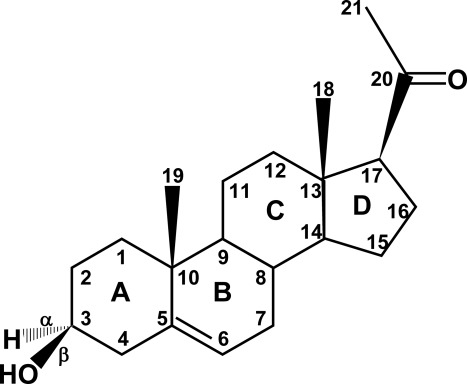
Fig. 1.
Structure of pregnenolone, illustrating the cycloperhydropentano-phenanthrene structure common to all steroids. The carbon atoms are indicated by numbers, and the rings are designated by letters according to standard convention. Substituents and hydrogens are labeled as α or β if they are positioned behind or in front of the plane of the page, respectively. Pregnenolone is derived from cholesterol, which has a six-carbon side chain attached to carbon no. 20. Pregnenolone is a “Δ5 compound,” having a double bond between carbons no. 5 and 6; the action of 3β-hydroxysteroid dehydrogenase/isomerase moves this double bond from the B ring to carbons 4 and 5 in the A ring, forming Δ4 compounds. Most of the major biologically active steroid hormones are Δ4 compounds. [© R. J. Auchus.]