Abstract
Dendritic cell (DC) antigen cross-presentation is generally associated with immune responses to tumors and viral antigens and enhancing this process is a focus of tumor vaccine design. In this study, we found that the myeloid cell surface peptidase CD13 is highly and specifically expressed on the subset of DCs responsible for cross-presentation, the CD8+ murine splenic DCs. In vivo studies indicated that lack of CD13 significantly enhanced T cell responses to soluble OVA antigen, although development, maturation, antigen processing and presentation of DCs are normal in CD13KO mice. In vitro studies showed that CD13 regulates receptor-mediated, dynamin-dependent endocytosis of antigens such as OVA and transferrin but not fluid-phase or phagocytic antigen uptake. CD13 and antigen are co-internalized in DCs but CD13 did not co-immunoprecipitate with antigen receptors, suggesting that CD13 does not control internalization of specific receptors but regulates endocytosis at a more universal level. Mechanistically, we found that phosphorylation of the endocytic regulators p38MAPK and Akt was dysregulated in CD13KO DCs and blocking these kinases perturbed CD13-dependent endocytic uptake. Therefore, CD13 is a novel endocytic regulator that may be exploited to enhance antigen uptake and T cell activation to improve the efficacy of tumor-targeted vaccines.
Introduction
Production of an efficient immune response to tumor or viral antigens requires the presentation of antigen in the context of MHC class I in a process called antigen cross-presentation (1). In the mouse, a subset of professional antigen presenting cells, the CD8+ DCs, are primarily responsible for cross-presentation and activate antigen specific cytotoxic T cells to initiate an effective immune response (2). Recent evidence has indicated that a human counterpart of these cells exists and can be distinguished by the expression of CD11c and CD141 (3, 4, 5). The mechanisms of DC antigen uptake, processing and presentation are complex and are determined largely by both the nature of the antigen and the receptor that it encounters (6, 7, 8). While antigens destined to be cross-presented are often internalized by phagocytic or macropinocytic mechanisms, our understanding of this process has recently been advanced by investigations of soluble exogenous antigens internalized by receptor-mediated endocytosis that are cross-presented in an MHC class I manner (6, 8). These studies showed that antigen binding to its cognate receptor triggers sorting of the antigen receptor complex to kinetically and functionally distinct endosomal compartments, thus determining the fate of internalized antigen (6, 7). Antigen entering rapidly maturing endosomes that fuse with vesicles of the degradative lysosomal pathway are presented in the context of MHC II, while antigen targeted to a longer-lived, early endosomal population ultimately is cross-presented in the context of MHC I. Increased understanding of receptor-mediated uptake and identification of molecules that regulate this process is central for the development of more effective anti-tumor vaccines as well as therapies for autoimmune and infectious diseases.
CD13 or aminopeptidase N is a membrane bound metallopeptidase expressed on specific cell types in many tissues and on all cells of the myeloid lineage. It is a multifunctional protein that has both enzymatic-dependent and -independent functions (9). As an ectopeptidase, CD13 cleaves neutral amino-terminal residues from short peptides to regulate their biological activity. Independent of its enzymatic activity CD13 is the receptor for a subclass of coronaviruses in a number of species (10) and is a homotypic adhesion molecule regulating inflammatory monocyte trafficking (11). Other studies have implicated CD13 in a variety of key cellular processes such as angiogenesis (12), tumor cell invasion (13) and metastasis (14). Our recent studies have indicated that normal hematopoietic development is maintained in mice with a global deletion of CD13 (15) and also highlighted cell types in which CD13 is highly expressed and thus may contribute to tissue function. In the present investigation we confirmed that CD13 is very highly expressed on DCs derived from both bone marrow and spleen, suggesting it may play a role in antigen presentation, the primary function of DCs. Previous studies have implicated CD13 in macrophage phagocytosis (16) and antigen processing (17), but further studies render these associations less clear (15, 18). Thus, a direct role for CD13 in antigen presentation has not been defined. In the current study, we found that CD13 is highly expressed on the CD8+ subset of CD11c+ DCs that is principally involved in cross-presentation of viral and tumor antigens in the context of MHC I to induce cytolytic T cell responses. Additionally, the lack of CD13 leads to significantly increased DC antigen uptake and subsequent cross-presentation of antigen in vitro and in vivo. Because enhancing antigen cross-presentation is a common goal in optimizing tumor vaccines, CD13 may be an attractive therapeutic target to improve the efficacy of receptor-targeted vaccines.
Material and Methods
Mice
CD13 global null mice were generated at the Gene Targeting and Transgenic Facility at University of Connecticut (15) and backcrossed for 10 generations to C57Bl/6J strain (Jackson Laboratory, Bar Harbor, ME). For all experiments 6–8 week old mice were used in accordance with Institutional and Office of Laboratory Animal Welfare guidelines.
Reagents
Unless otherwise stated, all antibodies were purchased from Biolegend, eBiosciences and BD Biosciences. Anti-hCD13 mAb 452 was the kind gift of Dr. Meenhard Herlyn, University of Pennsylvania.
Generation of bone marrow-derived dendritic cells (BMDC)
Total bone marrow cells were obtained by flushing the femur and tibia, followed by lysis of red blood cells and cultured for 6 days in complete RPMI 1640 medium supplemented with 20ng/ml GM-CSF.
Isolation of CD8+ splenic DCs
Total splenocytes were isolated by mechanical shear and RBC lysis. A single cell suspension was incubated with CD3 and CD19-PE antibodies followed by treatment with PE-microbeads, passed through a MACS column to deplete CD3+ T-and CD19+ B-cells. Total splenic DCs (flow through fraction) were isolated with CD11c followed by CD8 microbeads to obtain CD8+ CD11c+ and CD8−CD11c+ populations. Purity was verified by flow cytometry.
Isolation of human peripheral blood monocytic DC
Buffy coats from healthy volunteers were isolated by Ficoll gradients (Amersham) and after 6–7 days in culture, immature hMoDC (human blood-derived monocytic DCs) were collected and verified for CD141+ CD13+ expression by flow cytometry using anti-human CD141-APC (Miltenyi Biotec) and anti CD13mAb 452-FITC.
In Vitro Antigen Cross-Presentation
B3Z T-cell hybridoma cells and bone marrow derived or splenic dendritic cells were seeded in a 96-well round bottom plate (19). The cultures were prepared in medium alone or in the presence of varying concentrations of SIINFEHL (SHL8) peptide, DOTAP loaded, OVA-bead loaded BMDCs or endofree soluble chicken ovalbumin (Biovendor). After an 18 hour incubation, cells were washed once with PBS followed by a chromogenic lacZ substrate (Calbiochem). After 8–24 hour incubation at RT in the dark, the absorbance at 595 nm was measured.
In vivo Antigen Cross-presentation
CD8+ T-lymphocytes were isolated by magnetic separation (Miltenyi Biotec) from the spleen and lymph nodes of Rag−/−CD45.1 OT-1 mice and labeled with 5μM (CFSE) dye for 10 min at RT, washed once and resuspended in sterile PBS to a final concentration of 1.0 × 107 cells/ml. On day 0, experimental animals were injected i.v. with 1.0 × 106 CFSE-labeled OT-1 T cells. On day 1, animals were primed with 10μg soluble OVA (0.5ml/mouse) intradermally. On day 4 spleen cells were analyzed by flow cytometry for CFSE content in the CD8+CD45.1+CFSE+ lymphocyte population.
Uptake of soluble OVA
CD11c+ BMDC or CD8+ CD11c+ spDCs were pretreated with either Mannan (300ng/ml), DMA (500μM) or Bestatin (100μm/ml) or kinase inhibitors for 30″ at 37°c followed by treatment with alexa488- or alexa594-labeled ovalbumin for an additional 45″ at 37°c. Untreated DC and DC treated with only ovalbumin served as controls. Cells were washed and stained with anti-CD11c Ab and analyzed by flow cytometry. Cells were incubated at 4°c to determine the extent of nonspecific binding which was subtracted from the experimental intracellular uptake.
Flow Cytometry
Flow cytometry was performed on either Calibur or LSRII (Becton Dickinson). Data were analyzed using Flow-Jo software (Tristar).
Immunofluorescence
Cell suspensions were fixed in 4% paraformaldehyde solution, permeabilized with 0.2% Triton X-100 and blocked with 5% BSA. For colocalization studies OVA treated cells were incubated with a rat polyclonal anti-CD13 Ab (AbD Serotec) overnight at 4°c. Cells were treated with TOPRO3 (for nuclear stain) and affixed to slides by cytospin. Slides were mounted with slow-fade (Molecular Probes) and analyzed by a Zeiss Axiovert fluorescence microscope.
Western blot analysis
MR protein was precipitated with protein G-conjugated agarose beads (Invitrogen) by constant rotation for 1 h at 4°C. The beads were washed with NP-40 lysis buffer and analyzed by immunoblot with either anti-MR or anti-CD13 antibodies.
Statistical analysis
All data are expressed as the mean +/− SD or SEM. Statistical differences between groups were analyzed by using unpaired, two-tailed t test. Differences were considered significant at p<0.05.
Results
CD13 is highly expressed on specific subsets of mouse and human DCs
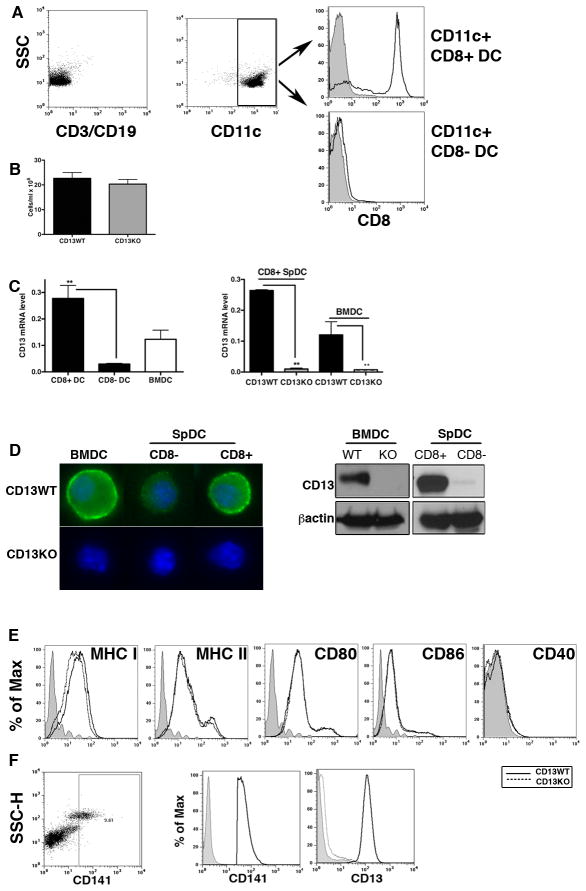
To obtain an initial clue to CD13’s contribution to DC function, we analyzed the expression level of CD13 in the two major murine spDC subsets, the CD8+ and CD8− populations, as well as in DCs differentiated from bone marrow with GM-CSF (CD11c+ BMDC). Splenocytes from WT and CD13KO mice (15) were separated into CD8+ or CD8−CD11c+ DC populations by sequential negative and positive immunoselection using Ab-bound microbeads (Fig 1A). Total numbers of isolated CD8+ spDCs (Fig 1B) and CD11c+ BMDCs (not shown) were comparable in mice of either genotype. Quantitative-RT-PCR analysis of mRNA isolated from spDC subsets showed a remarkable dichotomy in CD13 expression, with high levels of CD13 expressed on the CD11c+/CD8+ cells while expression in the CD11c+/CD8− population was significantly lower. Similarly, we detected appreciable amounts of CD13 in CD11c+ BMDC while cells from CD13KO animals were negative (Fig 1C) which was confirmed at the protein level by immunofluorescence and Western blot analysis (Fig 1D). Finally, flow cytometric characterization of the expression levels of differentiation and maturation markers on BMDC under basal conditions showed that the profiles of MHC and costimulatory molecules on WT and CD13KO cells were largely indistinguishable, suggesting that CD13 does not play a role in DC development (Fig 1E).
Fig 1. CD13 is highly expressed on the CD8+ subset of mouse spDC and CD11c+ BMDC.
A) Purification of CD8+ CD11c+ spDCs. Total splenocytes were magnetically depleted of CD3+ T-and CD19+ B-cells (left). CD11c+ spDCs (devoid of T-and B-cells, center) were further separated into CD8+ CD11c+ and CD8−CD11c+ spDCs with CD11c and CD8 microbeads and the purity of each population was verified by flow cytometry (right). B) Numbers of CD8+ CD11c+ spDCs from CD13WT and CD13KO mice, cells/ml +/− SEM (n=3 mice, 3 experiments). C) Levels of CD13 mRNA assayed by qRT-PCR in the indicated cell populations. Numbers indicate relative mRNA expression +/SEM (n=4 mice, 3 separate experiments). D) CD13 protein expression in BMDC and CD8+ spDC in CD13WT mice by immunofluorescence and immunoblot analysis. Images were acquired with Zeiss Axiocam camera (0.63 × magnification) and processed by Zeiss Axiovision software. Blue; DAPI (nucleus) and green; CD13. E) Flow cytometric analysis of expression of differentiation and activation markers in CD11c+ BMDC from CD13WT (solid line) and CD13KO (dotted line) under basal conditions (n=3 mice in each of 2 independent experiments). Grey area indicates unstained cells. F) Expression of CD13 on the CD141+ subset of human peripheral blood derived DCs. Representative images from two independent isolates. **; p<0.01.
Recently, a small (0.03% of human PBMC) but consistent subset of human peripheral blood DCs has been characterized as the phenotypic and functional counterpart of the murine CD8+ DC subset (3, 4, 5). Analysis of in vitro expanded human peripheral blood monocyte-derived DCs by flow cytometry showed that indeed, CD13 is highly expressed on this CD141+/CD11c+ subset of human monocyte derived DCs, consistent with our findings in murine DCs (Fig 1F). While suggesting a potential functional role for CD13 in these specific DCs subtypes in mice and humans, this observation also serves to reinforce the phenotypic connection between these two populations.
Lack of CD13 expression results in increased cross-presentation of soluble OVA in vivo
The CD8+ DC subset is unique in its ability to cross-present exogenous antigens on MHC I (2), suggesting a functional role for CD13 in antigen cross-presentation. To investigate this possibility, we chose an established in vivo adoptive transfer model which measures the efficiency of endogenous DC MHC I-restricted antigen presentation based on the extent of proliferation of adoptively transferred clonal CD8+ T cells. CD8+ T-cells purified from CD45.1+ OT-1 mice [capable of responding only to a specific OVA peptide in the context of MHC I (20)] were labeled with the proliferation-dependent dye CFSE and adoptively transferred into either WT or CD13KO mice (CD45.2+) and subsequently challenged with an intradermal injection of soluble OVA. Ex-vivo flow cytometric analysis of CD8/CD45.1+ donor lymph node T cells showed a clear reduction in CFSE levels in cells isolated from CD13KO animals, indicating that more T cells had divided in response to OVA immunization (Fig 2A). Similarly, the ‘division index’ (the average number of divisions of all donor cells) indicates a higher number of dividing cells in the CD13KO compared to WT animals (Fig 2B). Alternatively, in PBS control animals, CFSE levels were uniform between genotypes and determined the gates for ‘undivided’ and ‘divided’ donor T cells. Because we have previously implicated CD13 in leukocyte trafficking (11), we sought to determine if CD13 affected the ability of the DCs to encounter the adoptively transferred OT-1 T cells. However, co-injection of differentially labeled wild type and CD13 null DCs into the footpads of OVA immunized wild type mice showed that the two populations were equally represented in the draining popliteal lymph nodes (Figure 2C), suggesting that the lower numbers of proliferating T cells is not due to effects on trafficking. Taken together, these results suggest that CD13 may negatively regulate DC antigen cross-presentation.
Fig 2. In vivo cross-presentation of soluble OVA results in enhanced CD8+ T cell activation in the absence of CD13.
A) CFSE-labeled CD45.1+ OT-I cells were injected i.v. into CD13WT and CD13KO mice. Soluble OVA was then injected intradermally (100 μg OVA/g). Lymphocytes were isolated from spleen and lymph nodes, stained with CD8 and CD45.1 Abs and OT-I cell proliferation (indicated by dilution of CFSE) was analyzed by flow cytometry (CFSE+ CD8+ CD45.1+ cells). The CFSE content of the OT-1 cells (CD8+ and CD45.1+) in the total live population was assessed as a measure of proliferation. B) The average number of cell divisions in the original population as indicated by the division index. (n=4 mice per genotype, 6–8 wks old, in 3 separate experiments). *; p<0.05. C) Flow cytometric analysis of the number of CD11c+ PKH67-labeled wild type or PKH26-labeled CD13KO BMDCs that had migrated to the popliteal LN of wild type mice upon challenge with OVA (n=3/genotype, 2 experiments).
CD13 regulates cross-presentation of polypeptide, but not other forms of antigen by DCs to CD8+ T cells in vitro
Efficient antigen presentation involves a number of interrelated processes including antigen uptake, antigen processing, MHC loading and re-presentation of the MHC/antigen complex on the cell surface, each of which is important to productive T cell activation. To determine whether CD13 participates in one or more of these processes, we tested the T cell response to WT or CD13KO DCs treated with different forms of antigen designed to distinguish aspects of cross-presentation (Table I) in an in vitro system using the B3Z T cell hybridoma. Similar to T cells from the OT-1 mouse, this cell line specifically recognizes the SH8 OVA-derived peptide in the context of MHC I Kb but contains a lacZ reporter gene under the control of elements of the IL-2 enhancer (19). In this system, lacZ activity induced in B3Z/DC co-cultures parallels levels of IL-2 transcription and so directly reflects the efficiency of presentation of OVA-peptide-MHC I complexes by DCs. Interestingly, CD13null DCs provided with OVA polypeptide showed a nearly two-fold increase in B3Z activation while there was no difference between WT and CD13KO DCs in the T cell response to other forms of antigen, indicating that CD13 contributes to antigen uptake but not processing or presentation (Fig 3 and Table I) and provides further evidence that the increase in T cell activation is not due to differential trafficking in the absence of CD13. Finally, in agreement with our previous observations (15) DC antigen uptake by phagocytosis (bead associated OVA) is not affected by lack of CD13, suggesting that only particular mechanisms of uptake are CD13-dependent.
Table I.
Forms of antigen used and processes measured in Fig 3.
| Fig | Form of Antigen | Processes Involved |
|---|---|---|
| 3A | OVA peptide (SIINFEHL, SH8) | positive control- circumvents antigen uptake, processing and presentation (51) |
| 3B | intact OVA protein | antigen uptake via receptor-mediated endocytosis, requires processing and presentation (8) |
| 3C | OVA coated polystyrene beads | antigen uptake by phagocytosis, requires processing and presentation (52) |
| 3D | OVA loaded into DCs via liposomes | bypasses antigen uptake, requires antigen processing and presentation (53) |
Fig 3. CD13KO BMDCs show enhanced cross-presentation of soluble antigen in vitro.

DCs were loaded with increasing doses of the indicated forms of antigen, co-cultured with B3Z cells and the response measured by a colorimetric assay. A) H2Kb (SIINFEHL or SH8) peptide, B) soluble OVA, C) OVA adsorbed to polystyrene beads and D) OVA-loaded liposomes. Data represents absorbance +/− SD (n=3 mice/genotype, 3 independent experiments). *; p<0.05, **; p<0.01.
CD13null DCs internalize soluble antigen more efficiently
To confirm that CD13 regulates uptake of soluble antigen, we directly assessed the relative ability of WT and CD13KO DCs to internalize fluorescently labeled OVA protein in vitro. Consistent with enhanced cross-presentation in the absence of CD13 in vivo and in vitro, we observed significantly higher uptake of antigen by CD13null CD8+ spDCs over a wide range of antigen doses at 37°C, both in the amount of antigen internalized (increased mean fluorescence intensity, MFI) and the number of cells internalizing antigen (% cellularity, Fig 4A). This effect was not due to extracellular coating of the antigen, as it did not occur when performed at 4°C (not shown). Interestingly, it has been shown that at high OVA concentrations, DC uptake mechanisms switch and fluid phase uptake becomes predominant over receptor-mediated endocytosis (21), predicting that the CD13 dependent increase in OVA endocytosis may disappear at high antigen doses. Indeed, at OVA concentrations above 1μg/ml the increase in OVA uptake is no longer evident (Fig 4B), suggesting that CD13 does not participate in fluid phase uptake, further defining its specificity. The increase in uptake by CD13KO CD8+ spDCs was also verified by immunoflourescence using labeled OVA at standard doses (Fig 4C). Importantly, pretreatment of DCs with an anti-CD13 monoclonal antibody boosts OVA uptake by WT DCs to the level of CD13KO cells in a dose dependent manner (Fig 4D), confirming that the effect is indeed CD13-dependent. In contrast to intact OVA, CD13WT and CD13KO BMDCs primed with different doses of labeled SIINFEHL peptide showed nearly identical uptake profiles by flow cytometry (Fig 4E), confirming that CD13 specifically regulates particular mechanisms of antigen uptake. Finally, immunostaining for CD13 shows that it appears to co-localize and co-internalize with labeled OVA as indicated by overlapping fluorochromes in WT DCs (Fig 4F). Taken together these data imply that CD13 participates in antigen uptake and thus impacts subsequent antigen cross-presentation by DCs.
Fig 4. Lack of CD13 results in increased uptake of soluble OVA by CD8+ spDCs in vitro.
A and B) Uptake of FITC labeled soluble OVA by CD8+ spDCs by flow cytometry (n=4, *; p<0.05, **; p<0.01, 3 separate experiments). C) Immunoflourescence analysis of alexa594-labeled OVA uptake by CD8+ spDCs isolated from CD13WT and CD13KO mice. Blue; Topro3 (nucleus) and Red; Alexa-594 OVA. Representative images of two independent experiments. D) FACS analysis showed the effect of increasing doses of CD13mAb in CD13WT mice on OVA uptake. *; p<0.05. E) BMDCs were pulsed with either control or labeled SIINFEHL peptide at a dose of 30μM and analyzed by FACS (n=3 mice each genotype). CD13WT (solid line) and CD13KO (dotted line) or unstained (grey area). F) Co-localization and co-internalization of CD13 with OVA as indicated by the arrow (yellow dots). Blue; Topro3, Red; CD13-Texas red and Green; Alexa-488 OVA. Three independent experiments.
CD13 regulates MR-mediated endocytosis
Antigen uptake for cross-presentation involves cell surface carbohydrate-binding receptors, primarily the C-type lectins of the mannose receptor (MR) family including the MR (CD206) and DEC205 (CD205) which are expressed on DCs [reviewed in ref (22)]. Pertinent to our study, uptake of soluble ovalbumin that is targeted for cross-presentation by CD8+ DC predominantly occurs via MR-mediated endocytosis. This process targets the OVA to specific early endosomes that are distinct from antigen destined for class II presentation (6, 21). As previously reported (6, 7), we found that the levels of mRNA encoding the MR in CD8− spDCs are quite low, but are significantly elevated in the CD13 positive BMDC and CD8+ spDC populations (Figs 1 and 5A). Similarly, protein levels of MR are equivalent as assessed by immunofluorescence and flow cytometry (Figs 5B and C). Further, immunoprecipitation/Western blot analyses indicate that CD13 and MR are not present in a complex in WT DC extracts under these conditions (Fig 5D). Therefore, combined with our results showing that internalized OVA and CD13 co-localize in BMDCs (Fig 4E) it is likely that CD13 may occupy the same endosomal compartment as internalized antigen bound to its receptor. To further investigate the relationship between CD13 and MR-mediated uptake, we pretreated WT or CD13KO spCD8+ DCs with mannan [to block OVA binding to MR (21)] followed by Alexa488-labeled OVA. Mannan significantly diminished the increase in uptake of OVA protein in CD13KO DCs at all doses (Fig 5E), reducing it to a level equivalent to that of WT DCs. Conversely, the increase in OVA uptake persisted in the presence of an inhibitor of macropinocytosis, dimethyl amiloride, Fig 5F, in agreement with published reports (6) and our previous data (Fig 4B). Moreover, pretreatment of the DCs with the aminopeptidase inhibitor bestatin [blocks CD13 peptidase activity (23)], had no effect on the uptake of OVA by BMDCs or spDCs over a range of doses (Fig 5F and not shown), indicating that CD13 does not act in a bestatin-sensitive manner in this context. Finally, we confirmed that the MR-dependent increase in OVA uptake enhances T cell activation by pretreating WT or CD13KO CD8+spDCs with the inhibitors prior to adding soluble OVA and B3Z cells, resulting in markedly reduced T cell activation in the presence of mannan while DMA and bestatin had no effect (Fig 5G and not shown). Taken together, lack of CD13 enhances cross-presentation of soluble antigen by CD8+spDCs and BMDC by regulating MR-mediated OVA uptake.
Fig 5. CD13 regulates DC uptake of soluble OVA via the MR.
A) qRT-PCR analysis of MR mRNA expression in both CD13WT and CD13KO CD8+ spDCs and BMDCs. Data represent relative expression +/−SEM, n=3 mice. B) Immunoflourescence, Blue; DAPI (nucleus), green; (MR). C) Flow cytometry, and D) Immunoblot analysis of MR protein expression in BMDCs. Lower, co-immunoprecipitation (IP) of CD13 with MR in BMDCs D). E) Effect of increasing doses of mannan on uptake of soluble OVA. MFI +/−SEM, n=4 mice. *; p<0.05, **; p<0.01. F) DMA and Bestatin showed no effect on OVA uptake in either genotype. **; p<0.05. G) Lack of CD13 increases in vitro cross-presentation of soluble OVA in a dose dependent manner. Data represents absorbance +/−SD, n=4 mice. a, CD13WT (0.25μg OVA vs. Mannan+OVA); b, CD13KO (0.25μg OVA vs. Mannan+OVA); c, CD13WT (0.5μg OVA vs. Mannan+OVA); d, CD13KO (0.5μg OVA vs. Mannan+OVA). *; p<0.05, **; p<0.01. Data are representative of three independent experiments.
CD13 regulates endocytosis of dynamin-dependent antigens
We next sought to determine the effect of the lack of CD13 on endocytosis of another well characterized receptor-mediated ligand, transferrin, or the fluid-phase uptake of dextran (24). Treatment of DCs with fluorescently labeled versions of these ligands showed that similar to OVA uptake (Fig 6A), transferrin internalization (Fig 6B) was significantly increased in the KO cells compared to WT, while FITC-dextran uptake was equivalent in both genotypes (Fig 6C). Furthermore, treatment of cells with Dynasore, a small molecule inhibitor of the scission GTPases dynamin 1 and 2 (25) reduced the levels of internalized OVA and transferrin in CD13KO cells to those of WT while having no effect on the uptake of dextran (Fig 6C), suggesting that CD13 may regulate dynamin-dependent endocytic mechanisms in DCs.
Fig 6. CD13 regulates endocytosis of dynamin-dependent antigens.
Similar to OVA-FITC, CD13KO DCs are more efficient in the uptake of Transferrin-FITC. Increasing doses of Dynasore abrogates uptake of A) OVA-FITC and B) Transferrin-FITC but not C) Dextran-FITC in both CD13WT and CD13KO. Data represents MFI +/−SD, n=3 mice. *; p<0.05, **; p<0.01, 3 separate experiments.
To begin to decipher the mechanism by which CD13 regulates endocytosis, we investigated conditions where antigen uptake by DCs is perturbed. It is widely believed that upon maturation, DCs terminate all antigen uptake mechanisms to ensure presentation of a single antigen epitope to responding cells (26). Therefore, if CD13 is important to DC maturation, the increased antigen uptake we observed in CD13KO DCs may reflect a more immature phenotype. However, when we induced maturation in WT and CD13KO DCs with LPS, expression levels of a panel of standard DC maturation and activation markers were slightly, but not significantly, lower in the CD13KO cells as assessed by flow cytometry (Fig 7A). The possibility that this small shift reflects a more immature phenotype is unlikely as the increase in antigen uptake and subsequent cross-presentation in CD13KO DCs was abrogated upon maturation with LPS, suggesting these cells mature normally (Figs 7B, C). Alternatively, the maturation-dependent decrease in MR uptake has recently been attributed to the downregulation of MR levels in mature DCs (27). MR expression is clearly reduced in both WT and CD13KO DCs treated with LPS (Fig 7D) and thus persistent MR expression during maturation in the absence of CD13 is not responsible for the enhanced uptake in CD13KO cells.
Fig 7. CD13KO DCs mature normally but show aberrant levels of activated p38 MAPK and pAkt.
A) Effect of LPS stimulation on expression of activation and maturation markers in CD13WT (solid line) and CD13KO (dotted line) BMDCs, grey area indicate unstained cells. OVA uptake B) and B3Z response C) were significantly reduced in both genotypes upon LPS treatment. D) Immunoblot analysis of MR protein expression under these conditions in either genotype. E) Treatment of DCs with specific inhibitors of p38-MAPK, PI-3K/Akt, JNK and ERK on OVA uptake. Data represents MFI +/−SD, n=2 mice. *; p<0.05, **; p<0.01. In unstimulated BMDCs, increased phospho p38 MAPK F) but reduced phospho Akt G) levels were observed in CD13KO cells compared to CD13WT. Data are representative of three independent experiments.
MAPK signaling is dysregulated in CD13 null DCs
It is well recognized that endocytosis regulates signal transduction cascades. Conversely, the process of endocytosis itself is tightly regulated by kinases of distinct signaling cascades [reviewed in ref. (28)]. To examine whether the increase in endocytosis in CD13KO cells involves signaling molecules, we treated DCs with inhibitors of various kinases that have been implicated in the regulation of endocytosis. Interestingly, inhibition of the stress kinase p38MAPK reduced OVA uptake by the CD13KO cells to a level similar to that of WT (Fig 7E) suggesting that p38MAPK is a critical contributor to CD13-dependent endocytosis, while blocking ERK or JNK kinases had no effect. Alternatively, PI-3K/Akt inhibition increased uptake by both WT and KO DCs equally, suggesting a negative regulatory role for these kinases in endocytic uptake (Fig 7E and supplemental Figure S1). Commensurate with levels of increased antigen uptake in unstimulated CD13KO DCs, phosphorylated p38MAPK protein levels were enhanced over WT (Fig 7F). In addition, and in agreement with functional data, phospho-Akt levels were lower in CD13KO DCs (Fig 7G). Activation of p38 MAPK increased in both WT and CD13KO cells upon antigen treatment (Fig 7F) as previously described (29). Therefore, CD13 negatively regulates dynamin-dependent, receptor-mediated endocytosis of ligands by mechanisms involving the p38MAPK, PI-3K/Akt kinase pathways, leading to increased antigen uptake, antigen cross-presentation and T cell activation.
Discussion
CD13 is widely expressed but it is generally found only on specific cell types within a given tissue, enabling it to perform multiple functions. For example in the kidney it is highly expressed on the luminal membrane of epithelial cells lining the proximal tubules where it degrades peptides to facilitate amino acid resorption (9). Alternatively, on monocytes it is a homotypic adhesion molecule that regulates immune cell trafficking to sites of inflammation independent of its enzymatic activity (11). In the present investigation we explored the functional consequence of the high expression of CD13 on the CD8+ subset of murine DCs and its human counterpart, CD11c+/CD141+ peripheral blood cells. Our study demonstrated that the absence of CD13 significantly enhances cross-presentation of soluble antigen in vivo and in vitro via regulation of receptor-mediated uptake. While uptake of the full-length polypeptide of the prototypical antigen OVA was clearly increased in CD13KO DCs, other modes of antigen uptake such as phagocytosis or pinocytosis were not affected by loss of CD13 nor were antigen processing or presentation mechanisms. We found that CD13 and antigen appear to co-localize in DCs, however CD13 and the MR do not co-immunoprecipitate under these conditions, which is consistent with their occupying the same endosomal compartment and that CD13 regulation of endocytosis is not limited to the MR. Indeed, lack of CD13 also increased the endocytosis of transferrin in a dynamin-dependent manner but had no effect on the fluid phase, dynamin-independent uptake of dextran, supporting specificity in CD13 regulation of internalization. Mechanistically, we found that while CD13KO DCs mature normally, key mediators of endocytosis p38MAPK and Akt are dysregulated in these cells and their inhibition perturbs the enhanced CD13-dependent uptake. Thus, altered kinase activation may contribute to the significant and productive amplification of antigen endocytosis resulting in enhanced antigen presentation and T cell responses.
Relevant to our current findings, a previously published study described an association among CD13, the MR and antigen in a macrophage cell line where CD13 was localized and co-internalized with MR and OVA (30). However, while these investigators postulated a role for CD13 in MR uptake, that role remained undefined. In our study, investigation of the contribution of CD13 to DC function and dissection of the mechanism of CD13-dependent antigen cross-presentation led us to a similar observation with a novel and unforeseen functional outcome, inhibition of antigen uptake. In this regard, gain of function studies have suggested that CD13 is a phagocytic accessory molecule that enhances Fcγ-receptor-mediated phagocytosis upon crosslinking with monoclonal antibodies (11). However in studies using our CD13KO animals we have found that lack of CD13 had no effect on macrophage uptake of opsonized antigen (15) which is supported by our current results showing that DC presentation of phagocytosed OVA-coated polystyrene beads is independent of CD13. Alternatively, early studies implicated CD13 in trimming of antigenic peptides prior to MHC loading (17), but this function has subsequently been attributed to the endoplasmic reticulum resident peptidases ERAP1 and 2, [endoplasmic reticulum associated peptidase 1 and 2 (18, 31)]. Thus, CD13 may be primarily an endocytic regulator and may contribute to presentation of antigen in this context alone.
Not surprisingly, CD13 itself is frequently endocytosed either through physiological sorting mechanisms or following ligation by physiologic or targeted ligands where its mode of uptake appears to depend on the cell type and proteins associated with CD13. In polarized epithelial cells, CD13 is an apical membrane protein, although newly synthesized CD13 is first expressed on the basolateral surface and subsequently internalized into distinct sorting endosomes in a clathrin, dynamin and cdc42 dependent manner (32). These endosomes are subsequently transcytosed and fuse with the apical membrane to achieve polarization (33, 34). Alternatively in other cells, internalization of CD13 from the cell surface can be triggered by binding of antibodies (11), coronavirus (35), drugs (36) or CD13-targeted vesicles (37). Where the mechanisms have been characterized, it appears that when ligated, CD13 is internalized by lipid raft/caveolar mechanisms (35). In addition, CD13 internalization can be modulated by associated proteins as illustrated by studies showing that CD13 is internalized via clathrin dependent mechanisms in endothelial cells, but transfection of a construct expressing RECK (reversion-inducing cysteine-rich protein with Kazal motifs) induces CD13/RECK complexes that are internalized in an alternate, clathrin independent manner (38). Therefore, CD13 is associated with various mechanisms of internalization, consistent with a role as a regulator of endocytosis.
Inhibition of p38MAPK activity abrogated the CD13-dependent increase in antigen uptake, while blocking the PI-3K/Akt pathway increased uptake in both WT and KO cells. Accordingly, basal phospho-p38MAPK levels are increased and phospho-Akt levels decreased in CD13KO DCs to an extent consistent with the increase in antigen uptake, implicating these kinases in the phenotype of CD13KO cells. Previous studies in numerous cell types have implicated active p38MAPK as a positive regulator of receptor-mediated endocytosis and blocking this activity prevented cargo internalization. Pertinent to this study, p38MAPK-dependent activation of the Rsk family serine-threonine kinase MAPKAPK2 is necessary for TLR-mediated endocytosis in DCs (39). Similarly, in kidney epithelium, entry of the avian reovirus requires activated p38MAPK to gain entry to the cell (40) as does EGFR internalization in keratinocytes (41). Mechanistic studies in neutrophils and fibroblasts have shown that p38MAPK is required for endosomal formation and internalization by the phosphorylation of endosomal proteins EEA-1 and Rab5a (42, 43). These data are consistent with p38MAPK as a mediator of enhanced antigen uptake in CD13KO cells.
The mechanism by which p38MAPK activity is increased by the loss of CD13 remains to be determined. As members of the mammalian stress-activated MAPK family, p38MAPKs are activated by cellular stresses such as irradiation, hypoxia, osmotic and heat shock as well as the ligation of a wide variety of physiological receptors [reviewed in (44)]. Mechanistically, p38MAPK is activated by a triple-layered kinase cascade that sequentially phosphorylates intermediate kinases to result in actively phosphorylated p38MAPK. Conversely, p38MAPK is inactivated by the MAPK phosphatases (MKPs) whose differential expression and activity dictates MAPK signal intensity and the duration of the physiological response. Relevant to our study, MR-mediated antigen uptake itself has been shown to activate p38MAPK (29) as does maturation of DCs in response to a number of stimuli, where blocking p38MAPK activity inhibits DC maturation (45). Therefore, the lack of CD13 may affect any number of p38MAPK regulators to provoke its activation. Alternatively, we have previously shown that antibody crosslinking of CD13 on human monocytic cell lines to mimic ligand binding induces signal transduction cascades (11), including activation of p38 MAPK (unpublished data) and thus CD13 may be more directly linked to p38 MAPK activation. However, the relevance of this observation to the present study awaits elucidation of the physiological relationship between basal vs. crosslinked CD13 activities. Finally, antagonism between the p38MAPK and Akt pathways has been demonstrated in a number of studies [reviewed in (46)], which may explain reduced levels of activated Akt in CD13KO cells. Further investigation will be necessary to dissect the specific link between CD13 expression and CD13 crosslinking and their effects on kinase activation and endocytosis.
Why a molecule that ultimately inhibits antigen cross-presentation is highly and specifically expressed on the subset of dendritic cells dedicated for cross-presentation is not clear. However, it has been shown that the efficiency of antigen presentation is dictated by the mechanism of antigen uptake (47, 48) and thus it is possible that CD13 suppression of receptor-mediated endocytosis diverts antigen to an uptake mechanism that would produce a more effective response. Alternatively, while receptor-mediated antigen uptake is highly efficient (49), receptors differ in their ability to supply antigen to intracellular compartments (50) which is thought to reflect a mechanism of endocytic regulation by restricting access to antigens (48). CD13 may regulate uptake by particular receptors and so may be an underlying factor in differential receptor uptake. Finally, as discussed above, CD13 itself is endocytosed in response to a number of different stimuli and similarly, its membrane localization is also altered in response to various signals (51), suggesting that its expression level and/or localization is context dependent. Thus, downregulation or sequestration of CD13 under certain conditions would allow endocytosis to proceed. Further investigation is required to address these interesting questions.
Importantly, a universal goal in the design of effective anti-tumor and anti-viral vaccines is enhanced DC antigen cross-presentation to amplify the production of antigen specific cytotoxic T lymphocytes directed against tumor or viral antigens [reviewed in ref. (52)]. Targeting antigens to DC surface proteins, including the MR, by various means is an attractive strategy that is often used to achieve this goal (53, 54). Our identification of CD13 as a regulator of DC antigen cross-presentation suggests that design of vaccines to target CD13, in combination with receptor-specific delivery of antigen, may be an effective and novel strategy to augment CTL responses and have the potential to improve both targeted vaccine delivery as well as modulate other immune responses.
Supplementary Material
Acknowledgments
Supported by Public Health Service grants CA-106345, NCI and HL-70694, NLHBI.
The authors thank the laboratories of Drs. Claffey, Aguila, Srivastava, Ferrer, LeFrancois and Khanna. In addition we thank the Gene Targeting and Transgenic, Flow Cytometry and the Research Histology Cores.
Abbreviations
- spDC
splenic derived dendritic cell
- BMDC
bone marrow-derived dendritic cell
- MR
Mannose receptor
- WT
wild type
- KO
knockout
References
- 1.Bevan MJ. Antigen recognition. Class discrimination in the world of immunology. Nature. 1987;325:192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- 2.den Haan JMM, Lehar SM, Bevan MJ. Cd8+ but Not Cd8− Dendritic Cells Cross-Prime Cytotoxic T Cells in Vivo. The Journal of Experimental Medicine. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, Le Moine A, Faure F, Donckier V, Sancho D, Cerundolo V, Bonnet D, Reis e Sousa C. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJ, Hart DN, Radford KJ. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 7.Lakadamyali M, Rust MJ, Zhuang X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgdorf S, Scholz C, Kautz A, Tampe R, Kurts C. Spatial and mechanistic separation of cross-presentation and endogenous antigen presentation. Nat Immunol. 2008;9:558–566. doi: 10.1038/ni.1601. [DOI] [PubMed] [Google Scholar]
- 9.Shipp MA, Look AT. Hematopoietic differentiation antigens that are membrane-associated enzymes: cutting is the key! Blood. 1993;82:1052–1070. [PubMed] [Google Scholar]
- 10.Yeager CL, Ashmun RA, Williams RK, Cardellichio CB, Shapiro LH, Look AT, Holmes KV. Human aminopeptidase N is a receptor for human coronavirus 229E. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mina-Osorio P, Winnicka B, O’Conor C, Grant CL, Vogel LK, Rodriguez-Pinto D, Holmes KV, Ortega E, Shapiro LH. CD13 is a novel mediator of monocytic/endothelial cell adhesion. J Leukoc Biol. 2008;84:448–459. doi: 10.1189/jlb.1107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- 13.Fontijn D, Duyndam MC, van Berkel MP, Yuana Y, Shapiro LH, Pinedo HM, Broxterman HJ, Boven E. CD13/Aminopeptidase N overexpression by basic fibroblast growth factor mediates enhanced invasiveness of 1F6 human melanoma cells. Br J Cancer. 2006;94:1627–1636. doi: 10.1038/sj.bjc.6603157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii H, Nakajima M, Saiki I, Yoneda J, Azuma I, Tsuruo T. Human melanoma invasion and metastasis enhancement by high expression of aminopeptidase N/CD13. Clin Exp Metastasis. 1995;13:337–344. doi: 10.1007/BF00121910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winnicka B, O’Conor C, Schacke W, Vernier K, Grant CL, Fenteany FH, Pereira FE, Liang B, Kaur A, Zhao R, Montrose DC, Rosenberg DW, Aguila HL, Shapiro LH. CD13 is dispensable for normal hematopoiesis and myeloid cell functions in the mouse. J Leukoc Biol. 2010;88:347–359. doi: 10.1189/jlb.0210065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mina-Osorio P, Ortega E. Aminopeptidase N (CD13) functionally interacts with FcgammaRs in human monocytes. J Leukoc Biol. 2005;77:1008–1017. doi: 10.1189/jlb.1204714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen SL, Pedersen LO, Buus S, Stryhn A. T cell responses affected by aminopeptidase N (CD13)-mediated trimming of major histocompatibility complex class II-bound peptides. J Exp Med. 1996;184:183–189. doi: 10.1084/jem.184.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat Immunol. 2002;3:1177–1184. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 19.Karttunen J, Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci U S A. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Burgdorf S, Lukacs-Kornek V, Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 22.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 23.Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T. Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes. J Antibiot (Tokyo) 1976;29:97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 27.Platt CD, Ma JK, Chalouni C, Ebersold M, Bou-Reslan H, Carano RA, Mellman I, Delamarre L. Mature dendritic cells use endocytic receptors to capture and present antigens. Proc Natl Acad Sci U S A. 2010;107:4287–4292. doi: 10.1073/pnas.0910609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol. 2010;185:929–942. doi: 10.4049/jimmunol.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabrilovac J, Cupic B, Zivkovic E, Horvat L, Majhen D. Expression, regulation and functional activities of aminopeptidase N (EC 3.4.11.2; APN; CD13) on murine macrophage J774 cell line. Immunobiology. 216:132–144. doi: 10.1016/j.imbio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 32.Ait-Slimane T, Galmes R, Trugnan G, Maurice M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol Biol Cell. 2009;20:3792–3800. doi: 10.1091/mbc.E09-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renold A, Cescato R, Beuret N, Vogel LK, Wahlberg JM, Brown JL, Fiedler K, Spiess M. Basolateral sorting signals differ in their ability to redirect apical proteins to the basolateral cell surface. J Biol Chem. 2000;275:9290–9295. doi: 10.1074/jbc.275.13.9290. [DOI] [PubMed] [Google Scholar]
- 34.Ihrke G, Martin GV, Shanks MR, Schrader M, Schroer TA, Hubbard AL. Apical plasma membrane proteins and endolyn-78 travel through a subapical compartment in polarized WIF-B hepatocytes. J Cell Biol. 1998;141:115–133. doi: 10.1083/jcb.141.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen GH, Delmas B, Besnardeau L, Vogel LK, Laude H, Sjostrom H, Noren O. The Coronavirus Transmissible Gastroenteritis Virus Causes Infection after Receptor-Mediated Endocytosis and Acid-Dependent Fusion with an Intracellular Compartment. J Virol. 1998;72:527–534. doi: 10.1128/jvi.72.1.527-534.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jahne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, Orso E, Schmitz G. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor ezetimibe in the enterocyte brush border membrane. J Biol Chem. 2005;280:1306–1320. doi: 10.1074/jbc.M406309200. [DOI] [PubMed] [Google Scholar]
- 37.Garde SV, Forte AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, Wu JJ. Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD13-expressing cells and its antitumor effects. Anticancer Drugs. 2007;18:1189–1200. doi: 10.1097/CAD.0b013e3282a213ce. [DOI] [PubMed] [Google Scholar]
- 38.Miki T, Takegami Y, Okawa K, Muraguchi T, Noda M, Takahashi C. The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J Biol Chem. 2007;282:12341–12352. doi: 10.1074/jbc.M610948200. [DOI] [PubMed] [Google Scholar]
- 39.Zaru R, Ronkina N, Gaestel M, Arthur JS, Watts C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signaling response in dendritic cells and is activated through two distinct pathways. Nat Immunol. 2007;8:1227–1235. doi: 10.1038/ni1517. [DOI] [PubMed] [Google Scholar]
- 40.Huang WR, Wang YC, Chi PI, Wang L, Wang CY, Lin CH, Liu HJ. Cell entry of avian reovirus follows a caveolin-1-mediated and dynamin-2-dependent endocytic pathway that requires activation of p38 mitogen-activated protein kinase (MAPK) and Src signaling pathways as well as microtubules and small GTPase Rab5 protein. J Biol Chem. 2011;286:30780–30794. doi: 10.1074/jbc.M111.257154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert S, Frankart A, Poumay Y. p38 MAPK-regulated EGFR internalization takes place in keratinocyte monolayer during stress conditions. Arch Dermatol Res. 2010;302:229–233. doi: 10.1007/s00403-009-1020-0. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin NJ, Banerjee A, Khan SY, Lieber JL, Kelher MR, Gamboni-Robertson F, Sheppard FR, Moore EE, Mierau GW, Elzi DJ, Silliman CC. Platelet-activating factor-mediated endosome formation causes membrane translocation of p67phox and p40phox that requires recruitment and activation of p38 MAPK, Rab5a, and phosphatidylinositol 3-kinase in human neutrophils. J Immunol. 2008;180:8192–8203. doi: 10.4049/jimmunol.180.12.8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mace G, Miaczynska M, Zerial M, Nebreda AR. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J. 2005;24:3235–3246. doi: 10.1038/sj.emboj.7600799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 45.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 46.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 47.Belizaire R, Unanue ER. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc Natl Acad Sci U S A. 2009;106:17463–17468. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185:3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–155. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 50.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 51.Petrovic N, Schacke W, Gahagan JR, O’Conor CA, Winnicka B, Conway RE, Mina-Osorio P, Shapiro LH. CD13/APN regulates endothelial invasion and filopodia formation. Blood. 2007;110:142–150. doi: 10.1182/blood-2006-02-002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen TR, Dickgreber N, Hermans IF. Tumor antigen presentation by dendritic cells. Crit Rev Immunol. 2010;30:345–386. doi: 10.1615/critrevimmunol.v30.i4.30. [DOI] [PubMed] [Google Scholar]
- 53.Singh SK, Streng-Ouwehand I, Litjens M, Kalay H, Burgdorf S, Saeland E, Kurts C, Unger WW, van Kooyk Y. Design of neo-glycoconjugates that target the mannose receptor and enhance TLR-independent cross-presentation and Th1 polarization. Eur J Immunol. 2011;41:916–925. doi: 10.1002/eji.201040762. [DOI] [PubMed] [Google Scholar]
- 54.Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, Keler T, Odunsi K, Old LJ, Ritter G, Gnjatic S. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186:1218–1227. doi: 10.4049/jimmunol.1000808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.