Abstract
Mesenchymal stem cells (MSC) derived from bone marrow can potentially reduce the acute inflammatory response in spinal cord injury (SCI) and thus promote functional recovery. However, the precise mechanisms through which transplanted MSC attenuate inflammation after SCI are still unclear. The present study was designed to investigate the effects of MSC transplantation with a special focus on their effect on macrophage activation after SCI. Rats were subjected to T9–T10 SCI by contusion, then treated 3 days later with transplantation of 1.0×106 PKH26-labeled MSC into the contusion epicenter. The transplanted MSC migrated within the injured spinal cord without differentiating into glial or neuronal elements. MSC transplantation was associated with marked changes in the SCI environment, with significant increases in IL-4 and IL-13 levels, and reductions in TNF-α and IL-6 levels. This was associated simultaneously with increased numbers of alternatively activated macrophages (M2 phenotype: arginase-1- or CD206-positive), and decreased numbers of classically activated macrophages (M1 phenotype: iNOS- or CD16/32-positive). These changes were associated with functional locomotion recovery in the MSC-transplanted group, which correlated with preserved axons, less scar tissue formation, and increased myelin sparing. Our results suggested that acute transplantation of MSC after SCI modified the inflammatory environment by shifting the macrophage phenotype from M1 to M2, and that this may reduce the effects of the inhibitory scar tissue in the subacute/chronic phase after injury to provide a permissive environment for axonal extension and functional recovery.
Key words: bone marrow, macrophage, mesenchymal stem cell, spinal cord injury, transplantation
Introduction
Traumatic spinal cord injury (SCI) can cause neural tissue damage and sets off a series of events resulting in additional tissue loss. The cascade of secondary tissue damage following SCI includes an inflammatory response marked by infiltration of neutrophils and macrophages, along with resident microglia, activation of glial cells, and upregulated expression of proinflammatory cytokines (Popovich et al., 2002; Taoka et al., 1997). These intrinsic responses to tissue injury contribute to an environment that is inhibitory to axonal regrowth (Ramer et al., 2000), and could lead to an increase in cavitation and cyst formation at the center of the lesion, exacerbating neurological dysfunction (Carlson et al., 1998). One of the most promising therapeutic approaches for SCI is cell transplantation (Murray, 2004). A number of different cell types have been evaluated; these include adult mesenchymal stem cells derived from the bone marrow (MSC). MSC have been shown to promote anatomical and functional recovery in animal models of SCI by promoting tissue sparing (Himes et al., 2006; Sheth et al., 2008), and axonal regeneration (Wu et al., 2003). The therapeutic benefits of MSC are thought to be primarily related to their secretion of soluble factors and the provision of an extracellular matrix that provides neural protection and support, and secondarily to remyelination (Akiyama et al., 2002a,2002b), and neural differentiation (Ankeny et al., 2004; Zurita et al., 2008).
MSC are attractive candidates for transplantation into patients because they can easily be harvested, expanded, and stored, or obtained directly from the patient, allowing for autologous transplantation, avoiding the immunological and ethical problems associated with transplantation of other types of stem cells such as embryonic stem cells (McDonald et al., 1999) or neural stem cells (Ogawa et al., 2002). Evidence suggests that MSC are immunosuppressive (Di Nicola et al., 2002; Jiang et al., 2005), i.e., they possess anti-inflammatory activity and have been used to abrogate graft-versus-host disease (Wright et al., 2011). This property, in particular, may contribute to their capacity to reduce the acute inflammatory response to SCI and hence reduce cavity formation, as well as decrease astrocyte and microglia/macrophage reactivity (Himes et al., 2006; Neuhuber et al., 2005). Importantly, MSC transplantation in experimental SCI is reported to enhance tissue preservation after SCI, and reduce injury-induced sensitivity to mechanical stimuli, which is functionally indicative of anti-inflammatory activity (Abrams et al., 2009).
Considering the inflammatory response after SCI, different macrophage populations have been found locally, based on their phenotypes and activity, but two subtypes have become of great interest; classically-activated macrophages (termed the M1 phenotype), or alternatively-activated macrophages (M2 phenotype; Busch et al., 2011; Gordon, 2003; Laskin, 2009; Mantovani et al., 2004; Popovich et al., 1999; Schwartz et al., 1999). Classically-activated macrophages are the product of exposure to T-helper 1 (Th-1) cytokines (e.g., interferon-γ [IFN-γ] and tumor necrosis factor-α [TNF-α]). Alternatively-activated macrophages are the product of Th-2 cytokines (e.g., interleukin [IL]-4 and IL-13; Gordon, 2003; Ma et al., 2003).
Alternatively-activated macrophages do not only have enhanced phagocytic capacity, but they also possess anti-inflammatory activity, and hence have beneficial effects during recovery after SCI (Bomstein et al., 2003; Busch et al., 2011; Kigerl et al., 2009; Schwartz, 2010). MSC transplantation into SCI lesions also attenuates acute inflammation and this is beneficial to functional recovery following SCI. However, the precise mechanisms through which the transplanted MSC attenuate the inflammation after SCI are still unclear. The present study was designed to investigate the effects of MSC transplantation in rats with SCI. Specifically, we focused on the potential effects of MSC on alternatively-activated macrophages after SCI.
Methods
Preparation of MSC
Commercially obtained human MSC (lot. #PT-2501; Lonza, Walkersville, MD), which had a CD immunoprofile consistent with the MSC phenotypes (CD105+, CD166+, CD29+, CD44+, CD14−, CD34−, and CD45−), were cultured at 37°C in a humidified atmosphere of 5% CO2 in MSC basal medium (MSCBM) with 10% fetal calf mesenchymal cell growth supplement, 200 mM L-glutamine, 25 units penicillin, and 25 μg streptomycin (lot. #PT-3001; Lonza). When the proliferating colonies had reached near-confluence, the adherent cells were lifted by incubation in 0.25% trypsin solution for 5–10 min. After 3–4 passages, MSC were used for transplantation. For in vivo tracing, the MSC were pre-labeled with the membrane dye PKH26 according to the instructions provided by the manufacturer (Sigma-Aldrich, St. Louis, MO).
Animal model of spinal cord injury
Experiments were conducted in 57 adult male Sprague-Dawley rats, aged 8–10 weeks, with a mean body weight of 271±29.1 g (±SD). Following anesthesia using isoflurane (Forane®; Abbot, Tokyo, Japan), laminectomy was performed at the T10 level under a surgical microscope (VANOX-S; Olympus, Tokyo, Japan), taking utmost care to avoid dura matter laceration. At the T9–T10 vertebral level, the dorsal surface of the spinal cord was compressed extradurally using the Infinite Horizons Impactor (Precision Systems and Instrumentation LLC, Fairfax, VA), with an impact force of 200 kilodynes (kdyn). All rats were housed under a 12-h light-dark cycle in a bacteria-free biologically clean room, and all had free access to food and water ad libitum. They also received manual bladder expression twice daily until recovery of sphincter control. The experimental protocol was approved by the Ethics Committee for Animal Experimentation of Fukui University.
MSC transplantation
Three days after the injury, MSC were pre-labeled with the membrane dye PKH26, then 1.0×106 MSC in 5 μL MSCBM were injected into the contusion epicenter using a microsyringe in the treatment group. The control rats were injected with 5 μL of MSCBM alone (no cells) at 3 days after SCI. To evaluate the survival rate and the distribution of transplanted MSC, we examined midsagittal sections prepared from the injured portion of the spinal cord of three rats selected at random. The PKH26-positive area was calculated by a color image analyzer (MacSCOPE; Mitani, Fukui, Japan), and expressed as the relative area at 1 and 5 weeks compared with that at 3 days after SCI.
Immunofluorescence staining
Following injection of MSC or MSCBM, the rat spinal cord was perfused and fixed with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) and post-fixed in the same fixative (24 h), 10% sucrose in 0.1 M PBS (24 h), and 20% sucrose in 0.1 M PBS (24 h). Segments of the spinal cord (between the T8 and T12 cord segments) were embedded in optimal cutting temperature compound (OCT) and cut on a cryostat into serial 25-μm-thick sagittal frozen sections (n=21). For immunofluorescence staining, the sections were incubated at 4°C with anti-neuronal nuclei (NeuN) monoclonal antibody (1:400, mouse IgG; Chemicon International, Temecula, CA) for neurons, anti-reactive immunology protein (RIP) monoclonal antibody (1:400, rabbit IgG; Abcam plc, Cambridge, U.K.) as a mature oligodendrocyte-specific marker, anti-glial fibrillary acidic protein (GFAP) monoclonal antibody (1:500, rabbit IgG; Abcam plc) for astrocytes, anti-microglia/macrophage monoclonal antibody (OX42, CD11b, 1:100, mouse IgG; GeneTex, Irvine, CA) for microglia/macrophages, anti-iNOS polyclonal antibody (1:200, mouse IgG; Abcam plc), anti-CD16/32 polyclonal antibody (1:200, mouse IgG; Abcam plc), anti-arginase-1 polyclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD206 polyclonal antibody (1:200; Santa Cruz Biotechnology), anti-growth-associated protein (GAP)-43 polyclonal antibody (GAP-43, 1:500, rabbit IgG; Abcam plc), or monoclonal anti-neurofilament 200 kD (RT97, 1:1000, rabbit IgG; Abcam plc) diluted in Antibody Diluent with Background Reducing Components (Dako Cytomation, Carpinteria, CA). The secondary antibodies were donkey anti-goat Alexa-Fluor® 488-conjugated antibody, goat anti-rabbit Alexa-Fluor 488-conjugated antibody, goat anti-mouse Alexa-Fluor 488/fluorescein-conjugated antibody, or goat anti-mouse Alexa-Flour 350/fluorescein-conjugated antibody (1:250; Molecular Probes, Eugene, OR), applied for 1 h at room temperature.
All images were obtained using a fluorescence microscope (Olympus AX80; Olympus), or a confocal laser scanning microscope (model TCS SP2; Leica Instruments, Nusslosh, Germany). Furthermore, some sections were counterstained with nuclear marker 4,6-diamino-2-phenylindole (DAPI; Abbott Molecular, Des Plaines, IL). To count the number of inducible nitric oxide synthase (iNOS)-, CD16/32-, arginase-1-, and CD206-expressing OX42-positive cells, three midsagittal sections were selected randomly as mentioned above. From the epicenter area (0–1 mm caudal and rostral to the epicenter), 20 non-overlapping high-power fields were chosen at random for examination/analysis at 400×magnification, and the number of merged and non-merged OX42-positive cells was counted by a color image analyzer (MacSCOPE).
Flow cytometry analysis
Immediately after euthanasia by deep anesthesia, each rat was intracardially perfused with 200 mL of ice-cold 0.1 M PBS and the spinal cords were harvested (n=9). The injured portion of each spinal cord (1 cm around the epicenter) was surgically dissected and dissociated with collagenase (175 U/mL; Sigma-Aldrich) for 1 h at 37°C. The cells were washed in Dulbecco's modified Eagle's medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% fetal bovine serum, and filtered through a 40-μM nylon cell strainer (BD Biosciences, San Jose, CA) under centrifugation to remove tissue debris, and to obtain a single-cell suspension, based on the procedure described previously (Saiwai et al., 2010). The cells were subsequently incubated for 1 h on ice with the following fluorescently-tagged antibodies: APC anti-mouse CD45 (0.25 μg: 1 mL; BioLegend, San Diego, CA), Pacific Blue™ anti-mouse Ly-6G/Ly-6C (equivalent to Gr-1) (1.0 μg: 1 mL; BioLegend), and PerCP-CyTM 5.5 rat anti-mouse CD11b (0.25 μg: 1 mL; BD Pharmingen™, San Jose, CA). For intracellular staining (Stirling and Yong, 2008), the cells were resuspended in 1% buffered formalin (Fixation Buffer; Santa Cruz Biotechnology), and permeabilized with methanol (Permeabilization Buffer; Santa Cruz Biotechnology), followed by resuspension in ice-cold PBS to the proper concentration and incubation for 1 h with arginase-1 (1:200; Santa Cruz Biotechnology) secondarily conjugated to FITC (1:200; Santa Cruz Biotechnology), and PE iNOS antibody (3 μg/mL; Abcam plc). Samples with cells alone and cells incubated with isotype-matched irrelevant antibodies were prepared to be used as controls to eliminate non-specific binding and autofluorescence.
Flow cytometric analysis was performed using a FACS Canto™ II (BD Biosciences), using forward scatter to remove cellular debris. In each test, a minimum of 250,000 cells were analyzed, and the data were processed using BD FACSDiva software (BD Biosciences). The different cells in the suspension were classified according to the combination of the expressed antigens, stated in previous reports as follows: CD45 high/CD11b high/GR-1 high identified neutrophils (Fleming et al.,1993; Lagasse and Weissman, 1996); CD45 positive/CD11b high/GR-1 negative identified macrophages (Ho and Springer, 1983); and CD45 positive/CD11b low/GR-1 negative identified microglia (Sedgwick et al., 1991). The phenotype of each macrophage was corroborated through quantification of iNOS or arginase-1 expression.
Immunoblot analysis
MSC- or MSCBM-injected thoracic spinal cord segments (10 mm long) centered on the site of injury (epicenter) were obtained at 1 week after SCI. They were extracted and subjected to SDS PAGE (15% gels; n=3 in each group). Western blotting was conducted using standard techniques, with equal amounts of protein loaded into each well (Nakajima et al., 2007,2010). The membranes were probed for TNF-α (0.2 μg/mL; Abcam), IL-4, IL-6, and IL-13 (1:200; Santa Cruz Biotechnology), and the intensity of immunostaining was examined using HRP-conjugated secondary antibodies (Santa Cruz Biotechnology), and a commercially available kit for enhanced chemiluminescence (ECL; Amersham plc, Amersham, U.K.). The intensity of each band was expressed relative to that of β-actin (1:2000; Abcam plc). Kaleidoscope Prestained Standards (Bio-Rad Laboratories, Hercules, CA) were used as molecular weight controls.
Enzyme-linked immunosorbent assay (ELISA)
MSC- or MSCBM-injected thoracic spinal cord segments (10 mm long) centered on the site of injury (epicenter) were obtained 1 week after SCI. They were extracted and placed in 1 mL MSCBM and immediately homogenized (n=3 in each group). The homogenates were centrifuged at 12,000g for 20 min at 4°C. The protein concentration was analyzed by a Bio-Rad DC protein assay kit (no. 500-0116; Bio-Rad Laboratories). The concentrations of TNF-α, IL-4, IL-6, and IL-13 in the supernatant were determined using enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen) according to the instructions supplied with each kit. The level of each protein was determined by comparing the samples to the standard curve generated by the kit, and expressed as pg/mg of protein in the spinal cord.
Assessment of magnitude of injury and histological analysis
For semi-quantitative analysis of the extent of cavitation and demyelination at 5 weeks after SCI, images of axial sections stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB) (for myelination) were prepared (n=12). The cavitation area on H&E-stained sections and the extent of LFB-positive areas in the ventrolateral funiculus at the epicenter, and 4 mm rostral and caudal to the epicenter, were analyzed by a color image analyzer (MacSCOPE). The LFB-positive area in which the density of stain significantly exceeded the threshold of each background was calculated as a percentage of the cross-sectional area of residual tissue. To quantify RT97- and GAP-43-positive areas, images of three midsagittal sections around the epicenter (under 400×magnification) were selected randomly as mentioned above, and analyzed using grain counting with the light intensity automatically set by the color image analyzer (MacSCOPE; Uchida et al., 2002).
Assessment of locomotor behavior
To assess the behavior of each rat after SCI and recovery of locomotor function, 10 rats from each group were examined at 3 days, and 1, 2, 3, 4, and 5 weeks post-injury. Locomotor function of the hindlimbs was scored using the Basso-Beattie-Bresnahan (BBB) hindlimb locomotor rating scale. The BBB rating scale (Basso et al., 1995) is a 21-point system based on operationally-defined behavioral features that follow the recovery progression from complete paralysis to normal locomotion. The rating scale ranges from 0–21, with a score of 0 indicating complete hindlimb paralysis, while a score of 21 denotes completely normal locomotor function. Scores of 0–20 indicate an animal's altered ability to move the hindlimb joints, to bear weight, and to coordinate forelimb and hindlimb movement.
Statistical analysis
All values are expressed as mean±standard deviation (SD). Differences between groups were examined for statistical significance using one-way factorial analysis of variance (ANOVA). A p value<0.05 denoted the presence of a significant difference with Tukey's post-hoc analysis. The above tests were conducted using SPSS software version 11.0 (SPSS Inc., Chicago, IL).
Results
Distribution of transplanted MSC in injured spinal cord
The distribution of PKH26-labeled MSC in the injured spinal cord was assessed at 1 and 5 weeks after transplantation in harvested sagittal tissue sections. At 1 week post injury, the transplanted MSC were distributed only around the injured lesion, 2.6±0.5 mm rostral and 2.9±0.5 mm caudal from the epicenter (Fig. 1C and Table 1). On the other hand, the cells extended out of the injured lesion at 5 weeks, to 4.9±1.1 mm rostral and 5.8±1.4 mm caudal from the epicenter (Fig. 1A and B and Table 1). The PKH26-positive area after SCI was 1.23±0.29 mm2 at 1 week, and 0.21±0.06 mm2 at 5 weeks. Those were 72.1±16.8% and 11.9±3.6%, respectively, relative to the area at 3 days after SCI (Table 1).
FIG. 1.
Photomicrograph showing the distribution of PKH26-labeled transplanted mesenchymal stem cells (MSC) counterstained with 4,6-diamino-2-phenylindole (DAPI) for nuclei at 1 and 5 weeks after spinal cord injury (SCI; n=5 each). Transplanted PKH26 induced fluorescent MSC to survive and they appeared only around the injured site at 1 week after SCI (C), whereas a number of PKH-positive cells extended out of the injured lesion at 5 weeks after spinal cord injury (A and B). (B) High-power photomicrograph of the boxed area in A (scale bar=1 mm in A and C, 50 μm in B).
Table 1.
PKH26-Positive Area and Distribution of Transplanted MSC in the Injured Spinal Cord
| |
|
Time after SCI |
||
|---|---|---|---|---|
| 3 days (n=3) | 1 week (n=5) | 5 weeks (n=5) | ||
| Absolute PKH26-positive area (mm2) | 1.71±0.23 | 1.24±0.29 | 0.21±0.06 | |
| Relative PKH26-positive area (%)a | (100) | (72.1±16.8) | (11.9±3.6) | |
| Distance of transplanted MSC spread from epicenter (mm) | Rostral | 2.6±0.5 | 4.9±1.1 | |
| Caudal | 2.9±0.5 | 5.8±1.4 | ||
Relative PKH26-positive area compared to that at 3 days after SCI.
Values are mean±standard deviation (SD).
SCI, spinal cord injury; MSC, mesenchymal stem cells derived from bone marrow.
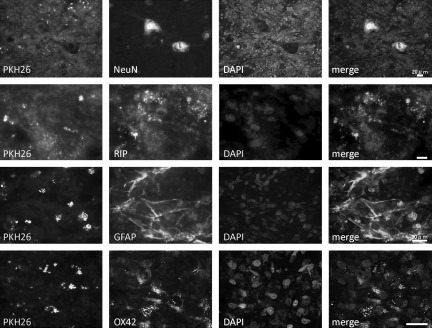
Immunostaining of spinal cord sections for NeuN, RIP, GFAP, and OX-42 was performed at 5 weeks after transplantation of MSC. The transplanted cells were identified by the distribution of PKH26. There was no obvious co-localization of NeuN, RIP, GFAP, or OX-42 with PKH26-identified MSC (Fig. 2). These results indicated that although the MSC were distributed in areas rich in glial and neuronal processes, there was no evidence that the MSC themselves had transdifferentiated into glial or neuronal cells.
FIG. 2.
Co-localization of cell-specific markers (second column; NeuN, RIP, GFAP, and OX-42), and MSC labeled with PKH26 (first column) in the injured spinal cord at 5 weeks after spinal cord injury (n=5). MSC did not express markers for neurons, oligodendrocytes, astrocytes, or microglia/macrophages (scale bars=20 μm; NeuN, neuronal nuclei; RIP, reactive immunology protein; GFAP, glial fibrillary acidic protein; MSC, mesenchymal stem cell).
Effects of MSC transplantation on phenotype of infiltrated macrophages after spinal cord injury
To evaluate the effects of transplanted MSC on the phenotype of infiltrated macrophages after SCI, tissues were immunostained for iNOS, CD16/32 for classically activated macrophages (M1 phenotype) and arginase-1, CD206 for alternatively activated macrophages (M2 phenotype) with OX-42 for microglia/macrophages. Higher numbers of classically-activated macrophages (OX-42 positive) were found in the control group, mainly located in the gray matter around the injury site, co-expressing iNOS (82.5±5.8%) and CD16 (78.7±7.1%); when compared to the MSC transplanted group, with less OX-42 positive cells co-expressing iNOS (27.4±5.1%) or CD16 (19.6±4.2%). These cells had small dense cytoplasm with occasional unipolar extensions (Fig. 3A and B). On the other hand, consistently higher numbers of alternatively-activated macrophages (OX-42 positive) were found in the MSC-transplanted group, co-expressing arginase-1 (38.9±8.1%) and CD206 (32.1±6.6%). These cells typically contained abundant cytoplasm encasing vacuolar structures. On the other hand, only a few arginase-1/CD206/OX42-positive cells were seen in the control group (1.2±0.1% and 0.8±0.05% of the OX42-positive cells, respectively; Fig. 3A and B).
FIG. 3.
(A) Immunofluorescence staining showing differences in the expression of iNOS and CD16/32 for classically-activated macrophages (M1 phenotype), and arginase-1 and CD206 for alternatively-activated macrophages (M2 phenotype) co-localized with OX-42 (blue) after MSC transplantation in the injured spinal cord at 1 week after spinal cord injury. In the MSC-transplanted group, the number of iNOS- and CD16/32-positive cells (arrows) decreased, and those of arginase-1 and CD206 (arrowheads) increased compared to those of the control group. (B) Percentage of merged cells of OX42-positive cells in the area 0–1 mm caudal and rostral to the epicenter (n=5 each; scale bars=50 μm; data are mean±standard deviation; *p<0.05; MSC, mesenchymal stem cell; iNOS, inducible nitric oxide synthase).
The profile of the identified macrophages (CD45positive/CD11bhigh/GR-1negative cells) was also analyzed quantitatively using flow cytometry. The proportions of CD45positive/CD11bhigh/GR-1negative cells (macrophages) within the injured spinal cord were higher in both the MSC (24.5±1.9%) and control group (30.4±2.1%) compared with the sham (laminectomy only) group (4.5±1.3%) at 1 week after SCI. In the control group, at 1 week after SCI, 93.4±5.6% of these cells were iNOSpositive classically-activated macrophages (M1 phenotype; 70,984±4256 cells), whereas no arginase-1positive alternatively-activated macrophages (M2 phenotype) were present. In comparison, in the MSC-transplanted group, only 11.7±1.3% of these cells were iNOSpositive (7166±796 cells), and 32.2±1.9% were arginase-1positive (19,723±1164 cells; Fig. 4A and B). The difference in the proportions of iNOSpositive and arginase-1positive cells between the MSC-transplanted and control group indicated that MSC transplantation seems to reduce the classically-activated macrophages (M1 phenotype), and shift the macrophage phenotype to the alternatively-activated macrophages (M2 phenotype).
FIG. 4.
Representative flow cytometry data at 1 week after injury. The number of iNOSpositive or arginase-1positive cells within the CD45positive/CD11bhigh/GR-1negative (macrophage) populations are different between the MSC-transplanted and control groups (n=3 each) (A). Numbers of iNOS- and arginase-1-positive cells in 250,000 analyzed cells. MSC transplantation reduced the proportion of iNOSpositive cells (M1 phenotype), and shifted the macrophage phenotype to arginase-1positive cells (M2 phenotype) at 1 week after spinal cord injury (B). Data are mean±standard deviation; *p<0.05; MSC, mesenchymal stem cell; iNOS, inducible nitric oxide synthase).
Changes in cytokine expression after MSC transplantation by immunoblot analysis and ELISA
Western blotting and ELISA were performed to evaluate the effects of MSC transplantation on TNF-α, IL-6, IL-4, and IL-13 protein levels in the region of the SCI at 1 week after injury. In the MSC-transplanted group, the intensities of the bands for TNF-α and IL-6 were attenuated, whereas those of IL-4 and IL-13 were increased compared with the control group (Fig. 5A and B). The amounts of TNF-α and IL-6 proteins were significantly lower (IL-6 dominant), while those of IL-4 and IL-13 were significantly higher (IL-13 dominant), in the MSC-transplanted group (TNF-α: 72.4±18.7; IL-6: 93.1±25.1; IL-4: 44.5±10.2; IL-13: 48.9±12.4 pg/mg protein) compared with the control group (TNF-α: 34.9±8.4; IL-6: 32.0±6.2; IL-4: 2.5±0.6; IL-13: 1.2±0.4 pg/mg protein; Fig. 5C).
FIG. 5.
(A) Representative Western blots of tumor necrosis factor-α (TNF-α), interleukin (IL)-4, IL-6, and IL-13 expression at 1 week after spinal cord injury (n=3 each). (B) Relative band intensities compared with that of β-actin. (C) Cytokine levels in the spinal cord (n=3 each). In the mesenchymal stem cell (MSC)-transplanted group, TNF-α and IL-6 levels were significantly lower, whereas IL-4 and IL-13 levels were significantly higher, compared to the control group. Data are mean±standard deviation; *p<0.05).
Histological evaluation of injured spinal cord after MSC transplantation
In order to examine the beneficial effects of MSC transplantation on spinal cord repair, the severity of trauma at the injury epicenter site was evaluated histopathologically at 5 weeks after SCI. H&E staining in the MSC-transplanted group showed a significant decrease in the total cavity areas at the epicenter and 4 mm rostral and caudal to the epicenter compared with the control group (Fig. 6A). On LFB-stained samples, images of the MSC-transplanted group demonstrated significantly smaller areas of cystic cavity formation and enhanced staining in both gray and white matter compared with those of the control group. Quantitative analysis of the myelinated areas revealed significant differences between the two groups at the injury epicenter and at 4 mm rostral and caudal to the epicenter (Fig. 6B). To determine the effects of MSC transplantation on axonal sparing, we examined sagittal sections of the spinal cords following immunostaining with anti-GAP-43 and anti-RT97 antibodies at 5 weeks after SCI. In the MSC-transplanted group, GAP-43-positive or RT97-positive fibers embedded within PKH26-labeled MSC were significantly increased, and their neurites were also significantly longer compared with the control group (Fig. 6C).
FIG. 6.
Hematoxylin and eosin (H&E) staining of midsagittal and axial sections of the lesion epicenter showed a remarkably smaller area of damage and cavity formation in the mesenchymal stem cell (MSC)-transplanted group. The total area of cavitation in the axial sections at the epicenter and at 4 mm rostral and caudal to the epicenter were significantly different between the two groups (n=3 each; A). The axial sections stained with Luxol fast blue (LFB) contained remarkably small areas of demyelination in the MSC-transplanted group compared with the control group. Quantification of LFB-positive myelinated areas showed a significant difference between the two groups at all examined sites (n=3 each; B). Representative images of midsagittal sections through an area 4 mm caudal to the epicenter showing greater abundance of growth-associated protein (GAP)-43-positive fibers and RT97-positive fibers in the MSC group compared with that in the control group (5 weeks after injury). (C) Quantification of GAP-43- and RT97-positive fiber areas showed a significant difference between the two groups at all examined sites (n=3 each; scale bar=100 μm).
Changes in locomotor BBB scores
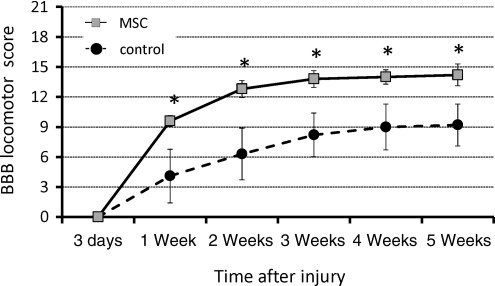
The degree of motor disturbance in the hindlimbs was assessed between day 3 and 5 weeks after SCI. Significant motor disturbance in the hindlimbs was noted in rats of the control group, though some degree of recovery was evident subsequently, which reached a functional plateau (BBB score 9.2±2.1) at 5 weeks after SCI. In contrast, the MSC-transplanted group showed a markedly better functional recovery such that the BBB locomotor score was significantly increased compared to the control group from 1 week after SCI (Fig. 7).
FIG. 7.
Serial changes in locomotor Basso-Beattie-Bresnahan (BBB) scores after spinal cord injury (SCI). A significant improvement in hindlimb motor function was observed in the mesenchymal stem cell (MSC)-transplanted group compared with the control group from 1 week and thereafter after injury (n=10 for each time point; *p<0.05).
Discussion
The aim of this research was to study the beneficial effects of MSC transplantation in the injured spinal cord, specifically with regard to the effects of MSC on macrophage phenotype and function. The major findings of our research were: (1) MSC transplanted into the site of spinal cord injury migrated into the adjacent nervous tissue to areas rich in glial and neuronal processes, but did not differentiate into glial or neuronal elements; (2) MSC transplantation favored the development of a population of alternatively-activated macrophages (M2 phenotype), while preventing the development of a population of classically-activated macrophages (M1 phenotype); (3) MSC transplantation was associated with a decrease in TNF-α and IL-6, and an increase in IL-4 and IL-13; (4) MSC transplantation reduced the size of the injury site and resulted in less scar tissue formation and increased myelin sparing; (5) these morphological findings correlated with increased axonal growth and improved locomotor function in the MSC-transplanted group compared with the control group.
Several studies reported that MSC can differentiate in vitro into neurons, astrocytes (Ankeny et al., 2004; Zurita et al., 2008), myocytes, and Schwann cells (Dezawa et al., 2005). In our study, transplanted MSC migrated to the neighborhood of the injured spinal cord, but did not differentiate into glial or neuronal elements. Current thinking is that the potential beneficial effects of MSC in SCI are not related to neuronal or glial differentiation of MSC, but rather from their secretion of growth factors and/or cytokines (Sasaki et al., 2009), which can provide neuroprotection (Chen et al., 2002; Parr et al., 2007), induction of axonal sprouting (Shen et al., 2006), neovascularization (Onda et al., 2008), and immunomodulation (Ohtaki et al., 2008; Bai et al., 2009). MSC may also promote axonal regeneration or encourage functional plasticity by establishing an environment that supports axonal growth, for example, by abrogating the inhibitory influence of the chondroitin sulfated proteoglycans (CSPG), and/or myelin debris present in the injury site and glial scar (Wright et al., 2011). MSC synthesize a number of neurotrophic cytokines that stimulate nerve growth, such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF; Crigler et al., 2006; Neuhuber et al., 2005). Furthermore, a recent study examined the response of MSC to environmental stimuli in the injured spinal cord tissue, and found increased synthesis by these cells of various cytokines, including IL-6, IL-7, and VEGF (Zhukareva et al., 2010). Because MSC can alter their gene expression profile in response to the surrounding environment (Ide et al., 2010; Mosser and Edwards, 2011; Yamaguchi et al., 2006), we hypothesize that transplanted MSC do not differentiate into neural cells, at least in the injured spinal cord environment, but instead they bring about CNS functional recovery by modifying the SCI environment to directly affect the endogenous cells present in that territory.
Hematogenous macrophages and microglia are the major players in the inflammatory pathology of SCI. Some investigators indicated that hematogenous macrophages are more detrimental, whereas microglia are believed to be relatively beneficial for spinal cord repair due to their higher phagocytic activity and expression of various neurotrophic factors (Mukaino et al., 2010). On the other hand, others indicated that the distinct hematogenous macrophage subset (i.e., alternatively-activated macrophages, M2 phenotype) is beneficial in spinal cord injury, but it also has a distinct role from activated resident microglia. The resident microglia are embedded in the CNS prior to injury and are immediately activated by the insult, whereas alternatively-activated macrophages (M2 phenotype) do not encounter the injured CNS tissue prior to their delayed arrival to the damage site (Shechter et al., 2009). Subsequent studies have correlated such divergent effects to the presence of different macrophage populations with contrasting functions, where classically-activated macrophages (M1 phenotype) are the predominant type after SCI, with deleterious effects on the injured tissues, and alternatively-activated macrophages (M2 phenotype) have only a short-term response, disappearing within 3–7 days after injury (Kigerl et al., 2009). That prevalence of the M1 phenotype over the M2 phenotype may be partially responsible for the lack of functional recovery after SCI.
Previous studies identified TNF-α and IL-6 as the most significant cytokines to generate classically-activated macrophages (Kigerl et al., 2009), and IL-4 and IL-13 to generate alternatively-activated macrophages (Loke et al., 2002), while identifying microglia as the main source of IL-4 (Ponomarev et al., 2007) and IL-13 (Offner et al., 2005). In our study, we found that 3 days after MSC transplantation following an SCI, the local cytokine profile changed into an alternative activating environment, with significant increases in IL-4 and IL-13 levels, and a simultaneous reduction in TNF-α and IL-6. The increased protein levels of Th-2 cytokines (IL-4 and IL-13) might activate resident microglia and hematogenous macrophages through the IL-4Rα/Jak1/STAT signaling pathway into alternatively-activated macrophages (M2 phenotype), where the reduced levels of Th-1 cytokines (TNF-α and IL-6) will prevent the macrophages from shifting back into classically-activated macrophages (M1 phenotype; Gordon, 2003; Mosser and Edwards, 2008).
MSC are thought to act as guiding strands for regenerating axons across the lesion site in the injured cord and along spinal cord tracts by expressing various cell adhesion molecules and receptors (Crigler et al., 2006). The transplanted MSC are reported to form bundles that bridged the lesion, which were also populated with immature astrocytes and nerve fiber outgrowths (Hofstetter et al., 2002; Wright et al., 2007). Alternatively-activated macrophages (M2 phenotype) exhibit high phagocytic abilities due to the presence of endosomes/lysosomes and pinocytic structures with digestive enzymes to remove scar tissue and growth inhibitors present in the myelin debris, thus allowing axonal regeneration (Kigerl et al., 2009; Schwartz, 2010). In our study, the MSC-transplanted group showed axon preservation and an enhanced spinal cord repair process, based on the increase in NF-H- and GAP-43-positive fibers at 5 weeks after injury, indicating anatomical improvement compared with the control group. The acute or subacute environment of the injured spinal cord may influence the mechanism by which the MSC graft induces tissue protection/repair in a manner that differs from the chronic phase (i.e., in the acute phase, MSC transplantation may have beneficial effects through their anti-inflammatory activity, whereas in the subacute/chronic phase after SCI, the MSC may be used for neurostimulatory and cell bridging effects; Wright et al., 2011).
In conclusion, our results suggest that the transplantation of MSC after SCI shifts the phenotype of macrophages after injury from classically-activated macrophages (M1 phenotype) to that of alternatively-activated macrophages (M2 phenotype) during the acute phase. This was associated with the presence of relevant cytokine profiles, reductions in inhibitory scar tissue/cavity formation in the subacute/chronic phase, and the provision of a permissive environment for axonal extension and functional recovery.
Acknowledgments
This work was supported in part by grants-in-aid to H.N., K.U., and H.B. for General Scientific Research of the Ministry of Education, Science, and Culture of Japan (grant no. 21591895, 21791389, 22390287, and 23791631). This study was also supported by a Grant from the Japan Orthopaedics and Traumatology Foundation, Inc., no. 218.
Author Disclosure Statement
No competing financial interests exist.
References
- Abrams M.B. Dominguez C. Pernold K. Reger R. Wiesenfeld-Hallin Z. Olson L. Prockop D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 2009;27:307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- Akiyama Y. Radtke C. Kocsis J.D. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 2002b;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama Y. Radtke C. Honmou O. Kocsis J.D. Remyelination of the spinal cord following intravenous delivery of bone marrow cells. Glia. 2002a;39:229–236. doi: 10.1002/glia.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankeny D.P. McTigue D.M. Jakeman L.B. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp. Neurol. 2004;190:17–31. doi: 10.1016/j.expneurol.2004.05.045. [DOI] [PubMed] [Google Scholar]
- Bai L. Lennon D.P. Eaton V. Maier K. Caplan A.I. Miller S.D. Miller R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Bomstein Y. Marder J.B. Vitner K. Smirnov I. Lisaey G. Butovsky O. Fulga V. Yoles E. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J. Neuroimmunol. 2003;142:10–16. doi: 10.1016/s0165-5728(03)00260-1. [DOI] [PubMed] [Google Scholar]
- Busch S.A. Hamilton J.A. Horn K.P. Cuascut F.X. Cutrone R. Lehman N. Deans R.J. Ting A.E. Mays R.W. Silver J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J. Neurosci. 2011;31:944–953. doi: 10.1523/JNEUROSCI.3566-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.L. Parrish M.E. Springer J.E. Doty K. Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Chen X. Li Y. Wang L. Katakowski M. Zhang L. Chen J. Xu Y. Gautam S.C. Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Crigler L. Robey R.C. Asawachaicharn A. Gaupp D. Phinney D.G. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 2006;198:54–64. doi: 10.1016/j.expneurol.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Dezawa M. Hoshino M. Nabeshima Y. Ide C. Marrow stromal cells: implications in health and disease in the nervous system. Curr. Mol. Med. 2005;5:723–732. doi: 10.2174/156652405774641070. [DOI] [PubMed] [Google Scholar]
- Di Nicola M. Carlo-Stella C. Magni M. Milanesi M. Longoni P.D. Matteucci P.E. Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Fleming T.J. Fleming M.L. Malek T.R. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Himes B.T. Neuhuber B. Coleman C. Kushner R. Swanger S.A. Kopen G.C. Wagner J. Shumsky J.S. Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil. Neural. Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- Hofstetter C.P. Schwarz E.J. Hess D. Widenfalk J. El Manira A. Prockop D.J. Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M.K. Springer T.A. Tissue distribution, structural characterization, and biosynthesis of Mac-3, a macrophage surface glycoprotein exhibiting molecular weight heterogeneity. J. Biol. Chem. 1983;258:636–642. [PubMed] [Google Scholar]
- Ide C. Nakai Y. Nakano N. Seo T.B. Yamada Y. Endo K. Noda T.E. Suzuki Y. Fukushima M. Nakatani T. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010;1332:32–47. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Jiang X.X. Zhang Y. Liu B. Zhang S.X. Wu Y. Yu X.D. Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A. Gensel J.C. Ankeny D.P. Alexander J.K. Donnelly D.J. Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E. Weissman I.L. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- Laskin D.L. Macrophages and inflammatory mediators in chemical toxicity: a battle of forces. Chem. Res. Toxicol. 2009;22:1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke P. Nair M.G. Parkinson J. Guiliano D. Blaxter M. Allen J.E. IL-4 dependent alternatively-activated macrophages have a distinctive in vivo gene expression phenotype. BMC Immunol. 2002;3:7. doi: 10.1186/1471-2172-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. Chen T. Mandelin J. Ceponis A. Miller N.E. Hukkanen M. Ma G.F. Konttinen Y.T. Regulation of macrophage activation. Cell. Mol. Life. Sci. 2003;60:2334–2346. doi: 10.1007/s00018-003-3020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Sica A. Sozzani S. Allavena P. Vecchi A. Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McDonald J.W. Liu X.Z. Qu Y. Liu S. Mickey S.K. Turetsky D. Gottlieb D.I. Choi D.W. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999;4:291–297. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- Mosser D.M. Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaino M. Nakamura M. Yamada O. Okada S. Morikawa S. Renault-Mihara F. Iwanami A. Ikegami T. Ohsugi Y. Tsuji O. Katoh H. Matsuzaki Y. Toyama Y. Liu M. Okano H. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp. Neurol. 2010;224:403–414. doi: 10.1016/j.expneurol.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Murray M. Cellular transplants: steps toward restoration of function in spinal injured animals. Prog. Brain. Res. 2004;143:133–146. doi: 10.1016/s0079-6123(03)43013-6. [DOI] [PubMed] [Google Scholar]
- Nakajima H. Uchida K. Kobayashi S. Inukai T. Horiuchi Y. Yayama T. Sato R. Baba H. Rescue of rat anterior horn neurons after spinal cord injury by retrograde transfection of adenovirus vector carrying brain-derived neurotrophic factor gene. J. Neurotrauma. 2007;24:703–712. doi: 10.1089/neu.2006.0004. [DOI] [PubMed] [Google Scholar]
- Nakajima H. Uchida K. Yayama T. Kobayashi S. Guerrero A.R. Furukawa S. Baba H. Targeted retrograde gene delivery of brain-derived neurotrophic factor suppresses apoptosis of neurons and oligodendroglia after spinal cord injury in rats. Spine. 2010;35:497–504. doi: 10.1097/BRS.0b013e3181b8e89b. [DOI] [PubMed] [Google Scholar]
- Neuhuber B. Timothy Himes B. Shumsky J.S. Gallo G. Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Offner H. Subramanian S. Wang C. Afentoulis M. Vandenbark A.A. Huan J. Burrows G.G. Treatment of passive experimental autoimmune encephalomyelitis in SJL mice with a recombinant TCR ligand induces IL-13 and prevents axonal injury. J. Immunol. 2005;175:4103–4111. doi: 10.4049/jimmunol.175.6.4103. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Sawamoto K. Miyata T. Miyao S. Watanabe M. Nakamura M. Bregman B.S. Koike M. Uchiyama Y. Toyama Y. Okano H. Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J. Neurosci. Res. 2002;69:925–933. doi: 10.1002/jnr.10341. [DOI] [PubMed] [Google Scholar]
- Ohtaki H. Ylostalo J.H. Foraker J.E. Robinson A.P. Reger R.L. Shioda S. Prockop D.J. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc. Natl. Acad. Sci. USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T. Honmou O. Harada K. Houkin K. Hamada H. Kocsis J.D. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J. Cereb. Blood. Flow. Metab. 2008;28:329–340. doi: 10.1038/sj.jcbfm.9600527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr A.M. Tator C.H. Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Ponomarev E.D. Maresz K. Tan Y. Dittel B.N. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J. Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. McGaughy V. Fisher L. Hickey W.F. Basso D.M. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. Wei P. Huitinga I. van Rooijen N. Stokes B.T. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Ramer M.S. Harper G.P. Bradbury E.J. Progress in spinal cord research—a refined strategy for the International Spinal Research Trust. Spinal Cord. 2000;38:449–472. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- Saiwai H. Ohkawa Y. Yamada H. Kumamaru H. Harada A. Okano H. Yokomizo T. Iwamoto Y. Okada S. The LTB4-BLT1 axis mediates neutrophil infiltration and secondary injury in experimental spinal cord injury. Am. J. Pathol. 2010;176:2352–2366. doi: 10.2353/ajpath.2010.090839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M. Radtke C. Tan A.M. Zhao P. Hamada H. Houkin K. Honmou O. Kocsis J.D. BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. J. Neurosci. 2009;29:14932–14941. doi: 10.1523/JNEUROSCI.2769-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Moalem G. Leibowitz-Amit R. Cohen I.R. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- Schwartz M. “Tissue-repairing” blood-derived macrophages are essential for healing of the injured spinal cord: from skin-activated macrophages to infiltrating blood-derived cells? Brain Behav. Immun. 2010;24:1054–1057. doi: 10.1016/j.bbi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Sedgwick J.D. Schwender S. Imrich H. Dorries R. Butcher G.W. ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R. London A. Varol C. Raposo C. Cusimano M. Yovel G. Rolls A. Mack M. Pluchino S. Martino G. Jung S. Schwartz M. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L.H. Li Y. Chen J. Zhang J. Vanguri P. Borneman J. Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Sheth R.N. Manzano G. Li X. Levi A.D. Transplantation of human bone marrow-derived stromal cells into the contused spinal cord of nude rats. J. Neurosurg. Spine. 2008;8:153–162. doi: 10.3171/SPI/2008/8/2/153. [DOI] [PubMed] [Google Scholar]
- Stirling D.P. Yong V.W. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J. Neurosci. Res. 2008;86:1944–1958. doi: 10.1002/jnr.21659. [DOI] [PubMed] [Google Scholar]
- Taoka Y. Okajima K. Uchiba M. Murakami K. Kushimoto S. Johno M. Naruo M. Okabe H. Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Uchida K. Baba H. Maezawa Y. Kubota C. Progressive changes in neurofilament proteins and growth-associated protein-43 immunoreactivities at the site of cervical spinal cord compression in spinal hyperostotic mice. Spine. 2002;27:480–486. doi: 10.1097/00007632-200203010-00008. [DOI] [PubMed] [Google Scholar]
- Wright K.T. Masri W. Osman A. Chowdhury J. Johnson W.E. Bone marrow for the treatment of spinal cord injury: Mechanisms and clinical application. Stem Cells. 2011;29:169–178. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K.T. El Masri W. Osman A. Roberts S. Chamberlain G. Ashton B.A. Johnson W.E. Bone marrow stromal cells stimulate neurite outgrowth over neural proteoglycans (CSPG), myelin associated glycoprotein and Nogo-A. Biochem. Biophys. Res. Commun. 2007;354:559–566. doi: 10.1016/j.bbrc.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Wu S. Suzuki Y. Ejiri Y. Noda T. Bai H. Kitada M. Kataoka K. Ohta M. Chou H. Ide C. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 2003;72:343–351. doi: 10.1002/jnr.10587. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. Kuroda S. Kobayashi H. Shichinohe H. Yano S. Hida K. Shinpo K. Kikuchi S. Iwasaki Y. The effects of neuronal induction on gene expression profile in bone marrow stromal cells (BMSC)—a preliminary study using microarray analysis. Brain Res. 2006;1087:15–27. doi: 10.1016/j.brainres.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Zhukareva V. Obrocka M. Houle J.D. Fischer I. Neuhuber B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine. 2010;50:317–321. doi: 10.1016/j.cyto.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Zurita M. Vaquero J. Bonilla C. Santos M. De Haro J. Oya S. Aguayo C. Functional recovery of chronic paraplegic pigs after autologous transplantation of bone marrow stromal cells. Transplantation. 2008;86:845–853. doi: 10.1097/TP.0b013e318186198f. [DOI] [PubMed] [Google Scholar]