique information about pathologic disruption of regional anatomic connectivity (166) (Fig 7). Fractional anisotropy and mean diffusivity measures scale with clinical disease severity in AD and can help predict future progression from MCI to AD (167,168). Different methods have been used to extract measures related to fractional anisotropy, ranging from manual delineation of regions of interest to tract-based statistics to tractography (169–172).
Figure 7:
Fractional anisotropy in AD. Diffusion-tensor MR imaging (repetition time msec/echo time msec, 68/12 200; 41 diffusion-encoding directions). Voxel-wise analysis with tract-based spatial statistics in patients with AD (n = 17) and control subjects (n = 34) (Mayo Clinic, unpublished pilot data, January 2011). Fractional anisotropy is reduced in the parahippocampal gyrus (cingulum tract) (white arrows) and fornix (yellow arrow) in patients with AD as compared with control subjects, as shown in colored regions.
Perfusion Imaging
A characteristic bilateral temporoparietal decrease in cerebral blood flow has been well documented in AD by using nuclear medicine techniques (173,174). MR imaging measures of tissue perfusion by means of arterial spin labeling techniques mirror the classically established topographic patterns on FDG PET and single photon emission computed tomography (SPECT) images (175,176). Perfusion MR imaging is attractive because disease-specific regional cerebral blood flow information currently obtained with SPECT or FDG PET can be obtained noninvasively with arterial spin labeling in an integrated examination that includes other MR imaging–based modalities (Fig 8). This is an active area of research, and it remains to be seen if perfusion MR imaging can achieve the same diagnostic sensitivity and specificity that has already been demonstrated with FDG PET (177–179).
Figure 8:
Perfusion MR imaging in AD patients (n = 33) and control subjects (n = 62). Pulsed arterial spin labeling MR imaging (1.5/5.2) shows significant hypoperfusion (red areas) in AD patients, as compared with control subjects. (Reprinted, with permission, from reference 175.)
Hydrogen 1 MR Spectroscopy
The metabolites that are consistently abnormal in hydrogen 1 MR spectroscopy are N-acetylaspartate (NAA) and myo-inositol (180–186). NAA is found in neurons, and a decrease in NAA follows logically from the neuron loss and dysfunction in AD (187). Longitudinal change in NAA has been proposed as a response measure in clinical trials (188–190). myo-Inositol is located primarily in astrocytes and is elevated in AD. One possible explanation for this finding is the fact that activated glial cells surround amyloid plaques (191–194). Elevation in choline level has also been described in AD, but this has not been found consistently.
Functional MR Imaging
Two imaging techniques carry the name functional MR imaging. One is task based, and the other is task free or resting state. Most investigators find decreased task activation in patients with dementia, as compared with age-matched control subjects. Initial functional MR imaging activation paradigms were focused on memory, but investigators have extended this to a variety of paradigms (195–199). The notion of increased activation strength in mildly impaired or asymptomatic subjects who are at elevated risk for developing dementia is now fairly well established (200,201). One explanation offered for increased task activation strength in at risk subjects is that they are able to compensate by “working harder” mentally to maintain cognitive function in the face of progressing disorder (201–203). An alternative explanation is that hyperactivation may not be compensatory but, instead, a pathologic state that may contribute to further damage (204,205).
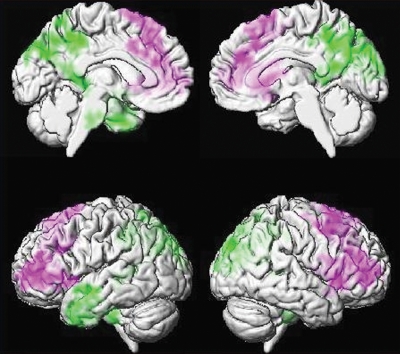
An alternative to task-activation functional MR imaging is task-free or resting-state functional MR imaging. Although first described by Biswal et al (206) in 1995, this approach has attracted a great deal of interest recently. Task-free functional MR imaging is based on detection of coincident intrinsic temporal fluctuations in blood oxygen level–dependent, or BOLD, signal intensity across areas of the brain. Temporal synchronicity is inferred to denote functionally connected networks (207). As compared with task-activation functional MR, task-free functional MR imaging has the obvious advantage in impaired subjects of not requiring active cooperation (208). Interest in applying task-free functional MR imaging to the study of cognitive impairment accelerated with the realization that areas that are active in the resting state (ie, default mode) coincide in part with areas that are particularly susceptible to amyloid deposition and to depressed glucose metabolism in AD (209,210). The default mode network undergoes changes not only in AD and MCI but also as a result of aging (211–216). Both increases and decreases in network connectivity have been described in cognitively impaired subjects (208,217–219). Specific reports vary, but converging evidence indicates a pattern of decreased connectivity in the task-negative (default mode) networks and increased connectivity in task-positive networks in AD dementia and MCI (210,211,213,215,216,220–222) (Fig 9).
Figure 9:
Resting-state functional connectivity in AD. Echo-planar MR imaging (2900/30) seed-based voxel-wise connectivity analysis in 56 APOEε4 noncarrier control subjects versus 28 AD subjects. Seed was placed in posterior cingulate gyrus for connectivity analysis. Purple indicates areas where AD patients have greater connectivity than control subjects; green, areas where AD patients have less connectivity than control subjects. Control subjects show greater connectivity with the seed within the posterior cingulate gyrus, precuneus, and left anterior temporal lobe (green) than AD subjects. AD subjects show greater connectivity than control to bilateral medial and lateral frontal regions (purple) (225).
Cognitively normal subjects who are at future risk for AD dementia by virtue of carrying the APOE ε4 allele have altered resting state connectivity patterns that are topographically similar to those seen in cognitively impaired subjects (223–225). Amyloid deposition measured with amyloid PET imaging is associated with disruption of default mode network functional connectivity in cognitively normal subjects with high amyloid burden (200,223,226–228). Network disturbances have also been described in cognitively normal elderly to middle-aged subjects who are APOE ε4 carriers but who have been documented to be amyloid PET negative (229). Disturbances have been described in APOE ε4 carriers in young adulthood (20s)—that is, subjects who are at elevated risk of AD later in life but who are too young to expect any AD pathophysiology to have developed (224).
Newer classification methods that use global functional connectivity metrics have shown potential for diagnostic use (214,222,230–233).
Applications of Imaging Biomarkers: New Diagnostic Criteria for AD
On the basis of the preceding material, one might assume that CSF and imaging AD biomarkers currently play a prominent role in daily clinical practice. It might be surprising, however, to realize that this is not the case. Several explanations are typically offered for this state of affairs. Perhaps the most common is the fact that the absence of approved disease-modifying therapies minimizes the impetus for early diagnosis because early diagnosis will not change clinical management. An obvious reason that biomarkers are not widely used in clinical practice is that until very recently, diagnostic guidelines for AD did not incorporate CSF or imaging biomarkers. Criteria for the clinical diagnosis of AD were originally established in 1984 by a work group comprising representatives from the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (234). In the 1984 criteria, AD dementia was a diagnosis made solely on clinical grounds. It was also operationalized as a diagnosis of exclusion: AD was diagnosed when other potential causes of dementia were excluded. The role of imaging in the 1984 criteria (234) was, therefore, to help exclude conditions like cerebral infarction, space-occupying lesions, or potentially treatable causes such as hydrocephalus or subdural hemorrhage.
Recently, however, diagnostic guidelines for AD have been updated (145–149). One of these efforts was organized by the National Institute on Aging–Alzheimer’s Association. In 2010 these organizations commissioned three work groups. Each group was assigned the task of defining or revising diagnostic criteria for one of three recognized phases of the disease: preclinical AD, symptomatic predementia or MCI, and AD dementia (146–149). Biomarkers are used in all three phases of the disease. In addition to their traditional exclusionary role, imaging studies are now expected to provide positive diagnostic information. In the preclinical phase biomarkers are by definition the only indicator of latent disease. They are used to establish the presence of AD pathophysiologic processes in subjects with no overt symptoms. In the symptomatic MCI and AD phases, biomarkers are used to establish the underlying cause for an observed clinical deficit. In addition to aiding in establishing a pathophysiologic etiology to aid diagnosis, imaging and CSF biomarkers provide two additional major classes of clinically useful information. These are prediction of future clinical course (235–243) and longitudinal measurement of disease progression (244).
Applications of Imaging Biomarkers: Clinical Trials
In addition to use as diagnostic criteria in the new diagnostic guidelines (145–149), a second major use category for imaging and CSF biomarkers is in therapeutic trials. Incorporation of imaging and CSF biomarkers into clinical trial design is more advanced than is their incorporation in clinical diagnosis. For several years, nearly every therapeutic trial in AD has incorporated one or more AD biomarkers in the study design, where they can serve one or more specific functions.
Subject Inclusion
As an indicator of AD pathophysiologic processes, AD biomarkers are used for subject inclusion—ie, to insure appropriate targeting of the therapeutic mechanism of action. For example, it is rational to base inclusion in antiamyloid therapeutic trials on biomarker evidence of the presence of Aβ in the brain by using either amyloid PET imaging or CSF Aβ42 measurement (245–247).
Subject Exclusion
Anatomic MR imaging is commonly used for exclusionary purposes in AD therapeutic trials. For example, hemispheric cerebral infarction, tumor, hydrocephalus, prior brain surgery, and major cerebral hemorrhage are common exclusionary findings on screening MR images. Studies on which a prespecified number of microhemorrhages (recently renamed amyloid-related imaging abnormalities [248]) are identified are also now an exclusionary finding in antiamyloid trials (249).
Stratification, Enrichment, and Covariates
The neurodegenerative biomarkers—MR imaging and FDG PET or CSF tau level—indicate the severity or stage of neuronal injury and neurodegeneration and can, therefore, be used as covariates in analyses to stratify subjects at baseline or as an enrichment strategy to improve efficiency (41,250).
Outcome
The idea that biomarkers may serve as surrogate outcome measures in AD clinical trials has received a great deal of attention (251). Outcomes are, by definition, longitudinal measures of change over time; therefore, measurement precision is of paramount importance in this context. Enthusiasm for biomarkers as surrogate outcome measures was initially driven by studies measuring the natural rates of brain atrophy with structural MR imaging. Led by Fox et al (252) investigators demonstrated that the lower variance in serial MR imaging measurements, compared with that of clinical measures of cognition and function, could potentially allow clinical trials to be performed with much smaller sample sizes than is possible with traditional clinical instruments (86,253–261). Similar data were generated with FDG PET (262). In addition, biomarkers are more objective and reliable quantitative measures of AD pathophysiologic processes than traditional cognitive and functional outcomes that are affected by subject motivation and extrinsic factors such as alertness, environmental stresses, and informant mood and distress.
The context in which amyloid biomarkers would serve as surrogate outcome measures differs somewhat. Change in Aβ load over time has little relationship to change in cognition in natural history studies (57). Also, evidence of therapeutic plaque removal in already demented subjects does not correlate with a change in the trajectory of cognitive deterioration (at least, not in every instance examined) (263). However, change in amyloid biomarkers would provide clear evidence of correct targeting of the intervention (264).
At present, no therapeutic intervention is available that delays or reverses progression of the disease. Currently available treatments for AD are limited to symptomatic intervention (265). Unfortunately, therapeutic attempts at disease modification focused on various targets in the amyloid cascade pathway have been uniformly unsuccessful. Some point to these failed antiamyloid trials as evidence that the amyloid hypothesis is incorrect. A more widely held point of view, however, is that the disease-modifying interventional trials have failed because the wrong phase of the disease has been targeted (245–247). All studies to date have been performed in symptomatic subjects—most with mild to moderate dementia and some with MCI. Note in Figures 5 and 6 that clinical symptoms appear toward the end of the lifetime course of the disease. Many in the field believe that to be effective, disease-modifying therapeutic intervention must occur much earlier in the disease cascade—before onset of clinical symptoms. Imaging and CSF biomarkers will play pivotal roles in future clinical trials targeting clinically asymptomatic or minimally symptomatic subjects.
Need for Standardization of Imaging
The clinical practice of radiology is based on visual assessment of images. This diagnostic assessment is converted to a narrative interpretation which becomes the radiologic contribution to a patient’s diagnostic evaluation. Visual assessment of the degree of hippocampal atrophy on MR images (266) (Fig 4) or the degree of temporoparietal hypometabolism (Fig 3) is easily implemented and widely available. However, anatomic or physiologic processes are continuous phenomena, and verbal encoding of a visual impression does not lend itself to accurate or reproducible assessment of fine incremental grades of atrophy or glucose uptake.
For years, academic efforts have focused on quantitative measurements—that is, turning gray-scale images into numbers—for statistical analysis in research papers. This is particularly true for MR imaging. Initial efforts at image quantification focused on methods based on regions of interest, which were often hand drawn. In MR imaging, these efforts often focused on specific named structures such as the hippocampus or entorhinal cortex, which are involved early and progressively in AD (267,268). More recently, methods have been developed to automatically parcellate (269) gray matter density or the thickness of cortical surfaces (270–272) into regions of interest. This approach is reproducible and does not require manual intervention. Several investigators have developed multivariate analysis and machine learning–based algorithms that use the entire three-dimensional MR imaging data set to form a disease model against which individual subjects may be compared. A new incoming study is scored according to the degree and topographic pattern of atrophy as compared with the images from a large database of well-characterized subjects (273–279). Such measures capture the severity of neuronal abnormality—that is, Braak staging over the whole brain is better than measurement of hippocampal volume for determining severity (95).
Of the five major biomarkers of AD, three are imaging studies. Although the value of imaging biomarkers in both clinical diagnosis and clinical trials is clear, major barriers exist to actual implementation. The most important barrier has been lack of standardized methods, particularly methods for extracting quantitative information from images. Although initiatives such as the Alzheimer’s Disease Neuroimaging Initiative have focused on standardization of image acquisition methods (280,281), universally accepted standards for image quantification have not yet emerged.
An important contribution that the imaging community could make would be to establish open-source methods for standardized quantification. If imaging is to take its place as a widely used, clinically relevant disease biomarker for AD, then imaging should transform itself to operate as do biomarkers in other fields. For example, measures of blood pressure, serum glucose, or serum lipids have been standardized. Experts in the fields of hypertension, diabetes, and lipid metabolism have agreed on and adopted diagnostic classification criteria that are based on standardized laboratory measurements. Treatment protocols are tied to these universal measures. For imaging to assume a similar role as an AD biomarker, similar standardization of quantitative measures should take place. In an ideal world, a subject could be imaged with a unit by any vendor. An analysis software pipeline would be used to analyze that subject’s scan, extract quantitative data, compare those data to a standardized database, and assign the subject a probabilistic differential diagnosis and an imaging-based disease severity rank (282). Ideally this would happen “online” on the imaging unit. These quantitative diagnostic imaging measures would be incorporated into universally recognized diagnostic criteria for AD.
Essentials.
• The five most well-established biomarkers of Alzheimer disease (AD) at this time can be divided into two mechanistic categories: (a) measures of brain amyloid-β (Aβ) peptide deposition (cerebrospinal fluid Aβ42 and amyloid PET imaging) and (b) measures of neuronal injury and degeneration (cerebrospinal fluid tau, fluorodeoxyglucose PET, and structural MR imaging).
• A recently developed hypothetical model of the temporal evolution of AD biomarkers proposes that these five AD biomarkers depart markedly from normal in a sequential manner, with amyloid biomarkers departing first 15 or more years before the first clinical symptoms appear, and neuronal injury and degeneration biomarkers departing later in the disease course but correlating better with concurrent clinical symptoms.
• Recently updated diagnostic guidelines for AD from the National Institute on Aging–Alzheimer’s Association incorporate imaging and cerebrospinal fluid biomarkers.
• A major unmet need in the field is biomarker standardization—in particular, the development of standardized quantitative metrics for imaging biomarkers of AD.
Disclosures of Potential Conflicts of Interest: Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Institution has received money from Pfizer, Johnson & Johnson, Janssen, Eisai, and Elan for consultancies. Other relationships: none to disclose.
Acknowledgments
The author acknowledges the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation and Samantha Willie for manuscript preparation.
Received March 3, 2011; revision requested April 11; revision received April 28; accepted May 12; final version accepted May 17; final review by the author February 1, 2012.
Funding: This work was supported by the National Institutes of Health (grant AG11378).
Abbreviations:
- Aβ
- amyloid-β
- AD
- Alzheimer disease
- APOE
- apolipoprotein E
- CSF
- cerebrospinal fluid
- FDG
- fluorodeoxyglucose
- MCI
- mild cognitive impairment
References
- 1.Alzheimer A. Uber eigenartige Krankheitsfalle des spateren Alters. Z Gesamte Neurol Psychiatr 1911;4(1):356–385 [Google Scholar]
- 2.Alzheimer’s Association 2010 Alzheimer’s disease facts and figures. Alzheimers Dement 2010;6(2):158–194 [DOI] [PubMed] [Google Scholar]
- 3.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep 2009;57(14):1–134 [PubMed] [Google Scholar]
- 4.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58(12):1985–1992 [DOI] [PubMed] [Google Scholar]
- 5.RC Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009;66(12):1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43(11):2412–2414 [DOI] [PubMed] [Google Scholar]
- 7.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA 1995;273(16):1274–1278 [PubMed] [Google Scholar]
- 8.St George-Hyslop PH, Tanzi RE, Polinsky RJ, et al. The genetic defect causing familial Alzheimer’s disease maps on chromosome 21. Science 1987;235(4791):885–890 [DOI] [PubMed] [Google Scholar]
- 9.Davies P. The genetics of Alzheimer’s disease: a review and a discussion of the implications. Neurobiol Aging 1986;7(6):459–466 [DOI] [PubMed] [Google Scholar]
- 10.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991;349(6311):704–706 [DOI] [PubMed] [Google Scholar]
- 11.Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995;269(5226):973–977 [DOI] [PubMed] [Google Scholar]
- 12.Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995;375(6534):754–760 [DOI] [PubMed] [Google Scholar]
- 13.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996;2(8):864–870 [DOI] [PubMed] [Google Scholar]
- 14.Roses AD. Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol 1994;53(5):429–437 [DOI] [PubMed] [Google Scholar]
- 15.Strittmatter WJ, Weisgraber KH, Huang DY, et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993;90(17):8098–8102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261(5123):921–923 [DOI] [PubMed] [Google Scholar]
- 17.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet 1994;7(2):180–184 [DOI] [PubMed] [Google Scholar]
- 18.Saunders AM, Strittmatter WJ, Schmechel D, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993;43(8):1467–1472 [DOI] [PubMed] [Google Scholar]
- 19.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009;10(5):333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A 1993;90(20):9649–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67(1):122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol 2010;67(3):308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 2002;297(5580):353–356 [DOI] [PubMed] [Google Scholar]
- 24.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999;400(6740):173–177 [DOI] [PubMed] [Google Scholar]
- 25.Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol 1981;10(2):184–192 [DOI] [PubMed] [Google Scholar]
- 26.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991;30(4):572–580 [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E. Morphological criteria for the recognition of Alzheimer’s disease and the distribution pattern of cortical changes related to this disorder. Neurobiol Aging 1994;15(3):355–356; discussion 379–380 [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci 1970;11(3):205–242 [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82(4):239–259 [DOI] [PubMed] [Google Scholar]
- 30.Dickson DW, Crystal HA, Bevona C, Honer W, Vincent I, Davies P. Correlations of synaptic and pathological markers with cognition of the elderly. Neurobiol Aging 1995;16(3):285–298; discussion 298–304 [DOI] [PubMed] [Google Scholar]
- 31.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 1984;120(3):885–890 [DOI] [PubMed] [Google Scholar]
- 32.Selkoe DJ. Molecular pathology of Alzheimer’s disease: the role of amyloid. In: Growden JH, Rossor M, eds. The dementias. Boston, Mass: Butterworth-Heinemann, 1998; 257–283 [Google Scholar]
- 33.Golde TE, Estus S, Younkin LH, Selkoe DJ, Younkin SG. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science 1992;255(5045):728–730 [DOI] [PubMed] [Google Scholar]
- 34.Selkoe DJ. Deciphering Alzheimer’s disease: molecular genetics and cell biology yield major clues. J NIH Res 1995;7:57–64 [Google Scholar]
- 35.Gómez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol 1997;41(1):17–24 [DOI] [PubMed] [Google Scholar]
- 36.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 2004; 61(3):378–384 [DOI] [PubMed] [Google Scholar]
- 37.Ingelsson M, Fukumoto H, Newell KL, et al. Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 2004;62(6):925–931 [DOI] [PubMed] [Google Scholar]
- 38.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 2003;62(11):1087–1095 [DOI] [PubMed] [Google Scholar]
- 39.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol 1999;45(3):358–368 [DOI] [PubMed] [Google Scholar]
- 40.Savva GM, Wharton SB, Ince PG, et al. Age, neuropathology, and dementia. N Engl J Med 2009;360(22):2302–2309 [DOI] [PubMed] [Google Scholar]
- 41.Hampel H, Frank R, Broich K, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 2010;9(7):560–574 [DOI] [PubMed] [Google Scholar]
- 42.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov 2007;6(4):295–303 [DOI] [PubMed] [Google Scholar]
- 43.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol 2004;55(3):306–319 [DOI] [PubMed] [Google Scholar]
- 44.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68(20):1718–1725 [DOI] [PubMed] [Google Scholar]
- 45.Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 2007;68(7):501–508 [DOI] [PubMed] [Google Scholar]
- 46.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain 2008;131(Pt 6):1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol 2007;64(3):431–434 [DOI] [PubMed] [Google Scholar]
- 48.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67(3):446–452 [DOI] [PubMed] [Google Scholar]
- 49.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65(11):1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59(3):512–519 [DOI] [PubMed] [Google Scholar]
- 51.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 2008;131(Pt 3):665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chételat G, Villemagne VL, Pike KE, et al. Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease. Brain 2011;134(Pt 3):798–807 [DOI] [PubMed] [Google Scholar]
- 53.Xiong C, Roe CM, Buckles V, et al. Role of family history for Alzheimer biomarker abnormalities in the adult children study. Arch Neurol 2011;68(10):1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging 2010;31(8):1275–1283 [DOI] [PubMed] [Google Scholar]
- 55.Vlassenko AG, Mintun MA, Xiong C, et al. Amyloid-beta plaque growth in cognitively normal adults: longitudinal [11C]Pittsburgh compound B data. Ann Neurol 2011;70(5):857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sojkova J, Zhou Y, An Y, et al. Longitudinal patterns of b-amyloid deposition in nondemented older adults. Arch Neurol 2011;68(5):644–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain 2009;132(Pt 5):1355–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011;305(3):275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Villemagne VL, Ong K, Mulligan RS, et al. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med 2011;52(8):1210–1217 [DOI] [PubMed] [Google Scholar]
- 60.Fleisher AS, Chen K, Liu X, et al. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol 2011;68(11):1404–1411 [DOI] [PubMed] [Google Scholar]
- 61.Barthel H, Gertz HJ, Dresel S, et al. Cerebral amyloid-b PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 2011;10(5):424–435 [DOI] [PubMed] [Google Scholar]
- 62.Clark CM, Xie S, Chittams J, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol 2003;60(12):1696–1702 [DOI] [PubMed] [Google Scholar]
- 63.Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003;60(4):652–656 [DOI] [PubMed] [Google Scholar]
- 64.Schoonenboom NS, van der Flier WM, Blankenstein MA, et al. CSF and MRI markers independently contribute to the diagnosis of Alzheimer’s disease. Neurobiol Aging 2008;29(5):669–675 [DOI] [PubMed] [Google Scholar]
- 65.Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology 2009;73(15):1193–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimmer T, Riemenschneider M, Förstl H, et al. Beta amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry 2009;65(11):927–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolboom N, van der Flier WM, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 2009;50(9):1464–1470 [DOI] [PubMed] [Google Scholar]
- 68.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 2008;29(10):1456–1465 [DOI] [PubMed] [Google Scholar]
- 69.Weigand SD, Vemuri P, Wiste HJ, et al. Transforming cerebrospinal fluid Aβ42 measures into calculated Pittsburgh Compound B units of brain Aβ amyloid. Alzheimers Dement 2011;7(2):133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buerger K, Ewers M, Pirttilä T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain 2006;129(Pt 11):3035–3041 [DOI] [PubMed] [Google Scholar]
- 71.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009;65(4):403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology 2009;73(4):287–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arai H, Terajima M, Miura M, et al. Tau in cerebrospinal fluid: a potential diagnostic marker in Alzheimer’s disease. Ann Neurol 1995;38(4):649–652 [DOI] [PubMed] [Google Scholar]
- 74.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol 1995;26(3):231–245 [DOI] [PubMed] [Google Scholar]
- 75.Tapiola T, Overmyer M, Lehtovirta M, et al. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer’s disease. Neuroreport 1997;8(18):3961–3963 [DOI] [PubMed] [Google Scholar]
- 76.Schwartz WJ, Smith CB, Davidsen L, et al. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science 1979;205(4407):723–725 [DOI] [PubMed] [Google Scholar]
- 77.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001;21(10):1133–1145 [DOI] [PubMed] [Google Scholar]
- 78.Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007;69(9):871–877 [DOI] [PubMed] [Google Scholar]
- 79.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997;42(1):85–94 [DOI] [PubMed] [Google Scholar]
- 80.Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med 2000;41(11):1920–1928 [PubMed] [Google Scholar]
- 81.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med 1996; 334(12):752–758 [DOI] [PubMed] [Google Scholar]
- 82.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 1995;273(12):942–947 [PubMed] [Google Scholar]
- 83.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 2009;106(16):6820–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 2000;95(3):721–725 [DOI] [PubMed] [Google Scholar]
- 85.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 1997;49(3):786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schuff N, Woerner N, Boreta L, et al. MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 2009;132(Pt 4):1067–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grundman M, Sencakova D, Jack CR, Jr, et al. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci 2002;19(1-2):23–27 [DOI] [PubMed] [Google Scholar]
- 88.Whitwell JL, Petersen RC, Negash S, et al. Patterns of atrophy differ among specific subtypes of mild cognitive impairment. Arch Neurol 2007;64(8):1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology 1999;52(8):1687–1689 [DOI] [PubMed] [Google Scholar]
- 90.Hua X, Leow AD, Parikshak N, et al. Tensor-based morphometry as a neuroimaging biomarker for Alzheimer’s disease: an MRI study of 676 AD, MCI, and normal subjects. Neuroimage 2008;43(3):458–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002;58(5):750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology 2003;61(4):487–492 [DOI] [PubMed] [Google Scholar]
- 93.Gosche KM, Mortimer JA, Smith CD, Markesbery WR, Snowdon DA. Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 2002;58(10):1476–1482 [DOI] [PubMed] [Google Scholar]
- 94.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology 2008;71(10):743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vemuri P, Whitwell JL, Kantarci K, et al. Antemortem MRI based STructural Abnormality iNDex (STAND)-scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage 2008;42(2):559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 2010;9(1):119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009;132(Pt 5):1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature 2009;461(7266):916–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990;27(5):457–464 [DOI] [PubMed] [Google Scholar]
- 100.Caroli A, Frisoni GB; Alzheimer’s Disease Neuroimaging Initiative. The dynamics of Alzheimer’s disease biomarkers in the Alzheimer’s Disease Neuroimaging Initiative cohort. Neurobiol Aging 2010;31(8):1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buchhave P, Minthon L, Zetterberg H, Wallin AK, Blennow K, Hansson O. Cerebrospinal Fluid Levels of β-Amyloid 1-42, but Not of Tau, Are Fully Changed Already 5 to 10 Years Before the Onset of Alzheimer Dementia. Arch Gen Psychiatry 2012;69(1):98–106 [DOI] [PubMed] [Google Scholar]
- 102.Jack CR, Jr, Vemuri P, Wiste HJ, et al. Evidence for ordering of Alzheimer disease biomarkers. Arch Neurol 2011;68(12):1526–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koivunen J, Scheinin N, Virta JR, et al. Amyloid PET imaging in patients with mild cognitive impairment: a 2-year follow-up study. Neurology 2011;76(12):1085–1090 [DOI] [PubMed] [Google Scholar]
- 104.Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann Neurol 2011;69(1):181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lomasko T, Lumsden CJ. One-hit stochastic decline in a mechanochemical model of cytoskeleton-induced neuron death III: diffusion pulse death zones. J Theor Biol 2009;256(1):104–116 [DOI] [PubMed] [Google Scholar]
- 106.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 2009;41(10):1088–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 2009;41(10):1094–1099 [DOI] [PubMed] [Google Scholar]
- 108.Hollingworth P, Harold D, Sims R, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet 2011;43(5):429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet 2011;43(5):436–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20(3 Suppl. 2):S69–S74 [DOI] [PubMed] [Google Scholar]
- 111.Reed BR, Mungas D, Farias ST, et al. Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 2010;133(8):2196–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Evans DA, Hebert LE, Beckett LA, et al. Education and other measures of socioeconomic status and risk of incident Alzheimer disease in a defined population of older persons. Arch Neurol 1997;54(11):1399–1405 [DOI] [PubMed] [Google Scholar]
- 113.Fitzpatrick AL, Kuller LH, Ives DG, et al. Incidence and prevalence of dementia in the Cardiovascular Health Study. J Am Geriatr Soc 2004;52(2):195–204 [DOI] [PubMed] [Google Scholar]
- 114.Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 2002;59(11):1737–1746 [DOI] [PubMed] [Google Scholar]
- 115.Rocca WA, Cha RH, Waring SC, Kokmen E. Incidence of dementia and Alzheimer’s disease: a reanalysis of data from Rochester, Minnesota, 1975-1984. Am J Epidemiol 1998;148(1):51–62 [DOI] [PubMed] [Google Scholar]
- 116.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 1994;271(13):1004–1010 [PubMed] [Google Scholar]
- 117.Roe CM, Xiong C, Miller JP, Cairns NJ, Morris JC. Interaction of neuritic plaques and education predicts dementia. Alzheimer Dis Assoc Disord 2008;22(2):188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roe CM, Xiong C, Miller JP, Morris JC. Education and Alzheimer disease without dementia: support for the cognitive reserve hypothesis. Neurology 2007;68(3):223–228 [DOI] [PubMed] [Google Scholar]
- 119.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol 2008;65(11):1467–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer’s disease biomarkers are independent determinants of cognition. Brain 2011;134(Pt 5):1479–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol 2010;67(3):353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Landau SM, Marks SM, Mormino EC, et al. Association of Lifetime Cognitive Engagement and Low β-Amyloid Deposition [Epub ahead of print]. Arch Neurol 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69(24):2197–2204 [DOI] [PubMed] [Google Scholar]
- 124.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol 2005;18(4):224–227 [DOI] [PubMed] [Google Scholar]
- 125.Chui H. Vascular dementia, a new beginning: shifting focus from clinical phenotype to ischemic brain injury. Neurol Clin 2000;18(4):951–978 [DOI] [PubMed] [Google Scholar]
- 126.Jack CR, Jr, O’Brien PC, Rettman DW, et al. FLAIR histogram segmentation for measurement of leukoaraiosis volume. J Magn Reson Imaging 2001;14(6):668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 2007;62(4):406–413 [DOI] [PubMed] [Google Scholar]
- 128.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004;62(7):1148–1155 [DOI] [PubMed] [Google Scholar]
- 129.Petrovitch H, Ross GW, Steinhorn SC, et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann Neurol 2005;57(1):98–103 [DOI] [PubMed] [Google Scholar]
- 130.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66(2):200–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.131. Chui HC, Brown NN. Vascular cognitive impairment. Continuum: lifelong learning in neurology 2007;13(2):109–143 [Google Scholar]
- 132.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol 2008;63(1):72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kantarci K, Weigand SD, Przybelski SA, et al. Risk of dementia in MCI: combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology 2009;72(17):1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hendrie HC, Albert MS, Butters MA, et al. The NIH Cognitive and Emotional Health Project. Report of the Critical Evaluation Study Committee. Alzheimers Dement 2006;2(1):12–32 [DOI] [PubMed] [Google Scholar]
- 135.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 2005;62(10):1556–1560 [DOI] [PubMed] [Google Scholar]
- 136.Yaffe K. Metabolic syndrome and cognitive decline. Curr Alzheimer Res 2007;4(2):123–126 [DOI] [PubMed] [Google Scholar]
- 137.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71(14):1057–1064 [DOI] [PubMed] [Google Scholar]
- 138.138. Galasko DR. Dementia with Lewy bodies. Continuum: lifelong learning in neurology 2007;13(2):69–86 [Google Scholar]
- 139.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain 2007;130(Pt 3):708–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010;20(1):66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51(6):1546–1554 [DOI] [PubMed] [Google Scholar]
- 142.Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology 2004;62(3):506–508 [DOI] [PubMed] [Google Scholar]
- 143.Boeve BF, Baker M, Dickson DW, et al. Frontotemporal dementia and parkinsonism associated with the IVS1+1G->A mutation in progranulin: a clinicopathologic study. Brain 2006;129(Pt 11):3103–3114 [DOI] [PubMed] [Google Scholar]
- 144.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6(8):734–746 [DOI] [PubMed] [Google Scholar]
- 145.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 2010;9(11):1118–1127 [DOI] [PubMed] [Google Scholar]
- 146.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stejskal EO, Tanner JE. Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 1965;42(1):288–292 [Google Scholar]
- 151.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 1986;161(2):401–407 [DOI] [PubMed] [Google Scholar]
- 152.Rose SE, McMahon KL, Janke AL, et al. Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry 2006;77(10):1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bozzali M, Falini A, Franceschi M, et al. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2002;72(6):742–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 2007;68(1):13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Medina D, DeToledo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging 2006;27(5):663–672 [DOI] [PubMed] [Google Scholar]
- 156.Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology 2007;243(2):483–492 [DOI] [PubMed] [Google Scholar]
- 157.Teipel SJ, Stahl R, Dietrich O, et al. Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. Neuroimage 2007;34(3):985–995 [DOI] [PubMed] [Google Scholar]
- 158.Salat DH, Tuch DS, van der Kouwe AJ, et al. White matter pathology isolates the hippocampal formation in Alzheimer’s disease. Neurobiol Aging 2010;31(2):244–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Walhovd KB, Fjell AM, Amlien I, et al. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry and diffusion of the temporal-parietal memory network. Neuroimage 2009;45(1):215–223 [DOI] [PubMed] [Google Scholar]
- 160.Chen TF, Lin CC, Chen YF, et al. Diffusion tensor changes in patients with amnesic mild cognitive impairment and various dementias. Psychiatry Res 2009;173(1):15–21 [DOI] [PubMed] [Google Scholar]
- 161.Choi SJ, Lim KO, Monteiro I, Reisberg B. Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer’s disease: a preliminary study. J Geriatr Psychiatry Neurol 2005;18(1):12–19 [DOI] [PubMed] [Google Scholar]
- 162.Chua TC, Wen W, Chen X, et al. Diffusion tensor imaging of the posterior cingulate is a useful biomarker of mild cognitive impairment. Am J Geriatr Psychiatry 2009;17(7):602–613 [DOI] [PubMed] [Google Scholar]
- 163.Damoiseaux JS, Smith SM, Witter MP, et al. White matter tract integrity in aging and Alzheimer’s disease. Hum Brain Mapp 2009;30(4):1051–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Teipel SJ, Reuter S, Stieltjes B, et al. Multicenter stability of diffusion tensor imaging measures: a European clinical and physical phantom study. Psychiatry Res 2011;194(3):363–371 [DOI] [PubMed] [Google Scholar]
- 165.Agosta F, Pievani M, Sala S, et al. White matter damage in Alzheimer disease and its relationship to gray matter atrophy. Radiology 2011;258(3):853–863 [DOI] [PubMed] [Google Scholar]
- 166.Canu E, McLaren DG, Fitzgerald ME, et al. Microstructural diffusion changes are independent of macrostructural volume loss in moderate to severe Alzheimer’s disease. J Alzheimers Dis 2010;19(3):963–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology 2000;55(2):210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kantarci K, Petersen RC, Boeve BF, et al. DWI predicts future progression to Alzheimer disease in amnestic mild cognitive impairment. Neurology 2005;64(5):902–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fellgiebel A, Müller MJ, Wille P, et al. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging 2005;26(8):1193–1198 [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y, Schuff N, Du AT, et al. White matter damage in frontotemporal dementia and Alzheimer’s disease measured by diffusion MRI. Brain 2009;132(Pt 9):2579–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O’Dwyer L, Lamberton F, Bokde AL, et al. Multiple indices of diffusion identifies white matter damage in mild cognitive impairment and Alzheimer’s disease. PLoS ONE 2011;6(6):e21745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Koikkalainen J, Lötjönen J, Thurfjell L, et al. Multi-template tensor-based morphometry: application to analysis of Alzheimer’s disease. Neuroimage 2011;56(3):1134–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jagust W, Thisted R, Devous MD, Sr, et al. SPECT perfusion imaging in the diagnosis of Alzheimer’s disease: a clinical-pathologic study. Neurology 2001;56(7):950–956 [DOI] [PubMed] [Google Scholar]
- 174.Johnson KA, Jones K, Holman BL, et al. Preclinical prediction of Alzheimer’s disease using SPECT. Neurology 1998;50(6):1563–1571 [DOI] [PubMed] [Google Scholar]
- 175.Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer’s disease by spin-labeled magnetic resonance imaging. Ann Neurol 2000;47(1):93–100 [PubMed] [Google Scholar]
- 176.Du AT, Jahng GH, Hayasaka S, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology 2006;67(7):1215–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology 2011;77(22):1977–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hu WT, Wang Z, Lee VM, Trojanowski JQ, Detre JA, Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology 2010;75(10):881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tosun D, Mojabi P, Weiner MW, Schuff N. Joint analysis of structural and perfusion MRI for cognitive assessment and classification of Alzheimer’s disease and normal aging. Neuroimage 2010;52(1):186–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology 1993;187(2):433–437 [DOI] [PubMed] [Google Scholar]
- 181.Shonk TK, Moats RA, Gifford P, et al. Probable Alzheimer disease: diagnosis with proton MR spectroscopy. Radiology 1995;195(1):65–72 [DOI] [PubMed] [Google Scholar]
- 182.Schuff N, Amend D, Ezekiel F, et al. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology 1997;49(6):1513–1521 [DOI] [PubMed] [Google Scholar]
- 183.Schuff N, Amend DL, Meyerhoff DJ, et al. Alzheimer disease: quantitative H-1 MR spectroscopic imaging of frontoparietal brain. Radiology 1998;207(1):91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Meyerhoff DJ, MacKay S, Constans JM, et al. Axonal injury and membrane alterations in Alzheimer’s disease suggested by in vivo proton magnetic resonance spectroscopic imaging. Ann Neurol 1994;36(1):40–47 [DOI] [PubMed] [Google Scholar]
- 185.Tedeschi G, Bertolino A, Lundbom N, et al. Cortical and subcortical chemical pathology in Alzheimer’s disease as assessed by multislice proton magnetic resonance spectroscopic imaging. Neurology 1996;47(3):696–704 [DOI] [PubMed] [Google Scholar]
- 186.Jones RS, Waldman AD. 1H-MRS evaluation of metabolism in Alzheimer’s disease and vascular dementia. Neurol Res 2004;26(5):488–495 [DOI] [PubMed] [Google Scholar]
- 187.Jessen F, Block W, Träber F, et al. Decrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patients. Neurology 2001;57(5):930–932 [DOI] [PubMed] [Google Scholar]
- 188.Krishnan KR, Charles HC, Doraiswamy PM, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry 2003;160(11):2003–2011 [DOI] [PubMed] [Google Scholar]
- 189.Jessen F, Traeber F, Freymann K, Maier W, Schild HH, Block W. Treatment monitoring and response prediction with proton MR spectroscopy in AD. Neurology 2006;67(3):528–530 [DOI] [PubMed] [Google Scholar]
- 190.Adalsteinsson E, Sullivan EV, Kleinhans N, Spielman DM, Pfefferbaum A. Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet 2000;355(9216):1696–1697 [DOI] [PubMed] [Google Scholar]
- 191.Kantarci K, Shin C, Britton JW, So EL, Cascino GD, Jack CR., Jr Comparative diagnostic utility of 1H MRS and DWI in evaluation of temporal lobe epilepsy. Neurology 2002;58(12):1745–1753 [DOI] [PubMed] [Google Scholar]
- 192.Shonk T, Ross BD. Role of increased cerebral myo-inositol in the dementia of Down syndrome. Magn Reson Med 1995;33(6):858–861 [DOI] [PubMed] [Google Scholar]
- 193.Parnetti L, Lowenthal DT, Presciutti O, et al. 1H-MRS, MRI-based hippocampal volumetry, and 99mTc-HMPAO-SPECT in normal aging, age-associated memory impairment, and probable Alzheimer’s disease. J Am Geriatr Soc 1996;44(2):133–138 [DOI] [PubMed] [Google Scholar]
- 194.Marjanska M, Curran GL, Wengenack TM, et al. Monitoring disease progression in transgenic mouse models of Alzheimer’s disease with proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A 2005;102(33):11906–11910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sperling RA, Bates JF, Chua EF, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2003;74(1):44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci 2000;12(Suppl 2):24–34 [DOI] [PubMed] [Google Scholar]
- 197.Johnson SC, Schmitz TW, Moritz CH, et al. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging 2006;27(11):1604–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rombouts SA, Goekoop R, Stam CJ, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage 2005;26(4):1078–1085 [DOI] [PubMed] [Google Scholar]
- 199.Petrella JR, Wang L, Krishnan S, et al. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology 2007;245(1):224–235 [DOI] [PubMed] [Google Scholar]
- 200.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009;63(2):178–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dickerson BC, Salat DH, Greve DN, et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005;65(3):404–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Machulda MM, Ward HA, Borowski B, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 2003;61(4):500–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med 2000;343(7):450–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage 2010;51(3):1242–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gallagher M, Bakker A, Yassa MA, Stark CE. Bridging neurocognitive aging and disease modification: targeting functional mechanisms of memory impairment. Curr Alzheimer Res 2010;7(3):197–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995;34(4):537–541 [DOI] [PubMed] [Google Scholar]
- 207.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005;102(27):9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fleisher AS, Sherzai A, Taylor C, Langbaum JB, Chen K, Buxton RB. Resting-state BOLD networks versus task-associated functional MRI for distinguishing Alzheimer’s disease risk groups. Neuroimage 2009;47(4):1678–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A 2001;98(2):676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25(34):7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004;101(13):4637–4642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lustig C, Snyder AZ, Bhakta M, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A 2003;100(24):14504–14509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 2009;29(6):1860–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 2011;76(6):511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang HY, Wang SJ, Liu B, et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology 2010;256(2):598–606 [DOI] [PubMed] [Google Scholar]
- 216.Jones DT, Machulda MM, Vemuri P, et al. Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology 2011;77(16):1524–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Qi Z, Wu X, Wang Z, et al. Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage 2010;50(1):48–55 [DOI] [PubMed] [Google Scholar]
- 218.Sorg C, Riedl V, Mühlau M, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A 2007;104(47):18760–18765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang K, Liang M, Wang L, et al. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp 2007;28(10):967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Machulda M, Avula R, Vemuri P, et al. Examination of default mode network activity along the cognitive continuum: Normal aging, MCI, and AD [abstr]. Alzheimers Dement 2009;5(4 suppl):P268 [Google Scholar]
- 221.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009;62(1):42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 2010;133(Pt 5):1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010;67(6):584–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Filippini N, MacIntosh BJ, Hough MG, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A 2009;106(17):7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Machulda MM, Jones DT, Vemuri P, et al. Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol 2011;68(9):1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 2009;29(40):12686–12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mormino EC, Smiljic A, Hayenga AO, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex 2011;21(10):2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Drzezga A, Becker JA, Van Dijk KR, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 2011;134(Pt 6):1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Ab42. J Neurosci 2010;30(50):17035–17040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen G, Ward BD, Xie C, et al. Classification of Alzheimer disease, mild cognitive impairment, and normal cognitive status with large-scale network analysis based on resting-state functional MR imaging. Radiology 2011;259(1):213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Koch W, Teipel S, Mueller S, et al. Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. Neurobiol Aging 2012;33(3):466–478 [DOI] [PubMed] [Google Scholar]
- 232.Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLOS Comput Biol 2008;4(6): e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sanz-Arigita EJ, Schoonheim MM, Damoiseaux JS, et al. Loss of ‘small-world’ networks in Alzheimer’s disease: graph analysis of FMRI resting-state functional connectivity. PLoS ONE 2010;5(11): e13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34(7):939–944 [DOI] [PubMed] [Google Scholar]
- 235.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999;52(7):1397–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005;65(8):1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zetterberg H, Wahlund LO, Blennow K. Cerebrospinal fluid markers for prediction of Alzheimer’s disease. Neurosci Lett 2003;352(1):67–69 [DOI] [PubMed] [Google Scholar]
- 238.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009;302(4):385–393 [DOI] [PubMed] [Google Scholar]
- 239.Desikan RS, Cabral HJ, Settecase F, et al. Automated MRI measures predict progression to Alzheimer’s disease. Neurobiol Aging 2010;31(8):1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 2009;72(12):1048–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology 2009;73(4):294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fleisher A, Grundman M, Jack CR, Jr, et al. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol 2005;62(6):953–957 [DOI] [PubMed] [Google Scholar]
- 243.DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol 2007;64(1):108–115 [DOI] [PubMed] [Google Scholar]
- 244.Freeborough PA, Fox NC. The boundary shift integral: an accurate and robust measure of cerebral volume changes from registered repeat MRI. IEEE Trans Med Imaging 1997;16(5):623–629 [DOI] [PubMed] [Google Scholar]
- 245.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med 2011;3(111):111cm33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med 2011;17(9):1060–1065 [DOI] [PubMed] [Google Scholar]
- 247.Hyman BT. Amyloid-dependent and amyloid-independent stages of Alzheimer disease. Arch Neurol 2011;68(8):1062–1064 [DOI] [PubMed] [Google Scholar]
- 248.Sperling RA, Jack CR, Jr, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement 2011;7(4):367–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Salloway S, Sperling R, Gilman S, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 2009;73(24):2061–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.McEvoy LK, Edland SD, Holland D, et al. Neuroimaging enrichment strategy for secondary prevention trials in Alzheimer disease. Alzheimer Dis Assoc Disord 2010;24(3):269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 2005;64(9):1563–1572 [DOI] [PubMed] [Google Scholar]
- 252.Fox NC, Cousens S, Scahill R, Harvey RJ, Rossor MN. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol 2000;57(3):339–344 [DOI] [PubMed] [Google Scholar]
- 253.Jack CR, Jr, Slomkowski M, Gracon S, et al. MRI as a biomarker of disease progression in a therapeutic trial of milameline for AD. Neurology 2003;60(2):253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Schott JM, Frost C, Whitwell JL, et al. Combining short interval MRI in Alzheimer’s disease: Implications for therapeutic trials. J Neurol 2006;253(9):1147–1153 [DOI] [PubMed] [Google Scholar]
- 255.Vemuri P, Wiste HJ, Weigand SD, et al. Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology 2010;75(2):143–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Holland D, Brewer JB, Hagler DJ, Fennema-Notestine C, Dale AM; Alzheimer’s Disease Neuroimaging Initiative. Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A 2009;106(49):20954–20959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Leung KK, Clarkson MJ, Bartlett JW, et al. Robust atrophy rate measurement in Alzheimer’s disease using multi-site serial MRI: tissue-specific intensity normalization and parameter selection. Neuroimage 2010;50(2):516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wolz R, Heckemann RA, Aljabar P, et al. Measurement of hippocampal atrophy using 4D graph-cut segmentation: application to ADNI. Neuroimage 2010;52(1):109–118 [DOI] [PubMed] [Google Scholar]
- 259.Hua X, Gutman B, Boyle CP, et al. Accurate measurement of brain changes in longitudinal MRI scans using tensor-based morphometry. Neuroimage 2011;57(1):5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Fox NC, Ridgway GR, Schott JM. Algorithms, atrophy and Alzheimer’s disease: cautionary tales for clinical trials. Neuroimage 2011;57(1):15–18 [DOI] [PubMed] [Google Scholar]
- 261.Hua X, Lee S, Yanovsky I, et al. Optimizing power to track brain degeneration in Alzheimer’s disease and mild cognitive impairment with tensor-based morphometry: an ADNI study of 515 subjects. Neuroimage 2009;48(4):668–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A 2001;98(6):3334–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008;372(9634):216–223 [DOI] [PubMed] [Google Scholar]
- 264.Rinne JO, Brooks DJ, Rossor MN, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 2010;9(4):363–372 [DOI] [PubMed] [Google Scholar]
- 265.Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56(9):1154–1166 [DOI] [PubMed] [Google Scholar]
- 266.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 1992;55(10):967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology 1992;42(1):183–188 [DOI] [PubMed] [Google Scholar]
- 268.Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer’s disease. Lancet 1996;348(9020):94–97 [DOI] [PubMed] [Google Scholar]
- 269.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15(1):273–289 [DOI] [PubMed] [Google Scholar]
- 270.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14(1):11–22 [DOI] [PubMed] [Google Scholar]
- 271.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9(2):179–194 [DOI] [PubMed] [Google Scholar]
- 272.Brewer JB, Magda S, Airriess C, Smith ME. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer disease. AJNR Am J Neuroradiol 2009;30(3):578–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Csernansky JG, Wang L, Joshi S, et al. Early DAT is distinguished from aging by high-dimensional mapping of the hippocampus. Dementia of the Alzheimer type. Neurology 2000;55(11):1636–1643 [DOI] [PubMed] [Google Scholar]
- 274.Davatzikos C, Xu F, An Y, Fan Y, Resnick SM. Longitudinal progression of Alzheimer’s-like patterns of atrophy in normal older adults: the SPARE-AD index. Brain 2009;132(Pt 8):2026–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fan Y, Shen D, Davatzikos C. Classification of structural images via high-dimensional image warping, robust feature extraction, and SVM. Med Image Comput Comput Assist Interv 2005;8(Pt 1):1–8 [DOI] [PubMed] [Google Scholar]
- 276.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage 2008;39(3):1186–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Stonnington CM, Tan G, Klöppel S, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer’s disease. Neuroimage 2008;39(3):1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Klöppel S, Stonnington CM, Chu C, et al. Automatic classification of MR scans in Alzheimer’s disease. Brain 2008;131(Pt 3):681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Vemuri P, Simon G, Kantarci K, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: Differential-STAND. Neuroimage 2011;55(2):522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27(4):685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Jack CR, Jr, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement 2010;6(3):212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jack CR, Jr, Barkhof F, Bernstein MA, et al. Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer’s disease. Alzheimers Dement 2011;7(4):474–485.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]