Abstract
The ability to learn, store, and retrieve information about relationships is impaired in schizophrenia. Here, we tested 38 control and 61 schizophrenia subjects for their ability to identify the novel pairing of stimuli, based on associations learned during training. Subjects were trained on 3 sets of paired associates: 30 face-house pairs (H-F1), 30 face-house pairs (H-F2, same house with new face), and 30 face-face pairs (F3-F4). After training, participants were tested on the 3 explicitly trained pair types, as well as 30 new face-face pairs (F1-F2), which could only be linked together via the same house during the H-F1/H-F2 training blocks. Of 99 subjects tested, 37 patients with schizophrenia and 36 age-matched healthy control subjects learned the premise pairs and performed the relational memory test. Healthy control subjects were significantly more accurate in identifying the inferential (F1-F2) pairs than the noninferential (F3-F4) pairs. In contrast, schizophrenia patients were equally accurate on inferential and noninferential pairs, providing evidence for a relational memory deficit in schizophrenia. However, the current version of the associative inference paradigm, suggested by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia initiative, has limited feasibility, calling into question the generalizability of the findings for the larger schizophrenia population.
Keywords: relational memory, associative inference, schizophrenia, CNTRICS initiative
Introduction
Cognitive impairment is a core feature of schizophrenia.1–3 It is present at the onset of illness, remains persistent throughout the patient’s lifespan, and predicts social functioning.4–8 Meta-analyses have documented large effect sizes for verbal learning and memory.1,9,10 Within the domain of memory, recent studies have pointed to a specific deficit of relational memory in schizophrenia.11–16 This provides the opportunity for more translational studies of cognitive impairment in schizophrenia because the neural circuitry and cellular mechanisms of relational memory have been elucidated in rodent and nonhuman primate studies.17,18
Relational memory provides the ability to remember names with faces, the locations of various objects or people, and the order in which various events occurred.19 This is in contrast to item-specific memory, ie, the memory for the individual elements of a scene or episode.20 Behavioral and neuroimaging studies support the view that relational and item-specific memory depend upon distinct encoding and retrieval operations within the medial temporal lobe, prefrontal cortex, posterior parietal cortex, and midbrain regions.15,21–29 For example, within the medial temporal lobe, relational memory formation is supported by the hippocampus and item-specific memory formation by the perirhinal and parahippocampal cortices.20,30,31
Here, we employed the associative inference paradigm (AIP), which was suggested by the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative for the study of relational memory in schizophrenia.32 The CNTRICS battery of tasks was chosen from the cognitive neuroscience literature, with the goal to inform the development of treatment targets in patients with schizophrenia.33 Several conceptual and practical challenges arise when translating cognitive neuroscience paradigms for use in schizophrenia patients.34 First, paradigms should distinguish specific from generalized deficits, eg, those due to sedation from medications, decreased motivation, inattentiveness, and poor test taking strategies. Second, the paradigm must be sensitive enough to detect individual and group differences in clinical populations. Finally, the paradigm should be associated with a meaningful outcome in patients, eg, social functioning.35 These conditions appeared to be met for the AIP, which led the CNTRICS investigators to include it in their recommended battery of tests.33
Given the previous evidence that relational memory is impaired in schizophrenia, we hypothesized that schizophrenia patients would be impaired during the recognition of previously learned paired associates and would not benefit from the relational binding of previously learned items, which healthy control subjects can employ to improve their recognition accuracy.
Methods
Subjects
We recruited 68 patients with a diagnosis of schizophrenia or schizoaffective disorder and 43 healthy control subjects. The study protocol was approved by the Vanderbilt University Institutional Review Board. Patients were recruited both from Vanderbilt Psychiatric Hospital and from outpatient clinics in the community. Healthy control subjects were recruited by advertisements from the community. Written informed consent was acquired from all subjects after a detailed explanation of the study procedures, and all subjects were paid for their participation. All subjects were assessed by a trained rater using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition36 and were administered the screen for cognitive impairment in psychiatry (SCIP),37 a brief neuropsychological battery.
Only subjects without a history of significant head injury, major medical or neurological illness, or current alcohol or other substance abuse within the past 3 months were included in the study. Healthy control subjects had no Axis I psychiatric disorder and no history of any major neurological or medical illness, substance abuse, or psychotropic medication use. Twelve subjects were excluded prior to analysis due to computer malfunction (2 patients and 2 control subjects), diagnostic uncertainty (4 patients), and failure to follow task directions (1 patient and 3 control subjects). This left a sample of 38 patients with schizophrenia, 23 patients with schizoaffective disorder, and 38 healthy control subjects. A senior clinician (S.H.) confirmed all psychiatric diagnoses through patient interview, review of all structured interviews, and available hospital records.
Twenty-six subjects were not included in the analysis of the associate inference test because they did not attain the training criterion for the premise pairs, set a priori at 80% accuracy (24 patients and 2 control subjects). The remaining 37 patients (21 with schizophrenia and 16 with schizoaffective disorder) and 36 control subjects were age matched and gender matched and did not differ in parental education (table 1).
Table 1.
Demographics, Clinical Characteristics, and Cognitive Measures of the Subjects. Data Are Shown for 3 Groups: Healthy Controls (HC), Schizophrenia Patients Who Achieved the Training Criterion, ie, Good Learners (SZ GL), and Schizophrenia Patients Who Did Not Achieve the Training Criterion, ie, Poor Learners (SZ PL).
| Characteristic | HC (n = 36), Mean ± SD | SZ (GL)a (n = 37), Mean ± SD | SZ (PL)b (n = 24), Mean ± SD | Analysis: HC vs SZ (GL) |
Analysis: SZ (GL) vs SZ (PL) |
||||
| t | df | P | t | df | P | ||||
| Demographics | |||||||||
| Age | 34.39 ± 11.31 | 38.43 ± 11.5 | 42.88 ± 10.56 | −1.51 | 71 | .136 | −1.51 | 59 | .135 |
| NAART IQ | 114.36 ± 5.34 | 107.86 ± 8.15 | 101.38 ± 9.12 | 3.99 | 69 | <.001 | 2.75 | 54 | .008 |
| Education | 15.78 ± 2.42 | 14.03 ± 2.47 | 11.58 ± 2.06 | 3.06 | 71 | .003 | 4.02 | 59 | <.001 |
| Parental education | 13.85 ± 2.18 | 13.53 ± 3.14 | 11.85 ± 2.36 | 0.50 | 69 | .620 | 2.22 | 57 | .031 |
| Genderc | 17/19 | 17/20 | 11/12 | .01 | 1 | .913 | 0.02 | 1 | .887 |
| Clinical characteristics | |||||||||
| Age of onset | 20.78 ± 7.48 | 25.52 ± 9.60 | −2.14 | 58 | .037 | ||||
| Duration of illness | 17.61 ± 11.18 | 17.80 ± 11.54 | — | — | — | −0.07 | 58 | .948 | |
| HAM-D | 6.86 ± 6.59 | 6.33 ± 5.40 | — | 0.33 | 59 | .743 | |||
| YMRS | 5.32 ± 5.01 | 4.46 ± 4.22 | — | — | — | 0.70 | 59 | .486 | |
| PANSS-positive | 15.84 ± 5.73 | 18.38 ± 6.13 | — | — | — | −1.64 | 59 | .106 | |
| PANSS-negative | 13.92 ± 5.88 | 16.25 ± 7.96 | −1.31 | 59 | .194 | ||||
| PANSS-general | 26.46 ± 6.32 | 30.50 ± 6.78 | — | — | — | −2.37 | 25 | .021 | |
| PANSS-total | 56.22 ± 12.40 | 65.13 ± 16.47 | — | −2.41 | 59 | .019 | |||
| AIMS | 1.67 ± 2.73 | 1.87 ± 2.01 | — | — | — | −0.31 | 59 | .760 | |
| Cognitive measures (z-scores)d | |||||||||
| Verbal learning—immediate | −0.28 ± 1.04 | −1.64 ± 1.74 | −2.90 ± 1.58 | 3.88 | 62 | .001 | 2.81 | 57 | .007 |
| Working memory | 0.07 ± 0.78 | −1.54 ± 1.69 | −2.91 ± 1.64 | 5.06 | 62 | <.001 | 3.09 | 57 | .003 |
| Verbal fluency | 0.28 ± 0.84 | −0.02 ± 0.98 | −0.67 ± 1.14 | 1.32 | 62 | .193 | 2.32 | 57 | .024 |
| Verbal learning—delayed | −0.30 ± 1.00 | −1.13 ± 1.25 | −2.38 ± 1.47 | 2.85 | 62 | .006 | 3.49 | 57 | .001 |
| Processing speed | −0.48 ± 0.95 | −2.12 ± 1.14 | −3.09 ± 1.08 | 6.12 | 32 | <.001 | 3.20 | 56 | .002 |
| Overall mean | −0.14 ± 0.58 | −1.29 ± 0.94 | −2.38 ± 0.99 | 5.99 | 62 | <.001 | 4.26 | 57 | <.001 |
Note: NAART, North American Adult Reading Test; HAM-D, Hamilton Rating Scale for Depression; PANSS, Positive and Negative Syndrome Scale; YMRS, Young Mania Rating Scale; AIMS, Abnormal Involuntary Movement Scale.
SZ (GL) = good learners, all schizophrenia subjects who met the training criterion.
SZ (PL) = poor learners, all schizophrenia subjects who did not meet the training criterion.
Chi-square test performed rather than t-test, and sample sizes provided rather than means and SD.
Data available for 28 of the 36 healthy controls on cognitive measures.
Demographic, clinical, and cognitive characteristics of the sample are presented in table 1. The schizophrenia patients were chronically ill (mean duration of illness: 17.61 ± 11.18 years) and moderately symptomatic (mean Positive and Negative Syndrome Scale [PANSS] total scores: 56.22 ± 12.40). Most schizophrenia patients were medicated at the time of testing with a mean dose in chlorpromazine equivalent: 589.06 mg ± 350.53. Two patients were not taking antipsychotic medication. Patients had significantly fewer years of education (P = .003) and lower estimated verbal Intelligence Quotient (IQ) (P < .001), as measured by the North American Adult Reading Test38 than control subjects.
Apparatus and Stimuli
The stimuli were presented on a personal computer using E-Prime software (version 2.0) (Psychology Software Tools, Inc., Pittsburg, PA), and subjects viewed images on a 17-inch liquid crystal display external monitor. Stimuli included 30 color photographs of houses sized to 190 × 142 pixels and 120 color photographs of faces (60 male and 60 female), centered by the bridge of the nose, sized to 142 × 190 pixels. All face and house images were gathered from online picture databases.
Experimental Task
During training, subjects received explicit training on 3 sets of paired stimuli (figure 1). Subjects completed 4 study-test sessions with house-face pairs (premise pairs H-F1 and H-F2). During study, subjects were instructed to learn 30 house-face pairings, presented for 4 seconds each. A self-paced forced-choice test followed each study session. For H-F1 pairs, subjects were instructed to pick 1 of 2 houses, of equal familiarity, that matched the target face, and for H-F2 pairs, subjects picked 1 of 2 faces of equal familiarity that matched the target house. Subjects received 4 study-test sessions with the first set of house-face pairs (H-F1) followed by identical training with the second set of house-face pairs (H-F2) consisting of the original set of houses paired with a new face of opposite gender. Each house had 2 associated faces (1 male and 1 female). The last part of training consisted of 1 study-test session, in the same manner as before, with a set of novel face-face pairings (F3-F4). Training of the F3-F4 pairs was limited to 1 block, in order to keep accuracy below the ceiling levels of performance, expected for the highly practiced face-house pairs. During test, the previously seen F3-F4 pairs served as a comparison for the novel F1-F2 pairs (see below).
Fig. 1.
Experimental Paradigm. Subjects were trained on 3 sets of paired associates: 30 face-house pairs (H-F1), 30 face-house pairs (H-F2, same house paired with a novel face), and 30 face-face pairs. Each set of 30 pairs was presented for either 4 study-test sessions (face-house pairs) or 1 study-test session (face-face pairs). In each study-test session, subjects first viewed all 30 pairs and then completed an immediate 2 alternative forced-choice test with no feedback. After training, subjects completed a final test on all explicitly trained pairs (H-F1, H-F2, and F3-F4) and an additional set of 30 novel face-face pairs (F1-F2), which could only be linked together via the same house during the H-F1/H-F2 training blocks.
Immediately following the completion of training for all series, subjects were tested on the 3 sets of explicitly trained pairs (H-F1, H-F2, and F3-F4) and a novel set of 30 face-face pairs (F1-F2) (figure 1). The novel face-face set was constructed from the faces of the premise pairs. The correct pair could only be inferred through overlapping representations that included the same house. However, subjects were not explicitly told how to solve the inference pairs, they were only told to pick the matching face. The test was self-paced forced-choice, and pairs were presented in pseudorandom blocks with 6 trials each. The order of blocks ensured that inferential pairs (F1-F2) were shown prior to the corresponding premise pairs, to eliminate possible learning during test.
Statistical Analysis
We used repeated measures ANOVA to test for (1) effects of group (between-subject) as well as block and pair type on accuracy during training, (2) effects of group and face type on accuracy during test for house-face pairs, (3) effects of group and face-face pair types (relational vs nonrelational) on accuracy—the crucial comparison, and (4) same effects as in (2–3) on reaction time.
Results
Accuracy During Training
We determined a priori that each study sample should include at least 35 subjects who can meet the accuracy criterion during training. To achieve this goal, we needed to recruit a substantially larger number of subjects, ie, 38 healthy control subjects and 61 schizophrenia subjects.
Figure 2 displays the accuracy scores during training for those subjects who did achieve the training criterion (n = 36 control subjects and 37 schizophrenia patients) and the schizophrenia subjects who did not achieve the training criterion (n = 24 or 39% of the schizophrenia cohort). Only 2 healthy control subjects did not achieve the training criterion (representing 5% of the control cohort). The 73 subjects who did achieve the training criterion became more accurate throughout the 4 blocks of training (main effect of block: F(3, 71) = 165.38, P < .001) and were more accurate in learning the second set of house-face pairs (H-F2) (main effect of pair type: F(1, 71) = 20.32, P < .001). Overall, healthy control subjects were more accurate than patients (main effect of group: F(1, 71) = 17.34, P < .001). We also found a block by group interaction (F (3, 71) = 7.35, P < .001), reflecting a significant difference between the 2 groups in the slope of the learning curve.
Fig. 2.
Training and Test Accuracy Data (Mean and SE Values) for House-Face Pairs (H-F1 and H-F2) for Healthy Controls (HC), Patients With Schizophrenia Who Met Training Criterion (Good Learners) and Patients With Schizophrenia Who did not Meet Training Criterion (Poor Learners). Test data for the poor learners are not shown.
Accuracy During Test
All subjects who achieved the training criterion were examined for their ability to correctly identify the 2 previously learned house-face pairs (H-F1 and H-F2), the previously learned nonrelational face-face pair (F3-F4), and the novel relational pair (F1-F2) (figure 3). Healthy control subjects were more accurate than the subjects with schizophrenia in recalling the previously learned house-face pairs during test (main effect of group: F(1, 71) = 18.38, P < .001), and both groups were more accurate in correctly identifying the more recently learned pair (H-F2) (main effect of pair type: F(1, 71) = 71.03, P < .001) (figure 2).
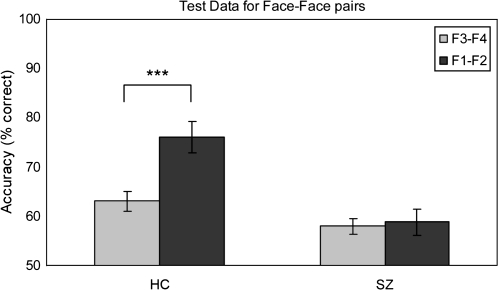
Fig. 3.
Test Accuracy Performance (Mean and SE values) for Face-Face Pairs (Trained F3-F4 Pairs and Novel-Related F1-F2 Pairs) for HC and Schizophrenia Subjects (SZ).
Overall, healthy control subjects were more accurate than patients in identifying both types of the face-face pairs (main effect of group: F(1, 71) = 34.54, P < .001) and performance was higher for F1-F2 pairs than F3-F4 pairs (main effect of pair type: F (1, 71) = 10.61, P < .01) (figure 3). The healthy control subjects were more accurate in correctly matching the novel relational (F1-F2) than the previously seen nonrelational (F3-F4) pairs (mean ± SD (F1-F2) = 76.02 ± 18.84, mean ± SD (F3-F4) = 63.06 ± 11.80; paired sample t-test; t (35) = 3.94, P < .001, Cohen’s d = 0.82). We will refer to this difference in accuracy as the relational memory effect. The schizophrenia patients did not show a relational memory effect (mean ± SD (F1-F2) = 58.83 ± 16.52, mean ± SD (F3-F4) = 58.02 ± 9.73; paired sample t-test; t (36) = .30, P = .76, Cohen’s d = 0.06), resulting in a significant group by condition interaction (F(1, 71) = 8.26, P < .01).
To explore in more detail the relational memory effect, we plotted the accuracy differences between (F1-F2) and (F3-F4) face-face pairs for each subject. The majority of control subjects (25 of 36) but less than half of schizophrenia patients (18 of 37) achieved a higher accuracy on the relational (F1-F2) pairs (figure 4). Using binomial statistics, we identified all subjects who were more accurate on the relational pairs. The cumulative binomial probability of scoring 66.67% correct or higher on this task is .049 (n = 30, P = .5); therefore, this score was used as the cut off for greater than chance performance. Of all the subjects with a positive accuracy difference, 24 of the 25 healthy control subjects but only 10 of the 18 schizophrenia subjects were considered to have achieved greater than chance relational memory performance.
Fig. 4.
Accuracy Differential for Relational (F1-F2) vs Nonrelational (F3-F4) Pairs for HC and Schizophrenia Subjects. Red bars indicate subjects who achieved greater than chance performance (≥66.67%) on the relational condition.
Response Latency During Test
We analyzed response latencies for correct trials only. The response latency was longer for relational pairs (F1-F2) (main effect of condition: F(1, 71) = 57.79, P < .001). Overall, response latencies did not differ between groups (main effect of group: F(1, 71) = 0.88, P = .35), but we found a significant group by condition interaction (F(1, 71) = 5.05,P < .05), driven by nonsignificantly slower responses to relational pairs (t (71) = 1.51, P = .14) and faster responses to nonrelational pairs in healthy control subjects (t (71) = 0.10, P = .92).
Predictors of Performance
The schizophrenia group who did not achieve the training criterion was more cognitively impaired and more symptomatic than the schizophrenia group who successfully learned during training, as assessed by the PANSS general and total scores (table 1). In addition, the 10 schizophrenia subjects with greater than chance relational memory performance demonstrated significantly better working memory ability, as measured by the SCIP, than the 27 subjects with poor relational memory performance (t (34) = 2.5, P = .018).
Discussion
This study of relational memory in schizophrenia is, to the best of our knowledge, the first report of the AIP in a clinical population. There were 3 major findings. First, the published version of the AIP, suggested by the CNTRICS initiative for the study of relational memory in schizophrenia,33 has limited feasibility due to high attrition of subjects in the training phase. Second, the patients with schizophrenia who completed training at the a priori criterion for accuracy on the premise pairs did show the hypothesized relational memory deficit: healthy subjects were more accurate in identifying novel relational pairs compared with previously studied nonrelational pairs, but the schizophrenia patients were not. Third, while the schizophrenia sample as a whole showed a relational memory deficit, a subset of patients did benefit from the relational binding acquired during training and showed normal relational memory performance.
Our study provides a good example for the challenge of translating cognitive paradigms for schizophrenia research.34 In our study, nearly half of the patients were unable to meet the training criterion. The subjects who were able to learn the face-house pairs sufficiently well showed significant increases in accuracy during the 4 training blocks. In contrast, the patients who did not learn up to criterion did not benefit from the repeated presentation of the face-house pairs during training, resulting in flat learning curves (figure 2). As sufficient learning of the paired associates is necessary to reliably evaluate relational memory, these patients could not be tested on the cognitive process of interest, ie, the reactivation and flexible use of representations during retrieval. Even though we are unable to comment further on the nature of their memory deficits, the SCIP data (table 1) indicates that this group did exhibit global cognitive impairments compared with the schizophrenia group who did reach the training criterion, including deficits on tasks of immediate and delayed item memory. The poor learners of the AIP in the schizophrenia group also had lower levels of subject and parental education and were more symptomatic than patients who were able to achieve the training criterion. Future studies of relational memory in schizophrenia should explore variants of the current paradigm in order to increase the feasibility of the AIP. Such attempts should focus on decreasing (1) the total number of face-house pairs to learn and (2) the number of face-house pairs to be learned before testing of relational memory with novel face-face pairs. This needs to be weighed against other parameters, such as the number of trials needed to perform event-related neuroimaging or pharmacological studies. Finally, as subjects were not explictly told how to complete the inference pairs, it is likely that the problem-solving demand contributed to the lower performance of the schizophrenia group. It would be helpful to test whether explict directions for the inference condition would improve inferential performance in schizophrenia subjects. But even improved versions of the current AIP will pose challenges for those who want to use it as an outcome measure for clinical trials because of the long administration time (roughly 75 min) and the implicit nature of the inference task, which is not explicitly stated in the task instructions. Because this is the first study to test the AIP with a clinical population, test-retest reliability is also not known.
Despite the high attrition of patients during the test phase, our results support the hypothesis that relational memory is impaired in schizophrenia. For all test trials, the accurate and inaccurate choice items were equally familiar. For novel inferential pairs (F1-F2), participants must flexibly retrieve the overlapping representations of the previously studied H-F1 and H-F2 pairs. Such flexible, yet accurate, associative inference decisions are the core cognitive mechanism that we attempted to study in our patient sample. We found that the relational memory effect, ie, greater accuracy on (F1-F2) vs (F3-F4) pairs, is significantly decreased in schizophrenia. This adds the AIP to the already existing experimental paradigms that have demonstrated relational memory deficits in schizophrenia.15,16,39–42
The schizophrenia group was not uniformly impaired on the associative inference task. Ten patients with schizophrenia completed all components of the task, including the relational F1-F2 condition. Similar heterogeneity of schizophrenia samples during the performance of memory tasks has been described by others.39,43 We found better working memory performance in those schizophrenia patients who performed well on the AIP. It is tempting to speculate that normal AIP performance in schizophrenia indicates normal prefrontal-hippocampal connectivity, whereas abnormal AIP performance indicates dysfunction in this circuitry. This needs to be explored in future studies.
In addition to the limited generalizability of our findings because of high attrition during training, our study has the additional limitation that all but 2 patients were treated with antipsychotic medication. While we cannot exclude the possibility that relational memory deficits are related to the pharmacological treatment, it is more likely that unmedicated patients would have performed worse during training, limiting feasibility even more. This is supported by previous studies, demonstrating that antipsychotic medication improves, not impairs, memory performance in schizophrenia, and by our own finding, that more clinically ill patients performed worse during training.44–49 However, future studies investigating the AIP in schizophrenia should include first-episode and unmedicated patients.
In conclusion, we examined the integrity of relational memory in schizophrenia using the AIP. While we were able to confirm the hypothesis of a relational memory deficit in schizophrenia, additional work is required to adapt the current version of the AIP for neuroimaging and pharmacological studies in patients with schizophrenia.
Funding
National Institutes of Health (R01 MH070560 to S.H.).
Acknowledgments
The authors would like to thank Dr Anita Must, Dr Neil Woodward, and Chris Shores for their help with this study. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 2.Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: from clinical assessment to genetics and brain mechanisms. Neuropsychol Rev. 2003;13:43–77. doi: 10.1023/a:1023870821631. [DOI] [PubMed] [Google Scholar]
- 3.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 4.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 5.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- 6.Matza LS, Buchanan R, Purdon S, Brewster-Jordan J, Zhao Y, Revicki DA. Measuring changes in functional status among patients with schizophrenia: the link with cognitive impairment. Schizophr Bull. 2006;32:666–678. doi: 10.1093/schbul/sbl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 8.Sponheim SR, Jung RE, Seidman LJ, et al. Cognitive deficits in recent-onset and chronic schizophrenia. J Psychiatr Res. 2010;44:421–428. doi: 10.1016/j.jpsychires.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- 10.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 11.Achim AM, Lepage M. Is associative recognition more impaired than item recognition memory in Schizophrenia? A meta-analysis. Brain Cogn. 2003;53:121–124. doi: 10.1016/s0278-2626(03)00092-7. [DOI] [PubMed] [Google Scholar]
- 12.Danion JM, Huron C, Vidailhet P, Berna F. Functional mechanisms of episodic memory impairment in schizophrenia. Can J Psychiatry. 2007;52:693–701. doi: 10.1177/070674370705201103. [DOI] [PubMed] [Google Scholar]
- 13.Leavitt VM, Goldberg TE. Episodic memory in schizophrenia. Neuropsychol Rev. 2009;19:312–323. doi: 10.1007/s11065-009-9107-0. [DOI] [PubMed] [Google Scholar]
- 14.Lepage M, Montoya A, Pelletier M, Achim AM, Menear M, Lal S. Associative memory encoding and recognition in schizophrenia: an event-related fMRI study. Biol Psychiatry. 2006;60:1215–1223. doi: 10.1016/j.biopsych.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 15.Ongur D, Cullen TJ, Wolf DH, et al. The neural basis of relational memory deficits in schizophrenia. Arch Gen Psychiatry. 2006;63:356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- 16.Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophr Res. 2004;68:235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- 17.Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3:166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- 21.Acuna BD, Eliassen JC, Donoghue JP, Sanes JN. Frontal and parietal lobe activation during transitive inference in humans. Cereb Cortex. 2002;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- 22.Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene AJ, Gross WL, Elsinger CL, Rao SM. An FMRI analysis of the human hippocampus: inference, context, and task awareness. J Cogn Neurosci. 2006;18:1156–1173. doi: 10.1162/jocn.2006.18.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- 25.Hinton EC, Dymond S, von Hecker U, Evans CJ. Neural correlates of relational reasoning and the symbolic distance effect: involvement of parietal cortex. Neuroscience. 2010;168:138–148. doi: 10.1016/j.neuroscience.2010.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Preston AR, Shrager Y, Dudukovic NM, Gabrieli JD. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- 28.Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waltz J, Knowlton B, Holyoak K, et al. A system for relational reasoning in human prefrontal cortex. Psychol Sci. 1999;10:119–125. [Google Scholar]
- 30.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 31.Wan H, Aggleton JP, Brown MW. Different contributions of the hippocampus and perirhinal cortex to recognition memory. J Neurosci. 1999;19:1142–1148. doi: 10.1523/JNEUROSCI.19-03-01142.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ragland JD, Cools R, Frank M, et al. CNTRICS final task selection: long-term memory. Schizophr Bull. 2009;35:197–212. doi: 10.1093/schbul/sbn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barch DM, Carter CS, Arnsten A, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schizophr Bull. 2009;35:109–114. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luck SJ, Gold JM. The translation of cognitive paradigms for patient research. Schizophr Bull. 2008;34:629–644. doi: 10.1093/schbul/sbn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter CS, Barch DM, Buchanan RW, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the SCID-I: Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 37.Purdon SE. The Screen for Cognitive Impairment in Psychiatry (SCIP): Instructions and three alternate forms. Edmonton, Canada: PNL Inc.; 2005. [Google Scholar]
- 38.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 39.Hanlon FM, Weisend MP, Yeo RA, et al. A specific test of hippocampal deficit in schizophrenia. Behav Neurosci. 2005;119:863–875. doi: 10.1037/0735-7044.119.4.863. [DOI] [PubMed] [Google Scholar]
- 40.Rowland LM, Griego JA, Spieker EA, Cortes CR, Holcomb HH. Neural changes associated with relational learning in schizophrenia. Schizophr Bull. 2010;36:496–503. doi: 10.1093/schbul/sbq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elvevag B, Egan MF, Goldberg TE. Paired-associate learning and memory interference in schizophrenia. Neuropsychologia. 2000;38:1565–1575. doi: 10.1016/s0028-3932(00)00074-9. [DOI] [PubMed] [Google Scholar]
- 42.Williams LE, Must A, Avery S, et al. Eye-movement behavior reveals relational memory impairment in schizophrenia. Biol Psychiatry. 2010;68:617–624. doi: 10.1016/j.biopsych.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman MJ, Titone D, Krastoshevsky O, et al. Reinforcement ambiguity and novelty do not account for transitive inference deficits in schizophrenia. Schizophr Bull. 2010;36:1187–1200. doi: 10.1093/schbul/sbp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Earle-Boyer EA, Serper MR, Davidson M, Harvey PD. Continuous performance tests in schizophrenic patients: stimulus and medication effects on performance. Psychiatry Res. 1991;37:47–56. doi: 10.1016/0165-1781(91)90105-x. [DOI] [PubMed] [Google Scholar]
- 45.Gilbertson MW, van Kammen DP. Recent and remote memory dissociation: medication effects and hippocampal function in schizophrenia. Biol Psychiatry. 1997;42:585–595. doi: 10.1016/S0006-3223(96)00435-0. [DOI] [PubMed] [Google Scholar]
- 46.Gold S, Arndt S, Nopoulos P, O'Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- 47.Jones HM, Brammer MJ, O'Toole M, et al. Cortical effects of quetiapine in first-episode schizophrenia: a preliminary functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:938–942. doi: 10.1016/j.biopsych.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Lahti AC, Holcomb HH, Weiler MA, et al. Clozapine but not haloperidol re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171–178. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- 49.Schuepbach D, Hill SK, Sanders RD, Hell D, Keshavan MS, Sweeney JA. Early treatment-induced improvement of negative symptoms predicts cognitive functioning in treatment-naive first episode schizophrenia: a 2-year followup. Schizophr Bull. 2004;30:837–848. doi: 10.1093/oxfordjournals.schbul.a007136. [DOI] [PubMed] [Google Scholar]