Background: FAD incorporation into bacterial flavoproteins is thought to be autocatalytic.
Results: The conserved hypothetical protein SdhE directly interacts with SdhA and is required for SdhA flavinylation.
Conclusion: SdhE is a co-factor chaperone that incorporates FAD into SdhA, which is required for succinate dehydrogenase function.
Significance: SdhE is the first protein identified as required for flavinylation of a flavoprotein in bacteria.
Keywords: Bacterial Metabolism, Electron Transport, FAD, Flavoproteins, Tricarboxylic Acid (TCA) Cycle, Co-factor, Prodigiosin, Sdh5, SdhA, Succinate Dehydrogenase
Abstract
Conserved uncharacterized genes account for ∼30% of genes in both eukaryotic and bacterial genomes and are predicted to encode what are often termed “conserved hypothetical proteins.” Many of these proteins have a wide phylogenetic distribution and might play important roles in conserved cellular pathways. Using the bacterium Serratia as a model system, we have investigated two conserved uncharacterized proteins, YgfY (a DUF339 protein, renamed SdhE; succinate dehydrogenase protein E) and YgfX (a DUF1434 protein). SdhE was required for growth on succinate as a sole carbon source and for the function, but not stability, of succinate dehydrogenase, an important component of the electron transport chain and the tricarboxylic acid cycle. SdhE interacted with the flavoprotein SdhA, directly bound the flavin adenine dinucleotide co-factor, and was required for the flavinylation of SdhA. This is the first demonstration of a protein required for FAD incorporation in bacteria. Furthermore, the loss of SdhE was highly pleiotropic, suggesting that SdhE might flavinylate other flavoproteins. Our findings are of wide importance to central metabolism because SdhE homologues are present in α-, β-, and γ-proteobacteria and multiple eukaryotes, including humans and yeast.
Introduction
The tricarboxylic acid (TCA)5 cycle is responsible for the oxidation of respiratory substrates to generate NADH and FADH2. The electron transport chain (ETC) couples the oxidation of NADH and FADH2 with the translocation of electrons by coenzymes through membrane-bound complexes I–IV to the reduction of a terminal electron acceptor (1, 2). This generates a proton gradient across bacterial and mitochondrial inner membranes to drive ATP synthesis via ATPase(s). This process of oxidative phosphorylation is the predominant means of energy generation among all organisms.
Both the TCA cycle and ETC are complex pathways that involve many participating proteins. The central elements of these pathways are well characterized, but important components might remain undiscovered. Conserved hypotheticals are uncharacterized proteins found in multiple species and may be involved in important steps in central cellular processes (3, 4). It has been hypothesized that the characterization of these proteins will give insights into complex cellular processes and potentially identify novel biochemical and genetic functions (4).
Gram-negative bacteria of the genus Serratia are members of the Enterobacteriaceae and are opportunistic pathogens of animal, plant, and insect hosts (5). Members of this genus are characterized by their ability to synthesize the red tripyrrole antibiotic, prodigiosin (2-methyl-3-pentyl-6-methoxyprodiginine; pig), which has antimicrobial, anticancer, and immunosuppressive properties (6, 7). Transposon mutagenesis to identify regulators of pig biosynthesis identified a putative membrane-bound protein, YgfX (previously termed PigV). Mutation of ygfX resulted in an ∼85% reduction in pig, and ygfX formed a putative operon with an upstream conserved hypothetical gene, ygfY (renamed sdhE; succinate dehydrogenase protein E) (8).
The aim of this current study was to investigate the function of the conserved hypothetical proteins SdhE and YgfX using the bacterium Serratia, a system that has been used previously to successfully characterize other conserved hypothetical proteins (9) and regulatory mechanisms (8, 10–15). Our bioinformatic analysis led to the hypothesis that SdhE was involved in succinate metabolism. The deletion of sdhE prevented growth in minimal succinate medium, and further analysis demonstrated that SdhE was required for succinate dehydrogenase (SDH) activity through the incorporation of the flavin adenine dinucleotide (FAD) co-factor into the flavoprotein, SdhA. Phylogenetic analysis of SdhE showed that it was found in both eukaryotes and bacteria, specifically the α-, β-, and γ-proteobacteria. The results from this study have identified a previously uncharacterized component of SDH in bacteria. Furthermore, this is the first report of a protein required for the incorporation of an FAD co-factor into a protein complex in any bacterial species.
EXPERIMENTAL PROCEDURES
Bacterial Strains, Plasmids, Phage, and Culture Conditions
Bacterial strains and plasmids are listed in supplemental Table S1, primers are in supplemental Table S2, and details of strain and plasmid constructions are described in the supplemental Experimental Procedures. Serratia sp. strain ATCC 39006 and Escherichia coli strains were grown at 30 and 37 °C, respectively, in Luria broth (LB; 5 g/liter yeast extract, 10 g/liter Bacto Tryptone, and 5 g/liter NaCl), in minimal medium (0.1% (NH4)2SO4, 0.41 mm MgSO4,40 mm K2HPO4, 14.7 mm KH2PO4, and 0.2% w/v glucose or 0.4% w/v succinate buffered with 75 mm HEPES, pH 6.9–7.1) at 180 rpm, or on LB agar or minimal agar supplemented with 1.5% (w/v) agar. For growth in minimal medium, strains were first grown overnight in LB, subcultured overnight in minimal glucose medium (0.2%), and then used to start growth in minimal medium in flasks. Bacterial growth was measured at an optical density of 600 nm (A600) in a Jenway 6300 spectrophotometer. When required, media were supplemented with antibiotics at the following concentrations unless otherwise stated: 50 μg/ml kanamycin, 50 μg/ml streptomycin, 50 μg/ml ampicillin, and 25 μg/ml chloramphenicol.
Biological Membrane Preparation
Membrane and soluble fractions for localization studies were prepared following protocols previously described with plasmids induced at an A600 of 0.6 with either 1 mm IPTG or 0.1% arabinose (9). Membrane fractions for Blue Native (BN)-PAGE analysis were prepared using the same methods with the addition of 10 μl of protease inhibitor mixture (Sigma) and 5 μl of DNase (Promega) to the resuspended culture prior to cell lysis. Membrane pellets for BN-PAGE were resuspended in 50 mm imidazole with 10% glycerol, and 400 μg of protein aliquots were frozen at −80 °C for future use. Protein concentration was determined using the Thermo Scientific BCA assay. When required, 400-μg aliquots were thawed and solubilized on ice with 0.5% Triton X-100 (Sigma-Aldrich) using 4× Invitrogen BN-PAGE sample buffer and prepared for BN-PAGE as described previously (16). NativePAGE Novex Bis-Tris gels (4–16%) (Invitrogen) were run following the manufacturer's guidelines and visualized with Coomassie Blue staining as described previously (17).
Purification of His-tagged Proteins
Five-ml LB overnight cultures of strains were used to inoculate 100-ml LB cultures grown in 1-liter flasks at a ratio of 1:50. Cultures were grown to an A600 of 0.6, induced with 1 mm IPTG, and grown overnight. Cultures were lysed under native conditions with 10 μl of protease inhibitor mixture (Sigma) and 5 μl of DNase (Promega) and purified using Ni-NTA resin and gravity feed columns using the batch purification protocol described in The QIAexpressionist (47). Proteins were eluted using an imidazole gradient of 45–250 mm imidazole.
Western Blotting
Western blotting of SDS-PAGE and BN-PAGE gels was carried out using a Tris/glycine buffer system following standard protocols. For BN-PAGE gels prior to immunodetection, PVDF membranes (0.45 μm) were washed in 20 ml of 8% acetic acid following Invitrogen protocols. Membranes were analyzed using mouse monoclonal anti-His (Sigma) or monoclonal anti-FLAG (Sigma) antibodies, and as a secondary antibody, goat anti-mouse IgG horseradish peroxidase (Santa Cruz Biotechnology). Bands were visualized by either the ChemiDoc imaging system (Bio-Rad) or x-ray film using the SuperSignal West Pico chemiluminescent substrate kit.
SDH and UV Fluorescence Flavin Assays
The enzymatic activity of SDH was measured by monitoring the decrease in absorbance at 600 nm over a period of 10 min in the presence of 0.1 m sodium succinate (pH 7.5) (Sigma), 0.05 mm 2,6-dichlorophenolindophenol (extinction co-efficient = 22 mm−1 cm−1) (Sigma), 0.1 m NaPO4 buffer (pH 7), and 50–100 μg of resuspended biological membranes in a 1-ml reaction volume. Experiments were carried out in technical triplicates, and activity was expressed relative to the background reduction of 2,6-dichlorophenolindophenol (membranes without succinate).
For FAD-UV assays, His-tagged purified proteins were separated by SDS-PAGE (15% polyacrylamide gels for SdhA and 20% gels for SdhE), destained, and exposed to UV light (FAD-UV) as described previously (18). Aliquots of the same samples were separated on additional gels for visualization of proteins by Coomassie Blue staining or Western blotting.
FAD Binding Assays
Purified proteins were incubated with the stated amounts of exogenous FAD from a 1 mg/ml stock in a 100-μl reaction volume buffered with 0.1 m NaPO4 (pH 7). Reactions were left overnight at room temperature and analyzed by FAD-UV and Coomassie Blue staining.
Cell lysates for FAD incorporation assays of wild type (WT) and ΔsdhE overexpressing SdhA N-His were prepared from 100-ml cultures grown in a 1-liter flask, induced with 1 mm IPTG at A600 of 0.5 and grown overnight. Cultures were harvested, lysed by lysozymes (20 μg/ml) and sonication (6 × 10-s bursts) in 0.1 m NaPO4 buffer (pH 7), and then centrifuged at 150,000 × g for 30 min with the resulting supernatant being kept at 4 °C for future use (19). FAD binding assays involved incubating 40 μl of WT + SdhA N-His (∼1 mg of total protein) or 83 μl of ΔsdhE + SdhA N-His (∼2 mg of total protein) with 10 μg of FAD with or without 35 μg of purified SdhE N-His in a 100-μl reaction buffered with 0.1 m NaPO4 (pH 7). Reactions were incubated at room temperature for 30 min and analyzed by FAD-UV.
Sample Preparation for UV-visible Spectra
Two ml of purified SdhE N-His (pMAT10) at 0.85 mg/ml from WT Serratia (90 mm imidazole elution fraction) was incubated with or without 300 μg of FAD overnight at 4 °C. To remove imidazole and unbound FAD, SdhE N-His with or without FAD was dialyzed overnight at 4 °C into elution buffer without imidazole (50 mm NaH2PO4, 300 mm NaCl, pH 8.0) with at least three buffer changes. One-ml volumes of each sample were analyzed by UV-visible spectra. Samples were analyzed by FAD-UV to confirm the loss of free FAD. SdhE N-His with or without FAD was also analyzed by intact protein mass spectrometry as described below, with the exception that samples were dialyzed into 0.1 m NaPO4 buffer (pH 7.0).
Trichloroacetic Acid Precipitation
Two-ml samples of purified SdhE N-His (pMAT10) at 0.6 mg/ml from WT Serratia (90 mm imidazole elution fraction) were incubated with or without 300 μg of FAD overnight at 4 °C. Free FAD was not removed from these samples. The UV-visible spectra of SdhE N-His with or without FAD in a total volume of 1 ml was measured. Sixty μl of 50% trichloroacetic acid was added to the 1-ml SdhE N-His samples, to give a final concentration of 3%, and incubated on ice for 30 min. The samples were centrifuged at 10,000 × g for 5 min. The resulting supernatants containing any free FAD were decanted, and the UV-visible spectra were measured as the acid precipitation spectra. The precipitated protein pellets were dissolved in 6 m guanidine hydrochloride in 0.1 m NaPO4, pH 7.0, and the UV-visible spectra were measured. The mol of FAD that precipitated per mol of SdhE N-His was determined using the extinction coefficients of FAD (ϵ450 = 11,300 m−1 cm−1) and SdhE N-His (ϵ280 = 12,615 m−1 cm−1).
Co-immunoprecipitation
Cultures were grown as described for the purification of His-tagged proteins. Cultures were induced with 1 mm IPTG and 0.1% arabinose. Lysates were passed through a Ni-NTA column, washed 8× in 10 mm imidazole wash buffer, and eluted using an imidazole gradient as described previously for the purification of His-tagged proteins.
Intact Protein Mass Spectrometry
SdhE N-His ± FAD was rebuffered either by drying and resolubilization in 30% (v/v) acetonitrile and 0.1% (v/v) trifluoroacetic acid (TFA) in water or by ZipTip purification on C4 material (>50 kDa) or C18 material (<50 kDa). One μl of sample was premixed with 10 μl of matrix (10 mg/ml α-cyano-4-hydroxycinnamic acid dissolved in 65% (v/v) aqueous acetonitrile containing 0.1% (v/v) TFA). Each sample was then spiked with 1 μl of a polypeptide/protein standard for internal mass calibration. An aliquot of 0.8 μl was then spotted onto a MALDI sample plate (Opti-TOF 384-well plate, AB SCIEX) and air-dried. Samples were analyzed on a 4800 MALDI tandem time-of-flight analyzer (MALDI-TOF/TOF, AB SCIEX). All mass spectrometry (MS) spectra were acquired in linear, positive-ion mode with 4000 laser pulses per sample spot. The mass range between 1000 and 25,000 was calibrated on the average masses of a four-polypeptide/protein calibration mix containing porcine insulin 1+ ion (m/z 5777.6), ubiquitin 1+ ion (m/z 8564.9), protein G 2+ ion (m/z 10,800.0), α-lactalbumin 1+ ion (m/z 14,070.1), and protein G 1+ ion (m/z 21,599.0).
Protein Identification by Mass Spectrometry
Excised protein bands were subjected to in-gel digestion with trypsin following previously described protocols (20). Eluted peptides were dried using a centrifugal concentrator. Samples were resolubilized in 5% (v/v) acetonitrile, 0.2% (v/v) formic acid in water and injected onto an Ultimate 3000 nano-flow UHPLC system (Dionex, Thermo Scientific) that was in-line-coupled to the nanospray source of an LTQ-Orbitrap XL hybrid mass spectrometer (Thermo Scientific). Peptides were separated on an in-house packed emitter-tip column (75-μm inner diameter PicoTip fused silica tubing (New Objectives, Woburn, MA) packed with C-18 material on a length of 8–9 cm) by a gradient developed from 5% (v/v) acetonitrile, 0.2% (v/v) formic acid to 80% (v/v) acetonitrile, 0.2% (v/v) formic acid in water over 35 min at a flow rate of 400 nl/min.
Full MS in a mass range between m/z 300–2000 was performed in the Orbitrap mass analyzer with a resolution of 60,000 at m/z 400. The strongest five signals were selected for collision-induced dissociation-MS/MS in the LTQ ion trap at a normalized collision energy of 35%.
For protein identification, MS/MS data were searched against both a user-defined and a Serratia amino acid sequence database (4417 sequence entries) using an in-house Mascot server. The search was set up for full tryptic peptides with a maximum of three missed cleavage sites. Carboxyamidomethyl cysteine, oxidized methionine, and pyroglutamate (Glu and Gln) were included as variable modifications where appropriate. The precursor mass tolerance threshold was 10 ppm, and the maximum fragment mass error was 0.8 Da. A conservative significance threshold using an individual ion score of greater than 36 was selected to account for the small database sizes.
Statistical Analysis
All experiments were performed in triplicate, and results are presented as the mean ± standard deviation. Statistical analysis was performed when appropriate using the Student's t test.
Construction of Phylogenetic Trees
Nucleotide sequences of sdhE (DUF339) homologues were imported into the MEGA4 software from MicrobesOnline (21, 22). Evolutionary history was inferred using the neighbor-joining method with bootstrap test (1000 replicates) used to determine the percentage of replicate trees in which the associated taxa clustered together. Branch lengths were represented in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated from the dataset. There were a total of 210 positions in the final dataset. Phylogenetic analyses were conducted in MEGA4 (21).
RESULTS
sdhEygfX Operon Is Conserved among Enterobacteriaceae
The Serratia ygfX gene encodes a predicted protein with 57% similarity/53% identity at the amino acid level to E. coli YgfX. The predicted translational start site of ygfX overlaps that of sdhE (previously termed ygfY) (encoding a protein with 84% similarity/80% identity at the amino acid level to the E. coli homologue), suggesting a putative operon (sdhEygfX) (supplemental Fig. S1A). Using primers specific to sdhE and ygfX and primers that span the predicted operon, RT-PCR analysis showed that sdhE and ygfX are transcribed as a bicistronic mRNA (supplemental Fig. S1B).
SdhE is an 88-amino acid protein, predicted to be cytoplasmic and a member of the DUF339 and COG2938 family of proteins, an uncharacterized domain present in eukaryotes and bacteria. YgfX is predicted to be a 143-amino acid inner membrane-bound protein and a member of the DUF1434 domain of unknown function. Use of Pfam and MicrobesOnline (22, 23) revealed that DUF1434 is present predominantly in the Enterobacteriaceae and several γ-proteobacterial families (Shewanellaceae and Vibrionaceae). Gene neighborhood analysis of DUF1434 (YgfX) using STRING (24) and the genome context viewer in MicrobesOnline (22) indicated that genes encoding DUF1434 proteins are found in putative operons with genes encoding DUF339 (SdhE) proteins in all Enterobacteriaceae species except Wigglesworthia glossinidia. This revealed that the sdhEygfX operon is highly conserved among this bacterial family.
SdhE Is Required for Succinate Dehydrogenase Activity
Putative functional linkages between SdhE and other proteins were identified using COG2938 and SdhE protein sequences in STRING (24). COG2938 proteins showed strong neighborhood and co-occurrence with SdhA (succinate dehydrogenase flavoprotein subunit) (supplemental Fig. S2A and supplemental Table S3). The Serratia SdhE sequence is absent from the STRING database, so E. coli K12 SdhE was chosen as a representative homologue. The strongest predicted functional partner was YgfX; however, multiple subunits of the SDH complex were present as putative functional partners (supplemental Fig. S2B and supplemental Table S3). Therefore, we hypothesized that SdhE had an involvement in succinate metabolism, by association or interaction with one or more of the subunits of SDH.
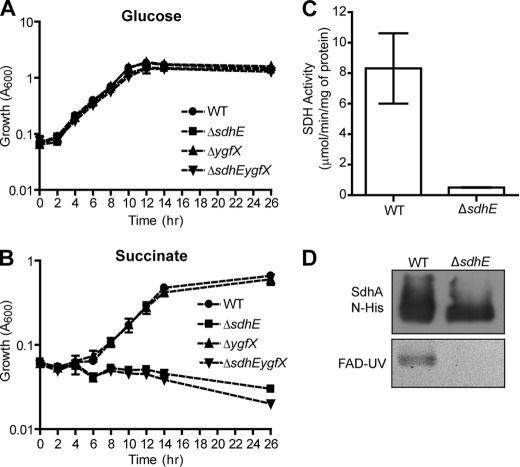
SDH is a key step in the TCA cycle, converting succinate to fumarate and donating electrons to the ETC (25, 26). If the loss of sdhE affected SDH activity, sdhE mutants would have impaired growth on minimal succinate, yet grow as well as wild type (WT; refers to parental strain LacA) on minimal glucose when glycolysis is the predominant means of energy generation and the sdhCDAB operon is repressed (26–28). To test this, clean single and double deletion mutations were constructed in sdhE and ygfX. Both ΔsdhE and ΔsdhEygfX deletion mutants had impaired growth on minimal succinate, yet grew as well as the WT on minimal glucose (Fig. 1, A and B). Impaired growth on minimal succinate could be complemented by sdhE in trans (supplemental Fig. S2C). The loss of YgfX alone did not impair growth on minimal succinate (Fig. 1B). SDH assays showed that the loss of SdhE led to a 90% reduction in SDH activity (Fig. 1C). This shows that the deletion of sdhE leads to a nonfunctional SDH complex.
FIGURE 1.
SdhE is required for succinate metabolism and flavinylation of SdhA. A and B, WT and mutant strains were grown in 25 ml (0.2%) of minimal glucose (A) and (0.4%) minimal sodium succinate medium in 250-ml flasks (B). C, SDH activity. SDH activity assays were carried out on Serratia WT and ΔsdhE membranes normalized to total protein. D, flavinylation of SdhA. SdhA N-His-tagged proteins purified from WT and ΔsdhE Serratia were assessed for FAD incorporation (lower panel), normalized to total protein (1.6 μg/lane) as shown by Western blotting (upper panel). Error bars in panels A–C indicate S.D.
SdhE Is Required for Incorporation of FAD into SdhA
FAD is a co-factor required for SDH activity (26, 29). During the course of our work, it was reported that a DUF339 protein (renamed Sdh5) in Saccharomyces cerevisiae was required for FAD incorporation into the SDH flavoprotein, Sdh1 (30). We hypothesized that SdhE homologues in bacteria would flavinylate the Sdh1 homologue SdhA. The presence of covalently bound FAD can be determined by running purified proteins on a denaturing gel as noncovalently linked FAD is released from the protein, whereas covalently linked FAD remains bound (31, 32). Visualization by UV light (FAD-UV) produces an illuminated band at the same molecular weight as the protein of interest (18, 31). To test FAD insertion, N-terminally His-tagged SdhA was overexpressed in WT or ΔsdhE Serratia, purified, and examined by FAD-UV. SdhA N-His purified from WT Serratia contained a detectable FAD co-factor, whereas SdhA N-His purified from ΔsdhE lacked FAD (Fig. 1D). This confirms that SdhE is required for the incorporation of FAD into SdhA.
Loss of SdhE Does Not Affect SDH Stability
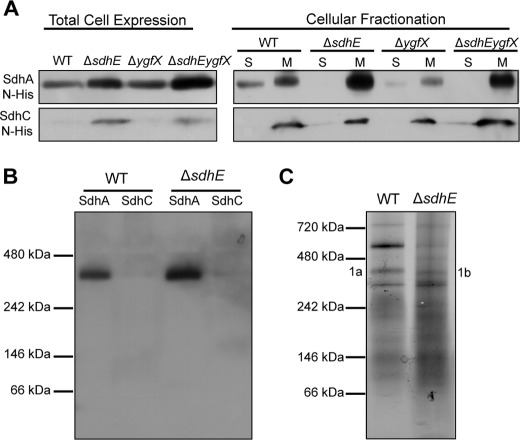
In S. cerevisiae, mutation of sdh5 affected FAD incorporation and resulted in SDH destabilization (30). This prompted investigations into whether SdhE and FAD incorporation also influenced SDH stability in bacteria. The stability of SDH was first assessed by examining the cellular localization of the N-terminal His-tagged SDH subunits SdhA and SdhC. Total levels of SdhA N-His and SdhC N-His were at higher levels in ΔsdhE and ΔsdhEygfX relative to the WT and ΔygfX strains (Fig. 2A). In all strains, SdhC N-His was correctly localized to the membrane (Fig. 2A). All strains correctly localized SdhA N-His to the membrane, with SdhA N-His more abundant in the membrane fractions of ΔsdhE and ΔsdhEygfX relative to the WT and ΔygfX (Fig. 2A). SdhA N-His was present in the soluble fraction of the WT and ΔygfX strains, but not in ΔsdhE and ΔsdhEygfX (Fig. 2A).
FIGURE 2.
Loss of SdhE does not affect SDH stability. A, cellular localization of SDH subunits. SdhA N-His and SdhC N-His were expressed without IPTG induction in Serratia WT and mutant strains that were separated into soluble (S) and membrane (M) fractions, and His-tagged proteins were detected by Western blotting. Twenty-five μl of A600 adjusted culture was loaded per lane for total cell expressions, whereas 30 μg of total protein was loaded per lane for cellular fractionations. B, integration of SDH subunits into SDH. Membrane fractions from Serratia WT and ΔsdhE expressing SdhA N-His and SdhC N-His were separated by BN-PAGE with 200 μg of total protein loaded per lane, and proteins were visualized by Western blotting. C, formation of SDH in native membranes. Membrane fractions from WT and ΔsdhE were separated by BN-PAGE with 200 μg of total protein loaded per lane, and proteins were visualized by Coomassie Blue staining. Bands of interest (1a and 1b) were analyzed by mass spectrometry.
The formation of the complete SDH complex was further assessed by separating membranes from WT and ΔsdhE strains expressing SdhA and SdhC N-His proteins by BN-PAGE. Both WT and ΔsdhE produced bands at ∼360 kDa for SdhA N-His in addition to a faint, but detectable, SdhC N-His (Fig. 2B). Crystal structure and BN-PAGE data have shown that functional SDH is trimeric with a molecular mass of 360 kDa (33, 34). Finally, native WT and ΔsdhE membranes were separated by BN-PAGE (Fig. 2C), and proteins present in bands at 360 kDa were identified by LC-coupled LTQ-Orbitrap MS. SdhA was the highest peptide match for both WT and ΔsdhE (protein score, 1537/1013; peptide matches, 40/24, respectively) with SdhB, SdhC, and SdhD also present at lower protein scores and peptide matches. Therefore, the loss of SdhE does not affect the stability or formation of SDH in Serratia.
SdhE Is a Soluble Monomer That Interacts with SdhA
To test whether SdhE was a soluble protein and YgfX was membrane-bound, C-terminal FLAG-tagged versions of both proteins were overexpressed in WT Serratia. SdhE C-FLAG was detected only in the soluble fraction, and YgfX C-FLAG was detected only in the membrane fraction (Fig. 3A). Previous structural analysis of E. coli SdhE has suggested that it is monomeric (35). Consistent with this, co-immunoprecipitation and bacterial two-hybrid studies revealed no self-interactions (data not shown).
FIGURE 3.
SdhE is a soluble monomer that interacts with SdhA. A, localization of SdhE and YgfX. SdhE C-FLAG and YgfX C-FLAG were grown in WT Serratia and separated into soluble (S) and membrane (M) fractions. Twenty μg of total protein was loaded per lane and analyzed by Western blotting. B, SdhE N-His interacts with native SdhA. Proteins were purified from WT Serratia expressing SdhE N-His or an empty vector control (pMAT15) with the resulting elution fractions (90 mm imidazole) analyzed by Coomassie Blue staining and FAD-UV. An asterisk represents bands analyzed by mass spectrometry. C, SdhE C-FLAG interacts with SdhA N-His. Affinity purification involved WT Serratia co-expressing SdhE C-FLAG with or without SdhA N-His. TC, total cell expression prior to affinity purification; FW, final wash; E1, 45 mm imidazole elution; E2, 90 mm imidazole elution.
S. cerevisiae Sdh5 interacted with Sdh1 (30). To test whether this occurred between the bacterial homologues, SdhE N-His was purified from WT Serratia under native conditions to determine whether native SdhA would co-purify. Analysis of all proteins co-eluting with SdhE N-His, by LC-coupled LTQ-Orbitrap MS, showed that although SdhA bound weakly to the Ni-NTA column in the vector control (peptide matches, 15; protein score, 584), a greater amount of SdhA was detected when SdhE N-His was purified on an Ni-NTA column (peptide matches, 47; protein score, 2017). FAD-UV analysis of SdhE N-His elution fractions provided further confirmation by showing a faint, but detectable, illuminating band at the size of SdhA (64 kDa), but absent from the vector control (Fig. 3B). However, when viewed by Coomassie Blue staining, a band at ∼64 kDa was present in both SdhE N-His and the vector control (Fig. 3B). MS of this band showed that the dominant product from both vector and SdhE N-His was glucosamine/fructose 6-phosphate aminotransferase (66 kDa). Interestingly, the SdhE N-His 64-kDa band contained SdhA (P score 916/peptide matches 12) but was absent from the vector control.
To further test this interaction, a reciprocal co-immunoprecipitation was used by purifying SdhA N-His (bait) on an Ni-NTA resin and screening the co-purification of co-expressed SdhE C-FLAG (prey). SdhE C-FLAG co-purified with SdhA N-His (Fig. 3C). However, the amount of SdhE C-FLAG that co-purified with SdhA N-His was low, suggesting that this might be a weak and/or transient interaction (Fig. 3C). Therefore, YgfX is membrane-bound, whereas SdhE is a soluble monomer that forms weak and/or transient interactions with the flavoprotein SdhA.
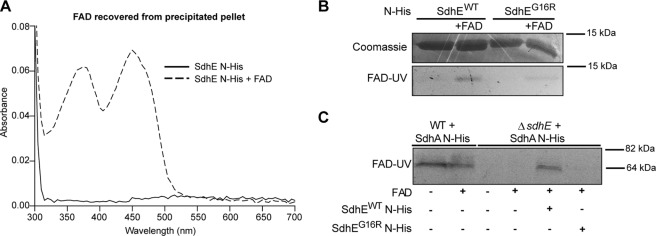
SdhE Binds FAD Co-factor
SdhE N-His purified from WT Serratia did not produce a detectable band when examined by FAD-UV (Fig. 4A). However, the addition of increasing amounts of exogenous FAD to SdhE N-His resulted in UV-illuminated bands at the same molecular mass, suggesting that SdhE is able to covalently bind the FAD co-factor (Fig. 4A). To try and detect very low levels of bound FAD, the FAD-UV method was altered by prolonging the incubation of the gel in acetic acid for 24 h. Using this method, low levels of bound FAD became visible in the SdhE sample without the addition of FAD (Fig. 4B). Binding of FAD to SdhE had not been previously observed for the S. cerevisiae homologue (30). Furthermore, SdhE lacks any conserved His, Tyr, or Cys residues, the usual amino acids for FAD binding (32, 36).
FIGURE 4.
SdhE binds FAD. A, SdhE N-His binds exogenous FAD. Purified SdhE N-His (36 μg) was incubated with increasing amounts of FAD and analyzed by FAD-UV and Coomassie Blue staining. B, SdhE N-His contains low amounts of FAD. Purified SdhE N-His (20 μg) and a vector control were analyzed by Coomassie Blue staining and FAD-UV. To detect low amounts of FAD, the SDS gel was washed with H2O following initial incubation in acetic acid and incubated overnight in H2O with residual acetic acid prior to UV-illumination. C, unbound FAD is removed by dialysis. FAD-UV of purified SdhE N-His and SdhE N-His preincubated with FAD prior to, and following, dialysis was performed. The molecular mass of free FAD is indicated. D and E, UV-visible spectrum of purified SdhE N-His (D) and SdhE N-His preincubated with FAD (dialyzed) (E). The inset represents the UV-visible spectra of SdhE N-His + FAD (dialyzed) minus SdhE N-His alone. F and G, peptide peaks identified from MS/MS analysis of intact SdhE N-His (F) and SdhE N-His preincubated with FAD (dialyzed) (G). Protein standards are indicated by an asterisk. The samples were prepared and dialyzed for UV-visible spectra and intact protein MS as described under “Experimental Procedures.”
If FAD is bound to a protein, UV-visible spectral analysis should produce peaks at ∼375 and 450 nm. Purified SdhE N-His was incubated with or without FAD, and then all unbound FAD was removed by dialysis, as confirmed by FAD-UV (Fig. 4C). The UV-visible spectrum of purified SdhE N-His without FAD added contained very minor peaks at 375 and 450 nm (Fig. 4D). However, the UV-visible spectrum of SdhE N-His preincubated with FAD and dialyzed to remove unbound FAD had a greater absorbance at 375 and 450 nm (Fig. 4E). The difference between the UV-visible spectra of these two samples indicate binding of FAD to SdhE (Fig. 4E, inset). Interestingly, dialysis resulted in a reduction in intensity of the FAD-UV band for SdhE N-His as compared with the sample prior to dialysis (Fig. 4C), suggesting that dialysis removes some FAD bound to SdhE N-His.
To further investigate possible SdhE-FAD binding, these samples were analyzed by intact protein MALDI-TOF MS. Purified SdhE N-His showed peaks of the singly and doubly charged ions at m/z 11,884.7 and 5941.04, respectively, corresponding to measured masses of the uncharged molecule of 11,880 and 11,883.7 Da (Fig. 4F). These measured masses match the expected average mass of SdhE N-His (11,885 Da) within the instrument error range of 5–10 Da for intact protein measurement by linear TOF MS in the mass range used here. The intact mass of SdhE N-His incubated with FAD and dialyzed also had a major peak at the mass for SdhE N-His alone (Fig. 4G). However, a distinct peak shift was observed with an increased mass of 795.4 Da for the singly charged species and 787.8 for the doubly charged ions (Fig. 4G). A similar peak shift was reproduced in multiple experiments. Considering the instrument error, this measured mass increase was in the range of the estimated mass of FAD (785 Da), which is consistent with SdhE binding a single FAD.
Covalently and noncovalently bound FAD can be discriminated not only by SDS-PAGE and FAD-UV assays (Fig. 4) but also by trichloroacetic acid precipitation. Covalently bound FAD should precipitate with the protein, whereas noncovalently bound FAD will be present in the supernatant. To further investigate FAD binding, SdhE N-His was incubated with FAD. Following acid precipitation of SdhE N-His + FAD, as expected, the supernatant contained free unbound FAD (data not shown). The precipitated protein pellet was bright yellow in color as compared with the control (supplemental Fig. S3) and was subsequently dissolved in 6 m guanidine hydrochloride. The UV-visible spectrum of the dissolved SdhE N-His pellet demonstrated the presence of FAD with distinct peaks at 375 and 450 nm (Fig. 5A). FAD precipitated with SdhE N-His at a ratio of 0.1 mol of FAD/mol of protein. SdhE N-His not preincubated with FAD contained no detectable FAD present in the acid-precipitated protein pellet (Fig. 5A). These results show that the majority of SdhE N-His exists in a non-FAD-bound form but that SdhE N-His is able to bind FAD when it is present.
FIGURE 5.
SdhE binds FAD and aids flavinylation of SdhA. A, SdhE N-His was preincubated with FAD. Protein samples were precipitated with trichloroacetic acid, and the protein pellet was redissolved in 6 m guanidine hydrochloride. The spectra of the redissolved SdhE N-His (solid line) and SdhE N-His preincubated with FAD (dashed line) were measured. B, a conserved Gly is required for FAD binding. N-His-tagged SdhEWT and SdhEG16R were purified from WT Serratia, and FAD binding was assessed by incubating 35 μg of either protein with or without 4 μg of FAD overnight at room temperature. C, SdhE aids incorporation of FAD into SdhA. Cell lysates of WT or ΔsdhE expressing SdhA N-His were incubated with FAD with or without 35 μg of purified His-tagged SdhEWT or SdhEG16R.
To determine whether FAD was interacting with SdhE or with the His tag, a tobacco etch virus-cleavable His tag was introduced. SdhE was still able to bind FAD despite the removal of the His tag, demonstrating that FAD binds to SdhE (supplemental Fig. S4). Furthermore, a conserved residue (Gly-16) that was required for Sdh5 interactions with Sdh1 in S. cerevisiae (30) was mutated (G16R) to investigate specific requirements for FAD binding. FAD-UV analysis of purified SdhEWT and SdhEG16R, with or without exogenous FAD, demonstrated that SdhEG16R contained lower levels of detectable FAD, relative to SdhEWT (Fig. 5B). Therefore, our data obtained from in-gel FAD-UV, UV-visible spectra, MS analysis, acid precipitation, and site-directed mutagenesis represent multiple lines of evidence that support the hypothesis that SdhE is able to bind FAD. However, it is hypothesized that SdhE exists predominately in a non-FAD-bound state, with the SdhE-FAD interaction possibly being weak or of low affinity.
SdhE Facilitates FAD Incorporation into SdhA
To determine whether SdhE directly incorporated FAD into SdhA, purified SdhEWT N-His was added back to cell lysates of ΔsdhE expressing SdhA N-His with or without exogenous FAD. SdhA expressed in ΔsdhE was unable to bind FAD when incubated with FAD alone (Fig. 5C). When incubated with FAD and purified SdhEWT, SdhA expressed in ΔsdhE was able to bind FAD (Fig. 5C). When SdhEG16R and FAD were added, SdhA expressed in ΔsdhE was unable to bind FAD. In conclusion, SdhE facilitates the incorporation of FAD into SdhA.
SdhE Mutants Are Pleiotropic
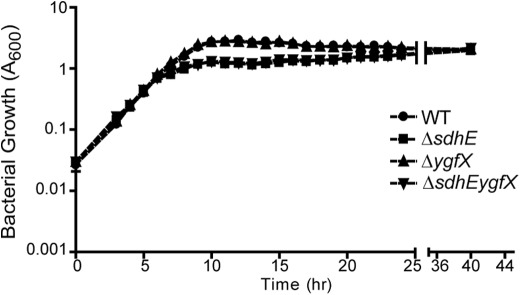
The role of SdhE in the TCA cycle and the ETC prompted further investigations to determine the phenotypic consequences of mutations in sdhE and ygfX. Both ΔsdhE and ΔsdhEygfX mutants showed reduced A600 throughout growth in LB liquid after ∼8 h growth, but continued to grow slowly until they reached an equivalent stationary phase A600 as the WT (Fig. 6). However, there was no difference in viable count, as determined by colony-forming units (cfu)/ml, throughout growth (supplemental Fig. S5A). This difference in A600 in stationary phase cultures (14 h) correlated with a small difference in cell length between WT and ΔsdhE (p < 0.05) as determined by transmission electron microscopy (supplemental Table S4). Similar differences in cell size were observed when grown in minimal glucose medium (supplemental Table S4).
FIGURE 6.
sdhE mutants have a growth defect in LB medium. WT and deletion mutants were grown in 25 ml of LB in 250-ml flasks. A600 was measured every hour for 24 h. Error bars indicate S.D.
Analysis of pig biosynthesis throughout growth showed that ΔsdhE, ΔygfX, and ΔsdhEygfX had a 50, 90, and 90% reduction in addition to a 70, 40, and 70% reduction in the transcription of the pig biosynthetic operon pigA::lacZ (supplemental Fig. S5, B and C). The reduction in pig biosynthesis could be complemented by adding back the deleted gene(s) in trans (supplemental Fig. S5D). Interestingly, ΔsdhE overexpressing SdhE produced a further 50% reduction in pig biosynthesis (supplemental Fig. S5D).
Analysis of other phenotypes showed that both ΔsdhE and ΔsdhEygfX had a 19 and 11% reduction in the production of the plant cell wall-degrading exoenzymes cellulase and pectate lyase, in addition to a 20% reduction in swimming motility and virulence in potato tuber rot assays (supplemental Table S4). Both ΔsdhE and ΔsdhEygfX showed reduced production of N-acyl homoserine lactones throughout growth (supplemental Table S4), whereas the production of the β-lactam antibiotic, 1-carbapen-2-em-3-carboxylic acid (a carbapenem) was completely abolished (supplemental Table S4 and supplemental Fig. S5, E and F). Caenorhabditis elegans survival curves showed no statistically significant decrease in virulence (supplemental Fig. S5G). Phenotypic alterations for ΔsdhE and ΔsdhEygfX could be complemented by adding sdhE in trans (supplemental Fig. S5, H–L). ΔygfX showed no difference in any phenotypes relative to the WT strain except for the reduction in pig. These results indicate that both genes affect pig biosynthesis at the level of transcription and that the lack of SdhE results in widespread defects.
SdhE Evolved Once Preceding the Evolution of Mitochondria
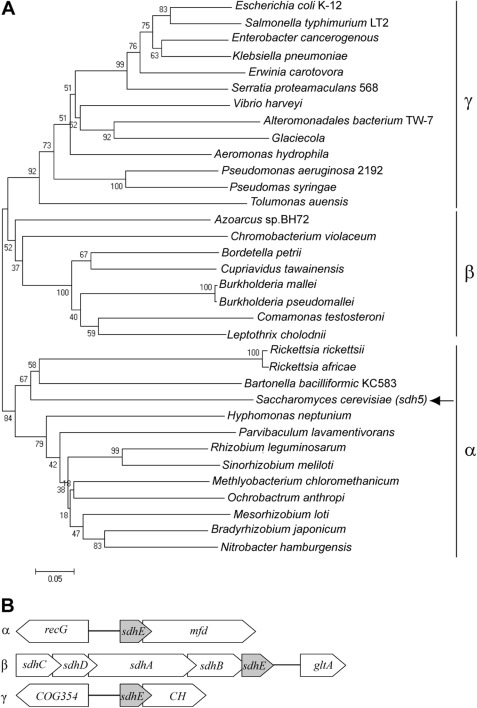
Analysis of DUF339 proteins using Pfam and the STRING occurrence viewer (COG2938 query) demonstrated that this domain is present in eukaryotes (124 species) and bacteria (593 species) (23, 24). Interestingly, bacteria that contain a DUF339 protein are restricted to the α-, β-, and γ-proteobacteria. Analysis of the mitochondrial proteome showed that a DUF339 protein (EM15/Sdh5) in S. cerevisiae is a nuclear-encoded mitochondrial protein (37). It is hypothesized that mitochondria are descendants of an ancestral α-proteobacterial Rickettsia-like species and that the proteobacteria have evolved in a linear manner following the progression of ϵ, δ, α, β, and γ (38, 39). We therefore hypothesized that sdhE evolved only once in the α-proteobacteria prior to the evolution of the mitochondria and remained in the majority of bacterial and eukaryotic descendants. Phylogenetic analysis of sdhE among bacterial species reveals three distinct clades representing the α-, β-, or γ-proteobacteria (Fig. 7A), with sdhE sequences from β and γ being the most closely related and α being distantly related (Fig. 7A). In agreement with our hypothesis, the Sdh5 sequence from S. cerevisiae groups with Rickettsia in the α-proteobacteria clade (Fig. 7A). These results suggest that DUF339 evolved once in an ancestral α-proteobacterial species and that it has remained in both bacterial and eukaryotic descendants.
FIGURE 7.
SdhE evolved once in an ancestral α-proteobacteria. A, phylogenetic analysis of sdhE homologues. All homologues are from the dominant organization with the exception of Rickettsia sp., which have a partially conserved organization. The arrow indicates S. cerevisiae. B, dominant genomic organizations of sdhE. Shown are the dominant genomic organization of sdhE (DUF339) genes and the surrounding genomic context in the α-, β-, and γ-proteobacteria. CH, conserved hypothetical (typically DUF1434/YgfX).
Each of the proteobacterial classes displayed a dominant sdhE genomic organization as determined by using the STRING neighborhood viewer (COG2938 as a search query) and the MicrobesOnline genomic context viewer (Fig. 7B). The dominant organization in α-proteobacteria, recG-DUF339-mfd, places sdhE in an operon with the transcription repair factor mfd. The dominant organization in β-proteobacteria, sdhCDAB-DUF339-gltA, is consistent with an involvement in succinate dehydrogenase. Finally, the dominant genomic organization in γ-proteobacteria is ygfZ (COG354)-DUF339-conserved hypothetical (frequently DUF1434/YgfX).
sdhEygfX Is Functionally Conserved among Enterobacteriaceae
The sdhEygfX operon is highly conserved among the Enterobacteriaceae. To test the functional conservation of SdhE and YgfX, E. coli sdhE (KmR marked) deletion mutants (40) were assessed for a functional SDH, and E. coli homologues of sdhE and ygfX were assessed for their ability to complement Serratia ΔsdhEygfX. E. coli sdhE mutants were impaired for growth in LB and minimal succinate (supplemental Fig. S6, A and B) as a result of an inactive SDH (supplemental Fig. S6C). Additionally, when added in trans, E. coli homologues complemented Serratia ΔsdhEygfX reduction in pig and A600, i.e. SdhE complemented A600, YgfX complemented pig, and SdhEYgfX complemented A600 and pig (supplemental Fig. S6, D and E). This demonstrates that SdhE and YgfX are functionally conserved among the Enterobacteriaceae and are likely to function through the same mechanism. Furthermore, this supports the role of SdhE as a key protein in SDH function in bacteria, such as in the model bacterium, E. coli.
DISCUSSION
SdhA covalently binds FAD via an 8α-N3-histidyl linkage. The flavinylation of SdhA is essential for activity of the SDH complex in both the TCA cycle and ETC and has been proposed to proceed through an autocatalytic FAD insertion (25, 32, 41). Our results contradict this autocatalytic flavinylation model and show that SdhE proteins are required for the incorporation of FAD into SdhA. SdhE is the first protein shown to be required for flavinylation of a flavoprotein in bacteria and is a key protein in controlling bacterial SDH activity. Interestingly, SdhE was listed previously as a conserved hypothetical protein, the characterization of which was considered to be of high priority (3).
We predicted a role for SdhE in succinate metabolism, based on bioinformatics. Subsequent deletion of sdhE impaired growth on succinate due to a defective SDH, which resulted from the failed incorporation of an FAD co-factor into SdhA. The absence of FAD, however, did not prevent correct assembly of SDH. This is consistent with previous studies of bacterial SDH showing that FAD is not required for assembly (42). S. cerevisiae sdh5 (a sdhE homologue) mutants failed to incorporate FAD into Sdh1, leading to an inactive SDH (30). However, S. cerevisiae SDH was destabilized and disassembled (30), reflecting possible differences in the role of flavinylation in maintaining the structural integrity of bacterial and eukaryotic SDH. In Serratia, deletion of sdhE resulted in increased SdhA incorporation into the membrane and an absence of cytoplasmic SdhA, relative to the WT strain. We hypothesize that in response to the loss of sdhE, cells incorporate more SdhA into the membrane in an attempt to compensate for the loss of active SDH. This could imply physiological or regulatory feedback.
The general mechanism of covalent FAD attachment is thought to require correct folding of the flavoprotein to allow for an attack by a nucleophilic amino acid side chain on a quinine methide form of the isoalloxazine moiety of FAD (25, 32, 41). This process was thought to be autocatalytic, with no accessory proteins required (25, 31, 32, 43). Recently, a DUF339 protein in S. cerevisiae was identified as being required for FAD incorporation in Sdh1 (30). Our work shows that SdhE (a DUF339 protein) in bacteria performs a similar role. Furthermore, our work has extended the characterization of DUF339 proteins providing initial mechanistic insights, suggesting that SdhE binds FAD and incorporates FAD into SdhA. The retention of the FAD-SdhE N-His complex in FAD-UV gels, intact protein MALDI-TOF, and following acid precipitation is suggestive of a covalent interaction. Although unusual, there is precedent for co-factor chaperones that bind co-factors through covalent interactions. One example is the heme chaperone CcmE that binds heme through a unique covalent interaction (44). Alternatively, without knowing the exact FAD binding site, a noncovalent interaction between FAD and SdhE cannot be unequivocally ruled out at this time. Furthermore, the amount of FAD that precipitated with SdhE and the reduction of FAD bound to SdhE following dialysis suggest that the SdhE-FAD interaction is weak or of low affinity.
SdhE homologues lack any conserved His, Tyr, or Cys residues, the usual amino acids for FAD binding (32). This suggests that SdhE binds FAD via a noncanonical mechanism. Mutation of the surface-exposed conserved Gly-16 to Arg (35) significantly reduced FAD binding and inhibited FAD incorporation in SdhA. It is unclear whether Gly-16 directly contributes to FAD binding. However, the presence of several highly conserved residues in the N terminus of SdhE in bacteria and eukaryotes suggests that this region is important for function and FAD interaction (35). Further investigation of the SdhE (DUF339 proteins)-FAD linkage is required for a more detailed understanding.
In S. cerevisiae, Sdh5 and Sdh1 directly interact. Furthermore, a G78R mutation (equivalent to G16R in SdhE), which is associated with the pathology of paraganglioma, abolishes this interaction (30). The bacterial SdhE is a soluble monomer that interacted weakly and/or transiently with SdhA. Alternatively, the low level of co-purified SdhE could relate to the flavinylated state of both proteins and the influence that this has on the SdhE-SdhA interaction. Given the requirement of SdhE for flavinylation of SdhA, we hypothesize that the interaction between SdhE and SdhA is necessary for FAD incorporation.
SdhE was initially identified as a gene neighboring a regulator of pig production (8). This study has demonstrated that sdhEygfX is a bicistronic operon, suggesting that these genes are functionally related, with both gene products affecting pig biosynthesis, directly or indirectly, at the level of transcription of the biosynthetic operon (pigA-O). This provides an additional link between carbon metabolism and the regulation of pig biosynthesis (11, 45). Furthermore, deletion of sdhE affected multiple phenotypes, suggesting that multiple biological pathways might be affected. Therefore, SdhE might have co-factor chaperone-like functions to assist FAD incorporation into other flavoproteins. Alternatively, it is possible that the disruption of SDH activity caused the growth aberration that led to a pleiotropic effect. Our current work has therefore encouraged a reassessment of the view that FAD incorporation occurs simply as a self-catalytic process.
The widespread conserved distribution of SdhE and its proposed evolutionary history are indicative of its functional importance. Our analysis of SdhE homologues in eukaryotes and bacteria supports the hypothesis that SdhE arose prior to the evolution of the mitochondria in an ancestral α-proteobacterium and was retained in almost all descendants. Dominant genomic organizations exist in bacteria, suggesting that genomic rearrangements of sdhE are relatively rare. Furthermore, this proposed evolutionary model for sdhE raises questions regarding flavinylation in other organisms that lack SdhE but contain SDH. In these organisms, is autocatalytic flavinylation sufficient, or is it assisted by other currently unidentified proteins?
We have shown that SdhE/DUF339 proteins are widespread in bacteria and eukaryotes. Furthermore, we have extended a recent study in eukaryotes (30) by showing that SdhE/DUF339 proteins control SDH activity in bacteria through a similar mechanism and can bind FAD. We speculate that SdhE exists predominantly in a non-FAD-bound state. When required, SdhE binds FAD and interacts with nonflavinylated SdhA. We hypothesize that due to a stronger affinity of SdhA for FAD, the co-factor is transferred from SdhE to SdhA. Alternatively, SdhE may alter the conformation of SdhA to allow FAD incorporation. These functions are not mutually exclusive and might both be performed by SdhE. Furthermore, SdhE may only bind FAD when in a complex with SdhA. Detailed investigations are underway to test these models.
The operonic arrangement of ygfX and sdhE in many bacteria suggests that YgfX has a role in the same pathway as SdhE and that this pathway is functionally important among the enterobacterial pathogens. In humans, mutations within the sdhE homologue (SDH5) increase susceptibility to tumor formation, specifically paraganglioma (30, 46). This genetically amenable bacterial system provides a unique opportunity to study the conserved mechanism of SdhE/DUF339 proteins, which will have implications for central metabolism and might also further our understanding of mitochondrial diseases. Finally, our work demonstrates the importance of research using bacterial systems on conserved hypothetical proteins for the elucidation of novel biochemical functions that are both physiologically important and of widespread significance in biology.
Supplementary Material
Acknowledgments
We thank Torsten Kleffmann for useful discussions, critically reading the manuscript, and MS analysis with Diana Carne and Simone Schönleben at the Centre for Protein Research at the University of Otago. We also thank Gregory Cook for helpful discussions, Richard Easingwood from the Otago Centre for Electron Microscopy for transmission electron microscope assistance, Wayne Patrick for the E. coli Keio mutants, and Kurt Krause for the tobacco etch virus protease.
This work was supported by a Marsden Fund, Royal Society of New Zealand grant (to P. C. F.). The C. elegans work was funded by the Biotechnology and Biological Sciences Research Council (to G. P. C. S.).

This article contains supplemental Experimental Procedures, Tables S1–S4, and Figs. S1–S6.
- TCA
- tricarboxylic acid
- pig
- prodigiosin
- ETC
- electron transport chain
- SDH
- succinate dehydrogenase
- BN-PAGE
- Blue Native-PAGE
- IPTG
- isopropyl-1-thio-β-d-galactopyranoside
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- N-His
- N-terminal hexahistidine tag
- C-FLAG
- C-terminal FLAG tag.
REFERENCES
- 1. Anraku Y. (1988) Bacterial electron transport chains. Annu. Rev. Biochem. 57, 101–132 [DOI] [PubMed] [Google Scholar]
- 2. Schultz B. E., Chan S. I. (2001) Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu. Rev. Biophys. Biomol. Struct. 30, 23–65 [DOI] [PubMed] [Google Scholar]
- 3. Galperin M. Y., Koonin E. V. (2004) “Conserved hypothetical” proteins: prioritization of targets for experimental study. Nucleic Acids Res. 32, 5452–5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts R. J. (2004) Identifying protein function: a call for community action. PLoS Biol. 2, E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grimont P. A., Grimont F. (1978) The genus Serratia. Annu. Rev. Microbiol. 32, 221–248 [DOI] [PubMed] [Google Scholar]
- 6. Williamson N. R., Fineran P. C., Gristwood T., Chawrai S. R., Leeper F. J., Salmond G. P. (2007) Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2, 605–618 [DOI] [PubMed] [Google Scholar]
- 7. Williamson N. R., Fineran P. C., Leeper F. J., Salmond G. P. (2006) The biosynthesis and regulation of bacterial prodiginines. Nat. Rev. Microbiol. 4, 887–899 [DOI] [PubMed] [Google Scholar]
- 8. Fineran P. C., Slater H., Everson L., Hughes K., Salmond G. P. (2005) Biosynthesis of tripyrrole and β-lactam secondary metabolites in Serratia: integration of quorum sensing with multiple new regulatory components in the control of prodigiosin and carbapenem antibiotic production. Mol. Microbiol. 56, 1495–1517 [DOI] [PubMed] [Google Scholar]
- 9. Gristwood T., McNeil M. B., Clulow J. S., Salmond G. P., Fineran P. C. (2011) PigS and PigP regulate prodigiosin biosynthesis in Serratia via differential control of divergent operons, which include predicted transporters of sulfur-containing molecules. J. Bacteriol. 193, 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burger S. R., Bennett J. W. (1985) Droplet enrichment factors of pigmented and nonpigmented Serratia marcescens: possible selective function for prodigiosin. Appl. Environ. Microbiol. 50, 487–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fineran P. C., Everson L., Slater H., Salmond G. P. (2005) A GntR family transcriptional regulator (PigT) controls gluconate-mediated repression and defines a new, independent pathway for regulation of the tripyrrole antibiotic, prodigiosin, in Serratia. Microbiology 151, 3833–3845 [DOI] [PubMed] [Google Scholar]
- 12. Fineran P. C., Williamson N. R., Lilley K. S., Salmond G. P. (2007) Virulence and prodigiosin antibiotic biosynthesis in Serratia are regulated pleiotropically by the GGDEF/EAL domain protein, PigX. J. Bacteriol. 189, 7653–7662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gristwood T., Fineran P. C., Everson L., Williamson N. R., Salmond G. P. (2009) The PhoBR two-component system regulates antibiotic biosynthesis in Serratia in response to phosphate. BMC Microbiol. 9, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gristwood T., Fineran P. C., Everson L., Salmond G. P. (2008) PigZ, a TetR/AcrR family repressor, modulates secondary metabolism via the expression of a putative four-component resistance-nodulation-cell-division efflux pump, ZrpADBC, in Serratia sp. ATCC 39006. Mol. Microbiol. 69, 418–435 [DOI] [PubMed] [Google Scholar]
- 15. Williamson N. R., Fineran P. C., Ogawa W., Woodley L. R., Salmond G. P. (2008) Integrated regulation involving quorum sensing, a two-component system, a GGDEF/EAL domain protein, and a post-transcriptional regulator controls swarming and RhlA-dependent surfactant biosynthesis in Serratia. Environ. Microbiol. 10, 1202–1217 [DOI] [PubMed] [Google Scholar]
- 16. Wittig I., Braun H. P., Schägger H. (2006) Blue native PAGE. Nat. Protoc. 1, 418–428 [DOI] [PubMed] [Google Scholar]
- 17. Schägger H. (2006) Tricine-SDS-PAGE. Nat. Protoc. 1, 16–22 [DOI] [PubMed] [Google Scholar]
- 18. Bafunno V., Giancaspero T. A., Brizio C., Bufano D., Passarella S., Boles E., Barile M. (2004) Riboflavin uptake and FAD synthesis in Saccharomyces cerevisiae mitochondria: involvement of the Flx1p carrier in FAD export. J. Biol. Chem. 279, 95–102 [DOI] [PubMed] [Google Scholar]
- 19. Brandsch R., Bichler V. (1989) Covalent co-factor binding to flavoenzymes requires specific effectors. Eur. J. Biochem. 182, 125–128 [DOI] [PubMed] [Google Scholar]
- 20. Shevchenko A., Jensen O. N., Podtelejnikov A. V., Sagliocco F., Wilm M., Vorm O., Mortensen P., Shevchenko A., Boucherie H., Mann M. (1996) Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two-dimensional gels. Proc. Natl. Acad. Sci. U.S.A. 93, 14440–14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tamura K., Dudley J., Nei M., Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24, 1596–1599 [DOI] [PubMed] [Google Scholar]
- 22. Alm E. J., Huang K. H., Price M. N., Koche R. P., Keller K., Dubchak I. L., Arkin A. P. (2005) The MicrobesOnline web site for comparative genomics. Genome Res. 15, 1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finn R. D., Mistry J., Tate J., Coggill P., Heger A., Pollington J. E., Gavin O. L., Gunasekaran P., Ceric G., Forslund K., Holm L., Sonnhammer E. L., Eddy S. R., Bateman A. (2010) The Pfam protein families database. Nucleic Acids Res. 38, D211–D222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen L. J., Kuhn M., Stark M., Chaffron S., Creevey C., Muller J., Doerks T., Julien P., Roth A., Simonovic M., Bork P., von Mering C. (2009) STRING 8: a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 37, D412–DD416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cecchini G. (2003) Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 72, 77–109 [DOI] [PubMed] [Google Scholar]
- 26. Hederstedt L., Rutberg L. (1981) Succinate dehydrogenase: a comparative review. Microbiol. Rev. 45, 542–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park S. J., Tseng C. P., Gunsalus R. P. (1995) Regulation of succinate dehydrogenase (sdhCDAB) operon expression in Escherichia coli in response to carbon supply and anaerobiosis: role of ArcA and Fnr. Mol. Microbiol. 15, 473–482 [DOI] [PubMed] [Google Scholar]
- 28. Cronan J. E., Laporte D. C. (1996) Escherichia coli and Salmonella. in Escherichia coli and Salmonella: Cellular and Molecular Biology (Neidhart F. C. ed.), Second Ed., pp. 206–215, ASM Press, Washington, D. C. [Google Scholar]
- 29. Robinson K. M., Rothery R. A., Weiner J. H., Lemire B. D. (1994) The covalent attachment of FAD to the flavoprotein of Saccharomyces cerevisiae succinate dehydrogenase is not necessary for import and assembly into mitochondria. Eur. J. Biochem. 222, 983–990 [DOI] [PubMed] [Google Scholar]
- 30. Hao H. X., Khalimonchuk O., Schraders M., Dephoure N., Bayley J. P., Kunst H., Devilee P., Cremers C. W., Schiffman J. D., Bentz B. G., Gygi S. P., Winge D. R., Kremer H., Rutter J. (2009) SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science 325, 1139–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edmondson D. E., Newton-Vinson P. (2001) The covalent FAD of monoamine oxidase: structural and functional role and mechanism of the flavinylation reaction. Antioxid. Redox Signal. 3, 789–806 [DOI] [PubMed] [Google Scholar]
- 32. Mewies M., McIntire W. S., Scrutton N. S. (1998) Covalent attachment of flavin adenine dinucleotide (FAD) and flavin mononucleotide (FMN) to enzymes: the current state of affairs. Protein Sci. 7, 7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yankovskaya V., Horsefield R., Törnroth S., Luna-Chavez C., Miyoshi H., Léger C., Byrne B., Cecchini G., Iwata S. (2003) Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 299, 700–704 [DOI] [PubMed] [Google Scholar]
- 34. Sousa P. M., Silva S. T., Hood B. L., Charro N., Carita J. N., Vaz F., Penque D., Conrads T. P., Melo A. M. (2011) Supramolecular organizations in the aerobic respiratory chain of Escherichia coli. Biochimie 93, 418–425 [DOI] [PubMed] [Google Scholar]
- 35. Lim K., Doseeva V., Demirkan E. S., Pullalarevu S., Krajewski W., Galkin A., Howard A., Herzberg O. (2005) Crystal structure of the YgfY from Escherichia coli, a protein that may be involved in transcriptional regulation. Proteins 58, 759–763 [DOI] [PubMed] [Google Scholar]
- 36. Heuts D. P., Scrutton N. S., McIntire W. S., Fraaije M. W. (2009) What's in a covalent bond? On the role and formation of covalently bound flavin co-factors. FEBS J. 276, 3405–3427 [DOI] [PubMed] [Google Scholar]
- 37. Sickmann A., Reinders J., Wagner Y., Joppich C., Zahedi R., Meyer H. E., Schönfisch B., Perschil I., Chacinska A., Guiard B., Rehling P., Pfanner N., Meisinger C. (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. U.S.A. 100, 13207–13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andersson S. G., Zomorodipour A., Andersson J. O., Sicheritz-Pontén T., Alsmark U. C., Podowski R. M., Näslund A. K., Eriksson A. S., Winkler H. H., Kurland C. G. (1998) The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature 396, 133–140 [DOI] [PubMed] [Google Scholar]
- 39. Gupta R. S. (2000) The phylogeny of proteobacteria: relationships to other eubacterial phyla and eukaryotes. FEMS Microbiol. Rev. 24, 367–402 [DOI] [PubMed] [Google Scholar]
- 40. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robinson K. M., Lemire B. D. (1996) Covalent attachment of FAD to the yeast succinate dehydrogenase flavoprotein requires import into mitochondria, presequence removal, and folding. J. Biol. Chem. 271, 4055–4060 [DOI] [PubMed] [Google Scholar]
- 42. Hederstedt L. (1983) Succinate dehydrogenase mutants of Bacillus subtilis lacking covalently bound flavin in the flavoprotein subunit. Eur. J. Biochem. 132, 589–593 [DOI] [PubMed] [Google Scholar]
- 43. Jin J., Mazon H., van den Heuvel R. H., Heck A. J., Janssen D. B., Fraaije M. W. (2008) Covalent flavinylation of vanillyl-alcohol oxidase is an autocatalytic process. FEBS J. 275, 5191–5200 [DOI] [PubMed] [Google Scholar]
- 44. Stevens J. M., Uchida T., Daltrop O., Ferguson S. J. (2005) Covalent co-factor attachment to proteins: cytochrome c biogenesis. Biochem. Soc. Trans. 33, 792–795 [DOI] [PubMed] [Google Scholar]
- 45. Haddix P. L., Jones S., Patel P., Burnham S., Knights K., Powell J. N., LaForm A. (2008) Kinetic analysis of growth rate, ATP, and pigmentation suggests an energy-spilling function for the pigment prodigiosin of Serratia marcescens. J. Bacteriol. 190, 7453–7463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kunst H. P., Rutten M. H., de Mönnink J. P., Hoefsloot L. H., Timmers H. J., Marres H. A., Jansen J. C., Kremer H., Bayley J. P., Cremers C. W. (2011) SDHAF2 (PGL2-SDH5) and hereditary head and neck paraganglioma. Clin. Cancer Res. 17, 247–254 [DOI] [PubMed] [Google Scholar]
- 47. Qiagen, Inc. (2003) The QIAexpressionist, Qiagen Inc., Valencia, CA [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.