Abstract
Protein misfolding and aggregation is a critically important feature in many devastating neurodegenerative diseases, therefore characterization of the CSF concentration profiles of selected key forms and morphologies of proteins involved in these diseases, including β-amyloid (Aβ) and α-synuclein (a-syn), can be an effective diagnostic assay for these diseases. CSF levels of tau and Aβ have been shown to have great promise as biomarkers for Alzheimer’s disease. However since the onset and progression of many neurodegenerative diseases have been strongly correlated with the presence of soluble oligomeric aggregates of proteins including various Aβ and a-syn aggregate species, specific detection and quantification of levels of each of these different toxic protein species in CSF may provide a simple and accurate means to presymptomatically diagnose and distinguish between these diseases. Here we show that the presence of different protein morphologies in human CSF samples can be readily detected using highly selective morphology specific reagents in conjunction with a sensitive electronic biosensor. We further show that these morphology specific reagents can readily distinguish between post-mortem CSF samples from AD, PD and cognitively normal sources. These studies suggest that detection of specific oligomeric aggregate species holds great promise as sensitive biomarkers for neurodegenerative disease.
Introduction
Neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) will affect an increasing number of people as our population ages. For AD alone, over 5 million Americans currently are living with the disease, with nearly half a million new cases expected each year with total yearly economic costs of over $170 billion.1 Diagnosis of these diseases is challenging as other neurodegenerative diseases such as Lewy Body Dementia (LBD), frontotemporal dementia and vascular dementia may share similar symptoms, but have different mechanisms and pathology.2 Patients in early stages of dementia are often classified as having Mild-Cognitive Impairment (MCI), a term that describes early, nondisabling cognitive disorders.3,4 Although MCI describes a transitional state between normal aging and dementia, not all MCI cases progress to dementia.4,5 Pathological changes associated with these different dementias have been shown to occur long before symptoms are evident,6 suggesting that an appropriate set of biomarkers would have great promise to study toxic mechanisms and pathways in these different diseases and to facilitate early diagnoses.
Numerous studies have looked at key biomarkers for diagnosing neurodegenerative diseases, especially AD (reviewed in ref. 7) where levels of tau, phosphorylated tau and amyloid-beta 42 (Aβ42) have shown promise for predicting AD. A recent study using these biomarkers correctly identified 94% of autopsy verified AD cases and also accurately predicted (100%sensitivity) which MCI cases would progress to AD.8 These very promising studies indicate that CSF biomarkers can be a valuable tool to both facilitate diagnosis of neurodegenerative disease and to assess effectiveness of different therapeutic strategies.
While CSF protein biomarkers such as tau and Aβ hold promise as diagnostics for neurodegenerative diseases, a better more selective diagnostic biomarker set can potentially be obtained by detecting specific toxic protein species that are associated with each disease. Since many neurodegenerative diseases are correlated with misfolding and aggregation of different target proteins; amyloid-beta (Aβ) with AD, alpha-synuclein (a-syn) with PD, LBD and other synucleinopathies, and tau with AD and various tauopathies, specific detection and quantification of levels of each of these different toxic protein species may provide a means study mechanisms of toxicity and progression in these diseases and to presymptomatically diagnose and distinguish between these diseases. For example, a vast amount of literature implicates Aβ accumulation and plaque formation as being central to the progression of AD,9 however, while the presence of amyloid plaques does not correlate well with the progression of AD,10,11 the presence of various different soluble Aβ species does.12,13 Similarly, soluble aggregate forms of tau have been correlated with AD14–16 and soluble forms of a-syn have also been correlated with PD.17,18
Since protein misfolding and aggregation are closely associated with many neurodegenerative diseases, determining the CSF concentration profiles of selected key forms and morphologies of proteins involved in these diseases, including Aβ and a-syn, can facilitate development of an effective diagnostic assay to help study these diseases. Since specific soluble aggregate morphologies of these proteins are likely present at only very low concentrations in CSF, in order to detect and quantify levels of these proteins, highly selective reagents that specifically recognize each of the target species are needed along with a sensitive biosensor system. We have used novel protocols to isolate reagents that bind specific morphologies of target proteins by combining the imaging capabilities of AFM with the binding diversity of phage display antibody technology.19–23 These morphology specific reagents are ideal candidates to determine whether specific aggregate species of Aβ and a-syn can be detected in CSF and whether they have potential as markers to help study and diagnose neurodegenerative diseases. We have also designed and developed a sensitive label-free biosensor technology operating on the principle of electrochemical impedance spectroscopy that is well suited to identify protein biomarkers in clinical samples.24–26 The biosensor has several features that are ideally suited for detecting low concentrations of specific protein morphologies in human samples. First it uses a label-free technology so only a single binding event and no modification of target antigen are needed. Second, the nanoscale array includes a porous filter to prevent cells and other large material from blocking the antibody surface and to confine the target antigen in the porous wells. Third, the sensor can determine antigen concentrations over large ranges with detection limits down to low femtomolar or even attomolar levels. We used the NanoMonitor assay (US patent application number: 20070256941) as a clinical diagnostic tool for simultaneous detection of low nano- and picogram/ml levels of various target antigens in patient serum samples.24–26 The high sensitivity of the Nanomonitor combined with the high selectivity of the morphology specific nanobodies provides a uniquely powerful tool to determine whether CSF levels of various different aggregate morphologies of Aβ and a-syn have potential as biomarkers to study and diagnose neurodegenerative diseases. Here we show that morphology specific nanobodies in conjunction with an electronic impedence biosensor can readily distinguish post-mortem CSF samples taken from human AD, PD and age matched non-diseased sources.
Materials and methods
In this section, an overview of the biosensor design and operation is given, followed by the experimental methods utilized.
Fabrication of the biosensor device
The biosensor device is comprised of three integrated parts; (a) a printed circuit board platform, (b) a nanoporous alumina membrane and (c) a silicone micro fluidic chamber. The measurement surface for the biosensor device is a printed circuit board platform comprised of inter-digitated working and counter electrodes. The tin oxide electrodes are 800 μm in width, 5mm in length and 800 nm in thickness with rounded edges to minimize fringe effects during the application of a sinusoidal voltage input signal (Fig. 1A).
Fig. 1.
(A) Physical layout of the sensor platform on PCB surface (B) Integration of the alumina membrane (C) Incorporation of silicone chamber and (D) Changes to the electrical double layer within each nanowell due to the binding of the target antigens onto the nanobodies.
A nanoporous alumina membrane is soldered onto the inter-digitated electrodes generating a high density array of nanowells. The membrane is 250 nm thick, has a lateral diameter of 13 mm with pore diameters of 200 nm. The porosity of the membranes varies between 25% and 50%. An alumina membrane was utilized since it offers electrical isolation between each individual nanowell, as well as good biocompatibility (Fig. 1B).
Finally, a circular silicone chamber encloses the nanotextured electrode surface to confine the fluid onto the device surface and prevent evaporation which could lead to electrical signal instability. The chamber has a maximum working volume of 1.6 ml. Thus, the combination of the alumina membrane on a PCB substrate enclosed by a silicone chamber forms an inexpensive bio-sensing device capable of detecting various bio-molecules (Fig. 1C).
Detection methodology – electrochemical impedance spectroscopy (EIS)
The electronic biosensor measures impedance changes to the electrical double layer at the solid–liquid interface within the nanowells induced when target proteins contained in the sample bind to reagents such as antibodies immobilized on the sensor surface. Impedance measurements provide very detailed information about the electrical changes occurring at the conductive or semi-conductive interfaces.
When target antigens bind immobilized antibody inside the nanowells, the double layer capacitance changes due to the change in the surface charge concentrations. Thus, the double layer capacitance directly correlates to the amount of binding taking place at the solid–liquid interface, and the amount of binding is directly proportional to the concentration of the target species. Thus, by characterizing the double layer capacitance, we can get an accurate estimate of the concentration of the target species. The changes to the double layer capacitance can be represented as the measured impedance changes especially at low frequencies (i.e. below 1 kHz). In the sensor configuration used here, redox probes are not used, and it can be assumed that all the conduction occurring at the interface is non-faradaic in nature, so the charge distribution dynamics at the metal—solution interface characterizing the bio-molecular interactions at the surface can be modeled using the Helmholtz–Gouy–Chapman model with Sterns correction.27,28 Since binding of antigens to the nanobodies is free of any bio-chemical mediators, the impedance changes within the electrical double layer (Fig. 1D) is non-Faradaic, and the electrical circuit model of the sensor can be represented as a simple resistive-capacitive (RC) series circuit whose values are extracted by a frequency response analyzer potentiostat.24 For probing the impedance changes to the electrical double layer of the nanowell electrodes, a very small amplitude sinusoidal voltage is applied to the electrochemical system, and the output current response is sensed, the ratio of the applied voltage phasor to the output current phasor is the resulting impedance, which is characterized using a frequency response analyzer (Gamry Industries Reference 600 potentiostat).
Another commonly used electrochemical immuno-sensing technique is the pulsed amperometry. This method involves the immobilization of an immune-reagent component on the electrode transducer and the use of an electrochemical active substance produced by enzymatic reaction for signal generation.67 As simple as this appears, there can be numerous problems associated with an inadequate supply of enzyme inhibitors in the sample, instability of the enzyme over time, irreproducibility of the electrode kinetics for reoxidizing reagent or reducing oxidising agent, redox active interferences which either react at the electrode and/or couple with the reagent couple, and inadequate temperature control.68 The EIS technique eliminates most of these problems since it doesn’t rely on the redox properties of the analyte and doesn’t need an enzyme inhibitor. Another fundamental difference between the two techniques is the sensing mechanism. Amperometry involves detection of ions in the solution by applying a fixed voltage through electrodes, and measuring the current/change in current; whereas EIS involves characterizing the electrical double layer at the electrode by sweeping a range of frequencies, and measuring the current.
Nanobody immobilization to sensor surface
Nanobodies are immobilized onto the electrode sensor surface using a chemical linker. The electrode surface is first amine functionalized using 3-Aminopropyl Triethoxysilane (APTES, 2% in acetone buffer, Thermo Scientific Inc.). A 100 μl aliquot of 2% APTES is applied on the electrode surface and incubated at room temperature for 30 s. Excess APTES was then removed by flowing acetone over the surface. The alumina membrane is then soldered to the silanized electrode surface. Then 3,3′-dithiobis succinimidyl propionate (DSP, Thermo Scientific Inc.) dissolved in DMSO solvent (4 mg ml−1) is used to cross link the nanobodies to the electrode surface. The DSP (thiol linker) is added for 30 min to allow conjugation to the silanized electrode surfaces which form the base of the nanowells. After conjugation of the linker to the nanowell surfaces (primarily to the base of the nanowells), a 40 μl aliquot of the nanobody (1 mg ml−1) is added for 15 min, followed by addition of 40 μl of Bovine Serum Albumin (BSA) (2 mg ml−1) to block any unbound amine sites on the sensor surface. Phosphate buffered saline (1xPBS) is used to prepare antibody aliquots and for the wash steps due to its isotonic properties.
Preparation of CSF samples
Post mortem CSF Samples from AD, PD, MSA and non-diseased autopsy confirmed sources (Table 1) were generously provided by Dr Thomas Beach (Civin Laboratory for Neuropathology, Banner/Sun Health Research Institute, Sun City, AZ). The post-mortem interval for collection of samples is less than three hours. Samples were diluted to one-tenth of their original concentration in 1X PBS buffer and frozen.
Table 1.
Patient CSF sample data sheet giving PMI and diagnosis information
| Sample ID | Race (1 = white) | Gender (M/F) | Expired age | PMI | DX summary |
|---|---|---|---|---|---|
| ND1 | 1 | M | 71 | 3 | Control brain showing only very mild normal aging changes |
| ND2 | 1 | M | 80 | 2.4 | Control brain showing only mild, normal age-related changes |
| ND3 | 1 | M | 65 | 3.5 | Brain within normal limits for age (control brain); Alzheimer type II astrocytosis |
| AD1 | 1 | F | 82 | 4 | Alzheimer’s disease (AD); microscopic foci (2), cerebellar cortical sclerosis |
| AD2 | 1 | F | 57 | 2.16 | Alzheimer’s disease, presenilin 1 gene mutation (AD); Cerebral white matter rarefaction |
| AD3 | 1 | F | 84 | 1.83 | Alzheimer’s disease (AD); Cerebral white matter rarefaction; Etat crible, basal ganglia; Old infarct, right cerebellar hemisphere |
| PD1 | 1 | M | 80 | 2 | Parkinson’s disease (PD); non-diagnostic Alzheimer’s changes |
| PD2 | 1 | M | 70 | 1.83 | Parkinson Disease (PD); Dementia (history) |
| MSA | 1 | M | 83 | 2.16 | Parkinson’s disease (PD); dementia (history); Shy-Drager syndrome (autonomic failure) |
Generously provided by Dr Thomas Beach (Civin Laboratory for Neuropathology, Banner/Sun Health Research Institute, Sun City, AZ).
Expression and purification of nanobodies
D10, D5 and A4 nanobodies were produced and purified essentially as previously described.21,23 D10 was used to calibrate the sensor chip since it binds to all forms of a-syn including monomeric30 facilitating preparation of a-syn standards of known concentration. The D5 nanobody specifically recognizes a small oligomeric a-syn species,22 while A4 specifically recognizes a small oligomeric Aβ species.21 Nanobody specificity was demonstrated by several different assays including time course dot blot, ELISA, western blot and Atomic force microscopy based assays.21,22,31 The supernatant and cell lysate from a 1L culture were combined and concentrated in a tangential flow filter (Millipore) using a 10 kDa filter membrane (Millipore). Concentrated samples were purified using a protein A-Sepharose column (GE healthcare, NJ) as previously described.21 Fractions containing nanobody were pooled, dialyzed against PBS, lyophilized and stored at −20 °C. The purity of the nanobody sample was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 15% polyacrylamide gels (Bio-Rad, Hercules, CA) and western blotting, and the concentration was determined using bicinchonic acid (BCA) protein assay (Pierce, IL).
Sensor calibration
A-syn was prepared and purified in our lab as previously described.22,29 Purified stocks of a-syn were lyophilized and stored at −80 °C. Stocks were first dissolved in DI water and subsequent dilutions were made in Tris buffer (25 mM Tris, 150 mM NaCl, pH 7.4). A 40 μl aliquot of test sample is added, incubated for 10 min, and then impedance measurements are taken.
Results
Operating parameters and detectibility limits of biosensor with nanobodies as capturing agents
Specific aggregate morphologies of a-syn and Aβ are likely to be present in CSF samples only at very low concentrations (nanomolar or less), therefore successful detection of these targets requires a biosensor with very low detection limits. In order to determine the sensitivity of the biosensor, we utilized the D10 nanobody as a capture agent. D10 recognizes all forms of a-syn,30 and therefore we can accurately control target a-syn concentrations. The first step to determine biosensor sensitivity is to determine suitable electrical parameters which will enable detection of bound target using electrochemical impedance spectroscopy.
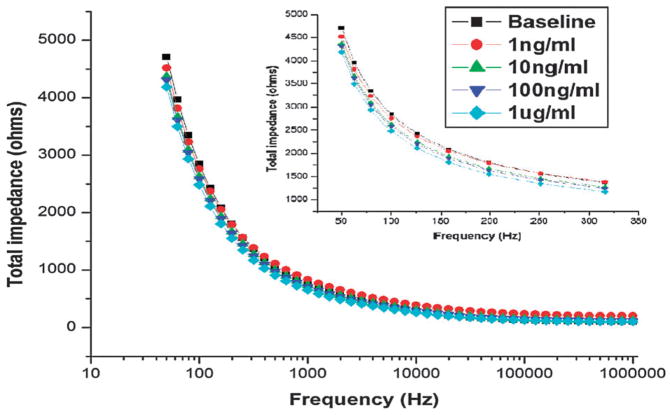
Since binding of antigen to target takes place over a high density array of nano-pores, the impedance signal we obtain correlates to the average signal obtained over all the pores, ensuring that even if some pores do not contain immobilized capture agent the measured impedance will be reproducible within an acceptable margin of error. The dimensions of the electrical double layer within the nanopores is approximately 50 nm. Based on previous studies,24,25 a 100 mv peak to peak pulse should be utilized to characterize changes to the capacitance of the electrical double layer induced by biomolecule binding. The second parameter that needs to be defined is the sensing frequency. Since double layer capacitance dominates the impedance spectrum for frequencies less than 1 KHz in order to determine the optimum frequency for these studies, we tested the frequency response by adding a range of monomeric a-syn concentrations (1 ng ml−1 to 1 ug/ml) to immobilized D10 using a frequency range from 50 Hz to 1MHz. A frequency of 100 Hz gave maximum visible shifts in the impedance induced by biomolecule binding (Fig. 2).
Fig. 2.
Impedance spectrum variation over the frequency range from 50 Hz to 1MHz. The inset shows a zoomed spectrum for various doses at relevant frequencies. 100 Hz showed maximum separation between the spectrums for individual doses, hence, 100 Hz was chosen as the sensing frequency.
Using the defined electrical parameters, we generated a calibration curve using different concentrations of a-syn using two controls; BSA was added to immobilized D10 to test for nonspecific binding to nanobody, and a-syn without immobilized D10 was used to measure background a-syn binding to the sensor surface.
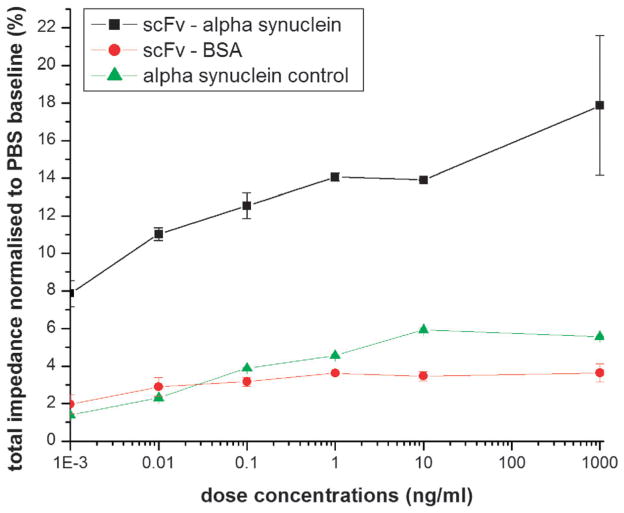
From the calibration curve, we can accurately detect antigen down to a limit of detection (LOD) of 1 picogram ml−1 (Fig. 3) indicating that it should be possible to detect low femtomolar concentrations of target antigen in clinical patient samples. The results also indicate that the target antigen, a-syn, binds to the immobilized nanobody and not the conjugation linker since the signal obtained with antigen binding to immobilized nanobody, even at antigen concentrations six orders of magnitude lower, is higher than the signal observed without nanobody (Fig. 3). The nanobody also specifically reacts with the a-syn target since no signal is observed with the control protein antigen, BSA (Fig. 3).
Fig. 3.
Calibration run of alpha-synuclein in pure samples. The plot also establishes the specificity of the biosensor in identifying the specific interaction between ScFv and alpha-synuclein.
After demonstrating that we can detect femtomolar concentrations of a-syn target using immobilized nanobodies, we next determined whether we could detect specific morphologies of a-syn in post-mortem CSF samples. While we utilized the D10 nanobody, which recognizes all forms of a-syn, for the calibration studies since we could accurately measure the concentration of monomeric a-syn present, in order to detect specific oligomeric forms of a-syn and Aβ in CSF samples, we next utilized two other nanobodies which selectively recognize either a specific oligomeric form of a-syn (D5) or of Aβ (A4). We immobilized either D5 or A4 to sensor chips and then analyzed nine different CSF samples, three taken from 3 AD patients, 3 PD-like (2 PD and one Multiple System Atrophy (MSA) which shares some similar symptoms to PD), and 3 aged matched non-demented control samples (ND). Since all nine samples could not be sequentially analyzed on a single chip, we divided the samples into groups of three, where each group contained one ND, one AD and one PD-like sample.
The experiments were repeated several times and performed simultaneously utilizing multiple sensor chips. In order to control for inter-sensor variability associated with the sensor manufacturing process where there is heterogeneous integration of multiple materials, we utilized a common sample in each data set (AD1) performed on each chip, and normalized data from different chips to this common reference point. An illustrative example of the comparison of percentage change in impedance values obtained in the raw data compared to normalized data is shown in Table 2. The percentage change in impedance values after normalization to a common reference point are shown for all nine CSF samples obtained with immobilized D5 (Fig. 4A) and A4 (Fig. 4B).
Table 2.
Percentage change in impedance from baseline for the D5 antibody. The normalized data set represents the raw data set normalized to group 1 AD reference point
| Raw data set
|
Normalized data set
|
|||||
|---|---|---|---|---|---|---|
| ND | AD | PD | ND | AD | PD | |
| Group 1 | 4.787248 | 6.108411 | 7.383063 | 4.787248 | 6.108411 | 7.383063 |
| Group 2 | 9.754458 | 15.3544 | 16.84676 | 3.815685 | 6.006235 | 6.590008 |
| Group 3 | 5.5921 | 7.9426 | 6.1691 | 2.871514 | 4.078518 | 3.16783 |
Note: All numbers are percentage change (%).
Fig. 4.
(A) Normalized data with D5 antibody (B) Normalized data with A4 antibody (C) Qualitative representation of the data.
Oligomeric species of both a-syn and Aβ are readily detected in post-mortem CSF samples even when assayed at 10-fold dilution (Fig. 4A and B). The results indicate that the levels of oligomeric a-syn and Aβ species in CSF varies depending on the disease, and that these oligomeric species have great promise as biomarkers for distinguishing between different neurodegenerative diseases.
Discussion
CSF levels of Aβ, tau and phosphorylated tau were shown to have promise as biomarkers for diagnosing AD.7,8 However increasing evidence indicate that various soluble aggregated oligomeric forms of Aβ, a-syn and tau are the relevant toxic species in different neurodegenerative diseases, and specific detection of different aggregate species in CSF may provide a more refined and powerful tool to facilitate early and accurate diagnosis of a variety of neurodegenerative diseases and to study the mechanisms involved in the onset and progression of these diseases. Protein aggregation is a common thread behind numerous neurodegenerative diseases including AD, PD, LBD, tauopathies and synucleinopathies. Aggregation of Aβ has been correlated with AD, aggregation of a-syn with PD, LBD and other synucleinopathies, and aggregation of tau with AD and various tauopathies. While the presence of fibrillar aggregates of these different proteins has been a classic diagnostic feature of the respective diseases, increasing evidence suggests that soluble oligomeric forms of these proteins are the relevant toxic species. During the polymerization process from monomeric to fibrillar form, each of the protein species must pass through different oligomeric states, suggesting that various oligomeric species may represent earlier biomarkers for these diseases compared to the presence of fibrillar forms.
A rapidly growing body of evidence indicates that oligomeric forms of Aβ are key factors in the onset and progression of AD. Aβ forms a number of soluble intermediate or metastable structures which may contribute to toxicity (reviewed in ref. 32 and 33). Cortical levels of soluble Aβ correlate well with the cognitive impairment and loss of synaptic function.12,13 Small, soluble spherical or annular aggregates of Aβ were shown to be neurotoxic,34–36 and oligomeric forms of Aβ, created in vitro or derived from cell cultures inhibit long term potentiation (LTP).35,37,38 The concentration of oligomeric forms of Aβ are elevated in transgenic mouse models of AD39 and human AD brain40 and CSF samples41 and the presence of an SDS stable dimeric form of Aβ associates well with dementia in AD patients.42 Disruption of neural connections near Aβ plaques was also attributed to oligomeric Aβ species,43 a halo of oligomeric Aβ surrounds Aβ plaques causing synapse loss,44 and oligomeric Aβ was shown to disrupt cognitive function in transgenic animal models of AD.45–47 Different size oligomers of Aβ have been correlated with AD, including a 56 kD aggregate48 and smaller dimeric, trimeric and tetrameric species.32,38,49,50 Therefore specific detection of soluble oligomeric Aβ species holds great promise as a biomarker for studying the progression of AD.
Similarly, formation of oligomeric aggregates of a-syn has also been correlated with PD and other synucleinopathies. A-syn is a major component of Lewy bodies and neurites.51,52 While wild-type and mutant forms of a-syn associated with familial cases of PD, A30P, E46K and A53T, can assemble into Lewy body like fibrils in vitro,53–57 the mutations increase the total rate of oligomerization compared to the wild-type form of a-syn.53,55,58 Different morphologies of a-syn have different affinities for various membranes, and both oligomeric and fibrillar forms59–62 have been shown to disrupt membrane permeability and integrity. Aggregated forms of a-syn were shown to induce toxicity in dopaminergic neurons in vivo63 and several different oligomeric morphologies were shown to each have different toxic mechanisms and effects on cells.64 Oligomeric forms of a-syn were shown to be toxic to neuronal cells,22 and toxic oligomeric a-syn forms were identified in living cells,65 in human plasma from PD patients,18 and in human PD brain tissue.22,23 Elevated levels of soluble oligomeric a-syn species were also detected in post-mortem brain extracts from patients with LBD,17 even though monomeric a-syn levels in CSF were not able to discriminate between LBD and AD.66 Therefore the presence of various oligomeric a-syn species in CSF is also a very promising biomarker for studying the progression of various neurodegenerative diseases.
We developed a novel biosensor for sensitive detection of biomolecules from clinical samples24–26 and show here that the nanomonitor has sufficient sensitivity to detect low femtomolar concentrations of target directly from human CSF samples using single chain antibody fragments or nanobodies as the capture agent. We also developed technology which enables us to isolate nanobodies that selectively recognize specific protein morphologies,19 and have isolated nanobodies that bind two different oligomeric a-syn species,22,23 and two different oligomeric Aβ species.20,21 The different oligomer specific nanobodies do not show cross-reactivity, so the nanobodies binding oligomeric Aβ do not bind oligomeric a-syn and vice versa. The different aggregate species recognized by each of the morphology specific nanobodies can be detected in post-mortem human AD or PD tissue, and can distinguish between AD, PD and healthy brain tissue.20–23 Therefore these nanobodies represent excellent tools to detect specific oligomeric aggregates of Aβ and a-syn in both clinical and animal model samples.
Here we show that detection of an oligomeric Aβ species in CSF samples using the A4 nanobody very sensitively distinguishes the AD CSF samples from the PD and ND samples (Fig. 4B). The oligomeric a-syn species recognized by the D5 nanobody, is present in highest concentrations in the PD samples, lower concentrations in the AD samples and lowest concentrations in the ND and MSA samples (Fig. 4A). Interestingly, the a-syn species recognized by D5 readily distinguishes between the PD and MSA CSF samples indicating the value of this technology in studying disease mechanisms. Using the results obtained with both the A4 and D5 nanobodies, we can clearly distinguish between AD, PD, and ND or MSA samples, where ND cases do not show above threshold levels of target with either nanobody, PD cases are distinguished by positive binding signal with D5, but not A4, and AD cases show a positive signal with A4 and also D5 in some cases (Fig. 4C). In order to determine whether detection of specific oligomeric forms of proteins are useful as biomarkers for studying and diagnosing neurodegenerative disease, a much larger CSF sample size will need to be analyzed using the various differentmorphology specific nanobodies against a-syn22,23 and Aβ.20,21
Conclusion
Diagnosis of neurodegenerative diseases including Alzheimer’s, Dementia with Lewy Bodies, frontotemporal dementia and Parkinson’s diseases is a challenging prospect. Since misfolding and aggregation of proteins including Aβ, a-syn and tau have been correlated with these neurodegenerative diseases, the presence of different forms of these target proteins in clinical samples has great promise as potential biomarkers to both study and diagnose these diseases. CSF levels of different Aβ and tau species can correctly predict progression to AD validating this concept. Since increasing evidence indicates that small soluble oligomeric forms of proteins including Aβ and a-syn are the relevant toxic species associated with neurodegeneration, oligomeric forms of these proteins may be sensitive biomarkers to study the onset and progression of these different neurodegenerative diseases. Here we show that the presence of different protein morphologies in human CSF samples can be readily detected using highly selective morphology specific reagents in conjunction with a sensitive electronic biosensor. We further show that using morphology specific reagents, we can readily distinguish between post-mortem CSF samples from AD, PD and cognitively normal sources. These studies suggest that detection of specific oligomeric aggregate species holds great promise for use as sensitive biomarkers for neurodegenerative disease.
Insight, innovation, integration.
Misfolded and aggregated protein variants contribute to many human diseases including Alzheimer’s and Parkinson’s diseases. Detection of small toxic oligomeric aggregates of proteins such as beta-amyloid or alpha-synuclein in human cerebral spinal fluid (CSF) or serum samples may provide a means to study disease mechanisms and progression and a means to presymtomatically diagnose these diseases. To detect the presence of different protein variants in CSF, we coupled the use of novel reagents that selectively recognize specific protein isoforms with a novel electronic biosensor that has femtomolar sensitivity and is compatible with untreated human samples. We show that the presence of specific oligomeric protein species in post-mortem human CSF samples can readily distinguish between different neurodegenerative diseases, and this technology has potential to facilitate early diagnosis of these diseases.
Acknowledgments
This work was supported by grants from the Arizona Biomedical Research Commission, and the Arizona Department of Health Services for the Arizona Alzheimer’s Consortium, the Michael J. Fox Foundation and Office of Naval Research under grant number N00014-07-1-0457. We are grateful to the Banner/Sun Health Research Institute Brain Donation Program of Sun City, Arizona for the provision of human brain tissue. The Brain Donation Program is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Prescott Family Initiative of theMichael J. Fox Foundation for Parkinson’s Research.
Contributor Information
Michael R. Sierks, Email: sierks@asu.edu.
Shalini Prasad, Email: shalini.prasad@utdallas.edu.
References
- 1.Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6(2):158–94. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, Pickering E, Kuhn M, Chen Y, McCluskey L, Elman L, Karlawish J, Hurtig HI, Siderowf A, Lee VM, Soares H, Trojanowski JQ. Novel CSF biomarkers for Alzheimer’s disease and mild cognitive impairment. Acta Neuropathol. 119(6):669–78. doi: 10.1007/s00401-010-0667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment-beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Cedazo-Minguez A, Winblad B. Biomarkers for Alzheimer’s disease and other forms of dementia: clinical needs, limitations and future aspects. Exp Gerontol. 45(1):5–14. doi: 10.1016/j.exger.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132(Pt 5):1355–65. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu WT, Chen-Plotkin A, Arnold SE, Grossman M, Clark CM, Shaw LM, McCluskey L, Elman L, Karlawish J, Hurtig HI, Siderowf A, Lee VM, Soares H, Trojanowski JQ. Biomarker discovery for Alzheimer’s disease, frontotemporal lobar degeneration, and Parkinson’s disease. Acta Neuropathol. 120(3):385–99. doi: 10.1007/s00401-010-0723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Meyer G, Shapiro F, Vanderstichele H, Vanmechelen E, Engelborghs S, De Deyn PP, Coart E, Hansson O, Minthon L, Zetterberg H, Blennow K, Shaw L, Trojanowski JQ. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch Neurol. 67(8):949–56. doi: 10.1001/archneurol.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 10.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62(11):1087–95. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm Suppl. 1998;53:127–40. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 12.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155(3):853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res. 2006;54(3):197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Brunden KR, Trojanowski JQ, Lee VM. Evidence that non-fibrillar tau causes pathology linked to neurodegeneration and behavioral impairments. J Alzheimers Dis. 2008;14(4):393–9. doi: 10.3233/jad-2008-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meraz-Rios MA, Lira-De Leon KI, Campos-Pena V, De Anda-Hernandez MA, Mena-Lopez R. Tau oligomers and aggregation in Alzheimer’s disease. J Neurochem. 112(6):1353–67. doi: 10.1111/j.1471-4159.2009.06511.x. [DOI] [PubMed] [Google Scholar]
- 17.Paleologou KE, Kragh CL, Mann DM, Salem SA, Al-Shami R, Allsop D, Hassan AH, Jensen PH, El-Agnaf OM. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132(Pt 4):1093–101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- 18.El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20(3):419–25. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 19.Barkhordarian H, Emadi S, Schulz P, Sierks MR. Isolating recombinant antibodies against specific protein morphologies using atomic force microscopy and phage display technologies. Protein Eng, Des Sel. 2006;19(11):497–502. doi: 10.1093/protein/gzl036. [DOI] [PubMed] [Google Scholar]
- 20.Kasturirangan S, Lin L, Emadi S, Boddapati S, Schulz P, Sierks MR. Nanobody specific for oligomeric beta-amyloid stabilizes non-toxic form. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Zameer A, Kasturirangan S, Emadi S, Nimmagadda SV, Sierks MR. Anti-oligomeric Abeta single-chain variable domain antibody blocks Abeta-induced toxicity against human neuroblastoma cells. J Mol Biol. 2008;384(4):917–28. doi: 10.1016/j.jmb.2008.09.068. [DOI] [PubMed] [Google Scholar]
- 22.Emadi S, Barkhordarian H, Wang MS, Schulz P, Sierks MR. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368(4):1132–44. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emadi S, Kasturirangan S, Wang MS, Schulz P, Sierks MR. Detecting morphologically distinct oligomeric forms of alpha-synuclein. J Biol Chem. 2009;284(17):11048–58. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bothara M, Venkatraman V, Reddy RK, Barrett T, Carruthers J, Prasad S. Nanomonitors: electrical immunoassays for protein biomarker profiling. Nanomedicine. 2008;3(4):423–36. doi: 10.2217/17435889.3.4.423. [DOI] [PubMed] [Google Scholar]
- 25.Reddy RK, Bothara MG, Barrett TW, Carruthers J, Prasad S. Nanomonitors: Protein biosensors for rapid analyte analysis. IEEE Sens J. 2008;8(5–6):720–723. [Google Scholar]
- 26.Venkatraman VL, Reddy RK, Zhang FY, Evans D, Ulrich B, Prasad S. Iridium oxide nanomonitors: Clinical diagnostic devices for health monitoring systems. Biosensors & Bioelectronics. 2009;24(10):3078–3083. doi: 10.1016/j.bios.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 27.Chang BY, Park SM. Electrochemical impedance spectroscopy. Annu Rev Anal Chem. 3:207–29. doi: 10.1146/annurev.anchem.012809.102211. [DOI] [PubMed] [Google Scholar]
- 28.Lisdat F, Schafer D. The use of electrochemical impedance spectroscopy for biosensing. Anal Bioanal Chem. 2008;391(5):1555–67. doi: 10.1007/s00216-008-1970-7. [DOI] [PubMed] [Google Scholar]
- 29.Volles MJ, Lansbury PT., Jr Relationships between the sequence of alpha-synuclein and its membrane affinity, fibrillization propensity, and yeast toxicity. J Mol Biol. 2007;366(5):1510–22. doi: 10.1016/j.jmb.2006.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C, Emadi S, Sierks MR, Messer A. A human single-chain Fv intrabody blocks aberrant cellular effects of overexpressed alpha-synuclein. Mol Ther. 2004;10(6):1023–31. doi: 10.1016/j.ymthe.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 31.Wang MS, Zameer A, Emadi S, Sierks MR. Characterizing Antibody Specificity to Different Protein Morphologies by AFM. Langmuir. 2008 doi: 10.1021/la8025914. [DOI] [PubMed] [Google Scholar]
- 32.Selkoe DJ. Alzheimer disease: mechanistic understanding predicts novel therapies. Ann Intern Med. 2004;140(8):627–38. doi: 10.7326/0003-4819-140-8-200404200-00047. [DOI] [PubMed] [Google Scholar]
- 33.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44(1):181–93. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274(36):25945–52. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 35.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper JD, Wong SS, Lieber CM, Lansbury PTJ. Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 37.Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924(2):133–40. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 39.Chang L, Bakhos L, Wang Z, Venton DL, Klein WL. Femtomole immunodetection of synthetic and endogenous amyloid-beta oligomers and its application to Alzheimer’s disease drug candidate screening. J Mol Neurosci. 2003;20(3):305–13. doi: 10.1385/JMN:20:3:305. [DOI] [PubMed] [Google Scholar]
- 40.Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer’s disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100(18):10417–22. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102(7):2273–6. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 133(Pt 5):1328–41. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai J, Grutzendler J, Duff K, Gan WB. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat Neurosci. 2004;7(11):1181–3. doi: 10.1038/nn1335. [DOI] [PubMed] [Google Scholar]
- 44.Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009;106(10):4012–7. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 46.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of Secreted Oligomers of Amyloid {beta}-Protein on Hippocampal Synaptic Plasticity: A Potent Role for Trimers. J Physiol. 2006;572(2):477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh DM, Klyubin I, Shankar GM, Townsend M, Fadeeva JV, Betts V, Podlisny MB, Cleary JP, Ashe KH, Rowan MJ, Selkoe DJ. The role of cell-derived oligomers of Abeta in Alzheimer’s disease and avenues for therapeutic intervention. Biochem Soc Trans. 2005;33(Pt 5):1087–90. doi: 10.1042/BST20051087. [DOI] [PubMed] [Google Scholar]
- 48.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 49.Klein WL. Synaptic targeting by Abeta oligomers (ADDLS) as a basis for memory loss in early Alzheimer’s disease. Alzheimer’s Dementia. 2006;2:43–55. doi: 10.1016/j.jalz.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Walsh DM, Selkoe DJ. Abeta Oligomers—a decade of discovery. J Neurochem. 2007;101(5):1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 51.Mezey E, Dehejia AM, Harta G, Tresser N, Suchy SF, Nussbaum RL, Brownstein MJ, Polymeropoulos MH. Alpha synuclein is present in Lewy bodies in sporadic Parkinson’s disease [see comments] [published erratum appears in Mol Psychiatry 1999 Mar;4(2):197] Mol Psychiatry. 1998;3(6):493–9. doi: 10.1038/sj.mp.4000446. [DOI] [PubMed] [Google Scholar]
- 52.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies [letter] Nature. 1997;388(6645):839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 53.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4(11):1318–20. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 54.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human alpha-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274(12):7619–22. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 55.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson’s disease mutations accelerate alpha-synuclein aggregation [published erratum appears in J. Biol. Chem. 1999 May 7;274(19):13728] J Biol Chem. 1999;274(14):9843–6. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 56.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry. 2000;39(10):2552–63. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 57.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55(2):164–73. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 58.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson’s disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97(2):571–6. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41(14):4595–602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 60.Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40(26):7812–9. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 61.Lashuel H, Petre B, Wall J, Simon M, Nowak R, Walz T, Lansbury P. alpha-Synuclein, Especially the Parkinson’s Disease-associated Mutants, Forms Pore-like Annular and Tubular Protofibrils. J Mol Biol. 2002;322(5):1089. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 62.Zhu M, Li J, Fink AL. The association of alpha-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278(41):40186–97. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 63.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27(12):3338–46. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Danzer KM, Haasen D, Karow AR, Moussaud S, Habeck M, Giese A, Kretzschmar H, Hengerer B, Kostka M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27(34):9220–32. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Outeiro TF, Putcha P, Tetzlaff JE, Spoelgen R, Koker M, Carvalho F, Hyman BT, McLean PJ. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One. 2008;3(4):e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noguchi-Shinohara M, Tokuda T, Yoshita M, Kasai T, Ono K, Nakagawa M, El-Agnaf OM, Yamada M. CSF alpha-synuclein levels in dementia with Lewy bodies and Alzheimer’s disease. Brain Res. 2009;1251:1–6. doi: 10.1016/j.brainres.2008.11.055. [DOI] [PubMed] [Google Scholar]
- 67.Lei CX, Gong FC, et al. Amperometric immunosensor for Schistosoma japonicum antigen using antibodies loaded on a nano-Au monolayer modified chitosan-entrapped carbon paste electrode. Sens Actuators, B. 2003;96(3):582–588. [Google Scholar]
- 68.Kissinger PT. Introduction toAmperometric Biosensor Configuration. CURRENTSEPARATIONSCOM and Drug Development. 1997;16(3) [Google Scholar]