Abstract
Cell polarity is defined as asymmetry in cell shape, protein distributions and cell functions. It is characteristic of single-cell organisms, including yeast and bacteria, and cells in tissues of multi-cell organisms such as epithelia in worms, flies and mammals. This diversity raises several questions: do different cell types use different mechanisms to generate polarity, how is polarity signalled, how do cells react to that signal, and how is structural polarity translated into specialized functions? Analysis of evolutionarily diverse cell types reveals that cell-surface landmarks adapt core pathways for cytoskeleton assembly and protein transport to generate cell polarity.
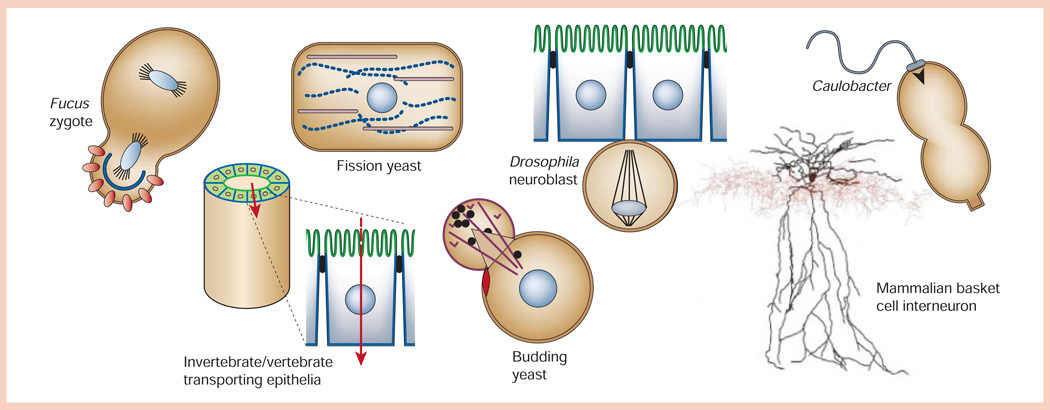
Most cells are polarized, including simple single-cell organisms (for example, budding yeast Saccharomyces cerevisiae and fission yeast Schizosaccharomyces pombe) and cells in multicellular invertebrates (the nematode Caenorhabditis elegans or fruitfly Drosophila) and vertebrates (mammals); even bacteria are polarized (Fig. 1). There is also an extraordinary diversity in the shapes of polarized cell. Consider the extremely attenuated shape of a mammalian neuron, which can be many metres long, the short rectangular shapes (5–30 µm long) of the single-cell S. pombe and cells in epithelial tissues in multicellular organisms, and the asymmetric shape of S. cerevisiae or Drosophila neuroblasts preparing to divide (Fig. 1). Different cell shapes are not just a quirk of nature, but are coupled to specialized cell functions, for example, to communicate between tissues over long distances (neurons), to provide barriers that regulate ionic homeostasis between different biological compartments (epithelia), and to disperse cellular components to daughter cells upon cell division.
Figure 1.
Diversity of shapes of polarized cells (not to scale). Fucus zygote exposed to a light gradient showing polarized distributions of ion channels/dihydropyridine receptors (red circles) and F-actin (blue line) in the rhizoid cell (bottom) compared to the thallus cell (top). Fission yeast (Schizosaccharomyces pombe) showing polarized distributions of actin (purple) and microtubules (blue dotted line) in the long axis of the cell, and the nucleus positioned in the centre of the cell. Drosophila neuroblast delaminated from the ventral neuroectoderm with an asymmetric plane of division that will yield a large ‘apical’ neuroblast stem cell and a small ‘basal’ ganglion mother cell. Caulobacter crescentus predivisional cell showing polarized distributions of the flagellum (swarmer cell, top) and stalk (stalked cell, bottom). Invertebrate/vertebrate transporting epithelium showing organization of polarized epithelial cells (apical membrane, green; basolateral membrane, blue) in a tube that separates two biological compartments and regulates vectorial transport of ions/solutes (red arrow) between those compartments. Budding yeast (Saccharomyces cerevisiae) forming a daughter cell ‘bud’ from the mother cell next to the previous site of cytokinesis (bud scar, red disc), and orienting actin cables (purple) for transport of vesicles (black circles) from the mother to daughter cell. Mammalian basket cell interneuron showing the distribution of the soma/dendrite (black) and axon (red; image courtesy of D. Madison, Stanford University School of Medicine).
At first glance, this diversity of cell shapes and functions suggests that each cell type must have evolved completely different ways to generate cell polarity that distinguishes, for example, a budding yeast from a multi-cell epithelium. But although the final structure is quite different, the basic toolbox of core mechanisms used to organize the cytoskeleton and deliver membrane proteins is common to all eukaryotic cell types. This review focuses on how these mechanisms are used to generate polarity in evolutionarily diverse cell types with different shapes and functions.
Generating polarity in single cells for mitotic division
S. cerevisiae and S. pombe are simple, well-understood examples of how cells generate polarity and couple it to a specific function; simple because they are single-cell organisms that generate cell polarity in order to divide, and well understood because they are genetically tractable. Genetic and cell biological studies point to the importance of localized assembly of actin patches and regulatory proteins to mark one site on the mother cell for membrane growth, and the attachment of actin cables and microtubules to those sites for vesicle delivery and spindle orientation, respectively.
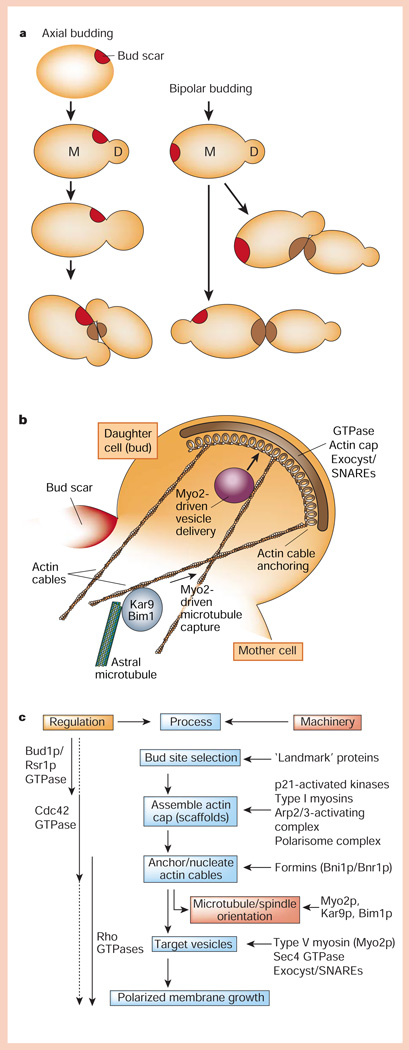
During vegetative growth, S. cerevisiae adopt genotype-dependent axial (haploid a or α cells) or bipolar (diploid a/α cells) growth patterns to produce a daughter cell bud1 (Fig. 2a). Two classes of genes prescribe ‘landmarks’ for axial (BUD3, BUD4 and BUD10/Axl2) and bipolar (BUD8 and BUD9) budding of the daughter cell2,3, and a third class is required for both budding patterns (BUD1/RSR1, BUD2 and BUD5)2,4. The landmark is retained on the plasma membrane close to (axial or bipolar pattern) or opposite (bipolar pattern) the site (bud scar) of the preceding cytokinesis (Fig. 2a). Membrane growth is anisotropic, occurring only in the new bud (Fig. 2b).
Figure 2.
Protein pathways for generating cell polarity in budding yeast. a, Axial and bipolar budding patterns. b, A complex of proteins is assembled at the bud tip that orients the actin cytoskeleton, astral microtubules and vesicle delivery to the bud. c, Hierarchical organization of regulators, processes and cellular machinery linking bud-site selection to assembly of an actin cap, anchoring/nucleation of actin cables, and vesicle targeting that results in polarized membrane growth at the bud.
Members of the Ras superfamily of small GTPases (Bud1p/Rsr1p, Cdc42p, and Rho1p/2p, Rho3p and Rho4p) coordinate the selection of the membrane growth site (the bud), and orientation of the cytoskeleton and targeting of vesicles to that site1,5 (Fig. 2b, c). Cdc42p is crucial in these events — in the absence Cdc42p the actin cytoskeleton is disorganized, and large, unbudded cells are formed as a result of isotropic growth of the mother cell6. A critical question is how is Cdc42p activity restricted to one site on the plasma membrane? One possibility is that Cdc42p is restricted to the landmark through the sequential recruitment of guanine-exchange factors (GEFs), which control GTPase activity locally7. Bud5p and Bud2p, the GEF and GTPase-activating protein for Bud1p/Rsr1p, respectively, co-localize with landmark proteins, and disruption of landmark proteins results in mislocalization of Bud5p8–10. Recruitment of Bud5p to the landmark would locally activate the uniformly distributed Bud1p/Rsr1p. In vitro studies11 show that the GTP-bound form of Bud1p/Rsr1p binds Cdc24p, the GEF for Cdc42p, which in the cell could restrict Cdc42p activity to the landmark. But cells lacking the Bud1p/Rsr1p machinery or any of the landmark genes form daughter cell buds, albeit randomly on the mother cell, in a process that requires activated Cdc42p2–4. Polarization of Cdc42p and actin polymerization could be controlled through a positive feedback loop initiated by a stochastic increase in Cdc42p on the membrane12, and landmarks normally adapt this feedback loop for bud formation at a prescribed, rather than random, site on the membrane.
Activation of Cdc42p at a site on the membrane unleashes global changes in cytoskeleton organization within the bud, involving assembly of a cap of actin filaments, and the organization of actin cables that extend into the mother cell1,13 (Fig. 2b, c). Ste20p and Cla4p are downstream effectors of Cdc42p that are members of the p21-activated kinase (PAK) family14. The type-I myosins Myo3p and Myo5p15 are PAK substrates, which together with homologues of Wiskott–Aldrich syndrome protein or WASP (Bee1p/Las17p) and WASP-interacting protein or WIP (Vrp1p) interact with the Arp2/3 complex to assemble and organize an actin cap at the bud site16–18 (for details of Arp2/3 function, see review in this issue by Gruenheid and Finlay, page 775).
A core scaffolding complex of Spa2p, Pea2p and Sph1p (the polarisome complex19) is assembled at the bud. The formin homologues Bni1p and Bnr1p bind this complex and, in turn, interact with proteins that assemble actin cables between the bud and mother cell20–22: profilin (Pfy1p)23, which promotes GDP to GTP exchange in monomeric actin and hence provides a local pool of GTP–actin for addition to the barbed end of growing actin filaments; Bud6p/Aip3p, an actin filament-binding protein24; and activated GTPases including Cdc42p, Rho1p/2p, Rho3p and Rho4p20,25. Loss of Bni1p and Bnr1p activity correlates with loss of actin cables throughout the bud and mother, and re-activation of Bni1p and Bnr1p results in re-growth of actin cables from the bud into the mother cell21,22. In vitro, Bni1p can also nucleate actin polymerization and bind the barbed (growing) end of actin filaments26,27. Taken together, Bni1p and Bnr1p and associated proteins seem to nucleate and anchor actin cables at the bud site (Fig. 2c).
Orientation of actin cables from the bud into the mother cell also drives microtubule capture in the bud and spindle orientation in the mother–bud axis for cytokinesis28 (Fig. 2b). Astral microtubules contain at their distal, plus ends a protein complex of Kar9p and Bim1p that binds to the type-V myosin Myo2p, which, in turn, binds to actin cables and moves the microtubule-attached complex into the bud29.
Actin cables between the bud and mother cell provide tracks for exocytic vesicle transport from the mother cell Golgi complex into the bud1,13 (Fig. 2b, c). This is critical for polarized membrane growth as SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) located on the target membrane (t-SNAREs; Sec9p, Sso1p/2p) are not restricted to the bud, but are localized over the surface of the mother cell and bud30. Vesicle delivery along actin cables requires Myo2p31,32. Vesicle docking/fusion complex at the bud is further specified by a large complex termed the exocyst, which is localized initially to the bud tip33; Sec4p, Rho1p/2p, Rho3p, Rho4p and Cdc42p regulate these late stages in vesicle delivery, and localization and function of the exocyst in the bud5,34. Disruption of genes encoding the exocyst complex results in accumulation of transport vesicles in the bud, indicating that the exocyst complex may tether arriving vesicles or activate the SNARE complex in preparation for SNARE-dependent vesicle fusion with the plasma membrane33. The yeast homologues of Drosophila lethal giant larva (a protein regulating epithelial polarity, see below), sro7/sro77, bind the t-SNARE Sec9p, and sro7/sro77 mutants accumulate post-Golgi transport vesicles in the cytoplasm35. Thus, both t-SNAREs and the exocyst are required for vesicle fusion at the bud, and localized vesicle docking/fusion in the bud is specified by vesicle delivery along actin cables to the exocyst.
Although these overlapping regulatory mechanisms involving different GTPases may seem overly complex, and in some cases redundant, they coordinate a series of processes that link, in time and space, the initial selection of the bud site to localized assembly and orientation of the actin and microtubule cytoskeletons in the bud–mother axis, and localized docking/fusion of vesicles at that site of membrane growth (Fig. 2c).
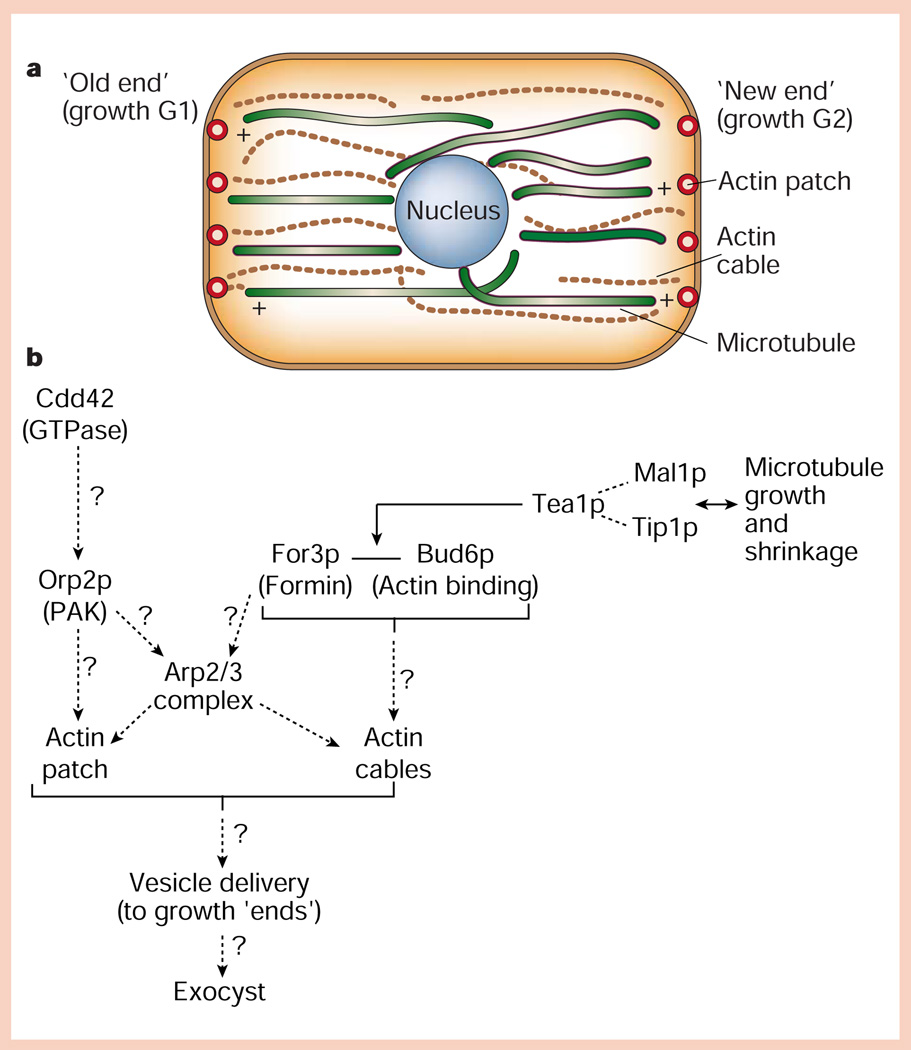
Compared to the highly polarized bud growth of S. cerevisiae, the rod-shaped fission yeast S. pombe divides in the middle by septation and, therefore, might be expected to grow in a more isotropic manner during the cell cycle. However, growth of fission yeast is also spatially polarized, first during G1 at the ‘old’ end opposite the site of septation, and then at the other end during G2 (Fig. 3a).
Figure 3.
Protein pathways for generating cell polarity in fission yeast. a, Distribution of actin (cables and cortical patches) and microtubule cytoskeletons relative to the ‘old’ and ‘new’ ends of the cell, which grow during the G1 and G2 phases of the cell cycle, respectively. b, Hypothetical organization of proteins in cortical actin patches, and interactions of microtubule plus ends with the cell end/cortical actin patch (for details, see text).
In fission yeast, a network of actin cables is oriented in the long axis of the cell, and in cortical patches at the cell ‘ends’36. Little is known about the machinery for localizing actin polymerization to cortical patches, although there are tantalizing indications of mechanisms similar to those in budding yeast (Fig. 3b). Cortical actin patches contain Orb2p, a member of the PAK family that may be downstream of Cdc42p37, the actin-binding protein Bud6p38, and myosin-I and Arp2/3 complex39 (Fig. 3b). For3p40, a member of the formin family (like Bni1p in budding yeast; see above), localizes to cell ‘ends’ and cortical actin patches and is required for actin cable assembly and organization of actin patches. In for3Δ mutants, most daughter cells grow at both ‘old’ and ‘new’ ends simultaneously, indicating that, as in budding yeast, localization of a formin (For3p) at cell ‘ends’ could be part of a pathway that interprets and builds upon ‘landmarks’ for polarized membrane growth41.
Microtubules, like actin cables, are organized in the long axis of the cell and are uniformly polarized with their plus ends at the cell ends and minus ends around the nucleus. Disruption of microtubules causes cells to bend and branch abnormally as they grow37, suggesting that they monitor the progress of cell growth. Microtubules have a 4–6 minute cycle of rapid polymerization towards a cell end, followed by catastrophic depolymerization ~100 seconds after contacting the end41,42. Several proteins seem to control this cycle (Fig. 3b). Tip1p42 and Mal3p43 bind to the plus ends of growing, but not shrinking microtubules, and may control microtubule length and dynamics.
How are polarized membrane growth and actin/microtubule cytoskeletons linked structurally and functionally? A candidate is the kelch repeat protein Tea1p, which localizes to microtubule plus ends and is deposited at cell ends when microtubules start to shrink44. Tea1p is in a complex with Bud6p38, which localizes to cell ends coincident with first ‘old’, and then ‘new’, end growth. Tea1p, Bud6p and perhaps For3p may provide a functional link between actin and microtubule cytoskeletons, and actin assembly and membrane growth at cell ‘ends’ (Fig. 3b). At present, little is known about how transport vesicle delivery is polarized for membrane growth in fission yeast, although the exocyst complex functions in vesicle delivery to cell ends and at the site of septation45 (Fig. 3b).
Baseline principles for cell polarity
Core mechanisms for actin polymerization and vesicle transport are adapted for polarized membrane growth around a landmark at the plasma membrane. The landmark is recognized and reinforced by localized assembly of a signalling complex of small GTPases (for example, Bud1p/Rsr1p, Cdc42p and Rho), which relays signals to modular protein complexes that concentrate machinery for actin cytoskeleton assembly (Arp2/3, WASP/WIP, type-I myosins and profilin), anchoring/nucleation of actin cables (formin proteins, including Bni1p and Bnr1p/For3p, and Bud6p), microtubule assembly/capture (Kar9p/Bim1p and Tea1p/Tip1p/Mal3p) and vesicle delivery (type-V myosins, Sec4p, SNAREs and exocyst). Assembly of this hierarchy of complexes links landmarks to local assembly and orientation of the cytoskeleton, vesicle delivery for membrane growth, spindle orientation and inheritance of landmarks by daughter cells.
Generation of cell polarity in a multi-cell epithelium
So far, I have considered cell polarity in the context of single cells preparing to divide. Do the same principles of cell polarity that emerged from studies of single cells apply to polarized epithelial cells in multi-cell organisms? Clearly, there is increased complexity in multicellular tissues, as more than one membrane domain is formed (Fig. 4a), often concurrently, and the cell must use additional mechanisms to initially distinguish these domains, target and organize different proteins in each domain and, ultimately, keep the identities of the domains separate. I focus here on two control points. First, the apical junctional complex, a structure conserved between Caenorhabditis elegans, Drosophila and vertebrates (Fig. 4a), which initiates cell–cell adhesion and regulates the identities of different membrane domains. Second, mechanisms involved in sorting and targeting proteins to different membrane domains. As examples, I will examine formation of polarized epithelia during de novo assembly of plasma membrane domains in the Drosophila blastoderm and cultured mammalian epithelial cells.
Figure 4.
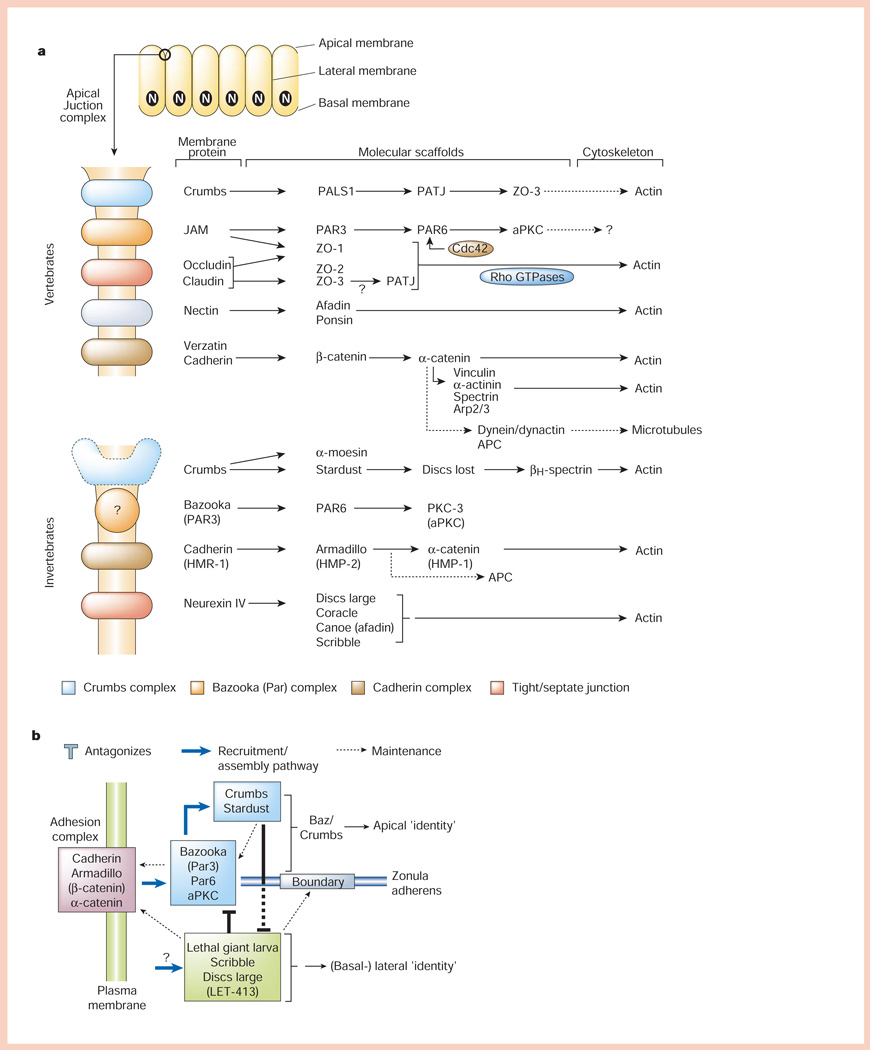
Organization of polarized epithelial cells and the apical junctional complex. a, Polarized epithelial cells form a monolayer in which the apical (unbounded surface) is separated at the boundary with the basal and lateral membranes (bounded surfaces) by the apical junctional complex (top). The main part of the panel shows molecular organization of the apical junctional complex. In vertebrates, the apical junctional complex is separated into structurally and functionally different sub-domains comprising membrane proteins (Crumbs, JAM (junctional adhesion molecule), nectin, occludin/claudin and cadherin) linked to modular protein scaffolds, which in turn bind mostly to the actin cytoskeleton, although links to microtubules are possible. In invertebrates (C. elegans and Drosophila), the apical junctional complex is similarly organized, except that the ‘tight junction’ function is provided by the septate junction localized below the cadherin (adherens) junction. b, Simplified scheme for how different protein complexes in the apical junctional complex regulate cell–cell adhesion (cadherin complex), and apical membrane (Bazooka and Crumbs complexes) and lateral membrane (Lethal giant larvae, Scribble and Disc large complex) identity. For details see text.
Extracellular contacts between epithelial cells define the bounded (basolateral) and free (apical) cell surface46. Although full development of apicobasal polarity requires organization cues from both cell–cell and cell–extracellular matrix adhesions47,48, assembly of the apical junctional complex, at the boundary between apical and (basal-)lateral membranes (Fig. 4a), recognizes and reinforces the principal landmark formed by cadherin-mediated cell–cell contacts. The apical junctional complex is a multifunctional, modular structure containing protein sub-complexes located at the boundary between the apical and lateral membrane domains49 (Fig. 4a). In general, each protein sub-complex comprises an integral membrane protein bound to scaffold protein modules, each of which has multiple protein–protein binding motifs that potentially could inter-link different membrane protein sub-complexes. Scaffold proteins generally bind to the actin cytoskeleton50–52, which is regulated by Rho-family small GTPases53, although links to microtubules may also be present54; some proteins at the apical junctional complex also act as transcriptional (co-)activators of gene expression55.
Genetic studies in Drosophila and C. elegans have identified classes of proteins in the apical junctional complex that regulate development of epithelial cell polarity56: cadherin/catenins57,58, Crumbs (Crb)/Stardust59, Bazooka (Baz/Par3)/Par6/atypical protein kinase C (aPKC)57,60,61 and Lethal giant larva (Lgl)62/Scribble (Scrib)63/Discs large (Dlg)64,65/LET-41366 (Fig. 4b). Recent studies show that the proteins in each class function in a common pathway, and the complexes seem to be integrated into a regulatory pathway that instructs apical and lateral membrane formation67,68. Cell–cell adhesion mediated by the cadherin/catenin complex57,58,67,68 is required to initiate these events, as maternal and zygotic deletion of one of the catenins, armadillo, in Drosophila inhibits formation of all cellular structures including the first epithelium58; note, however, the other complexes are required subsequently to maintain function and integrity of cadherin junctions67,68.
The Baz complex is recruited to cadherin junctions57,67,68 and initiates formation of the apical membrane. Spread of apical membrane identity down the lateral membrane is impeded by activity of the Lgl/Scrib complex60,67,68, which is recruited to a position below the Baz complex/cadherin junction60,67,68. The Lgl/Scrib complex seems to antagonize functions of the Baz and, later, Crb complexes, thereby maintaining lateral membrane identity67,68 below the adherens junction. The Crb complex is recruited apically to the Baz complex/cadherin junction57,59 and seems to further antagonize activity of the Lgl/Scrib complex by blocking the spread of lateral membrane, thereby maintaining apical membrane identity67,68 above the adherens junction (Fig. 4b).
Although these studies identify overarching roles for these different complexes in initiating polarity of the apical and lateral membrane domains, the underlying biochemical mechanisms remain unclear. It is noteworthy that many of the proteins involved contain protein-binding motifs, PDZ domains (for example, Baz (Par3), Par6, Scrib, LET-413 and Discs large)56 and leucine-rich domains (Scrib and LET-413)68 that act as scaffolds to bind and spatially order proteins in the apical junctional complex69–71. The Baz complex contains an aPKC60,72. In mammalian epithelia, dominant negative mutants of aPKC cause mislocalization of Par3 (Baz) and ZO-1 (a tight-junction protein), resulting in disruption of tight and adherens junctions, and defects in apical/basolateral polarity73. The Baz complex is also regulated by Rho-family GTPases that activate aPKC74,75. In addition, Lgl binds to the t-SNARE syntaxin-4 and the complex localizes to the lateral membrane76, but a role of Lgl in syntaxin-4 function has not been tested in detail. An attractive possibility is that complexes determining apical (Baz and Crb) and lateral (Lgl/Scrib) membrane domain identity may regulate delivery of transport vesicles containing apical and (basal-)lateral proteins, respectively, thereby contributing to the generation and maintenance of membrane domain identity.
Although specific protein complexes associated with the apical junctional complex control the identity of apical and basolateral membrane domains, how do cells determine the distribution of different proteins and functions in those domains? The generation of epithelial cell polarity during cellularization in the Drosophila embryo and in cultured mammalian cells provide mechanistic insights.
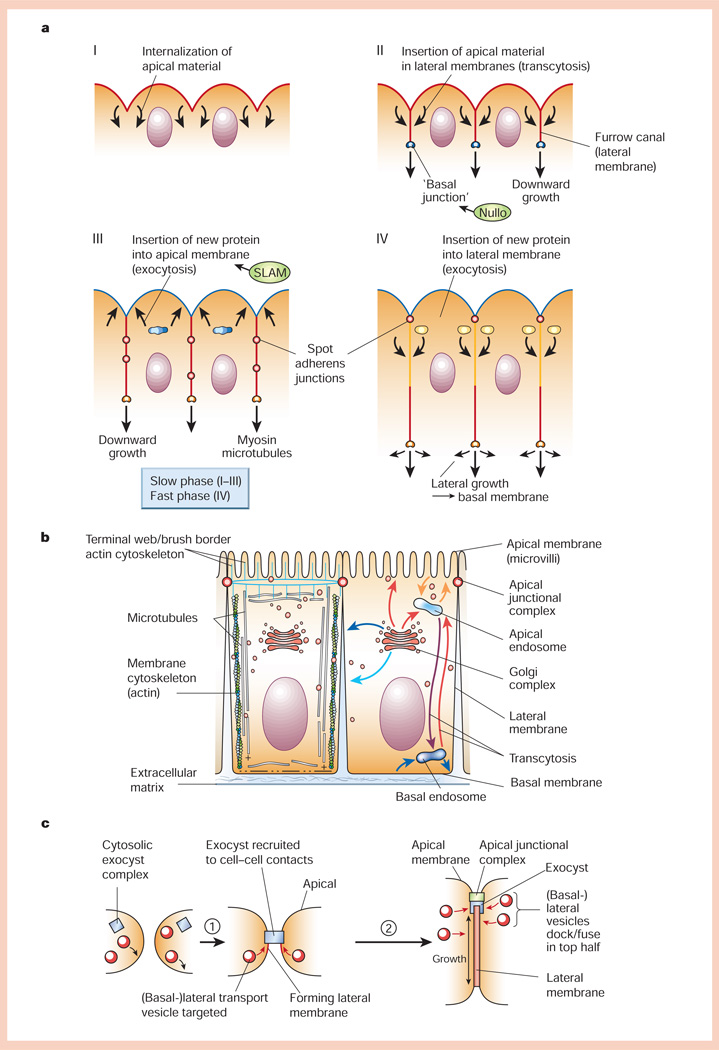
The first 13 cell divisions of the Drosophila embryo generate a syncytial blastoderm comprising ~6,000 nuclei that are localized beneath the plasma membrane. During cellularization, the surface area of the plasma membrane increases >25-fold as it forms between each nucleus to yield a sheet of columnar, polarized epithelial cells that are ~30 µm tall77 (Fig. 5a). Cellularization occurs in two phases, ‘slow’ and ‘fast’78. In the slow phase, lasting 35–40 minutes and accounting for ~10 µm of lateral membrane, growth occurs by rapid invagination of the apical surface and redistribution of internalized protein to the lateral membrane (furrow canal)78, perhaps by transcytosis. This phase of membrane growth is controlled by the product of the slam gene79, which coordinates localized membrane insertion and membrane growth. The growing end of the furrow canal is marked by a small cluster of cadherin/catenin complexes that form a ‘basal’ junction that is organized by the product of the nullo gene80; this junction also contains myosin-II and Discs lost80. Loss of microtubules also disrupts cellularization80. Thus, the force of membrane growth may be generated by insertion of membrane78, with actin–myosin contraction81 and microtubules guiding and stabilizing the lateral membrane as it grows downwards78.
Figure 5.
Generation of cell polarity in epithelia. a, Formation of the cellular blastoderm in early Drosophila embryogenesis (for details, see text). b, Schematic representation of polarized epithelial cells. Left, organization of the actin and microtubule cytoskeletons; right, organization of vesicle transport pathways to different plasma membrane domains either directly from the Golgi complex, or indirectly via apical or basal endosomes through endocytic or transcytotic pathways. c, Generation of the lateral membrane domain in cultured epithelial cells. Prior to cadherin-mediated cell–cell adhesion, the exocyst is cytosolic and vesicles fuse with the basal membrane. Upon cell–cell adhesion (step 1), the exocyst is recruited to cell–cell contacts and vesicles fuse with the forming lateral membrane. As more vesicles fuse, the lateral membrane increases in area around sixfold. Later (step 2), the exocyst and vesicle delivery are located in the apical region of the lateral membrane.
Towards the end of the slow phase of growth, the depleted apical membrane is replenished directly from intracellular pools in the secretory pathway78, and cadherin/catenin complexes begin to cluster towards a more apical aspect of the lateral membrane to form spot adherens junctions. During the subsequent fast phase of membrane growth, lasting 15–20 minutes and accounting for the remaining ~20 µm of membrane growth, new membrane is inserted into the more apical portion of the lateral membrane by direct transport of vesicles from the secretory pathway56,78 (Fig. 5a).
How are vesicles targeted to designated sites on different membrane domains, and how are proteins pre-sorted in the Golgi complex prior to their delivery in vesicles to the plasma membrane? Studies of cell polarity in cultured mammalian epithelial cells have provided some answers to these questions, as they can be used for detailed biochemical and cell biological analyses of protein sorting pathways46.
Most cells use endocytic pathways to route proteins between different domains of the plasma membrane and intracellular organelles82. In polarized epithelial cells, these pathways are coupled to sorting of proteins between different plasma membrane domains (termed transcytosis) (Fig. 5b). Transcytosis can be specific for transporting subsets of proteins between the apical and basolateral membranes (for example, ligand–receptor complexes83) or general for many classes of proteins78. In hepatocytes, for example, all membrane proteins are delivered from the Golgi to the basolateral membrane, and then apical proteins are specifically internalized and resorted by transcytosis to the apical membrane domain84.
In many epithelial cells, protein distributions in different membrane domains are also determined by direct sorting of apical and basolateral proteins in the trans-Golgi network (TGN) and subsequent targeting to the correct membrane domain85 (Fig. 5b). Sorting of proteins into the apical pathway seems to involve clustering membrane proteins into lipid rafts, and a glycosylphosphoinositide (GPI) domain in one class of proteins is sufficient to direct sorting into the apical pathway in most cells86. Additional studies in budding yeast also suggest that lipid rafts are important in protein sorting87. Sorting of proteins into the basolateral pathway requires sorting signals in the cytoplasmic domain and may be mediated by clustering through adaptor proteins and clathrin coats in the TGN or endosomes88,89. Note that protein sorting in the TGN seems to be a constitutive process that occurs in cells such as fibroblasts that have neither a defined apical or basolateral membrane domain, nor cadherin-mediated cell–cell adhesion90. Therefore, after cell adhesion, re-organization of the cytoskeleton and the generation of targeting patches for directed delivery of vesicles from the Golgi complex are critical from post-Golgi sorting of proteins to the correct membrane domain46.
Organization of the cytoskeleton is important in regulating epithelial polarity (Fig. 5b). The actin cytoskeleton localizes to the cell cortex of each membrane domain, and actin re-organization occurs in a bundle circumscribing the cell at the apical junctional complex. This reorganization depends, in part, on linkage of actin to components of the apical junctional complex, and regulation of actin polymerization by Rho-family small GTPases53, Arp2/3 and additional proteins91. Lateral membrane scaffolds containing actin-binding proteins regulate the distribution of some membrane proteins. For example, Na+,K+-ATPase is localized to the lateral membrane in mammalian and Drosophila epithelial cells through linkage to ankyrin and the spectrin scaffold92,93. The spectrin scaffold is recruited to cell–cell contacts by binding to the actin cytoskeleton that links to (cadherin) sub-complexes of the apical junctional complex46.
Microtubules are organized into bundles aligned in the apicobasal axis of the cell, with plus ends at the basal pole and intertwined mats of short filaments underneath the apical membrane and on the basal membrane46 (Fig. 5b). How microtubules adopt these distributions is not understood. Microtubules may also be attached to the apical junctional complex54, which may be important in orienting the mitotic spindle (this must be perpendicular to the apicobasal axis of polarity to maintain the monolayer organization of the epithelium). Actin may be important locally in vesicle delivery at the membrane, while microtubules seem to provide long-range pathways for vesicle delivery to plasma membrane domains, especially in transcytosis, and roles for both microtubule minus-end (dynein) and plus-end (kinesin family) motors has been implicated in vesicle targeting94,95.
Specification of vesicle docking/fusion with different plasma membrane domains involves the SNARE complex, in which binding between cognate vesicle v-SNAREs and target membrane t-SNAREs specifies vesicle delivery. In polarized epithelial cells, different t-SNAREs are localized to apical (syntaxin-3) and basolateral (syntaxin-4) membranes96 and inhibiting their function blocks vesicle delivery. Little is known about how t-SNAREs become localized to different membrane domains. Direct analysis of vesicle delivery to the basal and lateral membranes during polarization of Madin–Darby canine kidney (MDCK) cells revealed that, before cell–cell adhesion, vesicles containing either apical or basolateral membrane proteins fused with the basal surface97. But after induction of cadherin-mediated cell–cell adhesion, neither apical nor basolateral vesicles fused with the basal surface; observations of vesicle fusion in the apical membrane was technically impossible, but basolateral vesicles were found to fuse at the top half of the lateral membrane97. Thus, cell–cell adhesion may act as a signal for polarizing either vesicle docking/fusion machinery or vesicle/cytoskeleton transport pathways to the apical and lateral membranes (Fig. 5c). Interestingly, maintenance of syntaxin-3 depends on microtubules, and disruption of microtubules causes synaxin-3 to mislocalize to lateral membrane, with the consequence that apical vesicles fuse with the lateral membrane; no affect on syntaxin-4 distribution was observed97.
Like yeast, polarized epithelial cells express the exocyst complex (also referred to as the Sec6/8 complex)98. In polarized MDCK cells, the exocyst is localized to the apical aspect of the lateral membrane98, in the general region of basolateral vesicle docking/fusion97. Inhibition of exocyst function inhibits delivery of vesicles containing a basolateral protein to the plasma membrane, but delivery of apical vesicles is unaffected98. Cdc42 also has a role in delivery of basolateral, but not apical, transport vesicles99. The polarized distribution of the exocyst complex depends on the maintenance of cell–cell contacts, and cadherin-mediated cell–cell adhesion recruits the exocyst specifically to sites of cell–cell contact98. Similar to membrane growth during cellularization in the Drosophila embryo, polarization of MDCK cells results in a sixfold increase in the surface area of the lateral membrane, whereas those of apical and basal membrane domains do not increase100 (Fig. 5c).
Conclusions
Cell polarity is portrayed in a wide variety of shapes and functions in single-cell organisms and cells in multi-cell tissues. Each cell type has not evolved a different mechanism to generate polarity, but has instead adapted a basic set of evolutionarily conserved core mechanisms, including: localized assembly of signalling complexes, cytoskeleton assembly and recruitment, mobilization of proteins from intracellular pools, and targeted vesicle delivery to sites of membrane growth. Polarized cells generate asymmetry around a cell-surface landmark by localized assembly of modular protein scaffolds that direct assembly and orientation of the cytoskeleton and specify vesicle delivery for membrane growth at that site.
Several principles of cell polarity can be deduced from these diverse examples. The pathway is hierarchical. It is initiated by a cell-surface landmark or spatial cue (the point of cytokinesis in budding and fission yeast, and cell(–cell) adhesion in epithelial cells from worms, flies and mammals), and defines a point on the cell surface to which the cell orients (mother–daughter axis in yeast, and the apicobasal axis in epithelial cells). This axis of polarity is propagated from the landmark throughout the cell by adaptation of core mechanisms that assemble and orient actin and microtubule cytoskeletons around the landmark.
Cytoskeleton reorganization around the landmark is determined by the local assembly at the landmark of signalling complexes of Rho-family small GTPases (Rho, Cdc42 and Rac1), which concentrate machinery for localized assembly of actin (profilin, Arp2/3 complex and type-I myosins), and modular protein scaffolds (the polarisome and Bni1p/Bud6p, and For3p/Bud6p in budding and fission yeast, respectively, and the apical junctional complex comprising Crb, Baz and Lgl/Scrib complexes in epithelia). Actin polymerization and assembly of protein scaffolds also reinforce the organization, retention and inheritance of the landmark. Actin cytoskeleton orientation around the landmark may regulate capture/reorientation of microtubules (involving complexes of Kar9p, Tip1p/CLIP170, Bim1p/Mal1p/EB1 and APC) for spindle orientation in the axis of cell polarity (in budding yeast, Drosophila neuroblasts and epithelia) and for vesicle delivery to different membrane domains (in transcytotic and exocytic vesicle transport in epithelia).
The efficiency and fidelity of vesicle delivery to the landmark depends on cytoskeleton assembly and orientation, and recruitment to the landmark of vesicle docking/fusion machinery (small GTPases such as Sec4p and RalA, SNARE protein complexes and the exocyst); in epithelia, the machinery is separated to the apical and lateral membrane domains. Localized vesicle delivery results in rapid plasma membrane growth around the landmark. The identity of the new membrane domain is maintained by the subset of (pre-sorted) proteins that had been delivered there in vesicles either directly from the Golgi complex or by transcytosis, and by retention through membrane scaffolds. Assembly of this hierarchy of complexes links landmarks to local assembly and orientation of the cytoskeleton, vesicle delivery for membrane growth, spindle orientation, and the inheritance of landmarks by daughter cells.
Acknowledgements
This review is dedicated to I. Herskowitz (University of California, San Francisco) who first inspired me to think broadly about cell polarity.
References
- 1.Chant J. Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 1999;15:365–391. doi: 10.1146/annurev.cellbio.15.1.365. [DOI] [PubMed] [Google Scholar]
- 2.Chant J, Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991;65:1203–1212. doi: 10.1016/0092-8674(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 3.Zahner JE, Harkins HA, Pringle JR. Genetic analysis of the bipolar pattern of bud site selection in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 1996;16:1857–1870. doi: 10.1128/mcb.16.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl Acad. Sci. USA. 1989;89:9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pruyne D, Bretscher A. Polarization of cell growth in yeast I. Establishment and maintenance of polarity states. J. Cell Sci. 2000;113:365–375. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- 6.Adams A, Johnson D, Longnecker R, Sloat B, Pringle J. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etienne-Mannevile S, Hall A. Rho GTPases in cell biology. Nature. 2003;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 8.Kang PJ, Sanson A, Lee B, Park H-O. A GDP/GTP exchange factor involved in linking a spatial landmark to cell polarity. Science. 2001;292:1376–1378. doi: 10.1126/science.1060360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marston AL, Chen T, Yang MC, Belhumeur P, Chant J. A localized GTPase exchange factor, Bud5, determines the orientation of division axes in yeast. Curr. Biol. 2001;11:803–807. doi: 10.1016/s0960-9822(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 10.Park H-O, Sanson A, Herskowitz I. Localization of Bud2p, a GTPase-activating protein necessary for programming cell polarity in yeast to the presumptive bud site. Genes Dev. 1999;13:1912–1917. doi: 10.1101/gad.13.15.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Bender A, Cerione R. Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J. Biol. Chem. 1995;270:626–630. doi: 10.1074/jbc.270.2.626. [DOI] [PubMed] [Google Scholar]
- 12.Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- 13.Pruyne D, Bretscher A. Polarization of cell growth in yeast II. The role of the actin cytoskeleton. J. Cell Sci. 2000;113:571–585. doi: 10.1242/jcs.113.4.571. [DOI] [PubMed] [Google Scholar]
- 14.Eby J, et al. Actin cytoskeleton organization regulated by the PAK family of protein kinases. Curr. Biol. 1998;8:967–970. doi: 10.1016/s0960-9822(98)00398-4. [DOI] [PubMed] [Google Scholar]
- 15.Wu C, Lytvyn V, Thomas D, Leberer E. The phosphorylation site for Ste20p-like protein kinase is essential for the function of myosin-I in yeast. J. Biol. Chem. 1997;272:30623–30626. doi: 10.1074/jbc.272.49.30623. [DOI] [PubMed] [Google Scholar]
- 16.Evangelista M, et al. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 2000;148:353–362. doi: 10.1083/jcb.148.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WL, Bezanilli M, Pollard TD. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J. Cell Biol. 2000;151:789–800. doi: 10.1083/jcb.151.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechler T, Jonsdottir GA, Klee SK, Pellman D, Li R. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. J. Cell Biol. 2001;155:261–270. doi: 10.1083/jcb.200104094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheu YP, Santos B, Fortin N, Costigan C, Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol. Biol. Cell. 1998;18:4035–4069. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evangelista M, et al. Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–121. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- 21.Sagot I, Klee SK, Pellman D. Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nature Cell Biol. 2002;4:42–50. doi: 10.1038/ncb719. [DOI] [PubMed] [Google Scholar]
- 22.Evangelista M, Pruyne D, Amberg DC, Boone C, Bretscher A. Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nature Cell Biol. 2002;4:32–41. doi: 10.1038/ncb718. [DOI] [PubMed] [Google Scholar]
- 23.Imamura H, et al. Bni1p and Bnr1p: downstream targets of the Rho family of small GTPases which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 1997;16:2745–2755. doi: 10.1093/emboj/16.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amberg DC, Zahner JE, Mulholland JW, Pringle JR, Botstein D. Aip3p/Bud6p, a yeast actin-binding protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol. Biol. Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohno H, et al. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 1996;15:6060–6068. [PMC free article] [PubMed] [Google Scholar]
- 26.Sagot I, Rodal AA, Moseley J, Goode BL, Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nature Cell Biol. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- 27.Pruyne D, et al. Role of formins in actin assembly: nucleation and barbed-end association. Science. 2002;297:612–615. doi: 10.1126/science.1072309. [DOI] [PubMed] [Google Scholar]
- 28.Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liakopoulos D, Kusch J, Grava S, Vogel J, Barral Y. Asymmetric loading of Kar9 onto spindle poles and microtubules ensures proper spindle alignment. Cell. 2003;112:561–574. doi: 10.1016/s0092-8674(03)00119-3. [DOI] [PubMed] [Google Scholar]
- 30.Brennwald P, et al. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. doi: 10.1016/0092-8674(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 31.Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karpova TS, et al. Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell. 2000;11:1727–1737. doi: 10.1091/mbc.11.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.TerBush DR, Maurice T, Roth D, Novick P. The exocyst is a multi-protein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 34.Novick P, Guo W. Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 2002;12:247–249. doi: 10.1016/s0962-8924(02)02293-6. [DOI] [PubMed] [Google Scholar]
- 35.Lehman K, Rossi G, Adamo JE, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang F. Establishment of a cellular axis in fission yeast. Trends Genet. 2001;17:273–278. doi: 10.1016/s0168-9525(01)02279-x. [DOI] [PubMed] [Google Scholar]
- 37.Sawin KE, Hajibagheri MA, Nurse P. Mis-specification of cortical identity in a fission yeast PAK mutant. Curr. Biol. 1999;9:1335–1338. doi: 10.1016/s0960-9822(00)80058-5. [DOI] [PubMed] [Google Scholar]
- 38.Glynn J, Lustig R, Berlin A, Chang F. Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast. Curr. Biol. 2001;11:836–845. doi: 10.1016/s0960-9822(01)00235-4. [DOI] [PubMed] [Google Scholar]
- 39.Pelham RJ, Chang F. Role of actin polymerization and actin cables in the movement of actin patches in S. pombe. Nature Cell Biol. 2001;3:235–244. doi: 10.1038/35060020. [DOI] [PubMed] [Google Scholar]
- 40.Feierbach B, Chang F. Role of the fission yeast formin for3p in cell polarity, actin cable formation, and symmetric cell division. Curr. Biol. 2001;11:1656–1665. doi: 10.1016/s0960-9822(01)00525-5. [DOI] [PubMed] [Google Scholar]
- 41.Tran PT, Marsh L, Doyle V, Inoue S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brunner D, Nurse P. CLIP170-like Tip1p spatially organizes microtubular dynamics in fission yeast. Cell. 2000;102:695–704. doi: 10.1016/s0092-8674(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 43.Beinhauer JD, Hagan IM, Hegeman JH, Feig U. Mal3, the fission yeast homologue of the human APC-interacting protein EB1 is required for microtubule integrity and the maintenance of cell form. J. Cell Biol. 1997;139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, et al. The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe. Mol. Biol. Cell. 2002;13:515–529. doi: 10.1091/mbc.01-11-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol. Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- 47.Wang AZ, Ojakian GK, Nelson WJ. Steps in the morphogenesis of a polarized epithelium. I. Uncoupling the roles of cell-cell and cell-substratum contact in establishing plasma membrane polarity in multicellular epithelial (MDCK) cysts. J. Cell Sci. 1990;95:137–151. doi: 10.1242/jcs.95.1.137. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien LE, Zegers MM, Mostov KE. Building epithelial architecture: insights from three-dimensional culture models. Nature Rev. Mol. Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- 49.Knust E, Bossinger O. Epithelial polarity: composition and formation of intercellular junctions in different organisms. Science. 2003;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- 50.Dimitratos SD, Woods DF, Stathakis DG, Bryant PJ. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. BioEssays. 1999;21:912–921. doi: 10.1002/(SICI)1521-1878(199911)21:11<912::AID-BIES3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 51.Mohler PJ, Gramolini AO, Bennettt V. Ankyrins. J. Cell Sci. 2002;115:1565–1566. doi: 10.1242/jcs.115.8.1565. [DOI] [PubMed] [Google Scholar]
- 52.Tsukita S, Furuse M, Itoh M. Structural and signaling molecules come together at tight junctions. Curr. Opin. Cell Biol. 1999;11:628–633. doi: 10.1016/s0955-0674(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 53.Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nature Rev. Mol. Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- 54.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to β-catenin and may tether microtubules at adherens junctions. Nature Cell Biol. 2001;3:913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 55.Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu. Rev. Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 57.Muller HAJ, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophila embryogenesis. J. Cell Biol. 1996;134:133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tepass U, Knust E. crumbs and stardust act in a genetic pathway that controls the organization of epithelia in Drosophila melanogaster. Dev. Biol. 1993;159:311–326. doi: 10.1006/dbio.1993.1243. [DOI] [PubMed] [Google Scholar]
- 60.Wodarz A, Ramath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petronczki M, Knoblich J. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nature Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- 62.Bilder D, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 63.Bilder D, Li M, Perrimon N. Localization of apical determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 64.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized to septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- 65.Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1 the homologue of the Drosophila gene discs large. Dev. Biol. 2001;230:29–42. doi: 10.1006/dbio.2000.0113. [DOI] [PubMed] [Google Scholar]
- 66.Legouis R, et al. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nature Cell Biol. 2000;2:415–422. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- 67.Tanentzapf G, Tepass U. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nature Cell Biol. 2003;5:46–52. doi: 10.1038/ncb896. [DOI] [PubMed] [Google Scholar]
- 68.Bilder D, Schober M, Perrimon N. Integrating activity of PDZ protein complexes regulates epithelial polarity. Nature Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- 69.Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nature Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 70.Medina E, et al. Crumbs interacts with moesin and βHeavy-spectrin in the apical membrane skeleton of Drosophila. J. Cell Biol. 2002;158:941–951. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr. Biol. 2002;12:221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- 72.Izumi Y, et al. An atypical PKC directly associates and colocalizes at the epithelial junction with ASIP, a mammalian homologue of the Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 1998;143:95–103. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzuki A, et al. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J. Cell Sci. 2002;115:3565–3573. doi: 10.1242/jcs.00032. [DOI] [PubMed] [Google Scholar]
- 74.Joberty G, Petersen C, Gao L, Macara IG. A cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nature Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 75.Lin D, et al. A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signaling and cell polarity. Nature Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 76.Müsch A, et al. A mammalian homologue of the Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in MDCK cells. Mol. Biol. Cell. 2002;13:158–168. doi: 10.1091/mbc.01-10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Foe VE, Odell GM, Edgar BA. In: The Development of Drosophila melanogaster. Bate M, editor. Vol. 1. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 149–300. [Google Scholar]
- 78.Lecuit T, Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J. Cell Biol. 2000;150:849–860. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lecuit T, Samata R, Wieschaus E. slam encodes a developmental regulator of polarized membrane growth during cleavage of the Drosophila embryo. Dev. Cell. 2002;2:425–436. doi: 10.1016/s1534-5807(02)00141-7. [DOI] [PubMed] [Google Scholar]
- 80.Hunter C, Wieschaus E. Regulated expression of nullo is required for the formation of distinct apical and basal adherens junctions in the Drosophila blastoderm. J. Cell Biol. 2000;150:391–401. doi: 10.1083/jcb.150.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schejter ED, Wieschaus E. Bottleneck acts as a regulator of the microfilament network governing cellularization of the Drosophila embryo. Cell. 1993;75:373–385. doi: 10.1016/0092-8674(93)80078-s. [DOI] [PubMed] [Google Scholar]
- 82.Pfeffer S. Membrane domains in the secretory and endocytic pathways. Cell. 2003;112:507–517. doi: 10.1016/s0092-8674(03)00118-1. [DOI] [PubMed] [Google Scholar]
- 83.Mostov KE, Deitcher DL. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986;46:613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- 84.Bartles JR, Feracci HM, Stieger B, Hubbard AL. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J. Cell Biol. 1987;105:1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 86.Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. A glycosphingolipid membrane anchor acts as an apical targeting signal in polarized epithelial cell. J. Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bagnat M, Chang A, Simons K. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell. 2001;12:4129–4138. doi: 10.1091/mbc.12.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hunziker W, Harter C, Matter K, Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991;66:907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- 89.Areoti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J. Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J. Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 92.Nelson WJ, Veshnock PJ. Ankyrin (membrane-skeleton) binds to the Na+,K+-ATPase: implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- 93.Dubreuil RR, Wang P, Dahl S, Lee J, Goldstein LS. Drosophila β spectrin functions independently of α spectrin to polarize the Na,K ATPase in epithelial cells. J. Cell Biol. 2000;149:647–656. doi: 10.1083/jcb.149.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lafont F, Burkhardt JK, Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 1994;372:801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- 95.Kreitzer G, Marmostein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nature Cell Biol. 2000;2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- 96.Low SH, et al. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell. 1996;7:20007–22018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kreitzer G, et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nature Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- 98.Grindstaff KK, et al. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in polarized epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 99.Cohen D, Musch A, Rodriguez-Boulan E. Selective control of basolateral membrane proteins polarity by Cdc42. Traffic. 2001;2:556–564. doi: 10.1034/j.1600-0854.2001.20805.x. [DOI] [PubMed] [Google Scholar]
- 100.Vega-Salas DE, Salas PJ, Gundersen D, Rodriguez-Boulan E. Formation of the apical pole of epithelial (Madin-Darby canine kidney) cells: polarity of an apical protein is independent of tight junctions while segregation of a basolateral marker requires cell-cell interactions. J. Cell Biol. 1987;104:905–916. doi: 10.1083/jcb.104.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]